Abstract
Purpose
To compare ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) determination of diffuse liver steatosis.
Materials and methods
Quantification of liver steatosis on ultrasound, CT, and MRI was correlated with histopathology in 67 patients.
Results
Opposed-phase MRI demonstrated the highest correlation with steatosis (0.68 and 0.69, P<.01; intraclass correlation coefficient, 0.93). Spearman’s correlation (and intraclass correlation) coefficients were lowest for ultrasound [0.54, 0.33 (0.40)] and enhanced CT [0.33, 0.39 (0.97)].
Conclusion
Opposed-phase MRI demonstrated best overall performance for determining steatosis.
Keywords: Liver, steatosis, Ultrasound (US), Computed tomography (CT), Magnetic resonance imaging (MRI)
1. Introduction
Although diffuse liver fat or steatosis was previously considered to be a relatively benign and self-limiting entity, it is now known to commonly progress through necroinflammatory changes (known as nonalcoholic steatohepatitis or NASH) to cirrhosis. In the United States, an estimated 40 million adults are thought to have nonalcoholic fatty liver disease which is characterized by liver steatosis and is strongly associated with obesity [1–3]. Furthermore, it is thought that progression of steatosis to NASH and beyond is the primary cause of cryptogenic cirrhosis, which is the third most common indication for liver transplantation [2]. Liver steatosis is also associated with chronic viral hepatitis (especially hepatitis C), drug hepatotoxicity (e.g., antiretroviral therapy, lipid-lowering drugs), and alcohol excess [4–11]. It has been suggested that the presence of steatosis in many patients with Genotype 1 hepatitis C-related liver disease, which predominates in the United States, may be attributable to the coexistence of nonalcoholic fatty liver disease. Such coexistence of hepatitis C infection and nonalcoholic fatty liver disease has several important prognostic implications, including a predisposition to more aggressive liver fibrosis, reduced response rate to antiviral therapy, and possibly an increased risk of hepatocellular carcinoma [12]. Therefore, the assessment of liver fat in patients with diffuse liver disease may help identify patients who are at greater risk for developing more advanced liver disease and those who may not respond well to pharmacologic therapies.
Percutaneous liver biopsy is the current standard means of diagnosing and grading steatosis, but it is an invasive procedure with potentially serious complications including hemorrhage, infection, bile leak, and a mortality of up to 0.3% [13]. In view of the large population of subjects affected including children, liver biopsy is not an optimal means of detecting and monitoring liver steatosis. A number of studies have reported a correlation between histopathologic liver steatosis and different cross-sectional imaging modalities [11,14,15]. Radiologists are generally familiar with the hepatic cross-sectional imaging findings of liver fat or steatosis and probably consider the diagnosis to be relatively straightforward, but the diagnostic accuracy and inter-observer variability of these observations have been poorly explored. Liver steatosis has been shown to result in increased echogenicity at ultrasound, reduced attenuation at CT, and signal intensity loss on opposed-phase and fat-saturated magnetic resonance imaging (MRI). However, to date, there has been very limited work on the comparative analysis of liver steatosis detection with these modalities [16] and the optimal modality for clinically evaluating patients with suspected liver steatosis remains unclear. Therefore, we undertook this study to compare detection of liver steatosis with ultrasound, CT, and MRI.
2. Materials and methods
2.1. Patients
This was a retrospective, single-institution study. The study was approved by our Institutional Committee on Human Research with waiver of the requirement for written consent and was compliant with the Health Insurance Portability and Accountability Act. We searched our radiology and pathology information systems to identify patients who met the following inclusion criteria:
Liver tissue obtained for histopathologic analysis either by percutaneous biopsy or by surgical resection between 1998 and 2003.
Cross-sectional imaging (ultrasound, CT, or MRI) performed within 3 months (before or after) of liver biopsy.
Absence of significant therapeutic intervention that might affect hepatic steatosis, (pharmacologic or nonpharmacologic) in the period between imaging and tissue collection.
Sixty-five patients fulfilled these criteria. The study group consisted of 38 men and 27 women with a mean age of 49 years (range, 18–74 years). The indications for liver biopsy in these patients are given in Table 1. Only nontumorous tissue was analyzed for liver fat percentage in patients who underwent biopsy or resection for a focal mass. Eighteen of the 65 patients had a pathological diagnosis of cirrhosis. Fifty-one ultrasound, 47 CT, and 32 MRI studies were reviewed. Forty-nine of the 65 patients had more than one imaging study. The number of patients who underwent ultrasound, CT, and MRI is given in Table 2.
Table 1.
Indication for liver biopsy in all patients
| Indication | All patients (N=65) |
|---|---|
| Elevated liver enzymes | 16 |
| Hepatitis C | 16 |
| Potential liver donation | 12 |
| Liver mass | 10 |
| Metastases | 3 |
| Budd–Chiari syndrome | 3 |
| Liver transplant evaluation | 3 |
| Cryptogenic cirrhosis | 1 |
| Autoimmune hepatitis | 1 |
Table 2.
Distribution of imaging modalities in the 65 patients
| Modality | Number of patients |
|---|---|
| US+CT+MR | 16 |
| US+CT | 24 |
| US+MRI | 3 |
| CT+MRI | 6 |
| US only | 8 |
| CT only | 1 |
| MRI only | 7 |
2.2. Imaging technique
2.2.1. Ultrasound
Multiple transverse and longitudinal grayscale images of the abdomen were acquired by ultrasonographers using commercially available equipment (Acuson, Sequoia, Mountain View, CA, USA) with either a 4-MHz (n=41) or an 8-MHz (n=5) vector transducer. Patients were scanned in the supine and left lateral decubitus position, utilizing subcostal and intercostal approaches. Sonograms were performed under fasting conditions. The time-gain compensation was set to adjust the tissue echogenicity as constant as possible regardless of depth.
2.2.2. Computed tomography
Imaging was performed using a multidetector helical CT (Lightspeed Qx/i; GE Medical Systems, Milwaukee, WI, USA). Thirty of the 47 patients had only contrast-enhanced CT, 14 patients had both contrast-enhanced and unenhanced CT, and 3 patients had only unenhanced CT through the abdomen. All images were reviewed with 5-mm collimation. Contrast-enhanced CT imaging was performed after intravenous administration of 150 ml of iohexol (Omnipaque 350, Nycomed Amersham, Princeton, NJ, USA) injected at a rate of 2 to 5 ml/s. The portal venous phase images were obtained after a scan delay of 70 to 80 s.
2.2.3. Magnetic resonance imaging
MRI was performed with a 1.5-T scanner (Signa; GE Medical Systems) and a four-element torso phased-array coil (GE Medical Systems). Axial T1-weighted dual-phase breath-held spoiled gradient-echo sequence were acquired with the following parameters: TR/TE=90–150/2.1 and 4.2 ms; flip angle=75°; slice thickness/gap=8/1 mm; matrix=256×128–192; field-of-view=32–40 cm; and number of excitation=1. Axial (n=10) or coronal (n=20) non–fat-saturated T2-weighted breath-hold single-shot fast spin-echo sequence was acquired with the following parameters: effective TE=100 ms; slice thickness/gap=6/1 mm; field-of-view=32–40 cm; number of acquisitions=1; matrix=256×160–192. Axial fat-saturated T2-weighted breath-hold fast spin-echo images were acquired with the following parameters: TR=4000–5000 ms, effective TE=100 ms, slice thickness=8 mm, gap=1 mm, field-of-view=32–40 cm, number of acquisitions=2–3, matrix 256×256. Fat saturation was performed with manual frequency selection.
2.3. Image interpretation and analysis
2.3.1. Ultrasound
Two independent sonologists (—, —) with at least 15 years of abdominal ultrasound experience, unaware of clinical features and pathological findings, evaluated the degree of liver steatosis on a five-point scale: 0 (absent), 1 (minimal), 2 (mild), 3 (moderate), and 4 (severe) based on liver echogenicity. The ultrasound criteria for the five-point scale system were established and agreed between the two observers prior to viewing the images. A representative sagittal sonographic plane of section demonstrating the hepatic parenchyma and the adjacent right kidney was selected to determine liver echogenecity by the radiologist. The overall assessment of liver echogenicity was based on the combination of the echogenicity of the right renal cortex, beam attenuation with standard settings, visualization of the echogenicity of the walls surrounding intrahepatic vessels, and the degree of reflectivity from the diaphragm. Normal liver echotexture was recorded in the absence of steatosis. Minimal steatosis was indicated by a slightly increased liver echogenicity in relation to the right kidney, but echogenicity of the intrahepatic vessel walls and diaphragm was well visualized. Mild steatosis was defined by a liver echogenicity moderately greater than the right kidney with slight decreased visibility of the intrahepatic vessel walls and decreased reflectivity of the hemidiaphragm. Moderate steatosis was determined by liver echogenicity moderately greater than the right kidney with poor visualization of intrahepatic vessel walls and decreased reflectivity of the hemidiaphragm. Severe steatosis was determined by a significantly increased echogenicity of the liver compared to the right kidney, a lack of visualization of intrahepatic vessel walls, and markedly decreased reflectivity of the hemidiaphragm. Focal masses within the liver were not considered in the assessment. The liver was scored by the most affected area.
2.3.2. Computed tomography
Two independent fellowship-trained radiologists with 10-and 1-year post-fellowship experience in abdominal imaging (—, —) recorded the mean Hounsfield density measurement using regions of interest placed on the liver and spleen. For each scan, three regions of interest were obtained from the liver, one in the right hepatic lobe above the portal vein, one in the right hepatic lobe below the portal vein, and one in the left lobe. Three circular regions of interest were placed within the spleen at matched levels to the liver measurements in order to obtain the density of the spleen. The mean size of the regions of interest was 50 mm2 (range, 40–60 mm2). The area of the region of interest was kept constant for all measurements within each patient. Large vessels, focal masses, and artifacts were avoided. For patients who had both unenhanced CT and contrast-enhanced CT, the regions of interest were placed approximately in the same anatomical location. The mean density of the liver and spleen was calculated from the regions of interest. The mean liver to spleen attenuation difference for each patient was obtained by subtracting the mean splenic attenuation from the mean liver attenuation.
2.3.3. Magnetic resonance imaging
Two independent fellowship trained radiologists with 10- and 1-year post-fellowship experience in abdominal imaging (—, —) recorded the signal intensity measurement of the liver and spleen on matched in-phase and out-of-phase breath-held spoiled gradient-echo MR images and non–fat-saturated vs. fat-saturated T2-weighted images. Three circular regions of interest were placed in the liver, one in the right hepatic lobe above the portal vein, one in the right hepatic lobe below the portal vein, and one in the left lobe. The mean size of the regions of interest was 50 mm2 (range, 41–76 mm2). The regions of interest were placed at anatomically matched locations on paired T1- and T2-weighted sequences. Three regions of interest were placed within the spleen at anatomically matched levels to the right lobe of the liver for each sequence (to obtain similar distance from surface coil). The mean signal intensity for each patient was calculated from the regions of interest for both the liver and the spleen on each sequence. Vessels, focal masses, and artifacts were avoided. Since there is no objective signal intensity scale for MR, the spleen was used as an internal standard for calculating the percentage of relative signal loss of the liver [17], and relative liver signal intensity loss with opposed-phase imaging was calculated using the formula:
where SI=average liver signal intensity/average spleen intensity.
Relative liver signal intensity loss on fat-saturated T2-weighted MRI was calculated using the formula:
respectively, where SI=average liver signal intensity/average spleen intensity.
2.4. Histopathologic analysis
Liver tissue was obtained for histopathologic analysis 2 to 12 weeks after imaging (mean, 8) in 65 patients and 2 weeks prior to imaging in 2 patients. Specimens were obtained with a 16-gauge core needle biopsy (n=44), operative hepatic resection (n=22), or transjugular liver biopsy (n=1), and stained with hematoxylin and eosin. All histological specimens were retrospectively reviewed by a single pathologist (—) unaware of the radiological and clinical data. Non-tumorous tissue was evaluated in all samples from patients with focal masses. Liver fat was determined as the percentage of fat-containing hepatocytes on hematoxylin and eosin staining [18] using a standard visualization technique [19]. A standard grading system employed in clinical practice was used for determining steatosis grade ranging from 0 to 3, with 0 representing <5%; 1, ≥5% to <33%; 2, ≥33% to <66%; and 3, ≥66% of hepatocytes containing fat, respectively [16].
2.5. Statistical analysis
Statistical analysis was performed on a per patient basis. Spearman’s correlation was used to compare histopathologic liver steatosis grade with ultrasound, unenhanced and enhanced CT, opposed-phase MRI, and fat-saturated T2-weighted imaging. Intraclass correlations were calculated to determine reader agreement. Statistical analysis was performed using SAS 9.1 (SAS Institute, Cary, NC, USA) and S plus 6.2 (Insightful, Inc., Seattle, WA, USA). Histopathologic liver steatosis grade was used as the standard of reference and a 5% significance level was used for analyses.
3. Results
The mean histopathologic liver steatosis grade was 1 (range, 0–3). The number of patients with grades 0, 1, 2, and 3 were 24, 26, 7, and 8, respectively. The percentage of patients with Grade 0 steatosis for each imaging technique was 34% (11/32) for both opposed-phase and T2-weighted MRI, 37% (19/51) for ultrasound, 47% (8/17) for unenhanced CT, and 37% (16/43) for enhanced CT. Opposed-phase MRI demonstrated the highest Spearman’s correlation for both readers: opposed-phase (0.68, 0.69); fat-suppressed T2-weighted MRI (0.61, 0.54); ultrasound (0.54, 0.33); unenhanced CT (0.59, 0.62); enhanced CT (0.33, 0.39) (Figs. 1–5). A significantly greater correlation was observed with opposed-phase MRI than ultrasound, and a significantly lower correlation compared to all the other modalities was seen with enhanced CT (P<.01). The correlation coefficients with respective P values for each modality and reader are given in Table 3.
Fig. 1.
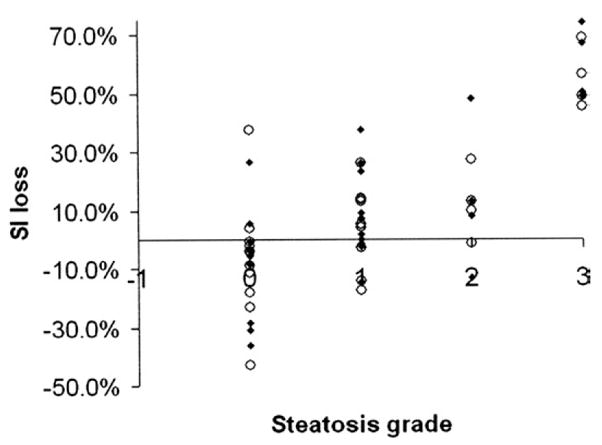
Correlation of relative liver signal intensity loss on opposed-phase imaging with liver steatosis grade (for Readers 1 and 2).
Fig. 5.
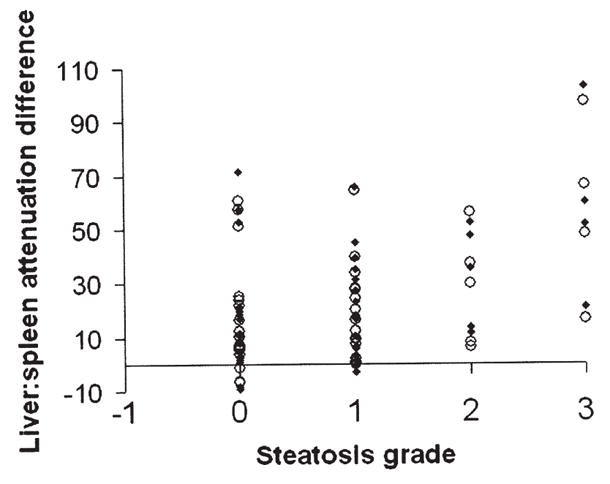
Correlation of liver: spleen attenuation difference on enhanced CT (portal venous phase) and liver steatosis grade (for Readers 1 and 2).
Table 3.
Spearman’s correlation coefficients for imaging correlation with histopathologic liver steatosis grade
| Ultrasound | Unenhanced CT | Contrast-enhanced CT | Opposed-phase MRI | Fat-saturated T2-weighted imaging | |
|---|---|---|---|---|---|
| r2 | 0.54, 0.33 | 0.59, 0.62 | 0.33, 0.39 | 0.68, 0.69 | 0.61, 0.54 |
| P value | <.01, .02 | .11, <.01 | .03, .01 | <.01, <.01 | <.01, .05 |
r2, Spearman’s correlation coefficient (Reader 1, Reader 2).
The intraclass correlation for each modality was opposed-phase MRI 0.93, fat-suppressed T2-weighted MRI 0.90, ultrasound 0.40, unenhanced CT 0.93, and enhanced CT 0.97. The intraclass correlation for ultrasound was significantly lower than that for other modalities (P<.05).
4. Discussion
The ability to reliably determine liver steatosis with imaging has been controversial [11,14,16,20–22]. According to Saadeh et al. [16], ultrasound, CT, and MRI have a low sensitivity for diagnosing liver fat and are only reliable when detecting moderate to high grades of steatosis (Grade 2 or higher). Levenson et al. [14] reported a correlation coefficient of 0.86 between opposed-phase MRI and liver steatosis in a small group of 16 patients. Other investigators reported correlation coefficients between opposed-phase MRI and liver steatosis of 0.9 (n=11) and 0.7 (n=13) in patients without cirrhosis or liver iron deposition, respectively [17,23]. A further recent study reported a correlation coefficient of 0.85 in 57 potential liver donors [24]. In this study, we found a good correlation (0.68, 0.69; P<.05) between opposed-phase MRI and histopathologic liver steatosis despite a low mean subject steatosis grade. The difference in correlation coefficients in our study compared to prior studies may reflect differences in patient populations [14,16,24]. A further explanation may be related to differences in histopathologic steatosis grading technique; one could hypothesize that the main problem may not lie with a particular imaging modality, but rather with the descriptors used for steatosis [20,21] and the qualitative nature of histopathologic steatosis grading.
A moderate to good correlation was observed with fat-saturated T2-weighted imaging (0.61, 0.54), which is concordant with prior observations [17]. However, we found a greater interobserver variability in liver fat estimation with fat-saturated T2-weighted sequences compared to opposed-phase imaging, which may be attributable to inhomogeneity in fat saturation techniques.
Joseph et al. [22] reported a high sensitivity (89%) and specificity (93%) for the imaging quantification of liver fat using ultrasound. We only observed a moderately good correlation between ultrasound and histopathologic liver steatosis grade for one of the two readers (0.54, 0.33). A possible explanation for the difference in ultrasound findings in our study compared to Joseph et al. [22] is the inherent limitation of the subjective nature of ultrasound scoring compared to the measurements made with CT and MRI, which is reflected in the relatively poor interobserver agreement we found in this study (0.40).
Limanond et al. [11] reported a correlation coefficient of 0.92 between unenhanced CT and liver biopsy estimation of liver steatosis in 27 liver donors. Park et al. [25] reported an 82% sensitivity for detection of greater than 30% steatosis on unenhanced CT in 154 potential liver donors. Unenhanced CT only correlated significantly with histopathology for one reader in our study, even though unenhanced CT is commonly considered to be a reliable modality for detecting liver fat [11,26]. The discrepancy with our study may be a reflection of the small number of subjects (n=17) who underwent unenhanced CT and also the lower steatosis grades in this patient group, which could reflect a relative insensitivity for detecting lower grades of liver steatosis with unenhanced CT. Other factors that may limit CT for fat quantification include the presence of iron and glycogen in the liver, the patient’s hematocrit, and drugs such as amiodarone. Some of these limitations may be overcome by the use of dual-energy CT, but this will require future investigation. The poor correlation we observed between contrast-enhanced CT and liver steatosis grade may be explained by the confounding effect of variable liver perfusion, which is influenced by cardiac output and bolus timing. Underlying liver disease, especially fibrosis, can also result in regional alterations of liver perfusion and hence liver attenuation after intravenous contrast administration.
A potential criticism of our study is the assumption of the homogeneous distribution of liver steatosis. However, in all cases we selected the most representative regions of liver and used multiple regions of interest to obtain a mean liver signal intensity. Second, there were only a small number of patients imaged with all three modalities (n=16), although histopathologic correlation was obtained for each patient imaged with any of the modalities. Third, we did not have healthy controls since liver biopsy performed for clinical work-up was necessary to be included in the study. However, a percentage (34% to 47%) of patients imaged with each technique did have Grade 0 steatosis. Fourth, patients imaged with unenhanced CT had lower liver steatosis grades which may have resulted in a lower correlation coefficient. This limitation is also a consequence of the retrospective nature of the study. Fifth, there was a variable range of time between imaging and liver biopsy or resection which was also due to the retrospective nature of the study. Sixth, our study only evaluated steatosis. The extent of other diffuse processes such as iron deposition or fibrosis was not assessed and may have affected imaging quantification of liver fat. However, our study included a mixed population of subjects with and without liver disease for the purpose of reflecting the performance of the different imaging modalities in clinical practice. Seventh, T2-weighted sequences with and without fat saturation were a combination of single-shot fast spin-echo and fast spin-echo sequences, but prior studies have reported the ability to quantify liver fat with this technique [17].
In conclusion, opposed-phase MRI demonstrated the highest correlation with histopathology, compared to T2-weighted MRI with and without fat saturation, CT, and ultrasound for quantification of diffuse liver fat.
Fig. 2.
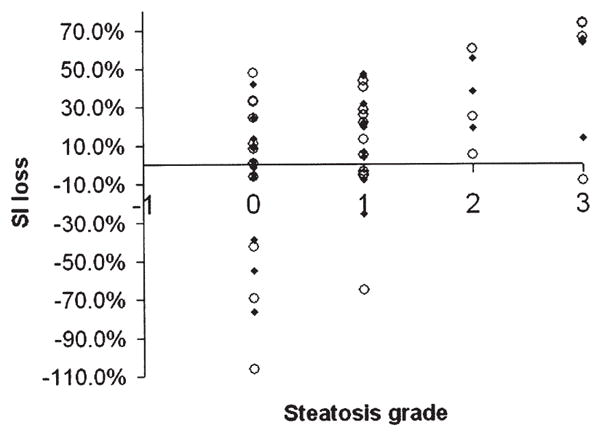
Correlation of relative liver signal intensity loss on fat-saturated T2-weighted imaging and liver steatosis grade (for Readers 1 and 2).
Fig. 3.
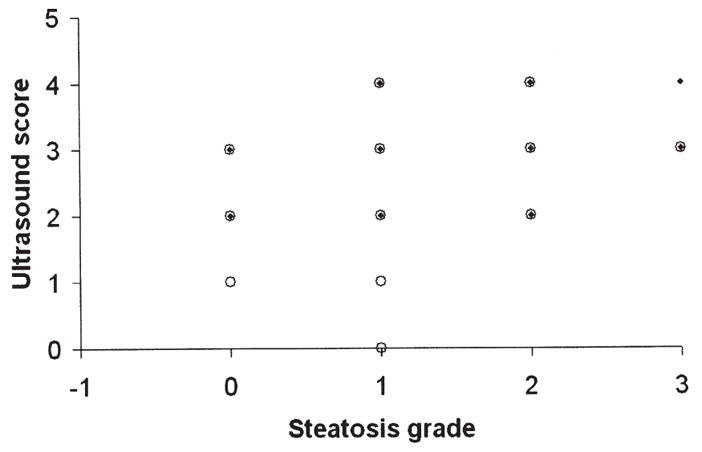
Correlation of ultrasound score with liver steatosis grade (for Readers 1 and 2).
Fig. 4.
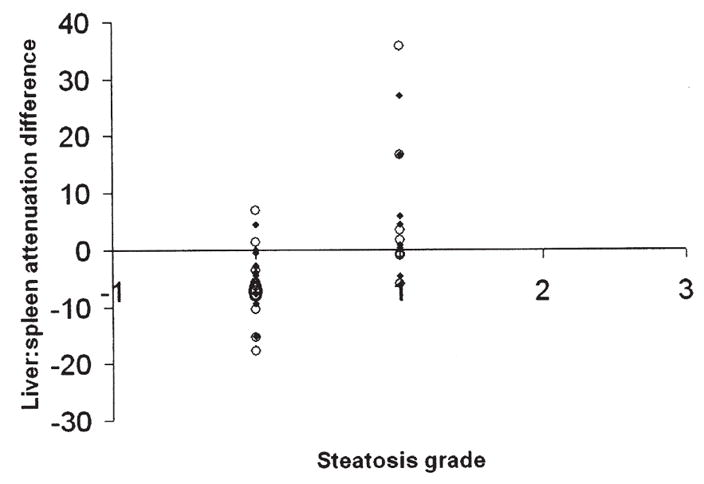
Correlation of liver: spleen attenuation difference on unenhanced CT and liver steatosis grade (for Readers 1 and 2).
Footnotes
This study was in part funded by RO1 DK074718-01A1.
References
- 1.Festi P, Colecchia A, Sacco T, Bondi M, Roda E, Marchesini G. Hepatic steatosis in obese patients: clinical aspects and prognostic significance. Obes Rev. 2004;5:27–42. doi: 10.1111/j.1467-789x.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 3.Mulhall BP, Ong JP, Younossi ZM. Non-alcoholic fatty liver disease: an over view. J Gastroenterol Hepatol. 2002;17:1136–43. doi: 10.1046/j.1440-1746.2002.02881.x. [DOI] [PubMed] [Google Scholar]
- 4.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–19. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 5.Clarke JM, Brancati FL, Diehl AM. Non-alcoholic fatty liver disease. Gastroenterology. 2002;122:1649–57. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 6.Miller CM, Gondolesi GE, Florman S, et al. One hundred nine living donor liver transplants in adult and children: a single-center experience. Ann Surg. 2001;234:301–11. doi: 10.1097/00000658-200109000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ristig M, Drechsler H, Powderly WG. Hepatic steatosis and HIV infection. AIDS Patient Care STDS. 2005;19:356–65. doi: 10.1089/apc.2005.19.356. [DOI] [PubMed] [Google Scholar]
- 8.Burke A, Lucey MR. Non-alcoholic fatty liver disease, non-alcoholic steatohepatitis and orthotopic liver transplantation. Am J Transplant. 2004;4:686–93. doi: 10.1111/j.1600-6143.2004.00432.x. [DOI] [PubMed] [Google Scholar]
- 9.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105–13. doi: 10.1055/s-2001-12933. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi M, Fujii K, Kiuchi T, et al. Effects of fatty infiltration of the graft on the outcome of living-related liver transplantation. Transplant Proc. 1999;31:403. doi: 10.1016/s0041-1345(98)01679-0. [DOI] [PubMed] [Google Scholar]
- 11.Limanond P, Raman SS, Lassman C, et al. Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings. Radiology. 2004;230:276–80. doi: 10.1148/radiol.2301021176. [DOI] [PubMed] [Google Scholar]
- 12.Negro F. Mechanisms and significance of liver steatosis in hepatitis C virus infection. World J Gastroenterol. 2006;12:6756–65. doi: 10.3748/wjg.v12.i42.6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 14.Levenson H, Greensite F, Hoefs J, et al. Fatty infiltration of the liver: quantification with phase-contrast MR imaging at 1.5T vs biopsy. AJR Am J Roentgenol. 1991;156:307–12. doi: 10.2214/ajr.156.2.1898804. [DOI] [PubMed] [Google Scholar]
- 15.Taylor KJ, Gorelick FS, Rosenfield AT, Riely CA. Ultrasonography of alcoholic liver disease with histological correlation. Radiology. 1981;141:157–61. doi: 10.1148/radiology.141.1.6270725. [DOI] [PubMed] [Google Scholar]
- 16.Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–50. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 17.Qayyum A, Goh JS, Kakar S, Yeh BM, Merriman RB, Coakley FV. Accuracy of liver fat quantification by MR imaging: comparison of out-of-phase gradient-echo and fat-saturated fast spin-echo techniques-initial experience. Radiology. 2005;237:507–11. doi: 10.1148/radiol.2372040539. [DOI] [PubMed] [Google Scholar]
- 18.Duerinckx A, Rosenberg K, Hoefs J, et al. In vivo acoustic attenuation in liver: correlations with blood tests and histology. Ultrasound Med Biol. 1988;14:405–13. doi: 10.1016/0301-5629(88)90076-2. [DOI] [PubMed] [Google Scholar]
- 19.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–74. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 20.Vehmas T, Kaukiainen A, Luoma K, Lohman M, Nurminen M, Taskinen H. Liver echogenicity: measurement or visual grading? Comput Med Imaging Graph. 2004;28:289–93. doi: 10.1016/j.compmedimag.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Tchelepi H, Ralls PW, Radin R, Grant E. Sonography of diffuse liver disease. J Ultrasound Med. 2002;21:1023–32. doi: 10.7863/jum.2002.21.9.1023. [DOI] [PubMed] [Google Scholar]
- 22.Joseph AE, Saverymuttu SH, al-Sam S, Cook MG, Maxwell JD. Comparison of liver histology with ultrasonography in assessing diffuse parenchymal liver disease. Clin Radiol. 1991;43:26–31. doi: 10.1016/s0009-9260(05)80350-2. [DOI] [PubMed] [Google Scholar]
- 23.Westphalen AC, Qayyum A, Yeh BM, et al. Liver fat: effect of hepatic iron deposition on evaluation with opposed-phase MR imaging. Radiology. 2007;242:450–5. doi: 10.1148/radiol.2422052024. [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Lee JM, Han JK, et al. Hepatic macrosteatosis: predicting appropriateness of liver donation by using MR imaging—correlation with histopathologic findings. Radiology. 2006;240:116–29. doi: 10.1148/radiol.2393042218. [DOI] [PubMed] [Google Scholar]
- 25.Park SH, Kim PN, Kim KW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105–12. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- 26.Raptopoulos V, Karellas A, Bernstein J, Reale FR, Constantinou C, Zawacki JK. Value of dual-energy CT in differentiating focal fatty infiltration of the liver from low-density masses. AJR Am J Roentgenol. 1991;157:721–5. doi: 10.2214/ajr.157.4.1892025. [DOI] [PubMed] [Google Scholar]


