Abstract
Clones of telomerized fibroblasts of adult human skin have earlier been obtained. It was shown that despite their fast growth in mass cultures, these cells poorly form colonies. Conditioned medium, antioxidants, and reduced partial oxygen pressure enhanced their colony formation, but not to the level characteristic of the initial cells. The conditioned medium of telomerized cells enhanced colony formation to a much greater extent than that of the initial cells. A study of proteome of the telomerized fibroblasts has revealed changes in the activities of tens of genes. A general trend consists in weakening and increased lability of the cytoskeleton and in activation of the mechanisms controlling protein degradation. However, these changes are not very pronounced. During the formation of immortal telomerized cells, selection takes place, which appears to determine changes in the expression of some genes. It was proposed that a decrease in the capacity of telomerized cells for colony formation is due to increased requirements of these cells to cell-cell contacts. The rate of cell growth reached that characteristic of mass cultures only in the largest colonies. In this respect, the telomerized fibroblasts resembled stem cells: they are capable of self-maintenance, but “escape” to differentiation in the absence of the corresponding microenvironment (niche), which is represented by other fibroblasts. Nondividing cells in the test of colony formation should be regarded as differentiated cells, since they have no features of degradation, preserve their viability, actively move, grow, phagocytize debris, etc. It was also shown that telomerization did not prevent differentiation of myoblasts and human neural stem cells. Thus, the results obtained suggest the existence of normal mechanisms underlying the regulation of proliferation in the telomerized cells, which opens possibilities of their use in cell therapy, especially in the case of auto-transplantation to senior people, when the cell proliferative potential is markedly reduced and accessibility of stem cells is significantly restricted.
Keywords: telomerization, immortalization, telomerase, human cells, cell senescence, fibroblasts, proteome, cell therapy, neural stem cells, lentiviruses, colony formation
Introduction
There are serious grounds to believe that medicine of the future will largely be based on cell technologies. The life span of humans significantly exceeded that elaborated as a result of natural selection due to the achievements of medicine. Hence, the resources of human body are insufficient for the normal life at the age, exceeding that (up to 40 years) of the Stone Age men (Fossel, 2004). During aging, cells die and this leads to weakened functions of organs and tissues, their decreased safety, and development of diseases related to aging. In addition to aging per se, any diseases, including specific ailments related to the progressive loss of cells, and traumas also lead to a decreased number of cells. It was shown in 1998 that introduction of the gene of telomerase catalytic component is capable of immortalizing human cells, i.e., they become capable of unlimited number of divisions in culture. Transient expression of the gene of telomerase catalytic component in human cells in vitro is, in our opinion, a promising approach allowing production of an amount of cells sufficient for cell replacement therapy (Narushima et al., 2005). In this case, telomerase elongates telomeres without genetic modification of the cells. This procedure makes it possible to increase the necessary cell mass and perform, if need be, a gene engineering correction of the defect. Thus, cell replacement therapies may prove to be essential for gene therapy as well. It is possible that the main manipulations on “therapy of diseased genes” will be performed ex vivo, outside the body of a patient, with subsequent introduction of the cells in the organism. The aim of this work was a comprehensive study of various telomerized cells.
Materials and Methods
Cells
Strains of diploid fibroblasts of the human adult skin no. 1608, human skeletal myoblasts no. 1603, and embryonic fibroblasts of human lung no. 1075 were obtained from the Collection of Cell Cultures, Medical-Genetic Research Center of the Russian Academy of Medical Sciences. The cells were obtained from normal donors and had normal karyotype. Fibroblasts were grown in DMEM medium and myoblasts in F-12 medium complemented by 10% embryonic calf serum (HyClone, USA), 5% funic human serum (PanEko, Russia), and 40 U/ml gentamycin.
Primary cultures of neural stem cells were obtained by I.N. Saburina (Institute of Gene Biology, Russian Academy of Sciences) from the forebrain of human embryos (medical abortuses) at the age of 6-11 weeks. The brain was minced, treated with trypsin and versene solutions, pipetted and transferred into culture medium. The cells were cultivated in a monolayer culture in plastic T25 culture vials (Costar, USA) in DMEM-F-12 medium complemented by 3% embryonic calf serum, growth factors FGF-=2 and EGF, 10 ng/ml each, 0.11 mg/ml sodium pyruvate, 0.32 mg/ml glutamate, and 40 U/ml gentamycin. The cells were replanted after the monolayer formation using versene solution and trypsin-versene mix (1 : 1). At the early stage of cultivation (2nd passage), the gene of human telomerase catalytic component was introduced in the cells via a lentiviral construct (lenti-hTERT).
The proportions of apoptotic and mitotic cells were counted using a Diaphot inverted phase contrast microscope (Nikon, Japan). The fibroblasts that lost their spread, were rounded, and had a nucleus of irregular shape were considered apoptotic.
Introduction of gene hTERT in human cells using a plasmid
Plasmid p190 containing the full sequence of gene hTERT under the control of cytomegalovirus (CMV) promoter/enhancer in vector pCI-neo (Promega, USA) was produced in the laboratory of Prof. R. Weinberg (Whitehead Institute of Biomedical Research, USA) and kindly provided by Prof. P. Donini (University of Rome, Italy). The plasmid comprises the gene of neomycin phosphotransferase responsible for resistance against antibiotic G-418. Plasmid pCI-neo was introduced as the control. Transfection was performed by means of electroporation (Electro Cell Manipulator BTX, USA).
Obtaining of preparations of infectional recombinant lentiviral particles
cDNA of the human gene hTERT including the full coding area (Meyerson et al., 1997) was cloned under CMV promoter in lentiviral vector pLU-PL3 (Sablina et al., 2005). For packaging of vector pseudoviral particles, DNA of lentiviral vectors was introduced in 293T cells (DuBridge et al., 1987; Pear et al., 1993) simultaneously with two packaging plasmids: pCMV-deltaR8.2 (expresses gag protein and reverse transcriptase of HIV; Dull et al., 1998) and pVSV-G (expresses protein G of vesicular stomatitis virus; Naldini et al., 1996). Three plasmids were mixed in equal proportions and introduced into cells using the method of lipofection and Lipofectamine-plus system according to the protocol recommended by the producer (Invitrogen, USA). Viral particles were collected starting from 48 h after the transfection every 8 h within 3 days. Just after collection of virus-containing supernatants, they were placed onto ice and 10% (volume) PEG-8000 (Sigma, USA) were added. After the virus collection was completed, aggregates of viral particles formed in the course of PEG precipitation were pelleted by low-speed centrifugation (5000 rpm, 10 min) and suspended in 1/20 of the initial supernatant volume of fresh DMEM medium complemented by 5% embryonic bovine serum. The preparation was divided into aliquots and stored at -70°C.
Introduction of gene hTERT in human cells by means of lentiviral vector
The genes can be introduced in both growing cultures and nondividing cells. In order to increase the proportion of transfected cells, it is recommended to decrease the number of cells and increase the virus titer. The titer of a nonconcentrated virus amounts usually to 3-5 × 106/ml. After the growth medium was removed at the stage of half-monolayer, the viral concentrate, 1 ml with a titer of 108/ml, diluted by the complete growth medium with serum at 1 : 2 was added to the cells growing in T25 flasks. This mix also contained 5 μg/ml polybrene. The cells were incubated overnight and the medium was then replaced by the standard medium. Expression of the gene developed at a slow rate within three to four days. The efficiency of lentiviral transfection, according to the parallel introduction of green fluorescent protein, was no less than 75%. No cloning was performed.
A study of proliferative potential
In order to determine the number of population duplications, the cells cultivated in 25 cm2 flasks (Nunclone, Denmark) were counted at each replanting. The numbers of attached cells per unit area of the culture flask were counted before and after replanting. For this purpose, the numbers of attached cells in a field of vision of a microscope were counted before and 2 h after replanting. Counts were made in 10 random fields of vision. The ratio of mean values obtained before and 2 h after previous replanting reflects the degree of cell multiplication between replantings. The logarithm of this value for base 2 is the number of population duplications.
The efficiency of colony formation was estimated as described elsewhere (Yegorov et al., 2004): 100 to 200 cells were planted onto Petri dishes and the numbers of cells were counted within 6 to 18 days.
Immunohistochemistry
The preparations of embryonic neural stem cells were fixed by 4% formaldehyde at the room temperature for 10 min. The cells were then incubated in phosphate salt buffer with 10% cattle serum, 0.1% Triton X-100, and 0.01% tween 20. The neuronal marker βIII-tubulin was visualized by means of monoclonal mouse antibodies (CYMBUS Biotechnology Ltd, USA). Secondary antibodies were conjugated with fluorescent dye Cy-3. The cells were photographed under a Leitz microscope (Germany).
Preparation of 2D gels
The preparations were made from cells that reached a density of about 80% of the closed monolayer. The cells were detached from the sub-layer by treating with a versene solution and washed twice by phosphate salt buffer. The preparation containing 40-210 | g protein was mixed with two volumes of a buffer containing 7 M urea, 2 M thiourea, 60 mM dithiothreitol, 65 mM CHAPS, and 5% ampholyte, pH 3.5-10. Isoelectric focusing was performed in glass capillaries (internal diameter 1.5 mm, length 170 mm). The capillaries were filled with a solution containing 3.5% (weight/volume) acrylamide, 8.5 M urea, 1.5% (weight/volume) CHAPS, 0.5% (weight/volume) NP40, 0.3% (volume) ampholyte, pH 3.5-10, 0.3% (volume) ampholyte, pH5-8, 0.05% (volume) of TEMED, and 0.05% (weight/volume) of APS. Only freshly made preparations were used. Fifty mM NaOH and 6 mM phosphoric acid served as cathode and anode buffers, respectively. The voltage was gradually elevated: 100, 200, 300, 400, and 500 V for 45 min each, and then 700 V were set overnight. Electrophoresis was performed in 9-16% PAAG, 1.5 mm thick, at 10°C and 30 mA. Proteins were visualized using standard silver staining.
Computer analysis of the 2D gel image
Silvered gels were scanned with a resolution of 300 dpi and analyzed using Melanie 3 (2D PAGE image analysis) software (GeneBio, Switzerland). Similar spots were compared in the control and experimental gels. Not less than three gels were analyzed.
Identification of 2D gel spots
Spots, ca. 2 mm3, were cut out and treated with trypsin in gel as described elsewhere (Shevchenko et al., 1996). The digested proteins were subjected to mass-spectrometry on a Reflex III MALDI-TOF mass-spectrometer (Bruker, USA) according to the producer's recommendations: samples of peptides (0.2-1 were mixed with an equal volume of a solution of dihydrobenzoic acid (20 mg/ml) (Sigma, USA) containing 20% CAN and 0.1% TFA and dried from air. Mass-specters were obtained for 8004000 Da in a regime of reflection and calibrated using internal calibration (MH+ 1046.54, 2212.10 Da). For protein identification we used database NCBI protein and Mascot software.
Results
Telomerized human fibroblasts
After the gene of telomerase catalytic component hTERT had been introduced in human adult skin fibroblasts, several cell clones were obtained (Yegorov et al., 2002). The cells somewhat slowed down their proliferation while reaching the Hayflick limit, but the rate of their growth then increased and remained unchanged later (Fig. 1a). Morphologically, the cells of different clones little differed from each other and resembled young human diploid fibroblasts. They were growing at a high rate and formed a very dense monolayer (Fig. 1b). These cells were characterized by telomerase activity and their telomeres were significantly longer than in the initial cells (Fig. 1c), they retained the diploid karyotype (Fig. 1d) and capacity of entering in the state of quiescence as a result of serum starvation and were characterized by contact inhibition of proliferation. Their saturating density of proliferation markedly increased (Fig. 1e): it became approximately twice that in young (at the level of 21 population duplications) diploid fibroblasts. The mechanisms of proliferative senescence were still active in the cells (Yegorov et al., 2003) and, when telomerase was blocked by azidothymidine, the telomeres were shortened and the cells passed to the state of artificial senescence (Fig. 1f).
Figure 1.
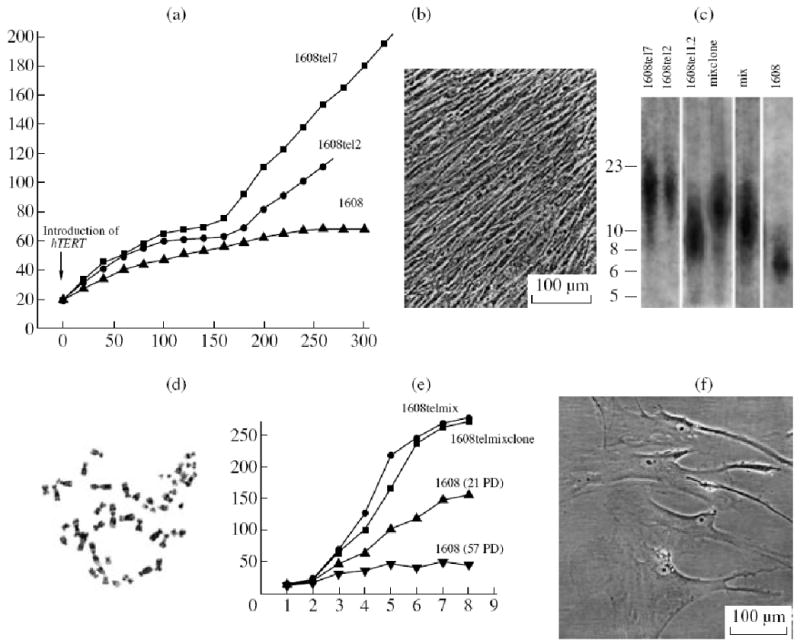
Properties of telomerized human fibroblasts. (a) Curves of cell growth after introduction of gene hTERT; abscissa: time, days; ordinate: number of population duplications. 1608, initial diploid fibroblasts; 1608tel7 and 1608tel12, clones of telomerized cells. (b) View of a closed monolayer of telomerized cells with a very high cell density. (c) Telomere lengths after telomerization, kB according to Southern hybridization. 1608, initial cells; other lanes represent different variants of telomerized cells. (d) Diploid karyotype of telomerized cells; (e) Curves saturation of cell proliferation reflecting the increase in cell numbers per unit area as the growth was inhibited due to contact inhibition of proliferation; abscissa: time, days; ordinate; number of cells, thousands. 1608telmix and 1608telmixclone, telomerized cells; 1608 (21 PD), young initial fibroblasts; 1608 (57 PD), old fibroblasts. PD, population duplication. (f) Telomerized cells in the state of artificial senescence.
Colony formation by telomerized cells
Despite an elevated growth rate in mass culture (Fig. 1a), the efficiency of colony formation by telomerized cells was significantly decreased: they formed much less colonies of smaller size (Fig. 2).
Figure 2.
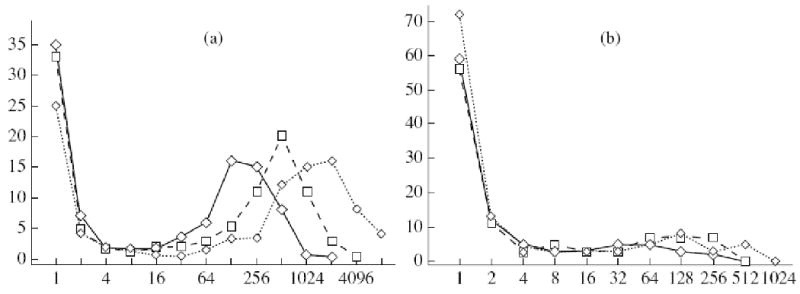
Lowered capacity of telomerized cells for colony formation: (a) 1608, (b) clone telMC. Abscissa: number of cells in colonies; ordinate: number of colonies, % (sizes of colonies expressed as cell numbers were approximate to degrees of two). Time of cultivation, days: (-<>-) 10, (-□-) 12, (-<>-) 14.
Since in all experimental variants we obtained a bimodal distribution of colonies by size, it was proposed that there is a positive feedback in the regulation of colony growth. Such a feedback may be realized via secretion of diverse growth-stimulating factors by the cells. This secretion may be effective in the direct vicinity of a colony. We studied the size distribution of separate colonies and compared it with the general distribution (Yegorov et al., 2005b). Separate colonies were defined as the colonies around which no other colonies were located at a distance of up to 5 mm. No significant differences were found (Fig. 3).
Figure 3.
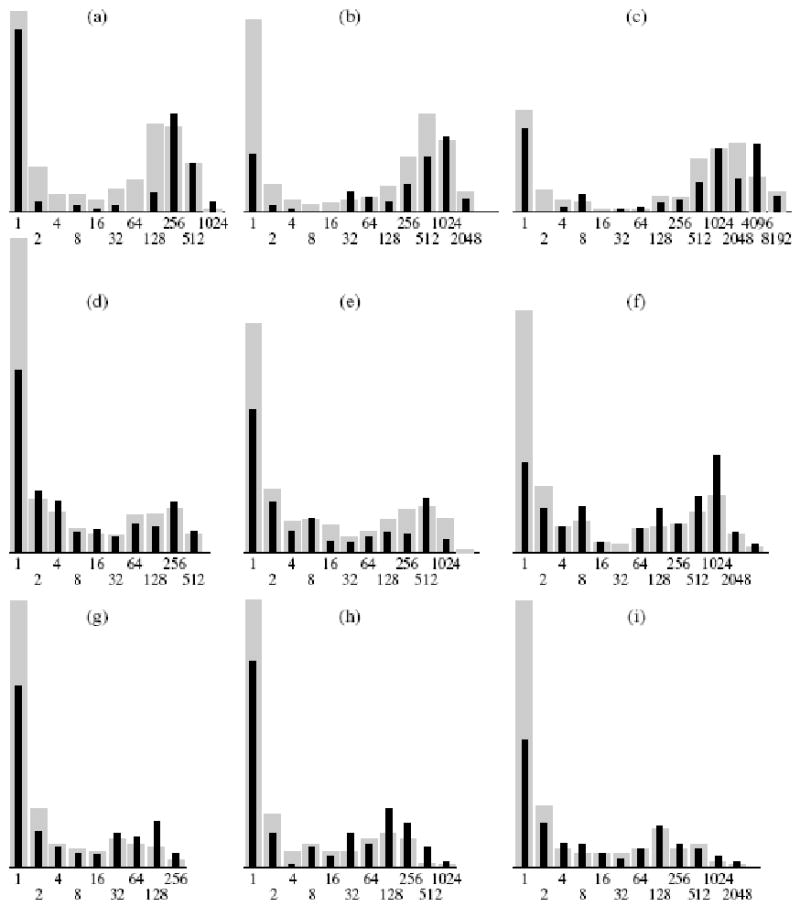
Size distribution of separate colonies. Abscissa: number of cells in colonies; ordinate: number of colonies, %. Cells within 10, 12, and 14 days of cultivation, correspondingly: (a-c) 1608; (d-f) 1075; (g-i) telMC (telomerized). Distribution of colonies: (  ) general, ( ▪ ) separate colonies.
) general, ( ▪ ) separate colonies.
Effect of conditioned medium
Since the colony size independence from the direct environment could be explained by significant stability of the factors secreted by the cells and their capacity for diffusion within the dish limits, we carried experiments with conditioned media using the previously described method (Yegorov et al., 2005b). Conditioned media exerted a significant growth-stimulating effect on colony formation. The number of large colonies increased and that of small colonies decreased at an elevated concentration of conditioned medium (Fig. 4a). The effect of a conditioned medium obtained as a result of cultivation of the telomerized cells was several fold stronger than that of the initial cells (Fig. 4b). The former affected also the initial cells, while the latter exerted no significant effect on the colony formation of telomerized cells (Fig. 4b).
Figure 4.
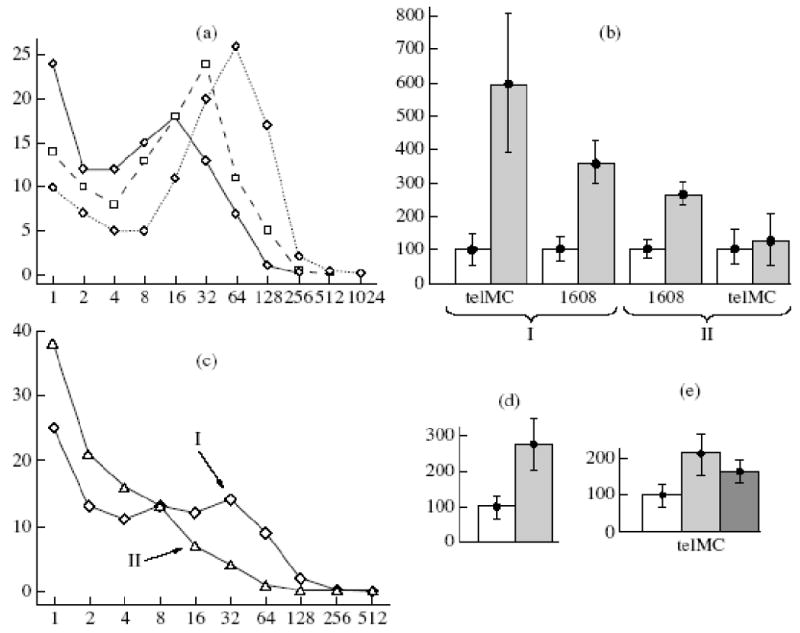
Increased colony formation of telomerized cells in the presence of conditioned medium, under decreased partial oxygen pressure, and upon addition of antioxidants. (a) Size distribution of 6-day colonies of 1608 cells in the presence of conditioned medium of telomerized cells at different concentrations, %: (-<>-) 0, (-□-) 20, (..<>..) 95. Abscissa: number of cells in colonies; ordinate: number of colonies,%. (b) Effect of conditioned media of the initial (1608, II) and telomerized cells (TelMC, I) on colony formation by 1608 and TelMC cells: ( ) 95% conditioned medium, (□) in the absence of conditioned medium. Ordinate: total amount of cells grown in a dish, %. (c) Effects of oxygen concentration on size distribution of 6-day colonies of telomerized cells. Abscissa: number of cells in colonies; ordinate, number of colonies, %. Cells were cultivated for week prior to the experiment at a corresponding oxygen concentration, %: I, 5, II, 212. (d) Increase in total number of cells in the experiments shown in (c). Oxygen concentration, %:
) 95% conditioned medium, (□) in the absence of conditioned medium. Ordinate: total amount of cells grown in a dish, %. (c) Effects of oxygen concentration on size distribution of 6-day colonies of telomerized cells. Abscissa: number of cells in colonies; ordinate, number of colonies, %. Cells were cultivated for week prior to the experiment at a corresponding oxygen concentration, %: I, 5, II, 212. (d) Increase in total number of cells in the experiments shown in (c). Oxygen concentration, %:  5, □ 21. (e) Effects of antioxidants on total increase in the number of telomerized cells in 6-day experiments of colony formation in the presence of 5% oxygen, %: (□) control, (
5, □ 21. (e) Effects of antioxidants on total increase in the number of telomerized cells in 6-day experiments of colony formation in the presence of 5% oxygen, %: (□) control, ( ) 20 mM carnosine, (
) 20 mM carnosine, ( ) 0.1 mM N-acetylcysteine
) 0.1 mM N-acetylcysteine
Role of oxidative stress
Also, we decided to check whether telomerized cells are more sensitive to oxidative stress. It has been proposed that the telomerized cells at a very low concentration (10/ml) are more sensitive to oxidative stress. Since the standard cultivation conditions are hyperoxic as compared to the in vivo conditions, experiments were carried out under the conditions of lowered partial oxygen pressure (Yegorov et al., 2005a). The decrease of partial oxygen pressure to 5%, instead of usual 21%, markedly stimulated colony formation (Fig. 4c): more large colonies were formed, while the number of small colonies decreased. As a result, the number of cells in dishes increased by a factor of 2.5 (Fig. 4d). Antioxidants acted similarly with decreased partial oxygen pressure (Fig. 4e): 20 mM carnosine enhanced colony formation at the atmospheric oxygen and at 5% oxygen and this effect was more pronounced at 21% oxygen.
Proteomic analysis
Thus, it was shown that the telomerized cells enhanced the production of growth-stimulating factors. In order to identify their chemical nature, we carried out a proteomic study of the proteins expressed by the telomerized cells.
We compared the expression of individual spots corresponding to certain proteins on images of 2D electrophoresis using Melanie 3 software. The spots that had significant differences in the telomerized and initial cells (no less than 2.5-fold) were subjected to mass-spectroscopy (MALDI-TOF) allowing protein identification. The differences were found for 69 proteins included in different databases (Table 1). Some differences, most interesting in our opinion, are presented in Table 2. When analyzing the described differences, we paid attention to the following features.
Table 1.
Differences in content of proteins between telomerized and initial cells
| Statmin 1 | + | 1p36.1-p35 |
| Cap Z | + | 1p36.1 |
| CRABP2, cellular retinoic acid binding protein 2 | - | 1q.21.3 |
| Lamin A/C, isoform 2 | - | 1q21.2-q21.3 |
| Peroxyredoxin 6 | + | 1q25.1 |
| Poly(rC) binding protein 1 | + | 2p13-p12 |
| NADH dehydrogenase (ubiquinone) Fe-S protein 1, 75 kDa, precursor | + | 2q33-q34 |
| Protein disulfide-isomerase (EC 5.3.4.1) ER60 precursor | - | 3q21.1 |
| Factor of translation initiation eIF-4A2 | + | 3q28 |
| SEC13-like 1 isoform α, SEC13-related protein | + | 3p25-p24 |
| Septin 11 | - | 4q21.1 |
| Succinate dehydrogenase complex, subunit A, flavoprotein, precursor | - | 5p15 |
| WD-40 repeat protein | + | 5q35, 5q31 |
| Karyopherin | + | 5q13.2 |
| Heat shock protein 4, isoform a, 70 kDa | + | 5q31.1-q31.2 |
| Heat shock protein 9B (precursor HSPA9B (mortalin-2)), 70 kDa | + | 5q31.1 |
| DPYSL3, protein dihydropyrimidinase-like 3 | + | 5q32 |
| G3BP, Ras-GTPase-activating protein SH3-domain-binding protein | + | 5q33.1 |
| Calnexin (CANX) | - | 5q35 |
| Nuclear chloride channel (CLIC1) | + | 6p22.1-p21.2 |
| β5-tubulin (tubulin β1 chain class Ivb) | - | 6p21.33 |
| Ecto-5′-nucleotidase | - | 6q14-q21 |
| Superoxide dismutase 2 (SOD2) | - | 6q25.3 |
| T-complex locus TSP-1 | + | 6q25.3-q26 |
| FSCN1, fascin homolog 1 | - | 7p22 |
| ACTB | - | 7p15-p12 |
| Heat shock protein 1, 27 kDa | - | 7q11.23 |
| ZYX | + | 7q32 |
| Aldosoreductase (EC 1.1.1.21) | + | 7q35 |
| Dihydropyrimidinase-like 2; collapsin response mediator protein hCRMP-2 | - | 8p22-p21 |
| Valosin containing protein | - | 8q13 |
| Eukaryotic factor of translation elongation eef1δ, isoform 2 | + | 8q24.3 |
| Transferrin | + | 8q32 |
| Thioredoxin | + | 9q31 |
| TXNDC4, thioredoxin domain containing 4 (endoplasmic reticulum) | - | 9q31.1 |
| Heterogeneous nuclear ribonucleoprotein K isoform a, uracil-DNA glycosylase | + | 9q21.32-q21.33 |
| Annexin 1 (lipocortin I) | - | 9q12-q21.2 |
| P4HA1, prolyl-4-hydroxylase protein | - | 10q21.3-q23.1 |
| Transaldolase (TALDO1, EC 2.2.1.2) | + | 11p15.5-p15.4 |
| Rcal, reticulocalbin 1 precursor | - | 11p13 |
| Nicotinamide N-methyl transferase | - | 11q23.1 |
| Zinc finger protein 259 | + | 11q23.3 |
| Heat shock protein 8, isoform 1, 70 kDa (HSPA8) | + | 11q24.1 |
| α2-Macroglobulin | - | 12p13.3-p12.3 |
| Triosophoisphate isomerase (Tim, EC 5.3.1.1) | + | 12p13 |
| FK506-binding protein 4 | + | 12p13.33 |
| Alkaline light chain of smooth muscle and nonmuscle myosin, isoform 1 | + | 12q13.2 |
| C12orf8, endoplasmic reticulum protein 29 precursor | + | 12q24.13 |
| Proteasome activator PA28, β-chain | + | 14q11.2 |
| hnRNP C2 | + | 14q11.2 |
| LGALS3 | - | 14q21-q22 |
| AHSA1, AHA1, activator of heat shock 90kDa protein ATPase homolog 1 | + | 14q23.3-31 |
| Phospholipase Cα, protein disulfide isomerase-associated 3 | - | 15q15 |
| Tropomyosin 3, fibroblastic | - | 1q22-q23 |
| Factor of translation initiation eIF-5A | - | 17p13-p12 |
| Lasp-1, LIM and SH3 protein 1 | - | 17q11-q21.3 |
| DEAD (Asp-Glu-Ala-Asp) box polypeptide 48 | + | 17q25.3 |
| Rho GDP dissociation inhibitor (GDI) α | + | 17q25.3 |
| TXNL1, thioredoxin-like protein | + | 18q21.2 |
| INB2, serine (or cysteine) proteinase inhibitor | + | 18q21.3 |
| R33729_1, chromosome 19 open reading frame 10 | - | 19p13.3 |
| Glyceraldehyde-3-phosphate dehydrogenase | - | 19q13.1 |
| Elongation factor 2 (EEF2, EF2) | - | 19pter-q12 |
| Proliferating cell nuclear antigen (PCNA) | + | 20pter-p12 |
| CCT8 Chaperonin containing TCP1, subunit 8 (theta) | - | 21q22.11 |
| Superoxide dismutase 1 (SOD1, Cuznsod) | - | 21q22.1, 21q22.11 |
| Type VI collagen, α1 | + | 21q22.3 |
| Pyridoxal kinas (PDXK) | - | 21q22.3 |
| Glucose-6-phosphate dehydrogenase | + | Xq28 |
* “+” elevated, “-” lowered.
Table 2.
Specific features of protein content* in telomerized cells
| Structural proteins | β5-tubulin | - | |
| Lamin A/C | - | ||
| Tropomyosin 3 | - | ||
| Maintenance of cell structure | Proteins of focal adhesion regions | Lasp-1 | - |
| ZYX | + | ||
| Light alkaline chain of myosin, isoform 1 | + | ||
| Destabilization of microtubules | Statmin 1 | + | |
| Growth of actin filaments | Protein capping F-actin, subunit β | + | |
| Protein metabolism | Proteins-chaperones | Heat shock protein 4, 70 kDa | + |
| Heat shock protein 9B, 70 kDa | + | ||
| T-complex 1 | + | ||
| AHSA1 | + | ||
| HSPA8 | + | ||
| Heat shock protein 1, 27 kDa | - | ||
| Increased presentation of MHC-1 | Proteasome activator PA28, β-chain | + | |
| Proliferation | Growth factor | Transferrin | + |
| Extracellular protease inhibitor | α2-Macroglobulin | - | |
| Cofactor of DNA-polymerase | Proliferating cell nuclear antigen (PCNA) | + | |
| Protection against reactive oxygen forms | Superoxide dismutase 1 (SOD1) | - | |
| Superoxide dismutase 2 (SOD2) | - | ||
| Extracellular protections | Thioredoxin | + | |
| Peroxyredoxin 6 | + | ||
| Protein resembling thioredoxin (TXNL1) | + | ||
| Production of NADP—key donor of electrons | Glucose-6-phosphate dehydrogenase | + | |
| Maintenance of glutathione in reduced state | Transaldolase | + | |
See Table 1.
(2) Intensity of protein metabolism
Note an increased amount of various chaperones in the telomer-ized cells (heat shock 70 kDa proteins 4 and 9B, t-com-plex 1, AHSA1, and HSPA8) versus a decreased amount of heat shock 23 kDa protein 1. In addition to chaperones, proteasomes (activator of proteasome PA28, β-chain) were activated in the telomerized cells, thus suggesting intensification of protein metabolism. Thus, the control over protein conformation was increased in the telomerized cells and pathways of their degradation were intensified.
(3) Changes related to proliferation
Changes related to proliferation were described. The content of the cofactor of DNA replicative synthesis PCNA was increased due, apparently, to increased proliferation at a decreased total content of cytoskeletal proteins. The telomerized cells produced actively transferrin, which can be considered as a growth factor. The content of extracellular protease inhibitor α2-macro-globulin was decreased, which can bind to growth factors and change cell sensitivity to these factors.
(4) Protection against reactive oxygen species
Protection against reactive oxygen species is changed in the telomerized cells. The content of superoxide dismutases 1 and 2 is decreased, while that of thioredoxin and peroxyredoxin 6 is increased. The content of transaldolase and glucose-6-phosphate dehydrogenase is also increased, thus suggesting an enhanced NADP production and maintenance of glutathione in a reduced state.
Relationship with genome structure
Having found so many distinctions of the telomerized cells, we decided to check whether they are related, at least partially, to the genome structure. The chromosome distribution of genes that changed their representation by proteins proved to be random (Fig. 5a). The distribution of such genes inside the chromosomes also looked accidental (Fig 5b): they were located predominantly on regions with high gene densities. However, we found that they were gathered in clusters (Fig. 5b) and non-randomness of such distribution, as compared to a random sample, was estimated as high as 90% (Fig. 5c). It may well be that increased clasterization is related to mutual influence of closely arranged genes, which can itself increase the number of genes that changed their protein representation.
Figure 5.
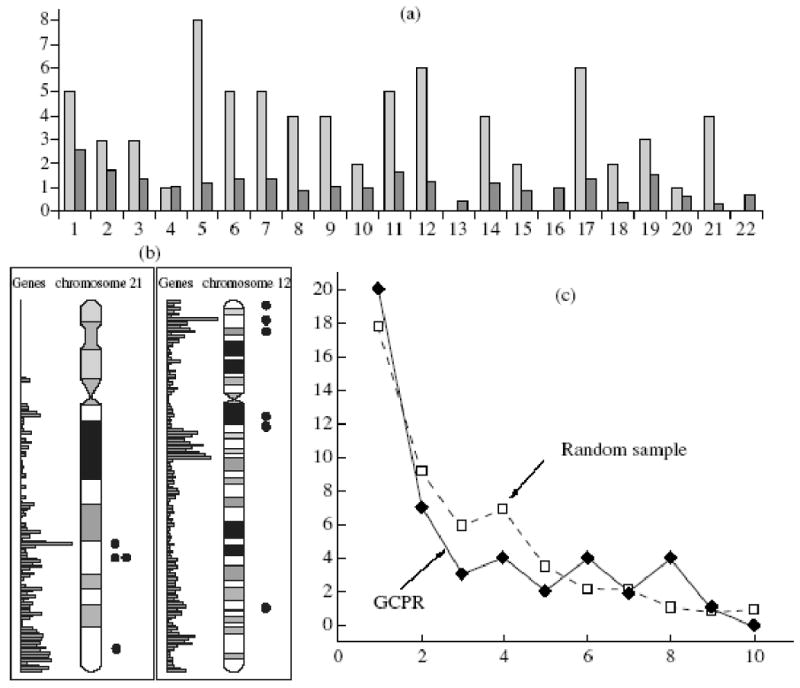
Relation of genes that changed their protein representation (GCPR) during telomerization to the genome structure. (a) Distribution of GCPR by chromosomes; Abscissa: ordinal number of chromosome; ordinate: amount of GCPR ( ) and total amount of genes on each chromosome, thousands (
) and total amount of genes on each chromosome, thousands ( ). (b) Examples GCPR distribution inside chromosomes. (c) total distribution of distances between GCPR (comparison to a random sample); abscissa: distance between GCPR, MB; ordinate: number of GCPR.
). (b) Examples GCPR distribution inside chromosomes. (c) total distribution of distances between GCPR (comparison to a random sample); abscissa: distance between GCPR, MB; ordinate: number of GCPR.
Selection in the course of telomerization
Many changes caused by the activity of one gene (hTERT) may be explained by selection in the course of production of telomerized cells, as a significantly delayed proliferation was noted in the region of Hayflick limit (Fig. 1a). Hence, we decided to test the suggestion of selection using a highly effective transfection of gene hTERT by a lentiviral vector. The amount of dying cells increased within several days after transfection (Table 3), thus suggesting unambiguously cell selection during telomerization.
Table 4.
Effect of gene hTERT expression on spontaneous neuronal differentiation in cultures of neural stem cells (NSC)
| Ordinal number of passage | Neuronally differentiated cells, %* | |
|---|---|---|
| NSC | NSC-hTERT | |
| 2 | 6.3 ± 2.4 | 6.7 ± 2.6 |
| 4 | 13.4 ± 2.7 | 16.5 ± 3.2 |
| 7 | 34.7 ± 3.5 | 43.2 ± 3.5 |
| 9 | 41.2 ± 4.2 | 49.3 ± 4.6 |
| 10 | 51.0 ± 4.4 | 54.5 ± 4.5 |
Differentiation of telomerized cells
It is of interest to follow the fate of cells that cease their proliferation in the test of colony formation. Firstly, the amount of such cells underwent no significant changes during testing for up to 14 days. We compared the total numbers of the colonies formed by adult human fibroblasts (no. 1608) and telomerized cells within different times after planting.
| Cells | Duration colony formation, days | ||
| 10 | 12 | 14 | |
| Initial fibroblasts | 108.8 ± 14 | 104.8 ± 12.4 | 88.8 ± 13.1 |
| Telomerized cells | 58.8 ± 9 | 61.4 ± 13.3 | 64 ± 10.8 |
Any groups of cells, including single cells, were considered as colonies. It can be seen that the number of colonies formed by different fibroblasts tends to decrease with time, but this decrease is statistically insignificant.
Secondly, we found that nondividing cells were subject to regular time-related changes (Fig. 6a): they are enlarged and spread, actively move, and phagocytize debris. It is hard to imagine that all these processes are expression of the beginning of degeneration of damaged cells. Since the immortal cells should not be subject to replicative senescence, the only cause of their transition into a lasting nondividing state is the process of differentiation. We are apt to consider non dividing cells in the test of colony formation as differentiated cells.
Figure 6.
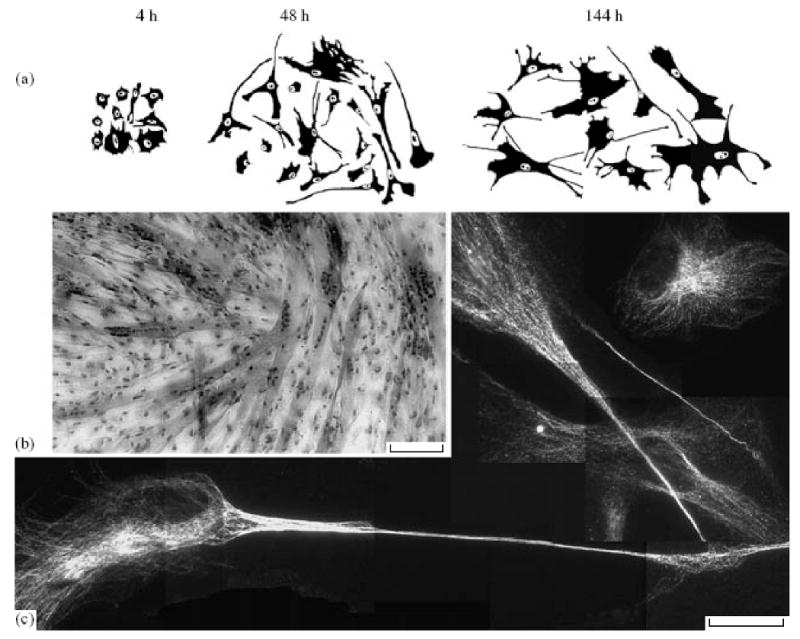
Differentiation of telomerized cells: (a) morphology of nondividing telomerized fibroblasts in the test of colony formation, time after planting; (b) formation of muscle fibers by telomerized myoblasts; polynuclear cells can be seen; (c) differentiation of human neural stem cells within nine passages after highly effective hTERT lentiviral transfection, staining for βIII-tubulin, typical neuron-like cells can be seen; Scale: (b) 80, (c) 40 μm.
In other experiments, we demonstrated that hTERT expression did not prevent differentiation. Firstly, clones of telomerized human myoblasts formed muscle fibers upon induction of myogenic differentiation (Fig. 6b). No significant differences were found in the proportion of cells involved in the muscle fiber formation for telomerized and initial myoblasts that passed through the same number of population duplications in culture.
Secondly, telomerized human neural stem cells in culture were subject to spontaneous neuronal differentiation to the same extent as the control cells (Fig. 6c; Table 4).
Discussion
In our opinion, it is most essential to account for the causes of decreased colony formation of the telomerized cells. We believe that for sustainable proliferation, the telomerized cells require a certain microenviron-ment to a greater extent than the initial cells. The telomerized cells, components of the largest colonies, grow at approximately the same rate as the cells in mass culture. The mean time of population duplication in culture for 70 days is 18 h. When a colony of 256 cells is formed (maximum size of 6-day colony), one duplication requires the same time, on average (18 h) (Yegorov et al., 2005a). It is very likely that for proliferation to occur, the cells should be in direct contact with each other. This requirement is not absolute; otherwise, no colonies could have been formed. Direct contact is required not in every cell cycle. Long-term (several cell cycles) absence of cell-cell contacts appears to serve a signal for exit from the cell cycle. In our opinion, it is most logical to term the exit of immortal undamaged cells cultured in a medium, optimal for their growth, from the cell cycle as differentiation (Yegorov et al., 2005b), since this resembles the behavior of other cells. In all likelihood, the spectrum of synthesized proteins undergoes changes in the case of such differentiation.
It has recently been shown that the well known strain of embryonic human lung fibroblasts MRC-5 is capable of expressing many features of stem cells (Rieske et al., 2005). It sufficed to change the traditional conditions of cultivation, for example, replace serum by a mix of certain growth factors, so that expression of the markers of embryonic stem cells and neuronal cells began in the cells, including the appearance of tyrosine hydroxylase, a marker of mature nerve cells. Although such examples surprise by possibilities of true or false transdifferentiation of the fibroblasts, they are unambiguously interpreted as phenomena directly related to differentiation. At the same time, transition of a fibroblast to the terminal nondividing state is usually not related to differentiation.
However, even if we propose that all human fibroblasts are identical, the transition of these cells to the terminal non-dividing state is, in its essence, differentiation and the forming cells should be called fibrocytes, like in other differentiations: chondroblast-chondrocyte, osteoblast-osteocyte, etc. Unfortunately, the term “fibrocyte” was assigned to definite type of fibroblast and its wider application may foul things up.
It is getting clear now that sustainable proliferation of stem cells requires their contacts with the cells of so-called niche (Scadden, 2006). It is possible that in the case of fibroblasts we have a similar situation: cells that lost contacts with other cells pass to differentiation, i.e. into a long-term nondividing state. Analogy with the stem cells becomes most evident for the telomerized cells, since they are capable of infinite self-maintenance and differentiate in response to a long-term loss of contact with other cells.
The proteomic data obtained fit quite well our concept of telomerized cells. The cytoskeleton of such cells is weaker (less lamins and tropomyosin), but more labile (more statmin destabilizing microtubules): growth of actin fibers (amount of F-actin capping protein β-subunit) is enhanced and proteins of focal contacts Lasp-1, ZYX, and isoform 1 of myosin light alkaline chain underwent changes. These changes agree with the outlook of telomerized cells: they are smaller and more mobile.
The changes in proteins related to the regulation of proliferation also agree with the earlier data: the cells grow at a faster rate and, therefore, the concentration of PCNA, a cofactor of polymerase α, is elevated and the amount of transferrin is increased; the latter is secreted into the external medium and can serve as growth factor. We demonstrated that the conditioned medium of telomerized cells possessed an elevated growth-stimulating property (Yegorov et al., 2005b), but the amount of α2-macroglobulin, a secreted protease inhibitor capable of binding various cytokines, is decreased (Mathew et al., 2003). It can be proposed that a decreased capacity of telomerized cells for colony formation is partly due to this change.
The changes in the amount of diverse heat chock proteins and chaperones (increased HSP70 - proteins 4 and 9B, t-complex 1, AHSA1, and HSPA8, but decreased CCT8, HSP27 protein 1, and calnexin) and increase in the amount of proteasome activator ((PA28, β-chain) favor intensification of protein metabolism. Taking into account the “immortal” and also unchanging character of telomerized cells, such alterations suggest an enhanced control over protein conformations. Suppression of proteasomes (slowing down of protein metabolism) is capable of inducing senescence in human fibroblasts (Torres et al., 2006). It has also been reported that promoters of heat shock protein HSP 16.2 possess an elevated activity in long living individuals of C. elegans (Rea et al., 2005). This activity allows specific individuals to endure lethal stresses. It is evident that the immortal cells should control protein conformation more carefully.
The increased activity of the proteasome activator may have additional significance: it should attract all internal proteins of telomerized cells into a complex with MHC-1, which should, in turn, increase the sensitivity of immune system to the telomerized cells.
The changes in the antioxidant system proved to be, on the surface, surprising. One could have expected total enhancement of the antioxidant protection in immortal invariable cells. However, we found a decreased content of key enzymes SOD1 and SOD2 that render superoxide anion innocuous. At the same time, the cell contents peroxyredoxin, thioredoxin, and a protein resembling thioredoxin (TXNL 1) increased. The decreased content of SOD1 and SOD2 may be explained by constitutively lowered production of reactive oxygen species in the telomerized cells, which are mostly derived from mitochondria. In addition, the mitochondria contain their own genome and its damage may restrict the proliferative potential to approximately the same extent as the shortening of telomeres. As a result of natural selection, the ability of the mitochondria replicate themselves should have been comparable to the cell proliferative potential. It is possible that telomerization affects significantly the functions of mitochondria either directly, or as a result of selection of the cells with less damaged mitochondria or with the mitochondria producing less reactive oxygen forms. It is known that expression of hTERT can somehow increase the potential on the mitochondrial membrane and prevent the increase in the concentration of reactive oxygen species (Kang et al., 2004).
Many distinctions inherent in the telomerized cells have already been described (Kanzaki et al., 2002, 2003; Lindvall et al., 2003; Young et al., 2003; Walter et al., 2003; Kang et al., 2004). The proteomic data, unlike most other data obtained using expression chips, possess their own advantages and drawbacks. On the one hand, application of mass-spectrometry allows us to obtain direct data on the presence or absence of specific protein products. Interpretation of the expression data is hampered due to different velocities of protein metabolism, different mRNA stability, diverse post-transcriptional modifications, etc. On the other hand, mass-spectroscopy data concern, above all, major protein fractions, rather that regulatory proteins represented by minor fractions. The mass-spectroscopy data make it possible to determine structural rearrangements of the cells and the general pattern of metabolism changes. Ideally, it is desirable to combine both methods for the full picture to be obtained.
So many differences (see RESULTS) can hardly be explained by the activity of a single gene. The mass-spectroscopy method we used is capable to identify no more than 2000 protein products. Human cells contain approximately 25000 genes and, as result of alternative splicing, diverse posttranscriptional modifications, and degradation processes, the total number of protein may amount to 100 000. This means that we found 69 differences out of thousands. In all likelihood, the described differences are due, in addition to the direct and indirect effect of hTERT expression, to long-term selection of immortal cells. The delay of proliferation of the telomerized cells in the region of Hayflick limit and increased amount of dying cells after mass hTERT transfection suggest unambiguously the selection in the course of cell immortalization.
Thus, although we found many changes in the protein composition of telomerized cells, they appear to be related to cell accommodation to the conditions of long-term proliferation and preservation all necessary cell mechanisms. The life of normal cells is accompanied by many changes in gene expression, which are not related to genetic modification, as exemplified by cell senescence (Zhang et al., 2003, 2004). It was shown by us and other researchers that the telomerized cells preserved all normal mechanisms of growth regulation and their spontaneous transformation was never recorded in our laboratory during seven years of observations. Hence, it is hopeful that telomerization ex vivo with subsequent careful analysis of the obtained cells will be used in future for autotransplantation in cell replacement therapy.
Table 3.
Effect of gene hTERT introduction in diploid fibroblasts with the help of lentiviruses within six days after transfection, p = 95%
| Effect, % | Fibroblasts | Proliferative age of cells, population duplications | |
|---|---|---|---|
| control | after transfection | ||
| Mitoses | 0.05 + 0.06 | 0.5 ± 0.2 | 40 |
| Apoptoses | 0.20 ± 0.13 | 2.0 ± 0.4 | |
| Mitoses Apoptoses |
2.00 ± 0.90 0.10 ± 0.10 |
5.0 ± 1.4 0.5 ± 0.3 |
13 |
| Apoptoses | 0.10 ± 0.10 | 0.5 ± 0.3 | |
Acknowledgments
We are thankful to E.V. Kazimirchuk, D.N. Karachentsev, T.D. Smirnova, T.G. Tsvetkova, and M.A. Eldarov for their help in this work.
This study was supported by the Russian Foundation for Basic Research, project 99-04-48073, 0204-49196, and 0.5-04-49443, Program “Leading Scientific Schools of Russian Federation,” projects NSh-1794.2003.4 and NSh-20182003.4, by Program of Fundamental Studies of the Russian Academy of Sciences “Stem Cells” and by NIH grants CA104903 and AG025278.
References
- DuBridge RB, Tang P, Hsia HC, et al. Analysis of Mutation in Human Cells by Using an Epstein-Barr Virus Shuttle System. Mol Cell Biol. 1987;7:379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dull T, Zufferey R, Kelly M, et al. A Third-Generation Lentivirus Vector with a Conditional Packaging System. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossel MB. Cells, Aging, and Human Disease. New York: Oxford Univ.; 2004. [Google Scholar]
- Kang HJ, Choi YS, Hong SB, et al. Ectopic Expression of the Catalytic Subunit of Telomerase Protects against Brain Injury Resulting from Ischemia and NMDA-Induced Neurotoxicity. J Neurosci. 2004;24:1280–1287. doi: 10.1523/JNEUROSCI.4082-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki Y, Onoue F, Ishikawa F, et al. Telomerase Rescues the Expression Level of Keratinocyte Growth Factor and Insulin-Like Growth Factor-II in Senescent Human Fibroblasts. Exp Cell Res. 2002;279:321–329. doi: 10.1006/excr.2002.5607. [DOI] [PubMed] [Google Scholar]
- Kanzaki Y, Onoue F, Sakurai H, et al. Telomerase Upreg-ulates Expression Levels of Interleukin (IL)-1alpha, IL-1beta, IL-6, IL-8, and Granulocyte-Macrophage Colony-Stimulating Factor in Normal Human Fibroblasts. Biochem Biophys Res Commun. 2003;305:150–154. doi: 10.1016/s0006-291x(03)00717-4. [DOI] [PubMed] [Google Scholar]
- Lindvall C, Hou M, Komurasaki T, et al. Molecular Characterization of Human Telomerase Reverse Tran-scriptase-Immortalized Human Fibroblasts by Gene Expression Profiling: Activation of the Epiregulin Gene. Cancer Res. 2003;63:1743–1747. [PubMed] [Google Scholar]
- Mathew S, Arandjelovic S, Beyer WF, et al. Characterization of the Interaction between Alpha2-Macroglobulin and Fibroblast Growth Factor-2: The Role of Hydrophobic Interactions. Biochem J. 2003;374:123–129. doi: 10.1042/BJ20021655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerson M, Counter CM, Eaton EN, et al. HEST2, the Putative Human Telomerase Catalytic Subunit Gene, Is Up-Regulated in Tumor Cells and during Immortalisation. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gallay P, et al. In vivo Gene Delivery and Stable Transduction of Nondividing Cells by a Len-tiviral Vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Narushima M, Kobayashi N, Okitsu T, et al. A Human Beta-Cell Line for Transplantation Therapy to Control Type 1 Diabetes. Nat Biotech. 2005;23:1274–1282. doi: 10.1038/nbt1145. [DOI] [PubMed] [Google Scholar]
- Pear WS, Nolan GP, Scott ML, et al. Production of High-Titer Helper-Free Retroviruses by Transient Transfec-tion. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea SL, Wu D, Cypser JR, et al. A Stress-Sensitive Reporter Predicts Longevity in Isogenic Populations of Caenorhabditis elegans. Nat Genet. 2005;37:894–898. doi: 10.1038/ng1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieske P, Krynska B, Azizi SA. Human Fibroblast-Derived Cell Lines Have Characteristics of Embryonic Stem Cells and Cells of Neuro-Ectodermal Origin. Differentiation. 2005;73:474–483. doi: 10.1111/j.1432-0436.2005.00050.x. [DOI] [PubMed] [Google Scholar]
- Sablina AA, Budanov AV, Ilyinskaya GV, et al. The Antioxidant Function of the P53 Tumor Suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadden DT. The Stem-Cell Niche as an Entity of Action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, et al. Mass Spectro-metric Sequencing of Proteins Silver-Stained Polyacryla-mide Gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Torres C, Lewis L, Cristofalo VJ. Proteasome Inhibitors Shorten Replicative Life Span and Induce a Senescent-Like Phenotype of Human Fibroblasts. J Cell Physiol. 2006;207:845–853. doi: 10.1002/jcp.20630. [DOI] [PubMed] [Google Scholar]
- Walter M, Davies JP, Ioannou YA. Telomerase Immortalization Upregulates Rab9 Expression and Restores LDL Cholesterol Egress from Niemann-Pick C1 Late Endosomes. J Lipid Res. 2003;44:243–253. doi: 10.1194/jlr.M200230-JLR200. [DOI] [PubMed] [Google Scholar]
- Yegorov YeE, Terekhov SM, Vishnyakova KS, et al. Immortalization of Normal Fibroblasts of Adult Human Skin by Incorporation of the Gene of Telomerase Catalytic Component. Biol Membr. 2002;19:483–490. [Google Scholar]
- Yegorov YeE, Terekhov SM, Vishnyakova KS, et al. Telomerization is a Way of Production of Immortal Human Cells Preserving Their Normal Properties. Ontogenez. 2003;34:183–192. [PubMed] [Google Scholar]
- Yegorov YeE, Moldaver MV, Terekhov SM, et al. Telomerization Does Not Enhance the Capacity of Human Fibroblasts to Colony Formation. Biol Membr. 2004;21:298–305. [Google Scholar]
- Yegorov YeE, Moldaver MV, Vishnyakova KS, et al. Effects of Oxygen on Culture of Human Fibroblasts. Biol Membr. 2005a;22:43–51. [Google Scholar]
- Yeorov YeE, Moldaver MV, Vishnyakova KS, et al. Evidence in Favor of Differentiation of Immortal Human Fibroblasts in vitro under Sparse Planting. Biol Membr. 2005b;22:458–465. [Google Scholar]
- Young JI, Sedivy JM, Smith JR. Telomerase Expression in Normal Human Fibroblasts Stabilizes DNA 5-Methylcytosine Transferase I. J Biol Chem. 2003;278:19904–19908. doi: 10.1074/jbc.M301685200. [DOI] [PubMed] [Google Scholar]
- Zhang H, Pan RH, Cohen SN. Senescence-Specific Gene Expression Fingerprints Reveal Cell-Type-Dependent Physical Clustering of Up-Regulated Chromosomal Loci. Proc Natl Acad Sci USA. 2003;100:3251–3256. doi: 10.1073/pnas.2627983100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Herbert BS, Pan RH, et al. Disparate Effects of Telomere Attrition on Gene Expression during Replicative Senescence of Human Mammary Epithelial Cells Cultured under Different Conditions. Oncogene. 2004;23:6193–6198. doi: 10.1038/sj.onc.1207834. [DOI] [PubMed] [Google Scholar]


