Abstract
Posterior permanent teeth with carious lesions radiographically extending no farther than halfway into dentin (n = 565) were restored using a resin-based composite by 38 dentists in a practice-based research network. Preoperative and 1-, 4-, and 13-week-posttreatment hypersensitivity was recorded using an 11-point visual analog scale filled out anonymously by subjects. Analyses were conducted to determine whether any correlation or association existed among several variables, including degree of carious activity, cavity extent, application of antimicrobial or desensitizing agents, application of liner, dentin bonding agent and resin-based composite employed, and composite placement method. Three results were fairly unexpected: only 36% of lesions were ranked as caries-active, 31% of teeth had appreciable preoperative hypersensitivity, and 16% of teeth with no preoperative hypersensitivity had appreciable hypersensitivity at 1 week posttreatment. Preoperative hypersensitivity was correlated with lesion visibility on radiographs but not with dentin caries activity (ranked on opening enamel), preparation depth, or preparation volume. Accrual to the study continues, and conclusions regarding other relationships awaits 13-week results.
Introduction
Postoperative hypersensitivity (POH) can be defined as pain in a tooth associated with mastication or with contact with hot, cold, sweet, or sour stimuli that occurs 1 week or more posttreatment. Pain associated with clenching, which may indicate a restoration in hyperocclusion, is typically excluded from definitions of POH.
A 2006 survey of the authors’ practice-based research network (PEARL: Practitioners Engaged in Applied Research and Learning) revealed that POH following posterior resin-based composite (RBC) restorations is a common concern among its member practitioner-investigators. The literature on POH is sparse, however, and it is difficult to draw firm conclusions from the few relevant published studies. Most studies that address POH have small sample sizes and are typically associated with evaluation of a particular bonding agent or resin-based composite formulation. Moreover, variables of interest differ from study to study, as do methods of measurement. The inconsistencies and singularly examined factors in POH studies thus limit our understanding both of the overall problem and of the influences and elements that may be key risk factors in the general practice setting.
To help improve our understanding of this phenomenon, the PEARL Network undertook an observational study among its membership to investigate the effectiveness of a range of techniques and materials in preventing or ameliorating postoperative hypersensitivity in Class I resin-based composite restorations for shallow carious lesions in dentin.
A protocol describing overall study conduct and a manual of procedures (MOP) detailing study procedures were developed by the PEARL Network Executive Management Team in conjunction with the PEARL Executive Committee (including PEARL practitioner-investigators), representatives of NIH’s National Institute of Dental and Craniofacial Research, the EMMES Corporation (PEARL’s data-coordinating center), and a panel of extramural consultants. Presented here are preliminary 1-week results for a projected 3-month follow-up study that as of this writing continues to enroll subjects.
Materials and Methods
Subject eligibility criteria included clinical diagnosis of one or more unrestored permanent posterior teeth with occlusal carious lesions judged on radiograph to be no more than one-half the distance from the dentinoenamel junction to the pulp (visibility of the lesion on the radiograph was not required but was recorded as visible, equivocal, or not visible in each instance). Not more than one tooth per quadrant was allowed in the study, nor more than two teeth per subject. Eligibility also required that each tooth of interest be in occlusion with a natural tooth, free of evidence of pulpitis (no report of lingering pain associated with any stimulus), and not periodontally involved (mobility <2 and no evidence of gingival inflammation). In keeping with the standards of study conduct in a practice-based research network, any case selected had to be one for which the practitioner would normally apply his or her standard of care, in this instance use of a resin-based composite. Beyond that, each dentist was expected to employ his or her routine methods for restoration. Use of the following was recorded: desensitizing agent, antimicrobial agent, liner, dentin bonding agent, flowable or other composite, and composite placement method (layering or bulk cure).
Baseline preoperative hypersensitivity in each subject’s tooth/teeth was established via a paper-based (11-point, 0–10) visual analog scale (VAS) questionnaire that collected the history of reactions to cold, hot, and sweet stimuli and to chewing and clenching; clenching was included to determine whether hyperocclusion might be responsible for tooth hypersensitivity. At the same time, subjects completed a 14-item oral health–related quality-of-life (OHRQoL) questionnaire, a modified version of the Oral Health Index Profile-14 (OHIP-14) that has been widely utilized elsewhere1–5 and that recorded such information as difficulty in pronouncing words and need to adjust diet because of the subject’s affected tooth or teeth.
Cavity preparation was initiated, and upon enamel removal dentin caries activity was ranked according to a modification of Kidd’s Dentin Caries Classification system:6,7
1 = Soft, serous
2 = Soft, dry
3 = Soft, dry, granular
4 = Leathery
5 = Firm but discolored
Upon completion of the preparation, cavity depth, width, and length were recorded8 as well as the extent of caries dentin removal (“all removed” or “some carious dentin left locally”). In addition, the details of dentin treatment, disinfection, desensitizing agent, liner, bonding agent, and resin-based composite used were recorded. Subjects were advised to contact the dentist if they experienced any level of POH.
At 6–7 days posttreatment, subjects completed postoperative hypersensitivity (POH) VAS and OHRQoL questionnaires and were reminded to report to the dentist any development of POH. Subject anonymity was ensured by having the reports filled out securely online or mailed to the PEARL clinical coordinating center (EMMES Corporation, Rockville, MD) and identified only by number.
Statistical Analysis
Descriptive statistics, including frequencies and percentages, were generated for variables of interest. For this analysis, “appreciable hypersensitivity” (AH) was defined as a VAS value of 3 or higher. Pearson correlation coefficients between sensitivity while chewing and sensitivity while clenching at baseline and at 1 week were obtained, and chi-square tests were used to test the relationship between baseline AH and active caries ranking as well as between baseline AH and lesion visibility on radiographs.
For this interim analysis, multiple teeth in a given subject were treated as independent cases.
Results
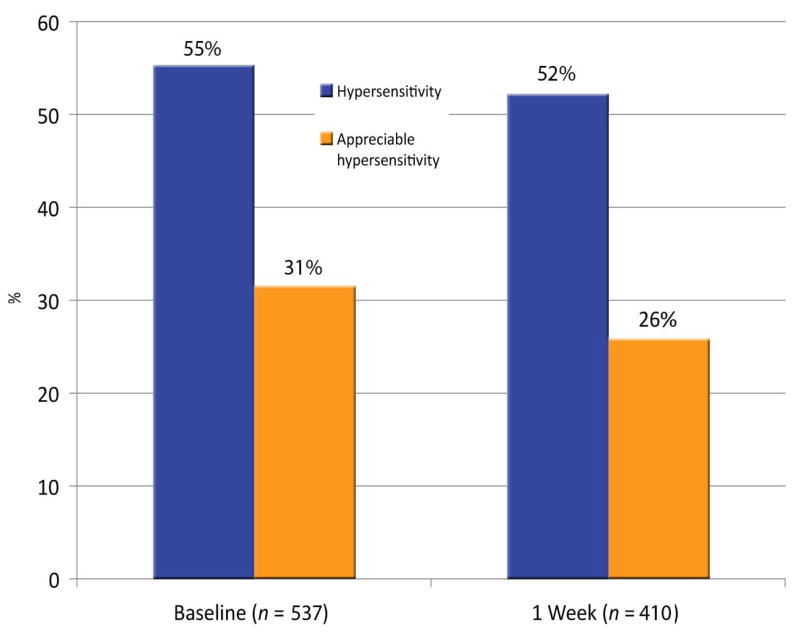
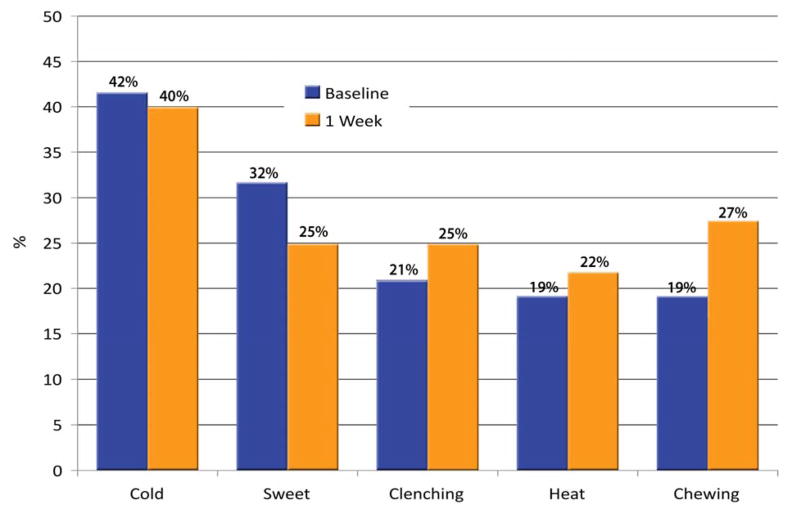
Thirty-eight PEARL practitioner-investigators enrolled 504 patients into the study (target enrollment: 610 patients). The median age of subjects was 25 years (range 10–59); 39% were male, 61% female. Of the 565 teeth represented, 88.3% were molars and 11.7% premolars; 55.7% were in lower quadrants. Among teeth with baseline sensitivity data, 54.6% (293/537) were reported to have some hypersensitivity, defined as a score of >0 on any VAS item; this proportion is 52.6% (283/538) if clenching is excluded. Teeth with appreciable hypersensitivity (AH; any score of ≥3) at baseline comprised 31.1% (167/537), or 29.0% (156/538) after excluding clenching (Fig. 1). At baseline, AH to cold was the most prevalent report (42%), followed by sweets (32%), clenching (21%), heat (19%), and chewing (19%); at 1 week posttreatment, the proportions were 40% for cold, 27% for chewing, 25% for sweets, 25% for clenching, and 22% for heat (Fig. 2). Pearson correlation coefficients between hypersensitivity while chewing and hypersensitivity while clenching at baseline and at 1 week were significant (0.56 and 0.63, respectively).
Fig. 1.
Percentage of teeth with some hypersensitivity (1 or 2 on any VAS score) and of teeth with appreciable hypersensitivity (AH: any one VAS score of ≥3), at baseline and at 1 week posttreatment.
Fig. 2.
Percentage of teeth with AH at baseline and at 1 week posttreatment, by stimulus.
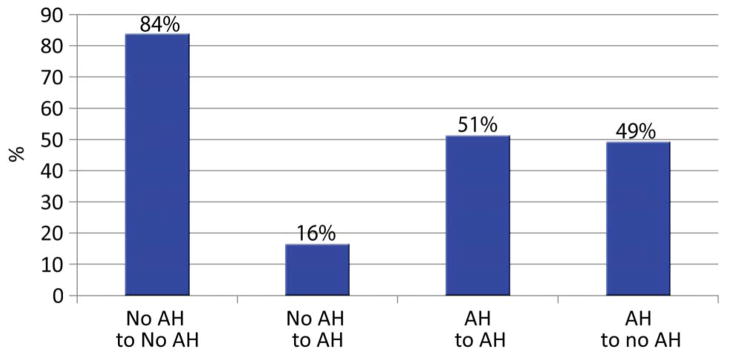
At 1 week postoperatively, 52.4% (215/410) of teeth left to follow-up had hypersensitivity and 26.1% (107/410) had AH (Fig. 1). Among teeth with baseline AH, the proportion with AH at 1 week posttreatment was reduced to 50.9% (58/114). Among teeth with no baseline AH, 16.0% (46/287) had AH at 1 week (Fig. 3). Neither baseline nor 1-week AH was correlated with either cavity depth or volume. Baseline AH was not associated with active caries ranking (<3 on the Dentin Caries Classification scale; chi-square test: p =.30). If we equate inactive caries with the highest three rankings on the Dentin Caries Classification scale (where the distribution was “3 = soft, dry, granular” 18.8%, “4 = leathery” 19.3%, and “5 = firm but discolored” 25.8%), then active caries was present in only 36.1% (196/543) of teeth.
Fig. 3.
Percentage of teeth undergoing change/no change in AH status from baseline to 1 week posttreatment. The finding that 18% teeth with no preoperative AH had developed AH by 1 week posttreatment (second column from left) was unanticipated.
These findings were analyzed for the influence of cofactors, but no relationship was found between 1-week maximum hypersensitivity and, for example, cavity volume or depth, liner use, dentin bonding agent use, or patient demographics. Nor was maximum hypersensitivity at baseline different between the antimicrobial subgroup and teeth with no antimicrobial treatment, further suggesting that these populations are similar in all respects other than level of 1-week AH. Caution in interpretation is nevertheless advised with regard to the effect of antimicrobial agents, as the numbers of these results are small, and possible variations in the details of application and removal have yet to be determined.
One cofactor that was found to correlate with preoperative AH was the radiographic appearance of the lesion. Among teeth with lesions visible on the radiograph, 40.4% (76/188) had preoperative AH as compared to 24.2% (16/66) of teeth whose radiographs were judged equivocal and 26.6% (54/203) in which a lesion was not visible (p < .01). Among teeth without POH at baseline, it was observed that those treated with an antimicrobial were more likely to report AH at week 1 than those not treated with an antimicrobial. Further analyses of this possible relationship are being conducted.
Discussion
There are few studies with which to compare the present investigation, which focuses on short-term postoperative hypersensitivity (POH) in Class I resin-based composite restorations of carious lesions that extend no more than halfway through the dentin toward the pulp using state-of-the-art materials and techniques. Perdigao et al.9 compared a self-etch to a total-etch adhesive system in Class I and Class II restorations by measuring responses to air and cold using a visual analog scale (VAS) as well as times to response. The authors reported no significant changes from baseline to periods up to 6 months in either measurement for either system. In a subsequent study by the same group using the same measurements but addressing only Class II restorations,10 a significant reduction was found in severity of cold response, as well as a significantly longer time to cold response, at 2 weeks postrestoration. In this latter study, however, no allowance was made for some of the large open lesions restored or for the thermal conductivity of the many amalgam restorations replaced. Moreover, while subjects reported mean sensitivity responses in the range 1.5–3.0, the fact that overall responses were in the range 0–10 for both air and cold stimulus suggests that at least some subjects experienced a noxious stimulus. It bears mentioning that sensitivity on mastication was reported as zero in both studies.
More recently, Casselli and Martins11 employed a split-mouth design to compare a total-etch to a self-etch system (neither using liners) in 104 Class I restorations placed by a single clinician. At 7 days postoperatively, 71% of the restorations were rated 0 on a 11-point VAS for sensitivity, 17% were rated 1.1–2, 2% were rated 2.1–3, and 4% were rated >3 (one rated 4 and one 5.1). At 6 months posttreatment, only 1% (1) of the restorations was rated in the range 1.1–2 and 2% (2) in the range 2.1–3 for sensitivity. No significant difference was found between bonding agents.
It is worth noting that in all these studies, subjects reported their perceptions directly to the treating clinician, raising the question of whether their responses can be considered forthright.
Reports of longer-term outcomes testify to the persistence of POH and the long-term implications of POH regardless of when it occurs. One representative study of 148 RBC restorations (mixed Class I and Class II) found that POH requiring restoration replacement occurred in 4% (5/140) of patients over 2 years.12 It was not reported whether the hypersensitivity in these restorations developed spontaneous or gradually over time.
The most comprehensive study of POH outcomes to date is a multicenter clinical trial involving 1101 restorations treated using a calcium hydroxide (CaOH) liner, a single bonding agent, and a single resin-based composite.13 The authors concluded that at 5 years, (1) restorations with POH were more likely to fail than restorations without POH; (2) restorations with POH in large cavities were more likely to fail than restorations in small cavities; and (3) regardless of cavity size, restorations were more likely to fail if POH occurred within the first recall (1 month after placement).
As this summary suggests, POH is often a secondary focus of studies that primarily examine restorative material and placement techniques, and thus measurement approaches have not been uniform. Due to the complexity involved in studying the underlying trigger(s) of POH and predicting its occurrence, sophistication and standardization of measurement have not evolved.
In the present study, the investigators were surprised by the finding that 31% of teeth had appreciable hypersensitivity (AH) pretreatment. Caries, unless extensive, is generally considered to be a “silent” disease, and the lesions included in this study were limited to no more than half the dentin depth. Frank caries with visibly open enamel was only reported in a few instances. Indeed, 30% of lesions were either not evident or equivocal on radiograph but diagnosed clinically. There was a positive correlation between caries diagnosed radiographically and AH. However, the lack of correlation between cavity depth following preparation and baseline maximum hypersensitivity was unanticipated.
Although appreciable hypersensitivity appears to have been significantly reduced by placement of a restoration (“AH to no AH,” Fig. 3), one should also take into account the 16% of no-baseline AH teeth reported to have AH at week 1. As can be seen in Fig. 1, the percentage of teeth with AH was reduced by only 5% from baseline to week 1 (31% to 26%), and the percentage of teeth reporting any hypersensitivity was only changed from 55% to 52%.
Our finding that 16% of teeth with no preoperative hypersensitivity were found to have AH at 1 week posttreatment was similarly unanticipated. As suggested earlier, this level of hypersensitivity may not be apparent in clinical settings if patients are reluctant to report it to their dentists unless it is severe. It is thus reasonable to speculate that hypersensitivity is underreported when patient outcomes are not solicited anonymously. We considered subtracting the number of subjects with clenching AH from our analysis of the group with acquired AH (i.e., those with 1-week posttreatment AH who had no baseline AH), as clenching AH may be related to the restoration being in hyperocclusion, but given the baseline correlation (0.56) between clenching and chewing AH, hyperocclusion may not be a factor in the POH observed.
Our finding that upon removing the enamel only 36% of teeth had caries ranked as active suggests that in many instances a caries risk management program rather than operative treatment is advised. This supports previous studies of early lesions14,15 and argues for methods to describe and record the stages of the carious process in enamel, such as the International Caries Detection and Assessment System (ICDAS),16 given that many of these lesions were not visible or were equivocal on radiographs. The ability to monitor changes in lesion appearance becomes important in managing caries as a disease.
As regards caries activity ranking, the modification of the Kidd Classification to include an intermediate ranking (“3 = soft, dry, granular”) as a descriptor of inactive caries was based on the authors’ clinical experience, where this state has occasionally been observed. The result that 18.8% of lesions were so ranked by PEARL P-Is compared to 19.1% described as “leathery” was unexpected and will be further explored.
The limited numbers of the variety of treatments and materials used in this effectiveness study require that caution be exercised interpreting some of our results. For example, while antimicrobial treatment appears to be associated with a reduction in AH among baseline-AH teeth, the exact opposite is true for no-baseline-AH teeth. The use of an antimicrobial may be attributed to the judgment by the dentist of the need for such an agent based upon the depth of the preparation, appearance of the remaining dentin, or estimated caries activity upon opening the lesion. Still, our analysis of the results of using an antimicrobial suggests that the negative impact of these agents may be real, but further results are needed before recommending a change in clinical practice.
Our study is unique not only in evaluating patients with regard to early caries and hypersensitivity but also in investigating several clinical variables linked with POH. Given the wide range of techniques employed by PEARL Network practitioner-investigators following cavity preparation, we are not yet in a position to attempt to correlate POH with use of antimicrobials, liners, dentin bonding agents, or restorative techniques. It is worth noting that, irrespective of baseline hypersensitivity, patients in some dental practices reported that nearly 100% of teeth experienced AH following treatment, while patients in other practices reported almost none. This reflects one of the strengths of conducting studies outside an academic environment: the ability of practitioner-investigators to compare, at the close of the study, their patient outcomes with the blinded outcomes of their fellow network members, which is an important aspect of participation in a practice-based research network.
It is hoped that, upon completion of this study, our 4-week findings will provide guidance for future studies of POH with the ability to positively impact the daily lives of patients. We are eager to review the final data to determine which if any of our preliminary results prove significant at longer term. Based on those results, the PEARL Network anticipates conducting a randomized controlled trial to determine which methods and techniques are best for reducing or eliminating POH in resin-based composite restorations.
Acknowledgments
This study was supported by the National Institute of Dental and Craniofacial Research, National Institutes of Health, through grant UO1 DE016755 awarded to the New York University College of Dentistry.
References
- 1.Forgie AH, Scott BJ, Davis DM. A study to compare the oral health impact profile and satisfaction before and after having replacement complete dentures in England and Scotland. Gerodontology. 2005 Sep;22(3):137–142. doi: 10.1111/j.1741-2358.2005.00066.x. [DOI] [PubMed] [Google Scholar]
- 2.McGrath C, Comfort MB, Lo EC, Luo Y. Can third molar surgery improve quality of life? A 6-month cohort study. J Oral Maxillofac Surg. 2003 Jul;61(7):759–763. doi: 10.1016/s0278-2391(03)00150-2. discussion 764–755. [DOI] [PubMed] [Google Scholar]
- 3.McGrath C, Hegarty AM, Hodgson TA, Porter SR. Patient-centred outcome measures for oral mucosal disease are sensitive to treatment. Int J Oral Maxillofac Surg. 2003 Jun;32(3):334–336. doi: 10.1054/ijom.2002.0377. [DOI] [PubMed] [Google Scholar]
- 4.Att W, Stappert C. Implant therapy to improve quality of life. Quintessence Int. 2003 Sep;34(8):573–581. [PubMed] [Google Scholar]
- 5.de Oliveira CM, Sheiham A. The relationship between normative orthodontic treatment need and oral health-related quality of life. Community Dent Oral Epidemiol. 2003 Dec;31(6):426–436. doi: 10.1046/j.1600-0528.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- 6.Kidd EA, Joyston-Bechal S, Beighton D. Microbiological validation of assessments of caries activity during cavity preparation. Caries. 1993 Res;27(5):402–408. doi: 10.1159/000261571. [DOI] [PubMed] [Google Scholar]
- 7.Zheng L, Hilton JF, Habelitz S, Marshall SJ, Marshall GW. Dentin caries activity status related to hardness and elasticity. Eur J Oral Sci. 2003 Jun;111(3):243–252. doi: 10.1034/j.1600-0722.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 8.Yip KH, Poon BK, Chu FC, Poon EC, Kong FY, Smales RJ. Clinical evaluation of packable and conventional hybrid resin-based composites for posterior restorations in permanent teeth: results at 12 months. J Am Dent Assoc. 2003 Dec;134(12):1581–1589. doi: 10.14219/jada.archive.2003.0103. [DOI] [PubMed] [Google Scholar]
- 9.Perdigao J, Geraldeli S, Hodges JS. Total-etch versus self-etch adhesive: effect on postoperative sensitivity. J Am Dent Assoc. 2003 Dec;134(12):1621–1629. doi: 10.14219/jada.archive.2003.0109. [DOI] [PubMed] [Google Scholar]
- 10.Perdigao J, Anauate-Netto C, Carmo AR, et al. The effect of adhesive and flowable composite on postoperative sensitivity: 2-week results. Quintessence Int. 2004 Nov-Dec;35(10):777–784. [PubMed] [Google Scholar]
- 11.Casselli DS, Martins LR. Postoperative sensitivity in Class I composite resin restorations in vivo. J Adhes Dent. 2006 Feb;8(1):53–58. [PubMed] [Google Scholar]
- 12.Lundin SA, Rasmusson CG. Clinical evaluation of a resin composite and bonding agent in Class I and II restorations: 2-year results. Quintessence Int. 2004 Oct;35(9):758–762. [PubMed] [Google Scholar]
- 13.Hayashi M, Wilson NH. Failure risk of posterior composites with post-operative sensitivity. Oper Dent. 2003 Nov-Dec;28(6):681–688. [PubMed] [Google Scholar]
- 14.Dennison JB, Hamilton JC. Treatment decisions and conservation of tooth structure. Dent Clin North Am. 2005 Oct;49(4):825–845. vii. doi: 10.1016/j.cden.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton JC, Dennison JB, Stoffers KW, Gregory WA, Welch KB. Early treatment of incipient carious lesions: a two-year clinical evaluation. J Am Dent Assoc. 2002 Dec;133(12):1643–1651. doi: 10.14219/jada.archive.2002.0114. [DOI] [PubMed] [Google Scholar]
- 16.Pitts N. “ICDAS”--an international system for caries detection and assessment being developed to facilitate caries epidemiology, research and appropriate clinical management. Community Dent Health. 2004 Sep;21(3):193–198. [PubMed] [Google Scholar]