Abstract
Treatment of C57BL/6 mice with cyclophosphamide (100 mg/kg) and fludarabine (200 mg/kg) induced non-myeloablative lymphodepletion without inhibiting D5 melanoma tumor growth. Utilizing this model, we found that induction of lymphopenia prior to adoptive transfer of ex vivo anti-CD3/CD28 activated and IL-2 expanded D5-G6 tumor draining lymph node cells enhanced the anti-tumor efficacy of the infused cells in both pulmonary metastases and subcutaneous D5 bearing mice. However, induction of lymphopenia did not promote intratumoral or extratumoral proliferation or accumulation of the infused cells. We have previously shown that radiotherapy enhances the therapeutic efficacy of intratumoral unpulsed dendritic cell vaccination in subcutaneous murine tumor models by augmenting the induction of anti-tumor cellular immune responses. Here, we confirmed this finding in a murine metastatic melanoma liver tumor model. Furthermore, local tumor irradiation combined with intratumoral dendritic cell administration significantly enhanced the therapeutic efficacy of tumor-reactive T cell adoptive transfer in this lymphodepleted liver tumor model. This was evident by reduced liver tumor size, decreased incidence of spontaneous intra-abdominal metastasis, and prolonged survival, resulting in 46% of mice cured. This enhanced anti-tumor activity was associated with a selective increase in proliferation, accumulation, and function of CD4+ rather than CD8+ infused cells. This multimodality regimen may have translational applications for the treatment of human cancers.
Keywords: Radiation, Dendritic Cell, Adoptive Transfer, Cancer Vaccine, Immunotherapy
INTRODUCTION
Cancer mortality is predominantly due to the outgrowth of metastatic disease. The liver constitutes a particularly frequent metastatic site for various human malignancies, including: gastro-intestinal, breast, lung, and melanoma (1). The survival rate for advanced-stage melanoma hasn’t changed over the past 30 years, and melanoma patients with liver metastases have an average life span of only 4 to 8 months (2). Experimental anti-tumor therapies are frequently tested for efficacy in subcutaneous (s.c.) tumor models since they provide convenient access to tumor induction and follow up. However, s.c. tumors may not adequately mirror orthotopic tumors, particularly in regard with response to immunological strategies. Whereas antigen presentation in s.c. tissues tends to elicit immunity, antigen presentation in the liver tends to induce tolerance (3, 4). This may be related to the physiologic functions of the liver which involve clearance of toxic metabolic products and foreign antigens derived from the gastrointestinal tract. To accommodate these functions, the microenvironment within the liver is characterized by local release of immunosuppressive mediators such as IL-10, prostaglandin E2, and transforming growth factor β. Therefore, compared with the skin, the liver represents a less permissive environment for induction of an effective anti-tumor immune response.
Adoptive cellular immunotherapy aims to eradicate metastatic cancer by transferring tumor-reactive T cells derived from the tumor-bearing host using either tumor-infiltrating lymphocytes, tumor-primed lymph node cells, or in vitro sensitized peripheral blood lymphocytes (5). Since this approach has shown promising results in animal models and in clinical trials, a major research effort has focused on designing strategies to enhance the anti-tumor activity of the infused cells (6). Part of this research effort has been devoted to optimizing in vitro methods used for generation of tumor-reactive T cells for adoptive transfer (AT) (7–9). Another approach has focused on modulating the immunological environment within the tumor-bearing host. One of the earliest examples is the exogenous administration of interleukin 2 (IL-2) after adoptive T-cell transfer which augments therapeutic efficacy (10). More recently, induction of lymphopenia prior to AT has been shown to enhance the anti-tumor activity of the infused cells (11, 12). In the clinical setting, non-myeloablative lymphodepletion is induced by administration of chemotherapy, of which cyclophosphamide plus fludarabine have been most frequently used. Most animal studies, on the other hand, have utilized either total body irradiation or genetically deficient mice. This enhancement in the therapeutic efficacy of AT has been attributed to several factors, including: homeostatic proliferation of the infused cells (13, 14), elimination of host regulatory T cells (15), enhanced availability of homeostatic cytokines (16), and removal of competition at the surfaces of antigen presenting cells (17). The effector function and the persistence of adoptively transferred tumor-reactive T cells within the tumor-bearing host are major factors contributing to treatment outcome.
Dendritic cells (DCs) loaded in vitro with tumor-derived antigens have the capacity to prime naïve T cells (18) as well as to stimulate activated, tumor-reactive T cells (19). Intratumoral (i.t.) vaccination with unpulsed DCs is based on in vivo-in situ priming of DCs. This approach offers the potential advantage of eliciting immunity against multiple relevant host-specific tumor antigens without major histocompatibility complex allele restrictions, while alleviating the need to obtain and load DCs with tumor-antigens. Utilizing various s.c. tumor models, we have previously shown that local tumor irradiation augments the capacity of unpulsed i.t. administered DCs to induce tumor-specific cellular immune responses resulting in enhanced therapeutic efficacy (20–23). In a s.c. melanoma model, radiotherapy combined with i.t. DC vaccination proved superior to tumor lysate pulsed-DC immunization in inhibiting tumor growth and prolonging survival (20). In this study, we examined whether radiotherapy could also enhance the therapeutic efficacy of DC administration in a metastatic melanoma liver tumor model. Furthermore, utilizing this liver tumor model, we evaluated whether the combination of radiotherapy and i.t. DC vaccination could stimulate adoptively transferred tumor-reactive T cells in vivo within a lymphodepleted host leading to improved anti-tumor efficacy of the infused cells.
To this end, we transferred T cells derived from ex vivo activated and expanded tumor draining lymph node (TDLN) cells. This source of effector cells has been studied extensively in animal models (7–9, 24–26), and its clinical counterpart, vaccine primed lymph node, has been evaluated in various clinical trials (27–30). TDLNs contain in vivo sensitized pre-effector cells that can be stimulated in vitro to generate tumor-specific CD8+ and CD4+ cells, both of which mediate tumor regression upon AT (31, 32).
MATERIALS AND METHODS
Mice
Female C57BL/6 mice with CD45.2 or CD45.1 phenotype were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were housed in specific pathogen-free conditions at the Animal Maintenance Facility of the University of Michigan Medical Center and used for experiments at 8 weeks of age or older. The University of Michigan Committee on Use and Care of Animals reviewed and approved all animal protocols.
Tumor Cells
D5 melanoma is a poorly immunogenic subclone of the B16-BL6 tumor of spontaneous origin in the C57BL/6 strain (24). D5-G6 is a D5 clone, transduced to express murine granulocyte macrophage colony-stimulating factor (24). Tumor cells were cultured in complete medium (CM) (20).
Depletion of Host Immune Elements
To induce lymphopenia, mice received two consecutive daily doses of cyclophosphamide (50 mg/kg; Sigma, St. Louis, MO) and fludarabine (100 mg/kg; Sicor, Irvine, Canada) intraperitonealy (i.p.).
In Vivo D5 Tumor Models
To induce lung metastases, mice were inoculated intravenously (i.v.) with 1×105 D5 cells. To establish subcutaneous tumors, mice were inoculated s.c. in the right flank with 2×106 D5 cells. To induce solitary metastatic liver tumors, mice were inoculated intrahepatically with 1×105 D5 cells. Tumor cells were injected subcapsularly into the ventral-caudal aspect of the left lateral lobe so that established tumors would be located ∼1 cm caudal to the xiphoid tip and along the mid-line. This procedure was performed under isoflurane anesthesia via mini-laparotomy.
Generation of T cells for AT
To induce TDLNs, mice were inoculated s.c. in bilateral flanks with 1×106 D5-G6 cells. After 9 days, inguinal lymph nodes were harvested. TDLN cells (1×106/ml CM) were activated in 6-well culture plates with immobilized anti-CD3 and anti-CD28 monoclonal antibodies (mAbs) (BD Biosciences, San Diego, CA) for 2 days. Culture plates were pre-coated with 1 µg of each mAb/ml PBS (3 ml/well) overnight at 4°C. After activation, cells (1×105/ml) were expanded in CM containing human recombinant IL-2 (60 IU/ml; Chiron, Emeryville, CA) for 3 days. Activated and expanded D5-G6 TDLN cells were injected i.v. to D5 tumor-bearing mice 7 days after tumor inoculation. Following AT, mice received IL-2 (i.p. 42,000 IU × 2/day for 8 doses). In some experiments, prior to AT, cells were stained with 5-(and 6-) Carboxyfluorescein Diacetate Succinimidyl Ester (CFSE) as previously described (20).
Flow Cytometric Analysis
Blood samples were drawn via the lateral saphenous vein. Spleens were harvested and disrupted to single cell suspensions. Erythrocytes in blood and spleens were depleted. After sacrificing mice, lungs were flushed prior to harvesting by injecting PBS into the right ventricle of the heart. Tumor-bearing lungs and s.c. tumors were mechanically disaggregated into single cell suspensions using a Medimachine (DakoUSA, Carpinteria, CA) as previously described (21, 33). All tissue samples were collected, processed, and analyzed individually.
All mAbs were purchased from BD Biosciences. Fc receptors were blocked, and tissue-derived single cell suspensions were stained as previously described (21). Cell staining was performed using fluorochrome-conjugated mAbs against CD3, CD4, CD8, CD45, CD45.1, and matching isotype control mAbs recommended by the manufacturer. Analysis was performed using a FACSCalibur cytometer with CellQuest software (Becton Dickinson, Mountain View, CA). To determine absolute number of cells in samples, a known quantity of 15 µm polystyrene microbeads (Bangs Laboratories, Fishers, IN) was added to each sample (21, 33). To determine the proliferation state of adoptively transferred cells within collected tissues, the geometrical mean of CFSE intensity of CD45.1+CD8+ and CD45.1+CD8− cells was recorded.
To assess IFNγ secretion in response to tumor cell stimulation, erythrocyte-depleted peripheral blood cells (1×106/ml CM) from tumor-bearing mice were placed into 24-well culture plates and stimulated with D5 cells (1×105/ml CM). After 4 hours, Brefeldin A (GolgiPlug, 1 µl/ml, BD Biosciences) was added. Cells were cultured for an additional 16 hours and then stained for CD4 and CD8. Intracellular staining for IFNγ was performed according to the manufacturer’s recommendations.
Tumor Irradiation
Liver tumors were irradiated by the Experimental Irradiation Core at the Cancer Center. Mice were placed in individual plastic restraints to ensure immobilization. Radiation was directed to the tumor site with the rest of the mouse being shielded. Radiation was delivered in 3 consecutive daily fractions of 4 Gy on days 3–5 after tumor inoculation, using an X-ray unit (Pantak Therapax DXT 300 Model; Pantak, East Haven, CT) at a dose rate of ∼3 Gy/min.
Generation of Bone Marrow-Derived DCs
DCs were prepared as previously described (20) and suspended at 1×106 cells/0.05 ml PBS for i.t. injection. DC vaccination was administered on day 6 and 10 after intrahepatic tumor inoculation via mini-laparotomy.
Monitoring Anti-Tumor Responses
On day 15 or 16 after induction of pulmonary metastases, mice were euthanized, and lungs were insufflated and fixed in Fekette’s solution. Metastases were enumerated under a magnifying glass. S.c. tumors were measured three times per week in the largest perpendicular diameters. Liver tumors were measured via exploratory laparotomy performed on day 14 after tumor inoculation. In some experiments, hepatic tumors were measured on day 19 by ultrasound imaging. This procedure was performed by the Center for Integrative Genomics under anesthesia using the high frequency-high resolution Vevo 770 ultrasound machine (VisualSonics, Toronto, ON, Canada) designed for imaging of mice (34). Images were acquired in sagittal and transverse planes. Three perpendicular tumor diameters were measured in the largest cross-section frame. Tumor volumes were calculated using the formula for the volume of an ellipsoid. Our preliminary studies and published reports (35, 36) established good correlation between liver tumor measurements obtained via ultrasound in mice and those obtained under direct vision. The abdominal cavity was explored for metastatic disease during laparotomy and at autopsy. Mice survival was monitored and recorded. Mice were euthanized when metastatic disease precluded peritoneal closure during laparotomy or once they have reached a moribund state as defined by the University Committee on Use and Care of Animals policy for end-stage illness and humane endpoints.
Statistical Analysis
Data were evaluated by unpaired t test (2 cohorts) or one-way analysis of variance (ANOVA) followed by Fisher’s protected least significant difference (PLSD) test for multiple comparisons (>2 cohorts). When data sets did not show a normal distribution, groups were compared using the Kruskal-Wallis test. Survival curves were compared using the log-rank test. P values < 0.05 were considered statistically significant.
RESULTS
Lymphodepletion augments the therapeutic efficacy of adoptive T-cell transfer without promoting proliferation or accumulation of the infused cells
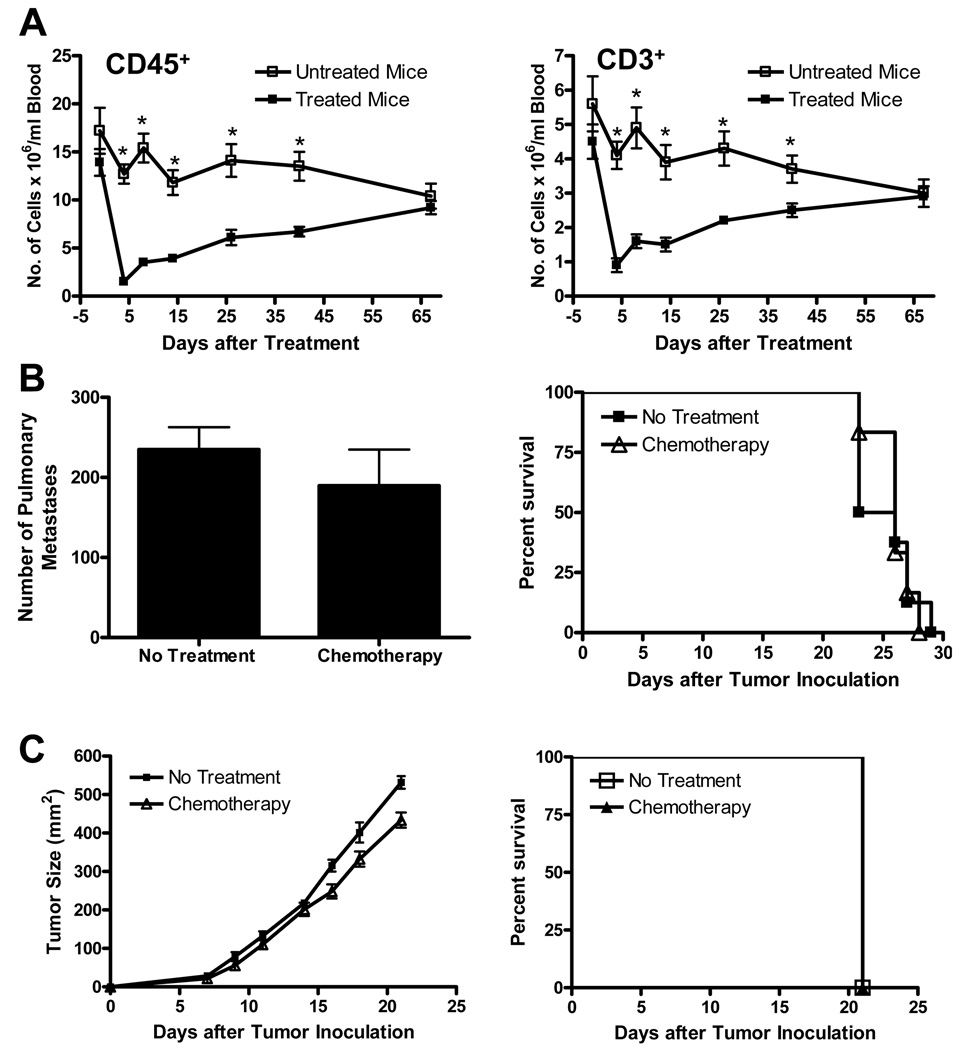
Treatment of C57BL/6 mice with cyclophosphamide (100 mg/kg) and fludarabine (200 mg/kg) mediated a profound, yet transient, reduction in total white blood cell (CD45) and T cell (CD3) counts in peripheral blood samples (Fig. 1A). CD45 and CD3 cell counts decreased by 90% and 80%, respectively, compared with pre-treatment values 4 days after initiation of treatment and gradually reverted to normal over a period of 67 days. Importantly, administration of this chemotherapy regimen to mice bearing 3-day D5 pulmonary metastases or s.c. tumors did not significantly inhibit tumor growth or prolong mice survival (Fig. 1B and C).
FIGURE 1.
Treatment of mice with cyclophosphamide and fludarabine induces a profound, yet transient, reduction in the number of CD45+ and CD3+ cells in peripheral blood samples without inhibiting D5 tumor growth or prolonging mice survival. A, Naïve mice received chemotherapy on days 0 and 1. Blood samples, drawn at the designated time points, were analyzed for CD45+ and CD3+ cells. Data represents mean ± SE (n=5). *P<0.02. B and C, Mice were inoculated i.v. (B) or s.c. (C) with D5 cells on day 0. Chemotherapy was administered on days 3 and 4. Anti-tumor responses were evaluated by enumerating pulmonary metastases on day 15 (B left), measuring s.c. tumor size (C left), and monitoring mice survival (B and C right). (n=6). All experiments were repeated two times with similar results.
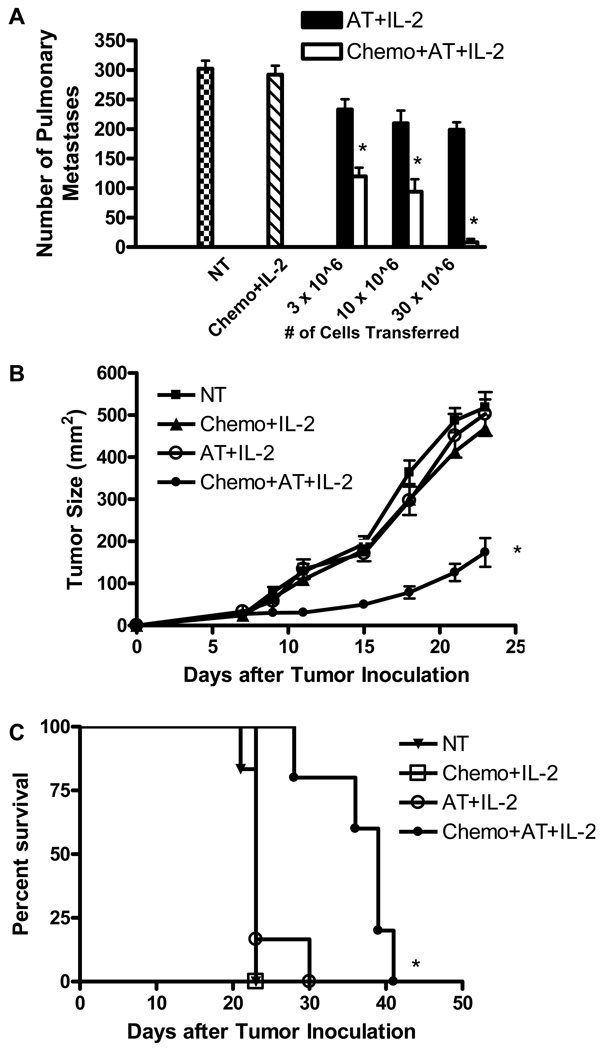
We utilized this clinically relevant murine model to examine the effects of a lymphopenic environment on AT of activated, tumor-reactive T cells. Our previous studies have shown that AT of ex vivo antibody-activated and IL-2-expanded D5-G6 TDLN cells followed by IL-2 administration mediates regression of 4-day D5 pulmonary metastases (24). Therefore, in this study, the anti-tumor efficacy of these effector cells was evaluated in a 7-day D5 lung metastases model and in a 7-day s.c. D5 tumor model. As shown in Fig. 2A, infusion of tumor-reactive T cells to mice bearing 7-day D5 pulmonary metastases followed by IL-2 treatment led to a modest reduction of lung tumors compared with control mice (P<0.02 for all three doses of infused cells). However, induction of lymphopenia prior to AT significantly enhanced therapeutic efficacy at all cell doses tested. AT of tumor-reactive T cells induced tumor regression in a dose dependent manner in both wild type and lymphodepleted hosts, but this trend reached statistical significance only in the latter group (P<0.002 comparing infusion of 30×106 versus 10×106 cells after administration of chemotherapy). Growth of 7-day s.c. D5 tumors was not inhibited by AT of 3×107 cells (Fig. 2B). In contrast, administration of chemotherapy before T cell infusion caused significant tumor growth inhibition and significantly prolonged mice survival (Fig. 2C).
FIGURE 2.
Induction of lymphopenia prior to adoptive transfer (AT) enhances the therapeutic efficacy of the infused cells. D5 pulmonary metastases (A) or s.c. tumors (B, C) were established in mice on day 0. Chemotherapy (chemo) was administered on days 3 and 4. AT (3×107 cells, unless otherwise specified) was given on day 7, followed by IL-2. Anti-tumor responses were evaluated by enumerating lung metastases on day 16 (A), measuring tumor size (B), and monitoring mice survival (C). Data represents mean ± SE (n=5). A,*P<0.001 comparing equal number of cells transferred with or without chemotherapy. B and C,*P<0.005 versus all other groups. All experiments were repeated two times with similar results. NT, no treatment.
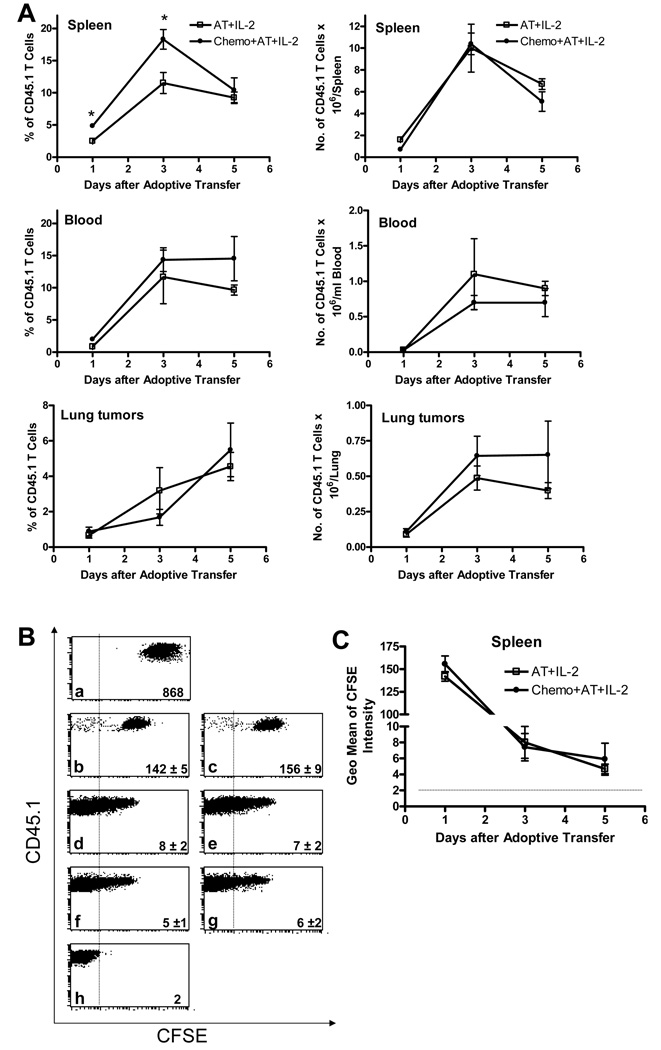
To assess in vivo accumulation and proliferation of the infused cells, CFSE-labeled CD45.1 tumor-reactive T cells were injected to CD45.2 tumor-bearing mice. In the D5 pulmonary metastases model, during the first 5 days after AT, accumulation of infused CD45.1 T cells within spleens, blood samples and lung tumors did not differ significantly between lymphodepleted and wild type mice (Fig. 3A). On day 1 and 3 after AT, the mean percentage of CD45.1 cells in spleens of mice treated with chemotherapy plus AT was significantly increased compared with mice treated with AT alone. However, this finding is probably due to depletion of host immune elements by the administration of chemotherapy since the absolute number of CD45.1 cells in the spleen did not differ significantly between the two treatment groups. Previous studies have established that 24 hours after AT, most of infused cells in the lung are localized within tumor nodules (33). Thus, evaluation of blood-flushed tumor-bearing lungs reflects intratumoral accumulation of the infused cells. Based on dye dilution within the above mentioned tissues, in vivo proliferation of CD45.1 infused cells occurred to the same extent in lymphopenic versus wild type mice (Fig. 3B, C, and data not shown). Similar results were observed in the s.c. D5 tumor model (data not shown). These findings confirm that a lymphopenic environment augments the anti-tumor efficacy of adoptively transferred tumor-reactive T cells. However, in this tumor model, lymphodepletion failed to facilitate proliferation and accumulation of the infused cells within tumor-bearing hosts.
FIGURE 3.
Induction of lymphopenia prior to AT does not enhance accumulation (A) or proliferation (B and C) of infused cells. Pulmonary metastases were induced in CD45.2 mice and treated as described in Fig. 2A. CFSE-labeled CD45.1 cells (3×107) were used for AT. Spleens, blood samples, and lungs were collected 1, 3, and 5 days after AT. A, Tissue-derived single cell suspensions were analyzed for CD45.1+ cells. Data are presented as mean percentage (left panels) or mean absolute number (right panels) of CD45.1 T cells ± SE (n=5). *P<0.02. B, Dot plots of CD45.1 T cells with (a) or without (h) CFSE labeling before adoptive transfer. Representative dot plots (gated on CD45.1+ cells) of spleen cells obtained 1 (b and c), 3 (d and e), and 5 (f and g) days after AT from mice treated with AT+IL-2 (b, d, and f) or Chemo+AT+IL-2 (c, e, and g). Numbers in dot plots designate mean ± SE of the geometrical mean of CFSE intensity (n=5). Dotted lines indicate lower limit of detection. C, The geometrical mean of CFSE intensity of CD45.1+ cells in spleen samples was recorded. Data represent mean ± SE (n=5). Dotted line indicates lower limit of detection based on unstained cells.
Radiotherapy enhances the therapeutic efficacy of DC vaccination in a liver tumor model
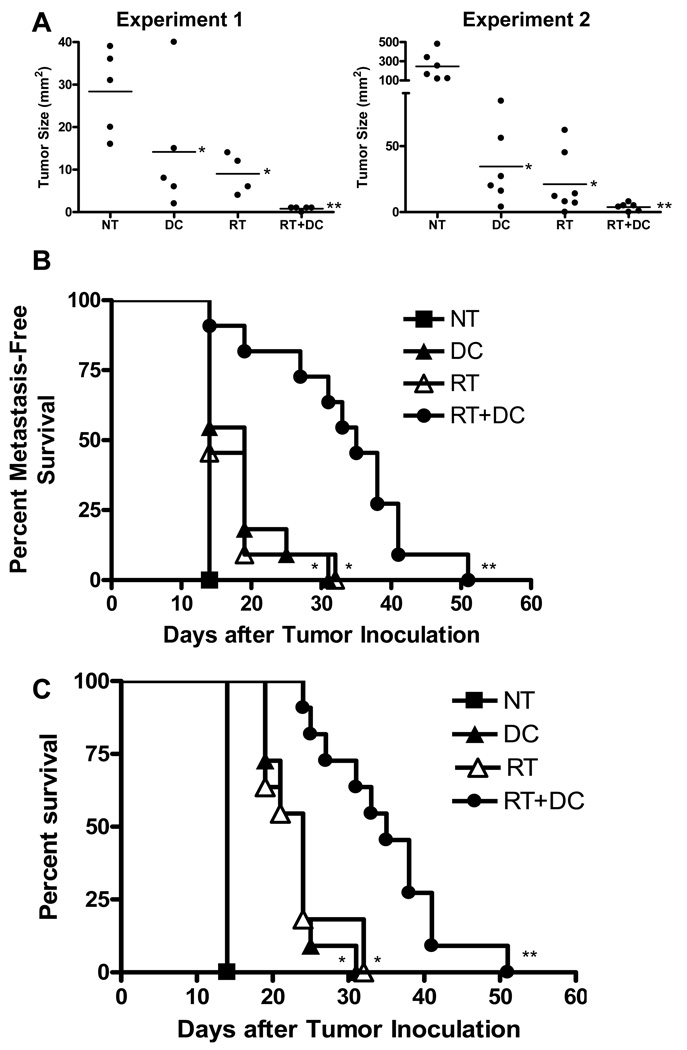
Utilizing s.c. tumor models, we have previously demonstrated that tumor irradiation augments the therapeutic efficacy of i.t. unpulsed DC administration (20, 22). To validate these results in a metastatic melanoma liver tumor model, mice were inoculated intrahepatically with D5 cells on day 0. Tumor irradiation was delivered on days 3–5. DCs were administered i.t. on days 6 and 10. Measurement of liver tumors on day 14 after tumor inoculation showed that both radiation therapy alone and DC vaccination alone significantly inhibited tumor growth compared with untreated mice (Fig. 4A). However, combining radiation with DC vaccination resulted in enhanced tumor regression (p < 0.01 versus all other groups). Mice were evaluated for intra-abdominal metastasis on day 14, 19, and after death. In all untreated mice, D5 hepatic tumors metastasized spontaneously into the peritoneal cavity by day 14 (Fig. 4B). Radiation therapy alone and DC vaccination alone slightly, albeit significantly, prolonged metastasis-free survival. Compared with both mono-therapies, radiation plus DC administration significantly inhibited the incidence of metastatic spread. Radiation alone, as well as DC vaccination alone, significantly prolonged overall survival (Fig. 4C). Further increase in mice survival was observed in response to the combined treatment (p <0.01 versus all other groups). These results suggest that an effective anti-tumor immune response can be elicited against liver tumors. However, despite the significant enhancement in therapeutic efficacy, all mice treated with radiotherapy plus DC vaccination eventually developed disseminated metastatic disease and succumbed to melanoma.
FIGURE 4.
Radiotherapy enhances the therapeutic efficacy of dendritic cell (DC) vaccination in a liver tumor model. D5 hepatic tumors were induced in mice and then treated with radiation (RT) plus i.t. DC injections. Anti-tumor responses were evaluated by measuring tumor size on day 14 (A), assessing for intra-abdominal metastatic spread on day 14, 19, and after death (B), and monitoring mice survival (C). Each group consisted of a total of 11 mice, treated in 2 independent experiments. A, Each data point represents an individual mouse; bars depict mean. B and C, Survival curves present cumulative data of 2 experiments. *P<0.02 versus no treatment (NT), **P<0.01 versus all other groups. Statistical analysis compared groups across the 2 experiments.
Radiotherapy combined with DC vaccination enhances the therapeutic efficacy of adoptive T-cell transfer
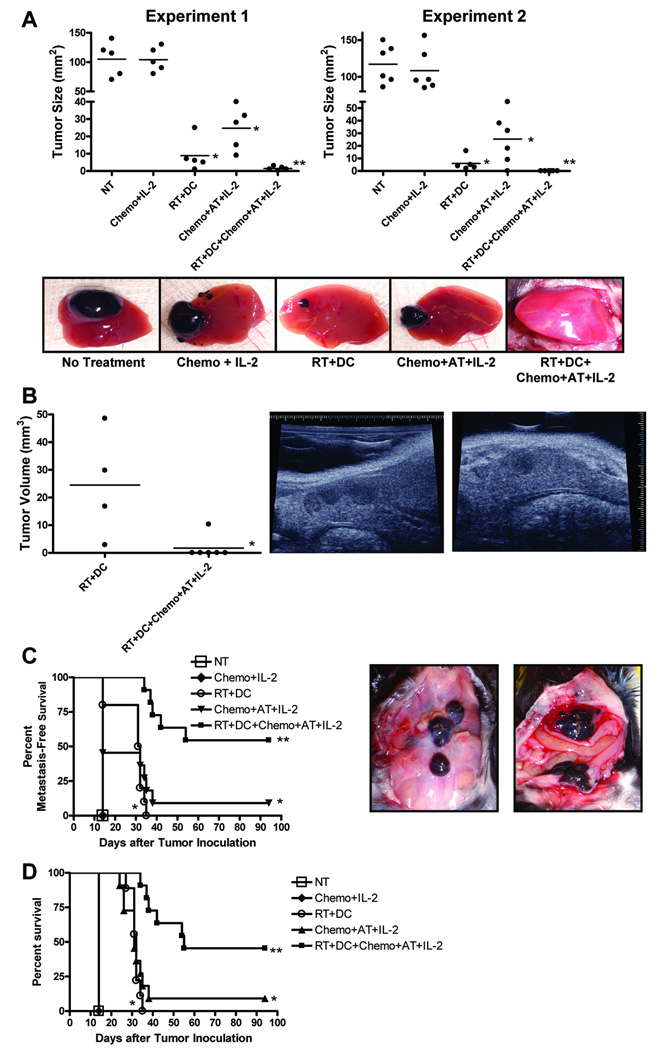
We hypothesized that tumor irradiation combined with DC administration might stimulate adoptively transferred T cells in vivo within a lymphodepleted host leading to improved anti-tumor efficacy of the infused cells. To test this hypothesis, D5 hepatic tumors were induced in mice and then treated with radiation plus DCs as described above. In addition, mice received chemotherapy on days 3 and 4. AT was given on day 7, followed by IL-2. Exploratory laparotomy, performed on day 14 after tumor inoculation, demonstrated that compared with control mice, chemotherapy plus AT plus IL-2 (Chemo+AT+IL-2) significantly inhibited tumor growth (Fig. 5A). However, in mice treated with radiation plus DC plus chemotherapy plus AT plus IL-2 (RT+DC+Chemo+AT+IL-2) tumors underwent further or complete regression. This reduction in mean tumor size reached statistical significance versus all other groups except RT+DC. Therefore, liver tumors in these two groups were further evaluated on day 19 via ultrasound imaging. Mean tumor volume in mice treated with RT+DC+Chemo+AT+IL-2 was significantly reduced (Fig. 5B).
FIGURE 5.
Radiotherapy (RT) plus dendritic cell (DC) vaccination enhances the therapeutic efficacy of adoptive transfer (AT). D5 hepatic tumors were induced in mice and then treated with RT+DC+Chemo+AT+IL-2. Anti-tumor responses were evaluated by measuring tumor size on day 14 via laparotomy (A), measuring tumor volume on day 19 via ultrasound imaging (B), assessing intra-abdominal metastatic spread on day 14 and after death (C), and monitoring mice survival (D). Each group consisted of a total of 11 mice, treated in 2 independent experiments. A, Each data point represents an individual mouse; bars depict mean. *P<0.0001 versus no treatment (NT) and chemotherapy plus interleukin-2 (Chemo+IL-2), **P<0.05 versus all other groups except RT+DC. Pictures of a representative D5 liver tumor from each group taken on day 14 are shown. B,*P<0.03. A sagittal (middle) and transverse (right) view of a representative ultrasound image of a D5 liver tumor from RT+DC are shown. C and D, Survival curves present cumulative data of 2 experiments. C,*P<0.02 versus NT and Chemo+IL-2, **P<0.002 versus all other groups. Representative pictures of disseminated intra-abdominal metastatic disease taken on day 14 from an untreated mouse (middle) and a mouse treated with Chemo+IL-2 (right) are shown. D,*P<0.0001 versus NT and Chemo+IL-2, **P<0.002 versus all other groups.
The presence of intra-abdominal metastasis was assessed during laparotomy on day 14, and after death. All untreated mice and mice treated with Chemo+IL-2 had disseminated metastatic disease on day 14 after tumor inoculation (Fig. 5C). RT+DC and Chemo+AT+IL-2 significantly prolonged metastasis-free survival. However, treatment of mice with RT+DC+Chemo+AT+IL-2 further increased metastasis-free survival (p<0.002 versus all other groups). RT+DC and Chemo+AT+IL-2 increased overall survival (Fig. 5D). Compared with these two treatment groups, RT+DC+Chemo+AT+IL-2 further prolonged survival and resulted in 46% of mice reaching long-term survival (>90 day). These findings demonstrate that in a lymphodepleted tumor-bearing host, radiotherapy combined with DC vaccination augments the therapeutic efficacy of adoptive T-cell transfer.
Radiotherapy combined with DC vaccination promotes proliferation, accumulation, and cytokine secretion of CD4+ cells
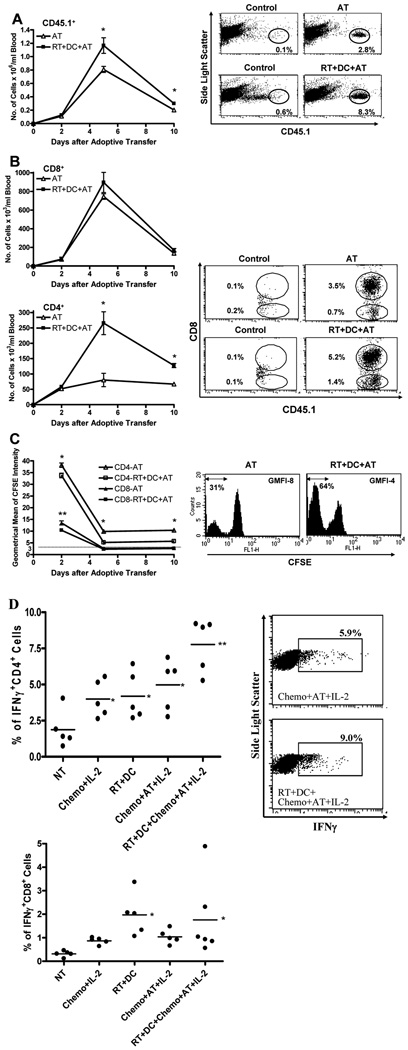
To elucidate the mechanisms by which radiation plus DC therapy enhances the anti-tumor activity of adoptively transferred T cells, we infused CFSE-labeled CD45.1 effector cells to CD45.2 liver tumor-bearing mice. Analysis of peripheral blood samples, using flow cytometry, disclosed a significantly higher number of CD45.1+ cells per ml of blood in mice treated with RT+DC+Chemo+AT+IL-2 compared with mice treated with Chemo+AT+IL-2 (Fig. 6A). This increase in the total number of donor cells was evident on day 5 and 10, but not 2, after AT. T cells used for AT consisted of ∼70% CD8+ cells and ∼30% CD4+ cells (data not shown). Further examination revealed that the number of CD45.1+CD8+ cells was not significantly elevated (Fig. 6B). In contrast, the number of CD45.1+CD4+ cells was markedly increased. At all time points tested, CD4+ donor cells detected in blood samples proliferated to a greater extent in mice treated with RT+DC+Chemo+AT+IL-2 versus mice treated with Chemo+AT+IL-2 (Fig. 6C). On day 2 after AT, CD8+ donor cells exhibited a similar trend. At later time points, dye dilution in both groups exceeded the detection limit precluding data interpretation.
FIGURE 6.
Radiotherapy (RT) plus dendritic cell (DC) vaccination promotes proliferation, accumulation, and IFNγ secretion of CD4+ cells. Liver tumors were induced in CD45.2 mice and then treated as described in Fig. 5. CFSE-labeled CD45.1 cells were used for adoptive transfer (AT). A, B, and C, All groups received chemotherapy plus IL-2 in addition to designated treatment. Erythrocyte-depleted peripheral blood cells, collected on days 2, 5, and 10 after AT, were analyzed for CD45.1+ (A), CD45.1+CD8+ (B), and CD45.1+CD8− (B) cells. The geometrical mean of CFSE intensity (GMFI) of CD45.1+CD8+ and CD45.1+CD8− cells was recorded (C). Dotted line indicates lower limit of detection based on mean value of unstained cells. Data on left represents mean ± SE (n=6). A, *P<0.02. B, *P<0.002. C, *P<0.01 versus CD4-RT+DC+AT, **P<0.02 versus CD8-RT+DC+AT. D, Blood samples were analyzed for IFNγ production of CD4+ and CD8+ cells in response to in vitro D5 tumor stimulation. Data points represent individual mice; bars depict mean. *P<0.05 versus no treatment (NT), ** P<0.02 versus all other groups. Representative dot plots or histograms acquired on day 5 after AT are shown in the right panels (A, no gate; B, gated on CD45.1+ cells; C, gated on CD45.1+CD8− cells; D, gated on CD4+ cells).
To evaluate whether this enhanced proliferation and accumulation of CD4+ infused cells correlates with augmented in vivo anti-tumor function, we examined the incidence of tumor-reactive IFNγ-producing CD4+ cells in blood samples of individual mice. This study, performed 5 days after AT, measured the percentage of donor and recipient CD4+ cells that produced IFNγ in response to in vitro D5 tumor stimulation. Blood samples of mice treated with Chemo+IL-2 contained a higher percentage of IFNγ+CD4+ cells versus untreated mice (Fig. 6D). However, this finding is probably due to the induction of lymphodepletion and therefore is unlikely to reflect an absolute increase in the number of IFNγ-producing cells. The increased incidence of IFNγ+CD4+ cells detected in blood samples of mice treated with RT+DC attests to the capacity of this combined modality to prime naïve T cells. Chemo+AT+IL-2 increased the incidence of IFNγ+CD4+ cells, but the difference did not reach statistical significance versus Chemo+IL-2. In contrast, RT+DC+Chemo+AT+IL-2 induced the highest level of circulating tumor-reactive IFNγ-producing CD4+ cells (p<0.02 versus all other groups). Using the same approach, we also examined the incidence of tumor-reactive IFNγ+CD8+ cells in peripheral blood samples of individual mice. As shown in the lower panel of Fig. 6D, treatment of mice with RT+DC induced a significant increase in the percentage of circulating IFNγ+CD8+ cells compared with untreated mice. The mean incidence of IFNγ+CD8+ cells in blood samples of mice treated with RT+DC+Chemo+AT+IL-2 was higher than that detected in mice treated with Chemo+AT+IL-2, but the difference between these two groups was not statistically significant. These results show that the enhancement in therapeutic efficacy of adoptive T-cell transfer mediated by radiation combined with DC vaccination is associated with a preferential increase in proliferation and accumulation of the CD4+ infused cells. Furthermore, this approach appears to augment the anti-tumor function of both endogenous and transferred CD4+ cells.
DISCUSSION
To the best of our knowledge, this is the first report to demonstrate that radiotherapy combined with DC vaccination enhances the therapeutic efficacy of adoptive T-cell immunotherapy of cancer. The enhancement in therapeutic efficacy was manifested by increased regression of tumors at the primary site as well as decreased incidence of metastatic spread leading to a pronounced benefit in survival. These findings indicate that this novel approach offers better control of both local and systemic disease.
The experimental design of this animal study was meant to enhance the clinical relevancy and applicability of the presented data. The identity of target antigens on tumor cells remains undefined when in vivo priming of DCs and T cells with whole tumor-derived antigens is implemented. This strategy, however, particularly in the clinical setting, provides the potential advantage of eliciting immunity against a broad and unique repertoire of tumor associated antigens that is characteristic of each individual host. A phase I clinical trial conducted in patients with advanced hepatoma demonstrated that administration of radiotherapy plus i.t. DCs is feasible, safe, and capable of eliciting tumor-specific immune responses as well as clinical responses (37). Therefore, addition of this combined treatment to adoptive T-cell transfer is unlikely to increase toxicity. The major morbidity associated with this approach is related to induction of lymphopenia (12). Further studies evaluating the capacity of radiation plus DCs to enhance the anti-tumor efficacy of AT in lymphodepleted versus wild type tumor-bearing hosts may address this concern.
Lou et al. (19) reported that immunization of s.c. B16-bearing mice with DCs pulsed with human gp100-derived peptide improves the anti-tumor efficacy of adoptively transferred pmel-1 cells. Park et al. (38) expanded this concept to a carcinoembryonic-expressing colon adenocarcinoma model. Recently, dendritic-tumor hybrid vaccination has been shown to augment the therapeutic efficacy of tumor-reactive T-cell AT (39, 40). Koike et al. (41) demonstrated that induction of lymphodepletion in tumor-bearing hosts does not enhance the anti-tumor efficacy of ovalbumin peptide-pulsed DC vaccination, but in this setting naïve T-cell AT induces tumor regression.
In this study, adoptively transferred T cells consisted of CD8+ and CD4+ cells, both of which have previously been shown to independently mediate tumor regression upon AT (31, 32). At the same time, DCs were loaded in vivo with the wide spectrum of whole tumor-derived antigens. This allowed us to directly compare the in vivo stimulatory effects of radiation plus DC vaccination on CD8+ versus CD4+ infused cells. Surprisingly, radiation plus DC vaccination preferentially increased expansion of CD4+, rather than CD8+, transferred cells. This pronounced proliferation and accumulation of CD4+ cells correlated with enhanced tumor-reactive IFNγ secretion. Although CD8+ CTLs are the cell subset known to mediate direct tumor cell kill (42), CD4+ T cells control the effector function, memory, and maintenance of these CD8+ T cells (15). Therefore, future studies will be aimed at elucidating the extent and mechanisms by which CD4+ tumor-reactive T cells contribute to the enhanced therapeutic efficacy of combined therapy with radiation, DC vaccines, and adoptive T-cell transfer.
Radiotherapy is already applied in the treatment of a wide array of human cancers due to its direct cytotoxic effect. However, accumulating data indicates that local tumor irradiation can also enhance the anti-tumor efficacy of DC-based immunotherapy by various pathways. Besides serving as an excellent source of tumor antigens (43), irradiated dying tumor cells have been shown to release the high-mobility-group box 1 (HMGB1) alarmin (44). Extracellular HMGB1 has been reported to act as a chemoattractant and activator of DCs (45). Binding of HMGB1 to Toll-like receptor 4 expressed on DCs was found to induce efficient processing and cross-presentation of tumor-derived antigens (44). We have reported that tumor irradiation augments the capacity of exogenously i.t. administered DCs to capture tumor antigens, home to the draining lymph node, and present processed antigens to T cells (21). Down-regulation of CCL21 gene expression within irradiated tumors may account for the facilitated migration of i.t. administered DC to the draining lymph node (21). Tumor irradiation may promote recruitment of effector T cells by inducing release of CXCL16 from cancer cells (46), and by facilitating normalization of the vasculature (47). Ionizing radiation may favorably alter the immunosuppressive microenvironment within solid tumors (48) leading to increased accumulation and improved function of DC-elicited and DC-stimulated tumor-reactive T cells. Exposure of tumor cells to radiation may render the cells more susceptible to T-cell killing by up-regulating cell surface expression of Fas (49). Tumor irradiation has also been shown to sensitize cancer stroma cells for efficient destruction by CTLs (50). These recent intriguing findings shed new light on the immunomodulatory capacity of ionizing radiation, and suggest that gaining better insight into the effects of radiotherapy on the tumor environment may allow future harnessing of the full potential of this modality to the combat against human cancer.
ACKNOWLEDGMENTS
We thank Anne-Michelle Noone (Department of Biostatistics, University of Michigan) for statistical consultation.
This work was supported by NIH grant CA59327, the Gillson Longenbaugh Foundation, and a gift from the Danto Family.
Footnotes
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
REFERENCES
- 1.Arciero CA, Sigurdson ER. Diagnosis and treatment of metastatic disease to the liver. Semin Oncol. 2008;35:147–159. doi: 10.1053/j.seminoncol.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Young SE, Martinez SR, Essner R. The role of surgery in treatment of stage IV melanoma. J Surg Oncol. 2006;94:344–351. doi: 10.1002/jso.20303. [DOI] [PubMed] [Google Scholar]
- 3.Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- 4.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 5.Li Q, Chang AE. Adoptive T-cell immunotherapy of cancer. Cytokines Cell Mol Ther. 1999;5:105–117. [PubMed] [Google Scholar]
- 6.Gattinoni L, Powell DJ, Jr, Rosenberg SA, et al. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q, Carr A, Ito F, et al. Polarization effects of 4-1BB during CD28 costimulation in generating tumor-reactive T cells for cancer immunotherapy. Cancer Res. 2003;63:2546–2552. [PubMed] [Google Scholar]
- 8.Li Q, Carr AL, Donald EJ, et al. Synergistic effects of IL-12 and IL-18 in skewing tumor-reactive T-cell responses towards a type 1 pattern. Cancer Res. 2005;65:1063–1070. [PubMed] [Google Scholar]
- 9.Iuchi T, Teitz-Tennenbaum S, Huang J, et al. Interleukin-21 augments the efficacy of T-cell therapy by eliciting concurrent cellular and humoral responses. Cancer Res. 2008;68:4431–4441. doi: 10.1158/0008-5472.CAN-07-5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shrikant P, Mescher MF. Opposing effects of IL-2 in tumor immunotherapy: promoting CD8 T cell growth and inducing apoptosis. J Immunol. 2002;169:1753–1759. doi: 10.4049/jimmunol.169.4.1753. [DOI] [PubMed] [Google Scholar]
- 11.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dummer W, Niethammer AG, Baccala R, et al. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185–192. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang LX, Shu S, Plautz GE. Host lymphodepletion augments T cell adoptive immunotherapy through enhanced intratumoral proliferation of effector cells. Cancer Res. 2005;65:9547–9554. doi: 10.1158/0008-5472.CAN-05-1175. [DOI] [PubMed] [Google Scholar]
- 15.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kedl RM, Rees WA, Hildeman DA, et al. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. 2000;192:1105–1113. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 19.Lou Y, Wang G, Lizee G, et al. Dendritic cells strongly boost the antitumor activity of adoptively transferred T cells in vivo. Cancer Res. 2004;64:6783–6790. doi: 10.1158/0008-5472.CAN-04-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teitz-Tennenbaum S, Li Q, Rynkiewicz S, et al. Radiotherapy potentiates the therapeutic efficacy of intratumoral dendritic cell administration. Cancer Res. 2003;63:8466–8475. [PubMed] [Google Scholar]
- 21.Teitz-Tennenbaum S, Li Q, Okuyama R, et al. Mechanisms involved in radiation enhancement of intratumoral dendritic cell therapy. J Immunother. 2008;31:345–358. doi: 10.1097/CJI.0b013e318163628c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang J, Wang Y, Guo J, et al. Radiation-induced apoptosis along with local and systemic cytokine elaboration is associated with DC plus radiotherapy-mediated renal cell tumor regression. Clin Immunol. 2007;123:298–310. doi: 10.1016/j.clim.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Teitz-Tennenbaum S, Li Q, Davis MA, et al. Dendritic cells pulsed with keyhole limpet hemocyanin and cryopreserved maintain anti-tumor activity in a murine melanoma model. Clin Immunol. 2008;129:482–491. doi: 10.1016/j.clim.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arca MJ, Krauss JC, Aruga A, et al. Therapeutic efficacy of T cells derived from lymph nodes draining a poorly immunogenic tumor transduced to secrete granulocyte-macrophage colony-stimulating factor. Cancer Gene Ther. 1996;3:39–47. [PubMed] [Google Scholar]
- 25.Chou T, Bertera S, Chang AE, et al. Adoptive immunotherapy of microscopic and advanced visceral metastases with in vitro sensitized lymphoid cells from mice bearing progressive tumors. J Immunol. 1988;141:1775–1781. [PubMed] [Google Scholar]
- 26.Geiger JD, Wagner PD, Cameron MJ, et al. Generation of T-cells reactive to the poorly immunogenic B16-BL6 melanoma with efficacy in the treatment of spontaneous metastases. J Immunother Emphasis Tumor Immunol. 1993;13:153–165. doi: 10.1097/00002371-199304000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Chang AE, Li Q, Bishop DK, et al. Immunogenetic therapy of human melanoma utilizing autologous tumor cells transduced to secrete granulocyte-macrophage colony-stimulating factor. Hum Gene Ther. 2000;11:839–850. doi: 10.1089/10430340050015455. [DOI] [PubMed] [Google Scholar]
- 28.Chang AE, Aruga A, Cameron MJ, et al. Adoptive immunotherapy with vaccine-primed lymph node cells secondarily activated with anti-CD3 and interleukin-2. J Clin Oncol. 1997;15:796–807. doi: 10.1200/JCO.1997.15.2.796. [DOI] [PubMed] [Google Scholar]
- 29.Chang AE, Li Q, Jiang G, et al. Phase II trial of autologous tumor vaccination, anti-CD3-activated vaccine-primed lymphocytes, and interleukin-2 in stage IV renal cell cancer. J Clin Oncol. 2003;21:884–890. doi: 10.1200/JCO.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 30.Chang AE, Li Q, Jiang G, et al. Generation of vaccine-primed lymphocytes for the treatment of head and neck cancer. Head Neck. 2003;25:198–209. doi: 10.1002/hed.10195. [DOI] [PubMed] [Google Scholar]
- 31.Arca MJ, Krauss JC, Aruga A, et al. Concurrent induction of CD4+ and CD8+ T cells during tumor growth with antitumor reactivity in adoptive immunotherapy. J Immunother. 1997;20:138–145. doi: 10.1097/00002371-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Li Q, Yu B, Grover AC, et al. Therapeutic effects of tumor reactive CD4+ cells generated from tumor-primed lymph nodes using anti-CD3/anti-CD28 monoclonal antibodies. J Immunother. 2002;25:304–313. doi: 10.1097/00002371-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Skitzki J, Craig RA, Okuyama R, et al. Donor cell cycling, trafficking, and accumulation during adoptive immunotherapy for murine lung metastases. Cancer Res. 2004;64:2183–2191. doi: 10.1158/0008-5472.can-03-2799. [DOI] [PubMed] [Google Scholar]
- 34.Foster FS, Zhang MY, Zhou YQ, et al. A new ultrasound instrument for in vivo microimaging of mice. Ultrasound Med Biol. 2002;28:1165–1172. doi: 10.1016/s0301-5629(02)00567-7. [DOI] [PubMed] [Google Scholar]
- 35.Schmitz V, Tirado-Ledo L, Tiemann K, et al. Establishment of an orthotopic tumour model for hepatocellular carcinoma and non-invasive in vivo tumour imaging by high resolution ultrasound in mice. J Hepatol. 2004;40:787–791. doi: 10.1016/j.jhep.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Graham KC, Wirtzfeld LA, MacKenzie LT, et al. Three-dimensional high-frequency ultrasound imaging for longitudinal evaluation of liver metastases in preclinical models. Cancer Res. 2005;65:5231–5237. doi: 10.1158/0008-5472.CAN-05-0440. [DOI] [PubMed] [Google Scholar]
- 37.Chi KH, Liu SJ, Li CP, et al. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J Immunother. 2005;28:129–135. doi: 10.1097/01.cji.0000154248.74383.5e. [DOI] [PubMed] [Google Scholar]
- 38.Park MY, Kim CH, Sohn HJ, et al. The optimal interval for dendritic cell vaccination following adoptive T cell transfer is important for boosting potent anti-tumor immunity. Vaccine. 2007;25:7322–7330. doi: 10.1016/j.vaccine.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 39.Savai R, Schermuly RT, Pullamsetti SS, et al. A combination hybrid-based vaccination/adoptive cellular therapy to prevent tumor growth by involvement of T cells. Cancer Res. 2007;67:5443–5453. doi: 10.1158/0008-5472.CAN-06-3677. [DOI] [PubMed] [Google Scholar]
- 40.Tamai H, Watanabe S, Zheng R, et al. Effective treatment of spontaneous metastases derived from a poorly immunogenic murine mammary carcinoma by combined dendritic-tumor hybrid vaccination and adoptive transfer of sensitized T cells. Clin Immunol. 2008;127:66–77. doi: 10.1016/j.clim.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Koike N, Pilon-Thomas S, Mule JJ. Nonmyeloablative chemotherapy followed by T-cell adoptive transfer and dendritic cell-based vaccination results in rejection of established melanoma. J Immunother. 2008;31:402–412. doi: 10.1097/CJI.0b013e31816cabbb. [DOI] [PubMed] [Google Scholar]
- 42.Breart B, Lemaitre F, Celli S, et al. Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J Clin Invest. 2008;118:1390–1397. doi: 10.1172/JCI34388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strome SE, Voss S, Wilcox R, et al. Strategies for antigen loading of dendritic cells to enhance the antitumor immune response. Cancer Res. 2002;62:1884–1889. [PubMed] [Google Scholar]
- 44.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 45.Yang D, Chen Q, Yang H, et al. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol. 2007;81:59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- 46.Matsumura S, Wang B, Kawashima N, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099–3107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganss R, Ryschich E, Klar E, et al. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002;62:1462–1470. [PubMed] [Google Scholar]
- 48.Demaria S, Formenti SC. Sensors of ionizing radiation effects on the immunological microenvironment of cancer. Int J Radiat Biol. 2007;83:819–825. doi: 10.1080/09553000701481816. [DOI] [PubMed] [Google Scholar]
- 49.Chakraborty M, Abrams SI, Camphausen K, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–6347. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 50.Zhang B, Bowerman NA, Salama JK, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]