INTRODUCTION
Multiple myeloma is a malignant plasma cell disorder that accounts for approximately 10% of all hematologic cancers.1,2 It is felt to evolve from an asymptomatic pre-malignant stage of clonal plasma cell proliferation termed monoclonal gammopathy of undetermined significance (MGUS). MGUS is present in over 3% of the population above the age of 50, and progresses to myeloma or related malignancy a rate of 1% per year.3,4 In some patients, an intermediate asymptomatic but more advanced pre-malignant stage referred to as smoldering multiple myeloma (SMM) can be recognized clinically.
The annual incidence of myeloma, age-adjusted to the 2000 United States population, is 4.3 per 100,000. 5 Almost 20,000 new cases and 11,000 new deaths are estimated to occur in the United States in 2007.6 Multiple myeloma is twice as common in African-Americans compared to Caucasians, and slightly more common in males than females.
The median age at diagnosis is 66 years.7 The most common presenting symptoms are fatigue and bone pain.7 Osteolytic bone lesions and/or compression fractures are the hallmark of the disease, and cause significant morbidity. Anemia occurs in 70% of patients at diagnosis and is the primary cause of fatigue. Renal dysfunction is occurs in 50% and hypercalcemia in 25% of patients.
HISTORICAL PERSPECTIVE
Initial Description of the disease
Although multiple myeloma has most likely been present for thousands of years, the first well-documented case was a patient, Sarah Newbury, described by Solly in 18448. Later, the case of Thomas Alexander McBean, led to the discovery of the Bence Jones protein in the late 1840s.9-12 Multiple myeloma is sometimes referred to as Kahler’s disease following a case report by Professor Otto Kahler. The term “plasma cell” was introduced by Waldeyer in 1875,13 but it was Ramon y Cajal, the neuroanatomist, to first accurately describe the plasma cell. In 1895, Marschalko, published the best description of plasma cells which included blocked chromatin, eccentric position of the nucleus, a perinuclear pale area (hof) and a spherical or irregular cytoplasm. Wright 14 was among the first to state that the tumor cells of myeloma consisted of plasma cells or immediate descendants of these cells. The first large case series of myeloma (n=412) was reported by Geschickter and Copeland.15
Monoclonal Proteins
Hyperproteinemia was first demonstrated in multiple myeloma in 1928 by Perlzweig et al 16. The term Bence Jones protein was first used by Fleischer in 1880.17 In 1922, Bayne-Jones and Wilson 18 described the existence of two distinct groups of Bence Jones protein. The group of light chains were later characterized by Korngold and Lipari using the Ouchterlony test in 1956.19 As a tribute to Korngold and Lipari, the two classes of Bence Jones proteins were subsequently designated kappa and lambda. In 1937, Tiselius20 separated serum globulins into three components: alpha, beta, and gamma. In 1939, Tiselius and Kabat 21 demonstrated that the gamma globulin fraction consisted of antibodies. The tall, narrow-based, “church spire” peak on serum protein electrophoresis characteristic of multiple myeloma was recognized in 1939 22. Immunoelectrophoresis was described by Grabar and Williams in 1953,23 and eleven years later, immunofixation was introduced by Wilson 24. Immunofixation is now the most commonly used approach for the identification of monoclonal proteins. The concept of monoclonal versus polyclonal gammopathies was enunciated by Jan Waldenstrom in 1961.25
Alkylator and Corticosteroid Based therapy
Rhubarb pill and infusion of orange peel is the first documented treatment for myeloma, administered to Sarah Newbury in 1844. Mr. McBean was treated with phlebotomy, with the application of leeches used as “maintenance therapy.” Later, he was treated with steel and quinine.8,9,26 Urethane introduced by Alwall in 1947 was the standard of therapy for more than 15 years.27 But urethane was discarded in 1966 when Holland et al. 28 randomized 83 patients with treated or untreated multiple myeloma to receive urethane or a placebo consisting of cherry and cola-flavored syrup and found no difference in the objective status or survival between the two treatment groups.
Melphalan (sarcolysin), a mainstay of therapy even today, was first introduced in 1958 by Blokhin et al.29 He found benefit in three of six patients with multiple myeloma. Later, in 1962, Bergsagel et al.30 reported significant improvement in 8 of 24 patients with multiple myeloma who were treated with melphalan.
In 1967, prednisone was reported by Maas et al to produce benefit in 8 of 10 patients with poor-risk myeloma.31 In a placebo-controlled double-blind trial, prednisone as a single agent produced significant decreases in serum globulin and an increase in hematocrit but no difference in survival when compared with a placebo.32 In a reanalysis of 2 CALGB myeloma treatment protocols, prednisone as a single agent produced a 44% objective response rate 33.
In 1969, Alexanian found in a randomized trial of 183 myeloma patients that survival was 6 months longer with melphalan and prednisone than melphalan alone, giving rise to the classic melphalan-prednisone (MP) regimen.34 For the next 30 years, numerous chemotherapy combinations were compared to MP in an attempt to improve on overall survival, without avail. In a large meta-analysis of over 20 randomized trials comparing melphalan and prednisone with various combinations of therapeutic agents, response rates were significantly higher with combination chemotherapy (60%) compared with melphalan and prednisone (53%) (P< 0.001). However, there was no significant difference in overall survival 35. It is only recently with the arrival of stem cell transplantation and novel agents as described below that improvements in overall have occurred in myeloma.
Stem Cell Transplantation
In 1957, Thomas et al. treated 6 human patients (1 of whom had multiple myeloma) with TBI or chemotherapy followed by an intravenous infusion of bone marrow cells.36. McElwain & Powles reported the first successful cases of autologous bone marrow transplantation in myeloma.37 Subsequently, Barlogie developed a series of treatment programs using autologous transplantation (“total therapy I, II and III”) that played a major role in establishing high-dose therapy and stem cell rescue as standard therapy for myeloma.
DISEASE DEFINITION
The disease definition for myeloma has undergone multiple revisions over the years. The current definition requires 10% or more clonal plasma cells on bone marrow examination (or biopsy proven plasmacytoma), M protein in the serum and/or urine (except in patients with true non-secretory myeloma) and evidence of end-organ damage (hypercalcemia, renal insufficiency, anemia, or bone lesions) that is felt to be secondary to the underlying plasma cell disorder.38,39 Table 1 provides the diagnostic criteria for myeloma, as well as related clonal plasma cell disorders that must be entertained in the differential diagnosis and distinguished from myeloma.
Table 1.
Diagnostic Criteria for Plasma Cell Disorders
| Disorder | Disease Definition | References |
|---|---|---|
| Monoclonal gammopathy of undetermined significance (MGUS) | All 3 criteria must be met:
|
38 |
| Smoldering multiple myeloma (also referred to as asymptomatic multiple myeloma) | Both criteria must be met:
|
38 |
| Multiple Myeloma | All 3 criteria must be met except as noted:
|
38,271 |
| Waldenström’s macroglobulinemia | Both criteria must be met:
|
272-276 |
Note: IgM MGUS is defined as
|
||
| Solitary Plasmacytoma | All 4 criteria must be met
|
277,278 |
| Systemic AL Amyloidosis | All 4 criteria must be met:
|
39 |
| Note: Approximately 2-3 percent of patients with AL amyloidosis will not meet the requirement for evidence of a monoclonal plasma cell disorder listed above; the diagnosis of AL amyloidosis must be made with caution in these patients. | ||
| POEMS Syndrome | All 3 criteria must be met:
|
279 |
| Note: Not every patient meeting the above criteria will have POEMS syndrome; the features should have a temporal relationship to each other and no other attributable cause. The absence of osteosclerotic lesions should make the diagnosis suspect. Elevations in plasma or serum levels of vascular endothelial growth factor and thrombocytosis are common features of the syndrome and are helpful when the diagnosis is difficult. | ||
Modified, reproduced with permission from Rajkumar SV, Dispenzieri A, Kyle RA. Mayo Clinic Proc 2006;81:693-703. © Mayo Clinic Proceedings39.
PATHOGENESIS
Evolution of MGUS
The first step in myeloma pathogenesis is the establishment of MGUS, a precursor lesion that precedes almost all cases of myeloma. The origins of MGUS are likely linked to antigenic stimulation. Studies show that human myeloma cell lines and primary myeloma cells express a broad range of Toll-like receptors (TLRs) which normally help B cells recognize infectious agents and pathogen-associated molecular patterns (PAMP).40-42 TLR-specific ligands cause increased myeloma cell proliferation, survival, and resistance to dexamethasone induced apoptosis. In addition, there is overexpression of CD126 (interleukin-6 receptor alpha-chain) in MGUS compared to normal plasma cells.43,44 Thus abnormal TLR expression and/or overexpression of IL-6 receptors in plasma cells may result in an enhanced response to infection and provide a sustained, autocrine IL-6 dependent, proliferative trigger for plasma cells which cause (or act in concert with) the certain cytogenetic events described below to establish the premalignant MGUS stage.
Approximately 50% of MGUS is associated with primary translocations in the clonal plasma cells involving the immunoglobulin heavy chain (IgH) locus on chromosome 14q32 (IgH translocated MGUS/SMM).1,45 The most common partner chromosome loci and genes dysregulated in these translocations are: 11q13 (CCND1 [cyclin D1 gene]), 4p16.3 (FGFR-3 and MMSET), 6p21 (CCND3 [cyclin D3 gene]), 16q23 (c-maf), and 20q11 (mafB)(Table 2).46-48 Most of the remaining cases of MGUS are associated with hyperdiploidy (IgH non-translocated MGUS).45,49-54
Table 2.
Primary translocations in Monoclonal Gammopathy of Undetermined Significance (MGUS) and Multiple Myeloma
| Chromosomal translocation | Gene dysregulated by translocation280 |
|---|---|
| t 11;14 | CCND1 (cyclin D1) |
| t 4;14 | FGFR-3 and MMSET |
| t 6;14 | CCND3 (cyclin D3) |
| t 14;16 | C-maf |
| t 14;20 | mafB |
Reproduced with permission from Rajkumar SV, Kyle RA2. Mayo Clinic Proc 2005;80:1371-82. © Mayo Clinic Proceedings.
Transformation of MGUS to Myeloma
The progression of MGUS to myeloma represents a simple, random, two-hit genetic model of malignancy.3 Several abnormalities such as Ras mutations, p16 methylation, abnormalities involving the myc family of oncogenes, secondary translocations, and p53 mutations have all been identified in clonal plasma cells in association with progression to the symptomatic stage.46 The bone marrow microenvironment also undergoes marked changes with progression, including induction of angiogenesis,55,56 suppression of cell-mediated immunity,57 and paracrine loops involving cytokines such as interleukin-6 and VEGF (vascular endothelial growth factor).58
Lytic bone lesions characteristic of myeloma are caused by an imbalance between the activity of osteoclasts and osteoblasts. There is an increase in RANKL (receptor activator of nuclear factor κB ligand) expression by osteoblasts (and possibly plasma cells) accompanied by a reduction in the level of its decoy receptor, osteoprotegerin (OPG).59,60 This leads to an increase in RANKL/OPG ratio, which causes osteoclast activation and bone resorption. In addition, increased levels of macrophage inflammatory protein–1α (MIP-1α), IL-3 and IL-6 produced by marrow stomal cells also contribute to the overactivity of osteoclasts. At the same time, increased levels of IL-3, IL-7 and dickkopf 1 (DKK1) inhibit osteoblast differentiation in myeloma.61
ADVANCES IN DIAGNOSIS
Myeloma is characterized by the presence of monoclonal immunoglobulins in the serum and/or urine. Monoclonal immunoglobulins are commonly referred to as monoclonal proteins, M proteins, or paraproteins. The presence of M proteins is indicative of a clonal plasma cell proliferative disorder such as myeloma, MGUS, Waldenstrom macroglobulinemia, etc; additional tests are required to distinguish between the various plasma cell disorders. M proteins are detected by serum protein electrophoresis (SPEP) in 82% of patients with myeloma, and by serum immunofixation (IFE) in 93%.7 The addition of urine protein electrophoresis and IFE (or the serum free light chain assay) increases the sensitivity of detecting monoclonal proteins in patients with myeloma to 97%.62 Currently only 1-2% of patients with myeloma will have no detectable M on any of these tests; these patients have true non-secretory myeloma.
Protein electrophoresis and immunofixation
Electrophoresis of serum and urine proteins (SPEP and UPEP, respectively) and immunofixation of these samples are the preferred methods of detection of monoclonal (M) proteins. UPEP requires a 24 hour urine collection for accuracy. M proteins on SPEP and UPEP appear as a localized band, and electrophoresis also allows measurement of their size.63 After recognition of a la possible M protein on electrophoresis, immunofixation is necessary for confirmation, and to determine the heavy- and light-chain class of the M protein. In addition, immunofixation is more sensitive than electrophoresis, and allows detection of smaller amounts of M protein and should therefore be performed whenever myeloma, amyloidosis, macroglobulinemia or a related disorder is suspected. Quantitation of serum immunoglobulins is performed with a rate nephelometer in patients in whom serum M proteins are detected as an added parameter that can be followed to assess response. Quantitative immunoglobulins are particularly useful in patients with myeloma and macroglobulinemia where at times the M protein size estimated on SPEP may be unreliable (eg., small beta migrating proteins, and IgA / IgM proteins that tend to polymerize)
Serum free light chain assay
The serum free light-chain (FLC) assay (Freelite™, The Binding Site Limited, Birmingham, U.K.) has been recently introduced into clinical practice.64 This automated nephelometric assay allows quantitation of free kappa (κ) and lambda (λ) chains (i.e., light chains that are not bound to intact immunoglobulin) secreted by plasma cells. An abnormal kappa/lambda FLC ratio indicates an excess of one light chain type versus the other, and is interpreted as a surrogate for clonal expansion based on extensive testing in normal volunteers, and patients with myeloma, amyloidosis and renal dysfunction.64,65 The assay is performed on automated chemistry analyzers, is widely available, and is commonly used to monitor patients with oligo-secretory or non-secretory myeloma and primary amyloidosis, as well as patients with the light-chain only form of myeloma.65-67
The normal serum free-kappa level is 3.3 to 19.4 mg/L and the normal free-lambda level is 5.7 to 26.3 mg/L.68 The normal ratio for FLC-kappa/lambda is 0.26-1.65. The normal reference range in the FLC assay reflects a higher serum level of free lambda light chains than would be expected given the usual kappa/lambda ratio of 2 for intact immunoglobulins. This occurs because the renal excretion of free kappa (which exists usually in a monomeric state) is much faster than free lambda (which is usually in a dimeric state). 64,65 Patients with a kappa/lambda FLC ratio <0.26 are typically defined as having monoclonal lambda free light chain and those with ratios >1.65 are defined as having a monoclonal kappa free light chain. If the FLC ratio is >1.65, kappa is considered to be the “involved” FLC and lambda the “uninvolved” FLC, and vice versa if the ratio is less than 0.26.
In a recent study, 428 patients with a monoclonal gammopathy and urine M protein at initial diagnosis of plasma cell dyscrasia who had also undergone serum immunofixation and serum FLC quantitation within 30 days of diagnosis were studied.62 The results of SPEP, serum immunofixation, serum FLC, UPEP, and urine immunofixation were then analyzed to determine if all patients could be accurately identified as having a monoclonal plasma cell disorder had the urine studies not been done. All 3 serum studies were normal in only 2 patients (0.5% of the cohort), indicating that urine studies can be eliminated when screening for the presence of monoclonal plasma cell disorders by using the serum FLC assay in combination with the SPEP and immunofixation. However, urine studies are required if a monoclonal process is identified since it helps in monitoring disease progression and response to therapy over time.
Evaluation of bone disease
Plain radiographic examination of all bones including long bones (skeletal survey) is the preferred method of detecting lytic bone lesions in myeloma. CT and MRI scans are more sensitive than conventional radiography in detecting bone disease and are indicated when symptomatic areas show no abnormality on routine radiographs. Fluoro-deoxyglucose positron emission tomography (FDG-PET) and bone densitometry studies have shown promise in the evaluation of bone disease in myeloma and need further investigation.
Bone marrow studies
By definition, all patients with myeloma have 10% or more clonal bone marrow plasma cells. If a lower extent of involvement is detected, one is either dealing with an erroneous diagnosis or a sampling error due to patchy marrow involvement. Occasionally an entity called “multiple” solitary plasmacytomas has been described in which there are clearly multiple plasmacytomas on clinical and radiographic examination, but marrow involvement is either minimal or absent. The monoclonal nature of bone marrow plasma cells is established by the demonstration of an abnormal kappa/lambda ratio by immunohistochemistry or flow cytometry. Plasma cells in multiple myeloma typically stain positive for CD38, CD56, and CD138, and are usually negative for surface immunoglobulin and CD19. Bone marrow samples should be studied conventional karyotyping studies and/or fluorescent in-situ hybridization studies to detect specific abnormalities such as t4;14, t14;16, and 17p-.
CURRENT STAGING AND PROGNOSTIC EVALUATION
Staging
Since 1975, the Durie-Salmon staging system has been used to stratify patients with multiple myeloma.69 However, this staging system has limitations especially in the categorization of bone lesions.70,71 Recently Greipp et al, developed an International Staging System (ISS) based on data from 11, 171 patients.72 The ISS overcomes the limitations of the Durie-Salmon staging, and divides patients into 3 distinct stages and prognostic groups based solely on the beta-2-microglobulin and albumin levels in the serum. Table 3 provides the details, and the advantages and disadvantages of these two staging systems.
Table 3.
Staging of Myeloma
| Staging System | Key Stage Characteristics | Comments |
|---|---|---|
| Durie-Salmon Staging System | Is more correlated with biologic stage and tumor burden of disease than the international staging system. Disadvantage is complexity, and subjective nature or interpretation of bone findings | |
| Stage I | All of the following:
|
Low M protein defined as IgG <5gm/dL, IgA <3g/dl, urine M spike >4000 mg/24 hours |
| Stage II | Not fitting stage I or II | |
| Stage III | One or more of the following:
|
High M protein defined as IgG >7gm/dL, IgA >5g/dl, urine M spike >12,000 mg/24 hours |
| Subclassification | ||
| A |
|
|
| B |
|
|
| International Staging System | Simple to use, but most renal failure patients will be stage III regardless of tumor burden. Does not apply to monoclonal gammopathy of undetermined significance or smoldering myeloma. | |
| Stage I |
|
|
| Stage II |
|
Prognostic Factors
Once patients are staged, several independent prognostic factors are helpful in predicting outcome in myeloma (Table 4).45,70,73-75 A combination of two or more of the independent prognostic factors listed on Table 4 provides adequate information to estimate prognosis. For example, Facon et al have shown that patients undergoing stem cell transplantation can be grouped into three clear prognostic categories using 2 prognostic factors; median survival was 25 months if patients had a high beta-2 microglobulin and deletion of chromosome 13 by fluorescent in situ hybridization.76 Patients with only one factor abnormal had a median survival of 47 months, while survival was in excess of 111 months in patients in whom both factors were normal.
Table 4.
Major Prognostic Factors in Myeloma
Independent Prognostic Factors
|
Additional Prognostic Factors
|
Prognostic factors such as age, hemoglobin concentration, creatinine, calcium, albumin, immunoglobulin class subtype, and extent of bone marrow involvement have demonstrated some prognostic value.70,73,75 However, they are of limited value once stage and the independent prognostic factors discussed earlier are known.77,78 Newer risk factors such as angiogenesis shed light on biology, but clinical utility is unclear.
Risk-stratification
Using some of the most important clinically available prognostic factors discussed above, a risk-stratification method has been adopted by the Mayo Clinic, called mSMART (Mayo Stratification for Myeloma and Risk-adapted Therapy; www.msmart.org). This model helps stratify patients into high-risk and standard-risk myeloma based on detection of deletion 13 or hypodiploidy on metaphase cytogenetic studies, deletion 17p- or immunoglobulin heavy chain (IgH) translocations t(4;14) or t(14;16) on molecular genetic studies, or plasma cell labeling index of 3% or higher on bone marrow immunofluorescent studies. Presence of any one or more of these high-risk factors (Table 5) classifies a patient as having high-risk MM.79 The median survival of high-risk MM is only 2-3 years even with tandem stem cell transplantation, compared to over 6-7 years in patients with average-risk MM.2
Table 5.
Mayo Risk-Stratification for Myeloma271
| High-risk characteristic | Percentage of newly diagnosed patients with the abnormality 7,45,281 |
|---|---|
| Conventional Cytogenetics: | |
| Deletion of chromosome 13 (monosomy) | 14% |
| Hypodiploidy | 9% |
| Either hypodiploidy or deletion 13 | 17% |
| Fluorescent in-situ hybridization (FISH): | |
| t(4;14) | 15% |
| t(14:16) | 5% |
| 17p- | 10% |
| Plasma cell labeling index (PCLI) studies: PCLI ≥3% | 6% |
| Any one of the above high-risk abnormalities | 25-30% |
Reproduced with permission from Rajkumar SV, Kyle RA2. Mayo Clinic Proc 2005;80:1371-82. © Mayo Clinic Proceedings.
RESPONSE ASSESSMENT
Several criteria have been used over the years to assess response to therapy in myeloma. In recent years, the European Group for Blood and Bone Marrow Transplant/ International Bone Marrow Transplant Registry/ American Bone Marrow Transplant Registry (EBMT/ IBMTR/ ABMTR criteria for the response and progression of MM treated by stem cell transplantation, commonly referred to as the Blade criteria or the EBMT criteria was adopted in many trials.80 Other commonly used response criteria were those developed by the Chronic Leukemia-Myeloma Task Force, Southwest Oncology group (SWOG), and the Eastern Cooperative Oncology Group (ECOG) have been largely abandoned. In 2006, the International Myeloma Working Group (IMWG) recognized the need for uniformity and published uniform response criteria that are to be used in future clinical trials.81
The IMWG uniform response criteria are similar to the EBMT criteria with a few major exceptions: addition of free light chain response and progression criteria for patients without measurable disease (see Table 6 for definition of measurable disease), modification of the definition for disease progression for patients in complete response, addition of very good partial response and stringent response categories, elimination of the minor response category, elimination of the mandatory 6 week wait time to confirm response, and some additional clarifications and correction of errors. The IMWG criteria for response and progression are listed on Table 7.
Table 6.
Definition of Measurable Disease
One or more of the following:
|
The FLC assay is not to be used for response assessment in patients who have evidence of measurable disease in the serum or urine by M protein levels.
Table 7.
International Myeloma Working Group Uniform Response criteria for multiple myeloma 81
| Response subcategory | Response criteria |
|---|---|
| Complete response* (CR) |
|
| Stringent complete response (sCR) | CR as defined above plus
|
| Very good partial response (VGPR)* |
|
| Partial Response (PR) |
|
| Stable disease (SD) |
|
| Progressive Disease (PD)* | Increase of 25% from lowest response value in any one or more of the following:
|
All response categories (CR, sCR, VGPR, PR) require two consecutive assessments made at anytime before the institution of any new therapy; complete and PR and SD categories also require no known evidence of progressive or new bone lesions if radiographic studies were performed. Radiographic studies are not required to satisfy these response requirements. Bone marrow assessments need not be confirmed.
Note clarification to IMWG criteria for coding CR and VGPR in patients in whom the only measurable disease is by serum FLC levels: CR in such patients a normal FLC ratio of 0.26-1.65 in addition to CR criteria listed above. VGPR in such patients is defined as a >90% decrease in the difference between involved and uninvolved free light chain FLC levels.
Adapted with permission from Durie et al. International uniform response criteria for multiple myeloma. Leukemia 2006;20:1467-73 81
NEW AGENTS IN THE TREATMENT OF MYELOMA
As described earlier, for decades, the mainstay of therapy has been alkylators and corticosteroids, specifically the oral regimen of melphalan and prednisone (MP). High dose therapy with autologous stem cell transplantation (ASCT) was subsequently shown to prolong survival compared to conventional chemotherapy, and adopted into standard practice. More recently, thalidomide,82 bortezomib,83,84 and lenalidomide,85,86 have emerged as effective agents in the treatment of myeloma (Table 8), and each has an interesting historical perspective.
Table 8.
New drugs with significant single-agent activity in multiple myeloma
| Agent | Usual Starting Dose | Postulated Mechanism of Action | Side-effects |
|---|---|---|---|
| Thalidomide | 50-200 mg orally days 1-28 every 4 weeks | Anti-angiogenesis, immunomodulation, and inhibition of tumor necrosis factor alpha. Direct cytotoxicity by inducing free radical mediated DNA damage. | Sedation, fatigue, skin rash, bradycardia, peripheral neuropathy and constipation are the most common side effects. Deep vein thrombosis when combined with dexamethasone or chemotherapy. Less frequent but serious side-effects are seizures, cardiac arrhythmia, and Stevens Johnson syndrome. |
| Bortezomib | 1.3mg/m2 intravenously days 1, 4, 8, 11 every 21 days | Inhibits the ubiquitin-proteasome catalytic pathway in cells by binding directly with the 20S proteasome complex. | The most common toxicities are gastrointestinal, transient cytopenias, fatigue, and peripheral neuropathy. |
| Lenalidomide* | 25 mg orally days 1-21 every 28 days | Analog of thalidomide. Immunomodulation and anti-angiogenesis | The most common adverse effects are grade 3 or 4 thrombocytopenia and neutropenia. |
Reproduced with permission from Rajkumar SV, Kyle RA2. Mayo Clinic Proc 2005;80:1371-82. © Mayo Clinic Proceedings.
In this section, the new agents for the treatment of myeloma will be reviewed. Subsequent sections will discuss in detail the current approach to initial therapy, stem cell transplantation, maintenance therapy, and treatment of relapsed refractory disease.
Thalidomide
Historical perspective
Chemie Grünenthal, a German pharmaceutical company introduced thalidomide(α-N-[phthalimido] glutarimide) into the market as a sedative on October 1, 1957.87-89 By 1960, it was sold in over 40 countries, and became popular both as a sedative and as treatment for morning sickness of pregnancy. It was marketed under various commercial names such as ‘Contergan’, ‘Distaral’, ‘Softenon’, ‘Neurosedyn’, ‘Isomin’, ‘Kedavon’, ‘Telargan’, and ‘Sedalis.’90
On November 18, 1961, Widukind Lenz, a German physician and geneticist determined that thalidomide was associated with severe teratogenic malformations.90 In December 1961, independent confirmation came from William McBride, an Australian obstetrician.91 Fetal malformations occurred inevitably when the drug was taken in the first trimester between days 35-49 after the last menstrual period.87,88 By the end of 1961, thalidomide was taken off the market in most countries but almost 10,000 infants had already been affected. The United States was spared, because the drug had been denied approval by Dr. Frances Kelsey at the FDA who was concerned about the lack of safety data.92
Shortly after the teratogenic properties of thalidomide were known, it was considered as a possible treatment against cancer.93,94 At least three clinical trials were undertaken in cancer,95,96 but no significant activity was seen. Although thalidomide was taken off the market and was unsuccessful against cancer, it nevertheless persisted as a therapeutic agent due to promising activity detected in leprosy (1964),97,98 Behcet’s Disease (1979),99 graft versus host disease (1988),100-104 and human immunodeficiency virus (HIV) associated oral ulcers105 and wasting (1989).106 Under pressure from HIV activists and to discourage illegal drug distribution networks, the FDA approved thalidomide for the treatment of erythema nodosum leprosum in the United States in July 1998 with a built in safety system termed the System for Thalidomide Education and Prescribing Safety (STEPS) program.107,108
Thalidomide is a chiral molecule and is dispensed as a racemic mixture of the (R)- (dextrorotatory) and (S)- (levorotatory) enantiomers.109,110 Although it is felt that the (R)- enantiomer is responsible for most of its sedative activities, and the (S)- enantiomer has a significant role in the teratogenic and anti-tumor properties, differences in enantio-selectivity are not clinically relevant because of rapid chiral interconversion in vivo at physiologic pH.111 As a result of this rapid racemization, the use of a pure (R)- enantiomer therefore would not have prevented the thalidomide tragedy. Most of the metabolism is by non-enzymatic hydrolysis, and no major dose reductions are generally needed in liver or renal failure.
Initial results in myeloma
Based on the significant anti-angiogenic properties of thalidomide,112,113 and evidence of increased angiogenesis in myeloma, Singhal, Barlogie and colleagues treated patients with relapsed and refractory multiple myeloma with the drug in late 1997. Thirty-two percent of 84 patients responded, making thalidomide the first new drug with single-agent activity for myeloma in over three decades.82,114 These results were confirmed by many other centers, in all phases of the disease.115-122
Studies show that response rates in relapsed disease are about 50% with the combination of thalidomide and steroids,123 and increase to over 65% with an oral three-drug combination of thalidomide, steroids and cyclophosphamide (CTD).124-126 Several other combination chemotherapy regimens containing thalidomide have been developed including DT-PACE (dexamethasone, thalidomide, cisplatin, adriamycin, cyclophosphamide and etoposide), BLT-D (clarithromycin, low dose thalidomide, and dexamethasone), and MTD (melphalan, thalidomide and dexamethasone).127
Dosage
Initially thalidomide was used in doses of up to 800 mg per day. However, most side-effects of thalidomide are dose-dependent, and there is no major improvement in clinical activity beyond a dose of 200 mg. Currently, thalidomide is usually given in a dosage of 50 to 200 mg daily; the dose is typically adjusted to the lowest dose that can achieve and maintain a response in order to minimize long-term toxicity. In most elderly patients (age >65) a dose of 50 to 100 mg is better tolerated.
Side-effects
The known toxicities of thalidomide include peripheral neuropathy, constipation, fatigue and sedation.128 Therapy for myeloma has revealed new toxicities of thalidomide that were previously unrecognized. These include deep vein thrombosis,129-132 toxic epidermal necrolysis,133 and hypothyroidism.134 The incidence of deep vein thrombosis (DVT) is only 1-3% in patients receiving thalidomide alone, but rises to approximately 10-15% in patients receiving thalidomide in combination with dexamethasone, and to about 25% in patients receiving the agent in combination with other cytotoxic chemotherapeutic agents, particularly doxorubicin.129-131,135,136 The management of thalidomide toxicity has been reviewed in detail by Ghobrial et al.128
Bortezomib
Historical Perspective
The multi-catalytic ubiquitin-proteasome pathway is responsible for the degradation of eukaryotic cellular proteins.137-140 The orderly degradation of cellular proteins by the ubiquitin-proteasome pathway is critical for signal transduction, transcriptional regulation, response to stress, and control of receptor function.141-144 The 26S proteasome (1500-2000 kDa) consists of a core 20S catalytic complex (approximately 700 kDa) and a 19S regulatory complex (Figure 1).137,141,144-148 Ubiquitin tagged proteins are recognized by the 19S regulatory complex, where the ubiquitin tags are removed proteins are fed into the inner catalytic compartments of the 20S proteasome cylinder for hydrolysis into small polypeptides ranging from 3 to 22 amino acids in length.149,150 151 152 137,150,153
Figure 1.
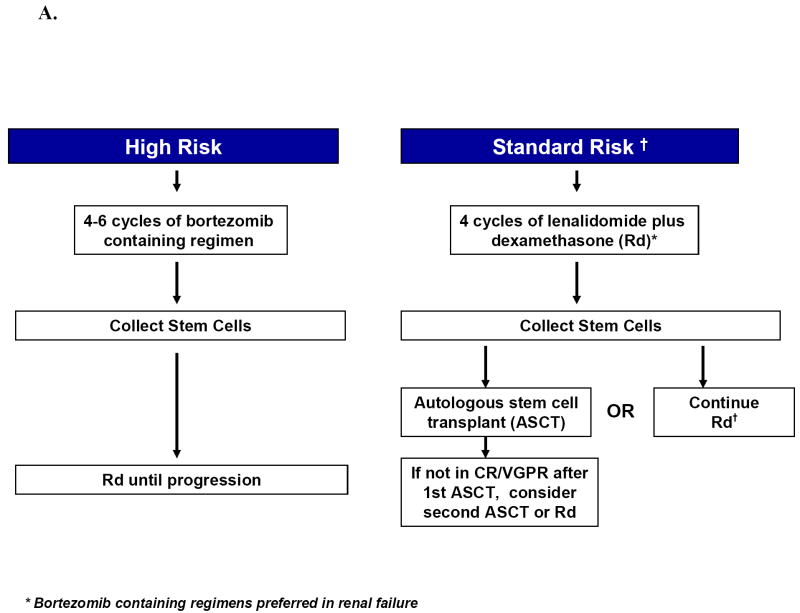
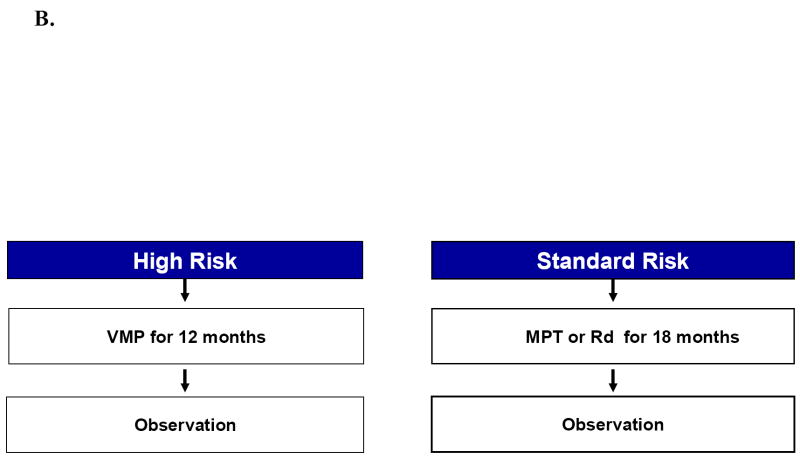
Approach to the treatment of newly diagnosed myeloma in patients who are candidates for transplant (A) and those who are not candidates for transplant (B). Footnote for Figure 1A. Rd, lenalidomide plus low dose dexamethasone; CR, complete response; VGPR, very good partial response; ASCT, autologous stem cell transplantation Footnote for Figure 1B. MPT, melphalan, prednisone, thalidomide; VMP, bortezomib, melphalan, prednisone.
Inhibition of the proteasome inhibition leads to cellular apoptosis, with malignant, transformed and proliferating cells being more succeptible.148,154 137,155-158 Numerous proteasome inhibitors were developed but none was suitable for clinical use.141,148,159,160 Subsequently, Adams and colleagues designed and developed several boronic acid derived compounds that inhibit the proteasome pathway in a highly specific manner.154 Bortezomib (N-pyrazinecarbonyl-l-phenylalanine-l-leucine boronic acid; previously known as PS-341 or MLN-341), a boronic acid dipeptide was then selected for preclinical and clinical testing.83,154 Bortezomib inhibits the proteasome pathway rapidly and in a reversible manner by binding directly with the 20S proteasome complex and blocking its enzymatic activity.
Preclinical studies demonstrated that bortezomib had potent cytotoxic and growth inhibitory effects across the panel of 60 cell lines from the NCI and in several murine models.139,161-166 Bortezomib also showed striking activity against myeloma cells in several preclinical models.167 168-170 Orlowski and colleagues conducted a phase 1 study in which responses were seen in several myeloma patients.
Initial results in myeloma
In the first phase II trial conducted in 202 patients with relapsed/refractory MM, approximately one-third responded to bortezomib therapy, with an average response duration of one year.83 These results were confirmed in a randomized phase II trial in patients who failed to respond or relapsed after front-line therapy for myeloma.171 Although initial trials only allowed a maximum of 8 cycles of bortezomib, responding patients were allowed to receive continued treatment on a compassionate use protocol, data from which indicate that it is safe to give at least an additional 5-6 cycles of therapy, without undue toxicity.171 In a recent randomized trial, progression free survival was superior with bortezomib compared to dexamethasone alone in patients with relapsed, refractory myeloma.84
Dosing
The starting dose of bortezomib is 1.3 mg/m2 given twice weekly on days 1, 4, 8, and 11 every 21 days. Dosage may need to be decreased to 1.0 mg/m2 or 0.7 mg/m2 based on toxicity. Although no clear maintenance dose has been defined, after 4-6 cycles, the dosage often needs to be decreased if there is a necessity to continue the drug in order to minimize the risk of neuropathy. One strategy is to lengthen the cycle length from 21 days to 28 or even 35 days. Another option is to administer bortezomib once a week.
Side-effects
The most common toxicities attributable to bortezomib therapy are gastrointestinal side effects, cytopenias, fatigue, fever, and peripheral neuropathy Peripheral neuropathy occurs in approximately 45% of patients, and is the most worrisome adverse effect. It can be painful. It is more frequent in patients who have received prior neurotoxic therapy and in those with pre-existing neuropathy. Neuropathy can be of grade 3 severity in about 10% of patients receiving therapy. Postural hypotension occurs in approximately 10% of patients.
Lenalidomide
Historical Perspective
Shortly after thalidomide was determined to be a teratogen in the 1960s, a number of analogs of thalidomide were synthesized to determine the mechanism of its teratogenicity. Similarly in the 1990s, a number of thalidomide analogs were synthesized in an attempt to increase efficacy and minimize toxicity. Lenalidomide, a 4-amino substituted analog of thalidomide formerly called CC-5013, is in a class of thalidomide analogs termed immunomodulatory drugs (IMiDs). It is safer and more effective than thalidomide in preclinical and clinical studies. In contrast to the clinical development of thalidomide, the development of lenalidomide can be considered an inevitable occurrence, commonly seen in various fields of medicine where pharmaceutical companies develop closely related and presumably more effective alternatives once an effective parent drug is identified.
Based on the preclinical studies conducted by the manufacturer and at the Dana-Farber Cancer Institute, lenalidomide was tested in phase I trials in relapsed refractory myeloma.172
Initial Results in myeloma
Lenalidomide first showed promising activity in MM in 2 phase I trials.172,173 In a phase II trial conducted at Mayo Clinic, 31 of 34 patients (91%) with newly diagnosed myeloma achieved an objective response with lenalidomide plus dexamethasone.85 Richardson et al reported on single-agent activity in a multi-center randomized phase II trial that enrolled 102 patients with relapsed/refractory myeloma.86 Overall response rate with single-agent lenalidomide was 17%. Two large phase III trials demonstrated significantly superior time to progression and overall survival with lenalidomide plus dexamethasone compared to placebo plus dexamethasone in relapsed myeloma.174,175 Based on these two randomized trials, lenalidomide plus dexamethasone was approved by the FDA in June 2006 for the treatment of myeloma in patients who have failed one prior therapy.
Dosing
Typical dosing of lenalidomide for myeloma is 25 mg per day on days 1-21 of a 28 day cycle, with dose adjustments based on toxicity. With prolonged therapy dose adjustments are usually needed. Most patients who receive lenalidomide for over one year are usually on lower doses of 5-15 mg per day given in the usual schedule. The dosing of lenalidomide in renal failure is unclear, and caution must be exercised since the drug is renally excreted.
Side-effects
The most common side-effects of lenalidomide are cytopenias. Fatigue, dryness of skin, and rash are other common side effects. The incidence of DVT is low (2-3%) with single-agent lenalidomide or lenalidomide plus low-dose dexamethasone, but rises markedly when the agent is combined with high-dose dexamethasone (approximately 20%). The risk of DVT can be decreased to less than 10% by reducing the dose of dexamethasone to 40 mg once a week.
Liposomal-doxorubicin
For many years, doxorubicin has been used in myeloma as part of the VAD regimen and other combination regimens. However, the single-agent activity of doxorubicin is minimal. Recently, liposomal doxorubicin has shown activity in newly diagnosed and relapsed myeloma. In a randomized trial, bortezomib plus liposomal doxorubicin was associated with a superior time to progression, resulting in the approval of liposomal doxorubicin for the treatment of relapsed myeloma.176 Further studies are needed to define the role of liposomal doxorubicin in myeloma.
Pomalidomide (CC-4047)
Pomalidomide (an analog of thalidomide and lenalidomide) has shown promising single-agent activity.177 In combination with low-dose dexamethasone pomalidomide has shown a response rate of over 60% in relapsed myeloma in a trial conducted by the Mayo Clinic.178 In this trial, pomalidomide was given orally 2 mg daily on days 1-28 of a 28-day cycle. Dexamethasone was given orally at a dose of 40 mg daily on days 1, 8, 15 and 22 of each cycle. Additional studies are ongoing. Pomalidomide is not approved by the FDA.
Carfilzomib
Carfilzomib, an irreversible proteasome inhibitor has shown significant single-agent activity in myeloma in two separate trials.179,180 The dose of carfilzomib was 20 mg/m2 intravenously days 1, 2, 8, 9, 15 and 16 every 28 days, for up to 12 cycles. There is a suggestion that carfilzomib may have less neurotoxicity compared with bortezomib. It is also not approved by the FDA so far. Clinical trials with carfilzomib are ongoing.
Other new drugs
There is a continued search for other active agents based on advances in myeloma biology.181 Other agents which have raised some interest and are undergoing testing include heat shock protein inhibitors, histone deacetylase inhibitors, perifosine, and bendamustine.
INITIAL TREATMENT OF MYELOMA
The median survival of symptomatic myeloma is 4 years. For over 3 decades, the mainstay of therapy was oral chemotherapy with melphalan and prednisone (MP). High dose therapy with autologous stem cell transplantation (ASCT) prolongs survival compared to conventional chemotherapy, and is now routinely incorporated into the treatment strategy either early in the disease course or at the time of relapse in eligible patients. As discussed earlier, thalidomide,82 bortezomib,83,84 and lenalidomide,85,86 have emerged as effective agents in the treatment of myeloma and have greatly altered the way myeloma is treated today.
Despite many advances, there is still no evidence that early treatment of patients with asymptomatic (smoldering) multiple myeloma prolongs survival compared to therapy at the time of symptoms. Similarly the use of bisphosphonates does not alter the natural history of smoldering myeloma, and are not indicated.182 Therapy is therefore initiated only in patients defined as having multiple myeloma based on the criteria listed on Table 1; patients with smoldering myeloma should be observed closely without treatment. In all stages of myeloma, clinical trials are preferred over standard therapeutic approaches, since the disease is still incurable. Over the years several landmark trials have greatly altered the treatment of myeloma, details of which are listed on Table 9.
Table 9.
Results Of Selected Randomized Trials That Have Had A Major Impact On Myeloma Therapy
| Trial | Disease Stage | Treatment comparison |
Total Number of patients studied |
CR/VGPR (%) |
Median EFS (months) |
Median OS (months) |
Comments |
|---|---|---|---|---|---|---|---|
| Stem Cell Transplantation | |||||||
| IFM 90206 | Newly Diagnosed | Conv. chemo | 100 | 13 | 18 | 44 | Established role of ASCT |
| ASCT | 100 | 38 | 28 | 57 | |||
| MRC VII207 | Newly Diagnosed | Conv. chemo | 201 | 8 | 20 | 42 | Confirmed role of ASCT |
| ASCT | 200 | 44 | 32 | 54 | |||
| MAG 90213 | Newly Diagnosed | Delayed ASCT | 94 | 21 | 13 | 65 | Demonstrated delayed ASCT as an alternative |
| Early ASCT | 91 | 32 | 39 | 64 | |||
| United States Intergroup S9321282 | Newly Diagnosed | Delayed ASCT | 255 | 15* | 21 | 64 | Demonstrated delayed ASCT as an alternative; dampened enthusiasm for allogeneic SCT |
| Early ASCT | 261 | 17* | 25 | 58 | |||
| Allogeneic SCT | 36 | 17* | NR | 6 | |||
| IFM 94283 | Newly diagnosed | Single ASCT | 199 | 42 | 25 | 48 | Established role of tandem ASCT if CR/VGPR not achieved with first ASCT |
| Double ASCT | 200 | 50 | 30 | 58 | |||
| PETHEMA284 | Post-induction (responding patients only) | Conv. chemo | 83 | 11* | 33 | 66 | Demonstrated that ASCT may have limited value in patients responding well to induction |
| ASCT | 81 | 30* | 42 | 61 | |||
| Italian232 | Newly diagnosed | Tandem ASCT | 82 | N/A | 29 | 54 | Demonstrated efficacy of single ASCT followed by non- myeloablative SCT in selected patients |
| Single ASCT followed by non-myeloablative SCT | 80 | N/A | 35 | 80 | |||
| New therapies | |||||||
| Italian GIMEMA group197 | Newly diagnosed | MP | 126 | 12 | 27 | 64% at 3yrs | Demonstrated potential value of MPT over the classic MP regimen |
| MPT | 129 | 36 | 54 | 80% at 3yrs | |||
| IFM 99-06199 | Newly diagnosed | MP | 196 | 7 | 18 | 33 | Changed standard of care in elderly to MPT (after 3 decades of MP) |
| MPT | 125 | 47 | 28 | 52 | |||
| Tandem intermediate-dose ASCT | 126 | 43 | 19 | 38 | |||
| APEX84 | Relapsed, refractory (1-3 prior therapies) | Bortezomib | 333 | 13 | 6** | NR | Led to full approval of bortezomib in the US |
| Dex | 336 | 2 | 3** | NR | |||
| MM-010174 | Relapsed, refractory (1-3 prior therapies) | Len/ Dex | 351 | 15* | 11** | Not reached | Pivotal trial establishing role of lenalidomide |
| Placebo/Dex | 3* | 5** | |||||
| MM-009175 | Relapsed, refractory (1-3 prior therapies) | Len/ Dex | 341 | 13* | 11* | 30 | Led to approval of lenalidomide in the US |
| Placebo/Dex | 1* | 5* | 20 | ||||
Abbreviations: ASCT, autologous hematopoetic stem cell transplantation; Conv. chemo, conventional chemotherapy; CR, complete response; Dex, dexamethasone; ECOG, Eastern Cooperative Oncology Group; EFS, event free survival; GIMEMA, Gruppo Italiano Malattie Ematologiche dell’Adulto; IFM, InterGroupe Francophone du Myélome; Len, Lenalidomide; MAG, Myélome Autogreffe; MM, multiple myeloma; MP, melphalan plus prednisone; MPT, melphalan, prednisone, thalidomide; MRC, Medical Research Council; NR, not reported; OS, overall survival; PETHEMA, Programa para el Estudio y Tratamiento de las Hemopatias Malignas; PFS, progression free survival; SCT, stem cell transplantation; US, United States; VGPR, very good partial response.
Denotes CR only; VGPR not reported.
PFS data not available; numbers reflect median time to progression
This Table was originally published in Blood. Kyle RA and Rajkumar SV. Multiple Myeloma. Blood. 2008; 111;2962-2972. © the American Society of Hematology
The choice of initial therapy depends on eligibility for stem cell transplantation and risk-stratification. The overall approach to treatment of symptomatic newly diagnosed multiple myeloma is outlined in Figure 1A and B. Eligibility for stem cell transplantation is determined by age, performance status, and coexisting comorbidities. Risk-Stratification is based on presence or absence of high-risk factors (Table 5)79 Table 10 lists the most common regimens used in the treatment of newly diagnosed myeloma, including recommended doses and activity.
Table 10.
Dosing Schedule and Response Rates of Commonly used Chemotherapy Regimens in Myeloma
| Regimen | Suggested Dosing Schedule* | Response rate in newly diagnosed myeloma | CR+VGPR‡ rate in newly diagnosed myeloma |
|---|---|---|---|
| Transplant Candidates | |||
| Thalidomide- Dexamethasone** (Thal/Dex)185,188 | Thalidomide 100-200mg oral days 1-28 | 63% | 44% |
| Dexamethasone 40mg oral days 1, 8, 15, 22 every 28 days | |||
| Repeated every 4 weeks × 4 cycles as pre-transplant induction therapy; or continued for up to one year or progression if used as primary therapy | |||
| Lenalidomide-Dexamethasone (Rev/low-dose Dex)285 | Lenalidomide 25mg oral days 1-21 every 28 days | 71% | 42% |
| Dexamethasone 40mg oral days 1, 8, 15, 22 every 28 days | |||
| Repeated every 4 weeks × 2-4 cycles as pre-transplant induction therapy; or continued till progression if used as primary therapy | |||
| Bortezomib-Dex (Vel/Dex)286 | Bortezomib 1.3mg/m2 intravenous days 1, 4, 8, 11 | 80% | 47% |
| Dexamethasone 40mg oral days 1-4, 9-12 | |||
| Reduce dexamethasone to days 1-4 only after first 2 cycles | |||
| Repeated every 3 weeks × 2-4 cycles as pre-transplant induction therapy | |||
| Bortezomib-Thalidomide-Dexamethasone (VTD)**287 | Bortezomib 1.3mg/m2 intravenous days 1, 4, 8, 11 | 93% | 60% |
| Thalidomide 200mg oral days 1-21 | |||
| Dexamethasone 20 mg day of and day after bortezomib | |||
| Repeated every 3 weeks × 3 cycles as pre-transplant induction therapy | |||
| VDT-PACE‡196 | Dexamethasone 40 mg/day PO daily days 1-4 | Not reported | Not reported |
| Thalidomide 100 mg/day days 1-28† | |||
| Cisplatin 10 mg/m²/day intravenous infusion over 24 hours days 1-4 | |||
| Cyclophosphamide 400 mg/m²/day intravenous infusion over 24 hours days 1-4 | |||
| Etoposide 40 mg/m²/day intravenous infusion over 24 hours days 1-4 | |||
| Doxorubicin 10 mg/m²/day intravenous infusion over 24 hours days 1-4 | |||
| Bortezomib 1 mg/m²/day intravenous on days 1,4,8,11 | |||
Note:
|
|||
| Non-Transplant Candidates | |||
| Melphalan-Prednisone | Melphalan 9mg/m2 oral days 1-4 | 35 | 8% |
| Prednisone 60mg/m2 oral days 1-4 | |||
| Repeated every 6 weeks until plateau | |||
| An alternative schedule is melphalan 8-10 mg oral days 1-7 with prednisone 60 mg days 1-7 repeated every 6 weeks until plateau | |||
| Melphalan-Prednisone- Thalidomide (MPT)199† | Melphalan 0.25mg/kg oral days 1-4 | 76% | 47% |
| Prednisone 2mg/kg oral days 1-4 | |||
| Thalidomide 100-200mg oral days 1-28 | |||
| Repeated every 6 weeks × 12 cycles | |||
| Borteozmib-Melphalan-Prednisone (VMP)204 | Melphalan 9 mg/m2 oral days 1-4 | 71% | 41% |
| Prednisone 60 mg/m2 oral days 1 to 4 | |||
| Bortezomib 1.3mg/m2 intravenous days 1, 4, 8, 11, 22, 25, 29, 32 | |||
| Repeated every 42 days × 4 cycles followed by maintenance therapy as given below: | |||
| Melphalan 9 mg/m2 oral days 1-4 | |||
| Prednisone 60 mg/m2 oral days 1 to 4 | |||
| Bortezomib 1.3mg/m2 intravenous days 1, 8, 15, 22 | |||
| Repeated every 35 days × 5 cycles | |||
Starting and subsequent doses need to be adjusted for performance status, renal function, blood counts, and other toxicities;
Dexamethasone dose listed is lower than used in trial.
Thalidomide dose listed is lower than used in trial
CR, complete response; VGPR, very good partial response; VDT-PACE, velcade, dexamethasone, thalidomide, cisplatin (platinum), Adriamycin, cyclophosphamide, and etoposide.
Transplant-Candidates
It is important to avoid protracted melphalan-based therapy in patients with newly diagnosed myeloma who are considered eligible for ASCT, since it can interfere with adequate stem cell mobilization, regardless of whether an early or delayed transplant is contemplated. Typically patients are treated with approximately 4 cycles of induction therapy prior to stem cell harvest. This includes patients who are transplant candidates but who wish to reserve ASCT as a delayed option for relapsed refractory disease. Such patients can resume induction therapy following stem cell collection until a plateau phase is reached, reserving ASCT for relapse.
Vincristine, doxorubicin, dexamethasone (VAD)
VAD was used for many years as pre-transplant induction therapy for patients considered candidates for ASCT. However, VAD requires an intravenous indwelling catheter, which predisposes patients to catheter related sepsis and thrombosis. Moreover, the neurotoxicity of vincristine can limit the future use of thalidomide and bortezomib, both of which also have neurotoxic potential. Most of the activity of VAD is from the high-dose dexamethasone component. Recently, Cavo and colleagues in a matched case-control study of 200 patients demonstrated that response rates with VAD were significantly lower compared to Thal/Dex; 76% versus 52%, respectively.183 Preliminary results from a randomized trial from France confirm these findings.184 As a result, VAD is not recommended anymore as initial therapy.
Pulsed Dexamethasone
Dexamethasone alone has also been used as induction therapy. It is typically used at a dose of 40 mg orally on days 1-4, 9-12, and 17-20 every 4-5 weeks. Objective response rates are approximately 45%,185 significantly lower compared to newer induction regimens. In randomized trials the early mortality rate associated with dexamethasone is over 10% in the first 4 months of therapy, reflecting the toxicity and ineffectiveness of this regimen. Consequently, single-agent dexamethasone is also no longer recommended as initial therapy.
Thalidomide-Dexamethasone (Thal/Dex)
In the last few years, Thal/Dex has emerged as the most commonly used induction regimen for the treatment of newly diagnosed myeloma in the United States. The use of Thal/Dex was initially based on three phase II clinical trials.118,186,187 Response rates with Thal/Dex range 64-76% in these studies, comparable or better than those obtained with infusional VAD.
The Eastern Cooperative Oncology Group (ECOG) recently reported the results of a randomized trial comparing Thal/Dex to dexamethasone (Table 7).185 Two hundred seven patients were studied. The best response within four cycles of therapy was significantly higher with Thal/Dex compared to dexamethasone alone; 63% versus 41%, respectively, P=0.0017. Adjusted response rates allowing for the use of serum M protein values alone in patients in whom a measurable urine protein at baseline was unavailable at follow-up were 72% with Thal/Dex versus 50% with dexamethasone alone. Stem cell harvest was successful in 90% of patients in each arm. DVT was more frequent with Thal/Dex (17% versus 3%). Overall, grade 3 or higher non-hematologic toxicities were seen in 67% of patients within four cycles with Thal/Dex and 43% with dexamethasone alone (P <0.001). Early mortality (first 4 months) was 7% with Thal/Dex and 11% with dexamethasone alone. Based on this trial, the United States Food and Drug Administration (FDA) has granted accelerated approval for Thal/Dex for the treatment of newly diagnosed myeloma.
Confirmatory results are available from a separate randomized, double-blind, placebo-controlled study comparing Thal/Dex versus placebo/dexamethasone (Placebo/Dex) as primary therapy in 470 patients with newly diagnosed myeloma.188 In this trial, the overall response rate was significantly higher with Thal/Dex compared with Placebo/Dex (63% v 46%), P < 0.001. Time to progression (TTP) was significantly longer with Thal/Dex compared with Placebo/Dex (median, 22.6 v 6.5 months, P < 0.001). As in the ECOG study, grade 4 adverse events were more frequent with Thal/Dex than with Placebo/Dex (30.3% v 22.8%).
A Dutch study compared thalidomide, Adriamycin, dexamethasone (TAD) with VAD as induction therapy.189 After induction patients in both arms proceeded to stem cell transplantation. A total of 556 patients were randomized and progression free survival was superior with TAD compared with VAD, median 25 months to 33 months (P<0.001). However no difference in overall survival was seen.
Patients receiving thalidomide in combination with high-dose steroids or chemotherapy need routine thromboprophylaxis with coumadin (target INR 2-3) or low-molecular weight heparin (equivalent of enoxaparin 40mg once daily). Aspirin can be used instead in patients receiving only low doses of dexamethasone (40mg once a week or lower) or prednisone in combination with thalidomide, provided no concomitant erythropoietic agents are used.
Lenalidomide-dexamethasone (Rev/Dex)
In a phase II trial conducted at the Mayo Clinic demonstrated remarkably high activity with the Rev/Dex regimen. Thirty-one of 34 patients (91%) achieved an objective response, including 2 (6%) achieving complete response (CR), and 11 (32%) meeting criteria for very good partial response (VGPR).85 Lacy et al recently updated the results of this study. With longer follow-up, 56% of patients achieved very good partial response or better. In the subset of 21 patients receiving Rev/Dex as primary therapy without ASCT, 67% achieved VGPR or better.190 Approximately fifty percent of patients experienced grade 3 or higher non-hematologic toxicity, similar to rates seen with dexamethasone alone.
ECOG recently reported preliminary findings of a randomized trial testing Rev/Dex as administered in the Mayo Phase II trial (and in the regulatory relapsed refractory myeloma studies) versus Rev/low-dose Dex (40 mg dexamethasone once weekly).191 Results so far show that toxicity rates are significantly higher with Rev/standard-dose Dex compared to Rev/low-dose Dex. Early (first 4 month) mortality rates were low in both arms, 5% and 0.5% respectively. The early mortality rate in the Rev/low-dose dexamethasone arm is probably the lowest reported in any large phase III newly diagnosed trial in which enrollment was not restricted by age or eligibility for stem cell transplantation; DVT rates are also low, making this one of the safest pre-transplant induction regimens for myeloma. Based on this Rev/low-dose dexamethasone is currently the regimen of choice in the Mayo Stratification for Myeloma and Risk-adapted Therapy (mSMART) protocol for the treatment of standard-risk myeloma in patients who are candidates for ASCT outside the setting of a clinical trial.79
Recommendations for thromboprophylaxis are similar to those discussed above with Thal/Dex; aspirin alone is probably sufficient for patients receiving lenalidomide plus low-dose dexamethasone.
Bortezomib-based regimens
In newly diagnosed myeloma, bortezomib has shown response rates of approximately 40% as a single-agent. 192 Significantly higher response rates (approximately 70-90%) have been observed with bortezomib plus dexamethasone (Vel/Dex),193,194 bortezomib, thalidomide, dexamethasone (VTD) and other bortezomib-based combinations. The CR plus VGPR rate is approximately 25-30% with Vel/Dex in one study. No adverse effect on stem cell mobilization was noted.
Harousseau and colleagues recently reported preliminary results of a randomized trial comparing VAD versus Vel/Dex as pre-transplant induction therapy.195 With over 400 patients enrolled, preliminary results show superior response rates and long-term outcome with Vel/Dex compared to VAD. DVT risk was low with bortezomib (<5%).
Bortezomib-based regimens are of particular value in patients with renal failure and in patients with high-risk myeloma (see below), and is not associated with high rates of thrombosis.
Other induction regimens
The role of other pre-transplant induction regimens, such as those containing doxorubicin or liposomal doxorubicin, need to be weighed in terms of the added side-effects that can affect quality of life, and should be considered investigational until future studies show that the addition of these agents improves long-term outcome compared to the regimens discussed above. One exception is patients presenting with very aggressive disease including plasma cell leukemia features or rapidly progressive disease with or without extramedullary features. In these patients, 2 cycles of the combination chemotherapy regimen VDT-PACE (bortezomib (Velcade), dexamethasone, thalidomide, cisplatin (platinum), doxorubicin (Adriamycin), cyclophosphamide, and etoposide) developed by Barlogie et al as part of total therapy III is highly effective in controlling the disease rapidly.196
Initial Therapy in Standard-Risk Patients Eligible for Transplantation
Patients who are not transplant candidates are treated with standard alkylating agent therapy. For decades this has meant therapy with melphalan plus prednisone (MP).1 Over the years, despite better response rates, no survival benefit has been reported with any of the more aggressive combination chemotherapy regimens compared to MP. Two recent randomized studies show that MPT (melphalan, prednisone, thalidomide) improves response and EFS compared to MP;197,198 an overall survival advantage has been observed in one of the two trials.198 As a result, MPT has emerged as the current standard of care for patients not eligible for ASCT.
Melphalan, prednisone (MP)
MP has been used for decades in myeloma. The use of MP has decreased due to more active regimens as discussed below. However, it is still a regimen of value in frail, elderly patients who may not tolerate bortezomib or thalidomide well. The response rate with MP is approximately 50%, and complete responses occur in fewer than 5% of patients. Prior to the arrival of new agents such as thalidomide and bortezomib, despite better response rates no survival benefit was found with any combination chemotherapy regimen compared to MP.35 The dosing of MP is given on Table 10.122 Blood counts are monitored 3 weeks after beginning therapy and the dose of melphalan is adjusted to produce mild cytopenia at mid-cycle.
Melphalan, prednisone, thalidomide (MPT)
Five randomized trials have compared MP to MPT.197,199-202 Only two show a clear overall survival benefit.199,200
Facon et al199 randomized 447 patients (ages 65-75) to MP versus MPT versus tandem autologous stem cell transplantation (ASCT) with reduced dose melphalan (Melphlan100 mg/m2) (Table 9). Early (first 3 months) mortality rate was 8% with MP compared to 3% with MPT. Significantly higher response and PFS rates were observed with MPT compared with either MP or tandem ASCT groups. More importantly the trial demonstrated a significant survival advantage with MPT; median overall survival not reached at 52 months, 33 months, and 38 months, respectively (p<0.001).
Hulin et al200 confirmed a survival advantage with MPT compared with MP in a randomized trial in patients over the age of 75. After numerous attempts to improve on MP over the years with a variety of combination chemotherapy regimes, the results of these 3 randomized trials finally changed the standard of care for elderly patients.
Palumbo et al randomized patients to either standard dose MP for 6 months or to MPT for six months followed by maintenance thalidomide (Table 9).197 Overall response rates were significantly higher with the MPT than the MP (76% versus 48%) as was the CR plus near CR rate (28% versus 7%). MPT also resulted in superior 2-year EFS rates (54% versus 27%, p=0.0006). No significant survival improvement has been seen even with longer follow up.203 Randomized trials led by Wijermans et al and Waage et al also do not show any significant survival benefit with MPT compared to MP201,202
MPT therapy is associated with greater toxicity than MP. Therefore not all elderly patients may be able to receive the regimen. Grade 3-4 adverse events occur in approximately 50% of patients treated with MPT, compared to 25% with MP. 197 As with Thal/Dex, there is a significant (20%) risk of DVT with MPT in the absence of thromboprophylaxis. However, this rate drops to approximately 3% with the use of thromboprophylaxis (eg., enoxaparin).197
Melphalan, Prednisone, Bortezomib (MPV)
Mateos et al have reported the first results of the novel combination MPV in newly diagnosed myeloma in patients 65 years or older. Therapy was associated with a response rate of 89%, including 32% CR rate. Approximately half of the patients with CR also had no residual bone marrow plasma cells by immunophenotypic studies. In addition, MPV appeared to overcome the poor prognosis conferred by deletion 13 and IgH translocations. Event-free survival (EFS) and overall survival at 16 months were 83% and 90%, respectively. Most common grade 3 or higher adverse events were thrombocytopenia (51%), neutropenia (43%), peripheral neuropathy (17%), diarrhea (16%) and infection (16%). Herpes zoster infection occurred without prophylaxis in 13%; and was reduced to 7% in with prophylactic acyclovir. MPV appears very promising with high CR rates.
A recent randomized trial compared VMP to MP in 682 patients (Table 9).204 The time to progression among patients receiving VMP was 24 months versus 16.6 months with MP, P<0.001. Partial response or better was seen in 71% with VMP and 35% with MP; corresponding complete-response rates were 30% versus 4%, respectively (P<0.001). Overall survival was significantly better with VMP, p=0.008. Grade 3 events occurred more often with VMP versus MP (53% vs. 44%, P=0.02). Grade 3 neuropathy was seen in 13% with VMP. This study established the superiority of VMP over MP in myeloma.
Melphalan, Prednisone, Lenalidomide (MPR)
Palumbo and colleagues have studied the addition of lenalidomide to MP in newly diagnosed patients older than 65 years of age.205 Fifty-four patients were studied. At the maximum tolerated dose, the overall response rate was 81%, with 48% of patients achieving at least very good partial response (VGPR) or better, and 24% of patients achieving CR. Major grade 3-4 adverse events were neutropenia, thrombocytopenia, anemia, rash and febrile neutropenia. All patients received prophylactic aspirin; DVT was uncommon, occurring in 3 patients 6% including two after aspirin discontinuation. Most patients needed growth factor support. Based on this trial, MPR appears to be a very effective oral regimen for the treatment of elderly patients with multiple myeloma who are not candidates for ASCT. An ECOG randomized trial is comparing MPR to MPT.
HEMATOPOIETIC STEM CELL TRANSPLANTATION
Autologous stem cell transplantation (ASCT)
Although not curative, ASCT improves complete response rates and prolongs median overall survival in myeloma by approximately 12 months.206-209 The mortality rate is 1-2%. The major studies that established the role of ASCT in myeloma are listed on Table 9. Melphalan, 200 mg/m2 is the most widely used preparative (conditioning) regimen for ASCT. It is superior to the older regimen of melphalan 140 mg/m2 and 8 Gy total body irradiation (TBI).210 Studies are ongoing to determine if the conditioning regimen can be improved with the addition of radioactive compounds (Holmium (HO166 DOTMP) or Samarium 153-SM-EDTMP)211,212 or bortezomib.
Three randomized trials show that survival is similar whether ASCT is done early (immediately following 4 cycles of induction therapy) or delayed (at the time of relapse as salvage therapy)(Table 9).213-215 In a Spanish PETHEMA group randomized trial, patients responding to induction therapy had similar overall and progression free survival with either ASCT or 8 additional courses of chemotherapy,216 suggesting that the greatest benefit from ASCT may be in those with disease refractory to induction therapy.217,218
There is little doubt that ASCT prolongs survival in myeloma, but its timing (early versus delayed) is controversial. Overall, given the inconvenience and side-effects of prolonged chemotherapy, insurance, and other issues we still favor early ASCT, especially for patients less than 65 years of age with adequate renal function.208 However, given effective new agents to treat myeloma, some patients and physicians may choose to delay the procedure. The need for early ASCT is an important question for future clinical trials.
Tandem transplantation
With tandem (double) ASCT, patients receive a second planned ASCT after recovery from the first procedure (Table 11).219,220 The recent IFM 94 randomized trial found significantly better event-free and overall survival in recipients of double versus single ASCT (Table 9).221 A similar benefit was also demonstrated in a randomized trial conducted in Italy;222 two other randomized trials are yet to show significant improvement in overall survival with tandem ASCT, but they have shorter follow up.223,224
Table 11.
Randomized Trials Comparing Conventional Chemotherapy Versus Single Autologous Stem Cell Transplantation
| Trial | No. of patients | CR/VGPR, % | PFS, mo | OS, mo | |||
|---|---|---|---|---|---|---|---|
| CCT | ASCT | CCT | ASCT | CCT | ASCT | ||
| IFM90206,288 | 200 | 13 | 38* | 18 | 28* | 44 | 57* |
| MRC7207 | 401 | 8 | 44* | 20 | 32* | 42 | 54* |
| MAG91289 | 190 | 4 | 6 | 19 | 25* | 48 | 48 |
| MAG90**213 | 185 | 57 | 20 | 13 | 39 | 64 | 65 |
| PEETHMA284 | 164 | 11 | 30* | 33 | 42 | 61 | 66 |
| S9321**282 | 516 | 17 | 15 | 7 yr 14% | 7 yr 17% | 7 yr 38% | 7 yr 38% |
| MMSG†,290 | 194 | 6 | 25* | 16 | 28* | 42 | 58+* |
| HOVON- 24‡,291 | 303 | 13 | 28* | 23 | 24* | 50 | 55 |
ASCT, autologous stem cell transplant; CCT, conventional chemotherapy; CR/VGPR, complete response or very good partial response; ORR, any response greater than or equal to a partial response; PFS, progression free survival; OS, overall survival;
Significant
Trials testing early versus delayed autologous stem cell transplant
Transplant arm is 2 low dose (melphalan 100 mg/m2) autologous stem cell transplants
Could be considered “double transplant” since both arms received melphalan 70 mg/m2 × 2 without stem cell support as induction therapy
Reproduced with permission from Dispenzieri, A et al. Mayo Clinic Proc 2007;82:323-341. © Mayo Clinic Proceedings.
In both the French and Italian trials, the benefit of a second ASCT was restricted to patients failing to achieve a complete response or very good partial response (>90% reduction in M protein level) with the first procedure. Based on these results, we now routinely collect enough stem cells for two transplants in all eligible patients. Patients achieving a complete response or very good partial response with the first transplant are observed or offered clinical trials investigating maintenance therapy, reserving the second ASCT for relapse.225 Patients not achieving such a response are offered a second ASCT.
Allogeneic Transplantation
The advantages of allogeneic transplantation are lack of graft contamination with tumor cells and presence of a graft versus myeloma effect.226,227 However, only 5%-10% of patients are candidates because of age, availability of a HLA matched sibling donor, and adequate organ function. Further, the high treatment related mortality (TRM), mainly related to graft versus host disease (GVHD) has made conventional allogeneic transplants unacceptable for most patients with myeloma.
Several recent trials have been conducted using non-myeloablative conditioning regimens (mini-allogeneic transplantation).228 Initial trials evaluating this approach included relapsed or refractory patients, which was thought to account at least in part for the poor outcomes. It also became apparent that this approach was less useful in patients who had significant residual tumor burden at the time of the non-myeloablative transplant, which led to the concept of a planned autologous SCT followed by a planned reduced intensity allogeneic stem cell transplant (RIC SCT) a couple of months later.229 The initial studies had treatment related mortalities approaching 25%, 3-year overall survival and progression-free survival rates were 41% and 21%, respectively.229 Adverse OS was associated with chemoresistant disease, more than 1 prior transplantation, and absence of chronic GVHD. Using the planned tandem autologous/nonmyeloablative allogeneic stem cell transplantation approach, outcomes have been better with treatment-related mortalities of 15% and 2 year overall survivals approaching 75%.230 However, there is also a high risk of acute and chronic GVHD, and the occurrence of GVHD appears necessary for disease control.
A French randomized trial addressed the role of allogeneic stem cell transplant in high risk patients as defined by the presence of deletion 13 by FISH and of beta-2 microglobulin greater than 3 mg/L.231 Based on biological randomization, patients were allocated to either double autologous stem cell transplant or to autologous SCT followed by a RIC allogeneic SCT. Though patients did better than expected in both arms, with median overall survivals of 41 and 35 months, respectively, there was no significant difference between the 2 arms with a median follow-up of 24 months. The major criticisms of this study have been that the criteria used to select ‘high-risk’ disease did not select for the highest risk patients; and that the reduced intensity conditioning used may have been too immunosuppressive, thereby abrogating the graft versus myeloma effect.
In contrast to the French trial, a significant survival advantage was seen with ASCT followed by a RIC allogeneic SCT compared to tandem ASCT in a randomized trial conducted in Italy,. On an intent to treat basis, the median overall and event-free survival were longer with autologous SCT followed by an RIC compared to tandem ASCT (80 months vs. 54 months, p=0.01; and 35 months vs. 29 months, p=0.02, respectively).232 However, the sample size was modest (n=162), and additional confirmation is needed.
A subsequent trial investigating non-myeloablative transplants in myeloma strikes a note of caution. In this Spanish trial reported by Rosinol et al,233 110 patients with myeloma failing to achieve near-complete remission (nCR) after first ASCT were assigned to a second ASCT (85 patients) or a reduced-intensity-conditioning allogeneic transplantation (allo-RIC; 25 patients), based on the human leukocyte antigen (HLA)–identical sibling donor availability. Although the CR rate was superior with allo-RIC (40% vs 11%, P = 0.001) transplantation-related mortality was higher (16% vs 5%, P = .07), and no benefit was seen with regards to overall survival. In addition, allo-RIC was associated with a 66% rate of chronic graft-versus-host disease. and no statistical difference in event-free survival and overall survival. At this time, mini-allogeneic transplantation remains investigational. It should only be considered in the context of clinical trials in standard-risk myeloma because current results with the tandem ASCT strategy described earlier yields 7-year survival rates in excess of 40%. In these patients the treatment related mortality and GVHD rates with non-myeloablative allogeneic transplantation are unacceptably high.
TREATMENT OF HIGH-RISK MYELOMA
Patients with high-risk myeloma tend to do poorly with median overall survival of approximately two years even with tandem ASCT. The main option for these patients is novel therapeutic strategies.2,79 Bortezomib containing regimens should be considered early in the disease course as primary therapy. In at least 3 separate studies, bortezomib appears to overcome the adverse effect of deletion 13.234-236 Due to the high risk of early relapse, stem cell transplantation is best reserved for relapse. Routine maintenance therapy could be considered (eg., lenalidomide plus low-dose dexamethasone) given the high risk of early relapse. Clearly clinical trials and new agents specifically designed for high-risk myeloma are needed.
MAINTENANCE THERAPY
Maintenance therapy with interferon-α is of limited value and is seldom used.237 Recent results from a large intergroup study showed no benefit with interferon as maintenance therapy.215 A study by Berenson and colleagues suggests that prednisone may be useful for maintenance therapy.238 Progression-free survival (14 versus 5 months) and overall survival (37 versus 26 months) were significantly longer with 50 mg versus 10 mg of prednisone orally every other day. Since this comparison included only those who responded initially to steroid-based therapy and since patients did not receive ASCT, it is difficult to generalize these results to current practice. Clinical trials are currently evaluating thalidomide, dendritic cell vaccination, and other novel approaches as maintenance therapy.
A French randomized trial (IFM 99-02) randomized 597 patients (age <65) following tandem autologous stem cell transplantation to no maintenance, pamidronate, or pamidronate plus thalidomide.239 There was a significant improvement in event-free survival with maintenance thalidomide plus pamidronate; the 3-year event-free survival rate from the time of randomization was 36% in arm A, 37% in arm B, and 52% in arm C (P <0.009). The corresponding 4-year overall survival rate was 77%, 74%, and 87%, respectively (P < 0.04). A similar overall survival benefit with thalidomide post-transplant has been noted in separate randomized trials conducted in Australia,240 Italy,241 and Tunisia.242 However, no significant overall survival benefit with maintenance thalidomide has been reported in a randomized trial conducted in Brazil,243 and in separate trials conducted by the Medical Research Council (MRC),244 and the University of Arkansas,245 Thus the results with thalidomide maintenance are conflicting. It also appears that most of the benefit is seen in patients who have failed to achieve a very good partial response with ASCT, and that thalidomide is functioning more as a fixed duration of consolidation therapy rather than maintenance. Further, it is not clear if all patients not receiving thalidomide maintenance had access to thalidomide at relapse in most trials. More data are needed before recommending thalidomide as maintenance particularly given the long term adverse effects of the agent. At present for standard risk patients, observation remains the standard following ASCT. Multiple studies addressing the use of lenalidomide post-stem cell transplantation are on-going.
TREATMENT OF RELAPSED MULTIPLE MYELOMA
Almost all patients with myeloma eventually relapse. If relapse occurs more than 6 months after stopping therapy, the initial chemotherapy regimen should be re-instituted. Patients who have cryopreserved stem cells early in the disease course can derive significant benefit from ASCT as salvage therapy.246
In general, patients who have indolent relapse can often be treated with single-agents or MP. These patients present with asymptomatic increases in serum and urine monoclonal protein levels, progressive anemia, or few small lytic bone lesions. In contrast, patients with more aggressive relapse often require therapy with a combination of active agents. Given the non-curative nature of myeloma, patients with relapsed disease typically continue on one drug/regimen until relapse or toxicity and then try the next option.
The activity of various new agents in relapsed disease was discussed earlier. Besides thalidomide, lenalidomide, and bortezomib, high-dose pulse dexamethasone or intravenous methylprednisolone are reasonable options, particularly if the patient has had an initial response to steroids and was off high-dose steroids when the relapse occurred.247,248 Patients relapsing more than 6-12 months following an auto-SCT can respond to MP or MPT, since most would not have been exposed to this regimen as induction therapy.
Combination chemotherapy regimens such as VDT-PACE, VAD or other alkylator-based regimens can be effective in relapsed and refractory disease. Intravenous melphalan at a dose of 25mg/m2 is another active regimen, but usually requires transfusion and growth factor support.
TREATMENT OF COMPLICATIONS
Hypercalcemia
Aggressive hydration with isotonic saline and corticosteroids are effective in most cases. A single dose of pamidronate, 60-90 mg intravenously over 2-4 hours,249 or zoledronic acid 4 mg intravenously over 15 minutes,250 will normalize the calcium levels within 24-72 hours in most patients
Skeletal Lesions
Surgical fixation of fractures or impending fractures of long bones may be needed. Local radiation should be limited to patients with disabling pain who have a well-defined focal process that has not responded to analgesics and/or chemotherapy. The administration of bisphosphonates significantly reduces the number of skeletal events (pathologic fracture, need for irradiation or surgery on bone and spinal cord compression).251 In a randomized trial of 392 patients with at least one lytic lesion, skeletal events (pathologic fracture, radiation or surgery to bone, and spinal cord compression) after 9 months of therapy were significantly fewer with pamidronate compared to placebo, 24% versus 41%, respectively, P <0.001.251 More recently, zoledronic acid has been shown to have comparable efficacy to pamidronate.252,253 Either pamidronate (90 mg intravenously over at least 2 hours every 4 weeks) or zoledronic acid (4 mg intravenously over 15-30 minutes every 4 weeks) is recommended in patients with multiple myeloma who have one or more lytic lesions on skeletal roentgenograms.252,254
The typical recommendation by initially was to continue bisphosphonates indefinitely at monthly intervals.254 However, by 2003, avascular osteonecrosis of jaw (ONJ) has been described as a new complication associated with their use255-259 The etiology of ONJ is unclear, but is likely multifactorial in origin. Although most patients who develop ONJ have had recent dental or oral surgical procedures (70%), the remainder develop spontaneous ONJ.259 Proposed mechanisms include that inhibition of osteoclast activity reduces bone turnover and remodeling and that bisphosphonates prevent release of bone-specific factors that promote bone formation.260
The incidence of ONJ ranges from 4.4% to 21% depending of they type and duration of bisphosphonate use.260,261 Risks increase with duration of therapy, and likely with use of zoledronic acid compared to pamidronate or other lower intensity bisphosphonates. A Mayo clinic consensus statement recommends pamidronate instead of zoledronic acid as the bisphosphonate of choice for long-term therapy.260 It also recommends 2 years of monthly bisphosphonate therapy for patients with myelomatous bone disease, followed by either cessation of therapy in patient who are off active treatment for their myeloma or continuation of therapy every 3 months for those who are receiving myeloma therapy. These recommendations are bolstered by two recent observations. The first is that the rates of myeloma bone disease in the pre-bisphosphonate error was highest during the first 2 years after diagnosis.262 The second supporting data element is the recent finding that pamidronate use after tandem transplant did not provide any significant reduction in skeletal events.239
Both vertebroplasty (injection of methylmethacrylate into a collapsed vertebral body) or kyphoplasty (introduction of an inflatable bone tamp into the vertebral body and after inflation the injection of methylmethacrylate into the cavity) have been successfully used to decrease pain and help restore height.263 Pain relief is generally rapid, and can be long-lasting.
Spinal cord compression from an extramedullary plasmacytoma should be suspected in patients with severe back pain, weakness or paresthesias of the lower extremities, or bladder or bowel dysfunction, or incontinence. The standard treatment for cord or cauda equina compression are corticosteroids (dexamethasone 10 mg to 40 mg IV followed by 4 mg orally/intravenously four times daily) and radiation therapy. On rare occasions, surgical decompression may be necessary.
Renal Insufficiency
Nonsteroidal anti-inflammatory agents can precipitate renal failure and should generally be avoided.264,265 Dehydration, infection, and radiographic contrast media may also contribute to acute renal failure. Maintenance of a high urinary output (3 L/day) is important in preventing renal failure in those with high levels of monoclonal light chains in the urine. Persons with acute or subacute renal failure due to light-chain cast nephropathy should be treated with Thal/Dex or dexamethasone to reduce the tumor mass as quickly as possible. A trial of plasmapheresis should be considered in these patients in an attempt to prevent irreversible renal damage.266
Anemia
Iron, folate, or B-12 deficiency may be responsible for the anemia as well and must be recognized and treated. Treatment of the underlying disease and renal failure often leads to improvement in the hemoglobin level. Erythropoietin (40,000 units subcutaneously weekly) or darbepoietin (200 micrograms subcutaneously every 2 weeks) are useful in patients with persistent symptomatic anemia,267,268 but should be restricted because of a higher risk of DVT when used in combination with thalidomide or lenalidomide. Blood transfusions are indicated for patients with symptomatic anemia who do not obtain benefit from other therapies.
Infections
Patients should receive pneumococcal and influenza vaccinations. Intravenously administered gamma globulin every 3-4 weeks is indicated if patients have recurrent serious infections associated with severe hypogammaglobulinemia. The use of prophylactic antibiotics in patients receiving chemotherapy for myeloma has not been settled. A small randomized placebo-controlled trial of trimethoprim-sulfamethoxazole in 57 patients with newly diagnosed myeloma demonstrated benefit with routine prophylaxis administered with the first two cycles of chemotherapy.269 Due to the high risk of infections, prophylactic antibiotics for 3-4 months should be considered for all patients starting therapy for newly diagnosed myeloma. Prophylaxis against Pneumocystis carinii pneumonia (PCP) should also be considered in all patients receiving high dose steroid therapy. However, due to the risk of serious skin toxicity, trimethoprim-sulfamethoxazole should be avoided in patients receiving Thal/Dex or Rev/Dex therapy. In these patients prophylaxis with other antibiotics (such as ciprofloxacin, levofloxacin or cephalosporins) and alternative agents for PCP prophylaxis should be considered. A randomized trial comparing no prophylaxis, trimethoprim-sulfamethoxazole, and ciprofloxacin in patients with newly diagnosed myeloma receiving chemotherapy has just completed accrual, and results are awaited.
Hyperviscosity Syndrome
Infrequently patients with multiple myeloma develop hyperviscosity syndrome. Plasmapheresis promptly relieves the symptoms and should be done regardless of the viscosity level if the patient has signs or symptoms of hyperviscosity.270
Acknowledgments
Supported in part by Grants CA62242 and CA107476 from the National Cancer Institute, Bethesda, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kyle RA, Rajkumar SV. Multiple Myeloma. N Engl J Med. 2004;351:1860–1873. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 2.Rajkumar SV, Kyle RA. Multiple Myeloma: Diagnosis and Treatment. Mayo Clin Proc. 2005;80:1371–1382. doi: 10.4065/80.10.1371. [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis of monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–569. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 4.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of Monoclonal Gammopathy of Undetermined Significance. N Engl J Med. 2006;354:1362–1369. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 5.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Melton LJ., 3rd Incidence of multiple myeloma in Olmsted County, Minnesota: Trend over 6 decades. Cancer. 2004;101:2667–2674. doi: 10.1002/cncr.20652. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer Statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 7.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1,027 patients with newly diagnosed multiple myeloma. Mayo Clinic Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 8.Solly S. Remarks on the pathology of mollities ossium with cases. Medica-Chirurgical Transactions of London. 1844;27:435–461. doi: 10.1177/095952874402700129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macintyre W. Case of mollities and fragilitas ossium, accompanied with urine strongly charged with animal matter. Medica-Chirurgical Transactions of London. 1850;33:211–232. doi: 10.1177/095952875003300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clamp JR. Some aspects of the first recorded case of multiple myeloma. Lancet. 1967;2:1354–1356. doi: 10.1016/s0140-6736(67)90935-x. [DOI] [PubMed] [Google Scholar]
- 11.Bence Jones H. Chemical pathology. Lancet. 1847;2:88–92. [Google Scholar]
- 12.Bence Jones H. On the new substance occurring in the urine of a patient with mollities ossium. Philos Trans R Soc Land. 1848;138:55–62. [Google Scholar]
- 13.Waldeyer W. Ueber bindegewebszellen. Archiv fur microbiologie and anatomie. 1875;11:176–194. [Google Scholar]
- 14.Wright JH. A case of multiple myeloma. Trans Assoc Am Phys. 1900;15:137–147. [Google Scholar]
- 15.Geschickter CF, Copeland MM. Multiple myeloma. Arch surg. 1928;16:807–863. [Google Scholar]
- 16.Perlzweig WA, Delrue G, Geschicter C. Hyperproteinemia associated with multiple myelomas: report of an unusual case. J Am Med Assoc. 1928;90:755–757. [Google Scholar]
- 17.Fleischer R., XXIV Ueber das Vorkommen des sogenannten Bence Jones’ schen Eiweisskorpers im normalen Knochenmark. Arch Pathol Anatom Physiol Klin Med. 1880;80:842–849. [Google Scholar]
- 18.Bayne-Jones S, Wilson DW. Immunological reactions of Bence-Jones proteins. II. Differences between Bence-Jones proteins from various sources. Bulletin of the Johns Hopkins Hospital. 1922;33:119–125. [Google Scholar]
- 19.Korngold L, Lipari R. Multiple-myeloma proteins. III. The antigenic relationship of Bence Jones proteins to normal gamma-globulin and multiple-myeloma serum proteins. Cancer. 1956;9:262–272. doi: 10.1002/1097-0142(195603/04)9:2<262::aid-cncr2820090210>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.Tiselius A. A new apparatus for electrophoretic analysis of colloidal mixtures. Transactions of the Faraday Society. 1937;33:524. [Google Scholar]
- 21.Tiselius A, Kabat EA. Electrophoretic study of immune sera and purified antibody preparation. J Exp Med. 1939;69:119–131. doi: 10.1084/jem.69.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longsworth LG, Shedlovsky T, MacInnes DA. Electrophoretic patterns of normal and pathological human blood, serum, and plasma. Journal of Experimental Medicine. 1939;70:399–413. doi: 10.1084/jem.70.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grabar P, Williams CA. Methode permettant l’etude conjuguee des proprietes electrophoretiques et immunochimiques d’un melange de proteines; application au serum sanguin. Biochim Biophys Acta. 1953;10:193–194. doi: 10.1016/0006-3002(53)90233-9. [DOI] [PubMed] [Google Scholar]
- 24.Wilson AT. Direct Immunoelectrophoresis. J Immunol. 1964;92:431–434. [PubMed] [Google Scholar]
- 25.Waldenstrom J. Studies on conditions associated with disturbed gamma globulin formation (gammopathies) Harvey Lectures. 19601961;56:211–231. [PubMed] [Google Scholar]
- 26.Kyle RA. Multiple myeloma: an odyssey of discovery. British Journal of Haematology. 2000;111:1035–1044. doi: 10.1046/j.1365-2141.2000.02318.x. [DOI] [PubMed] [Google Scholar]
- 27.Alwall N. Urethane and stilbamidine in multiple myeloma: report on two cases. Lancet. 1947;2:388–389. doi: 10.1016/s0140-6736(47)90375-9. [DOI] [PubMed] [Google Scholar]
- 28.Holland JR, Hosley H, Scharlau C, et al. A controlled trial of urethane treatment in multiple myeloma. Blood. 1966;27:328–342. [PubMed] [Google Scholar]
- 29.Blokhin N, Larionov L, Perevodchikova N, Chebotareva L, Merkulova N. Clinical experiences with sarcolysin in neoplastic diseases. Ann N Y Acad Sci. 1958;68:1128–1132. doi: 10.1111/j.1749-6632.1958.tb42675.x. [DOI] [PubMed] [Google Scholar]
- 30.Bergsagel DE, Sprague CC, Austin C, Griffith KM. Evaluation of new chemotherapeutic agents in the treatment of multiple myeloma. IV. L-Phenylalanine mustard (NSC-8806. Cancer Chemother Rep. 1962;21:87–99. [PubMed] [Google Scholar]
- 31.Salmon SE, Shadduck RK, Schilling A. Intermittent high-dose prednisone (NSC-10023) therapy for multiple myeloma. Cancer Chemother Rep. 1967;51:179–187. [PubMed] [Google Scholar]
- 32.Mass RE. A comparison of the effect of prednisone and a placebo in the treatment of multiple myeloma. Cancer Chemother Rep. 1962;16:257–259. [PubMed] [Google Scholar]
- 33.McIntyre OR, Pajak TF, Kyle RA, Cornwell GG, 3rd, Leone L. Response rate and survival in myeloma patients receiving prednisone alone. Medical & Pediatric Oncology. 1985;13:239–243. doi: 10.1002/mpo.2950130502. [DOI] [PubMed] [Google Scholar]
- 34.Alexanian R, Haut A, Khan AU, et al. Treatment for multiple myeloma. Combination chemotherapy with different melphalan dose regimens. JAMA. 1969;208:1680–1685. doi: 10.1001/jama.208.9.1680. [DOI] [PubMed] [Google Scholar]
- 35.Myeloma Trialists’ Collaborative Group. Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: An overview of 6,633 patients from 27 randomized trials. J Clin Oncol. 1998;16:3832–3842. doi: 10.1200/JCO.1998.16.12.3832. [DOI] [PubMed] [Google Scholar]
- 36.Thomas ED, Lochte HL, Jr, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491–496. doi: 10.1056/NEJM195709122571102. [DOI] [PubMed] [Google Scholar]
- 37.McElwain TJ, Powles RL. High-dose intravenous melphalan for plasma-cell leukaemia and myeloma. Lancet. 1983;2:822–824. doi: 10.1016/s0140-6736(83)90739-0. [DOI] [PubMed] [Google Scholar]
- 38.The International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–757. [PubMed] [Google Scholar]
- 39.Rajkumar SV, Dispenzieri A, Kyle RA. Monoclonal gammopathy of undetermined significance, Waldenstrom macroglobulinemia, AL amyloidosis, and related plasma cell disorders: diagnosis and treatment. Mayo Clinic Proceedings. 2006;81:693–703. doi: 10.4065/81.5.693. [DOI] [PubMed] [Google Scholar]
- 40.Jego G, Bataille R, Geffroy-Luseau A, Descamps G, Pellat-Deceunynck C. Pathogen-associated molecular patterns are growth and survival factors for human myeloma cells through Toll-like receptors. Leukemia. 2006;20:1130–1137. doi: 10.1038/sj.leu.2404226. [DOI] [PubMed] [Google Scholar]
- 41.Bohnhorst J, Rasmussen T, Moen SH, et al. Toll-like receptors mediate proliferation and survival of multiple myeloma cells. Leukemia. 2006;20:1138–1144. doi: 10.1038/sj.leu.2404225. [DOI] [PubMed] [Google Scholar]
- 42.Mantovani A, Garlanda C. Inflammation and multiple myeloma: the Toll connection. Leukemia. 2006;20:937–938. doi: 10.1038/sj.leu.2404229. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Andres M, Almeida J, Martin-Ayuso M, et al. Clonal plasma cells from monoclonal gammopathy of undetermined significance, multiple myeloma and plasma cell leukemia show different expression profiles of molecules involved in the interaction with the immunological bone marrow microenvironment. Leukemia. 2005;19:449–455. doi: 10.1038/sj.leu.2403647. [DOI] [PubMed] [Google Scholar]
- 44.Rawstron AC, Fenton JA, Ashcroft J, et al. The interleukin-6 receptor alpha-chain (CD126) is expressed by neoplastic but not normal plasma cells. Blood. 2000;96:3880–3886. [PubMed] [Google Scholar]
- 45.Fonseca R, Barlogie B, Bataille R, et al. Genetics and cytogenetics of multiple myeloma: a workshop report. Cancer Res. 2004;64:1546–1558. doi: 10.1158/0008-5472.can-03-2876. [DOI] [PubMed] [Google Scholar]
- 46.Kuehl WM, Bergsagel PL. Multiple myeloma: evolving genetic events and host interactions. Nature Rev Cancer. 2002;2:175–187. doi: 10.1038/nrc746. [DOI] [PubMed] [Google Scholar]
- 47.Bergsagel PL, Kuehl WM. Chromosome translocations in multiple myeloma. Oncogene. 2001;20:5611–5622. doi: 10.1038/sj.onc.1204641. [DOI] [PubMed] [Google Scholar]
- 48.Fonseca R, Bailey RJ, Ahmann GJ, et al. Genomic abnormalities in monoclonal gammopathy of undetermined significance. Blood. 2002;100:1417–1424. [PubMed] [Google Scholar]
- 49.Drach J, Angerler J, Schuster J, et al. Interphase fluorescence in situ hybridization identifies chromosomal abnormalities in plasma cells from patients with monoclonal gammopathy of undetermined significance. Blood. 1995;86:3915–3921. [PubMed] [Google Scholar]
- 50.Fonseca R, Aguayo P, Ahmann GJ, et al. Translocations at 14q32 are common in patients with the monoclonal gammopathy of undetermined significance (MGUS) and involve several partner chromosomes. Blood. 1999;94(Suppl 1):663a. A 2943. [Google Scholar]
- 51.Rajkumar SV. MGUS and Smoldering Multiple Myeloma: Update on Pathogenesis, Natural History, and Management. Hematology (Am Soc Hematol Educ Program) 2005;2005:340–345. doi: 10.1182/asheducation-2005.1.340. [DOI] [PubMed] [Google Scholar]
- 52.Chng WJ, Van Wier SA, Ahmann GJ, et al. A validated FISH trisomy index demonstrates the hyperdiploid and nonhyperdiploid dichotomy in MGUS. Blood. 2005;106:2156–2161. doi: 10.1182/blood-2005-02-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Magrangeas F, Lode L, Wuilleme S, Minvielle S, Avet-Loiseau H. Genetic heterogeneity in multiple myeloma. Leukemia. 2005;19:191–194. doi: 10.1038/sj.leu.2403555. [DOI] [PubMed] [Google Scholar]
- 54.Kaufmann H, Ackermann J, Baldia C, et al. Both IGH translocations and chromosome 13q deletions are early events in monoclonal gammopathy of undetermined significance and do not evolve during transition to multiple myeloma. Leukemia. 2004;18:1879–1882. doi: 10.1038/sj.leu.2403518. [DOI] [PubMed] [Google Scholar]
- 55.Rajkumar SV, Mesa RA, Fonseca R, et al. Bone marrow angiogenesis in 400 patients with monoclonal gammopathy of undetermined significance, multiple myeloma, and primary amyloidosis. Clin Cancer Res. 2002;8:2210–2216. [PubMed] [Google Scholar]
- 56.Kumar S, Fonseca R, Dispenzieri A, et al. Prognostic value of angiogenesis in solitary bone plasmacytoma. Blood. 2003;101:1715–1717. doi: 10.1182/blood-2002-08-2441. [DOI] [PubMed] [Google Scholar]
- 57.Galea HR, Cogne M. GM-CSF and IL-12 production by malignant plasma cells promotes cell-mediated immune responses against monoclonal Ig determinants in a light chain myeloma model. Clinical & Experimental Immunology. 2002;129:247–253. doi: 10.1046/j.1365-2249.2002.01929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vacca A, Ria R, Ribatti D, et al. A paracrine loop in the vascular endothelial growth factor pathway triggers tumor angiogenesis and growth in multiple myeloma. Haematologica. 2003 [PubMed] [Google Scholar]
- 59.Roodman GD. Role of the bone marrow microenvironment in multiple myeloma. Journal of Bone & Mineral Research. 2002;17:1921–1925. doi: 10.1359/jbmr.2002.17.11.1921. [DOI] [PubMed] [Google Scholar]
- 60.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. see comment. [DOI] [PubMed] [Google Scholar]
- 61.Tian E, Zhan F, Walker R, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. see comment. [DOI] [PubMed] [Google Scholar]
- 62.Katzmann JA, Dispenzieri A, Kyle R, et al. Elimination of the Need for Urine Studies in the Screening Algorithm for Monoclonal Gammopathies by Using Serum Immunofixation and Free Light Chain Assays. Mayo Clin Proc. 2006;81:1575–1578. doi: 10.4065/81.12.1575. [DOI] [PubMed] [Google Scholar]
- 63.Kyle RA, Rajkumar SV. Monoclonal gammopathy of undetermined significance. Br J Haematol. 2006;134:573–589. doi: 10.1111/j.1365-2141.2006.06235.x. [DOI] [PubMed] [Google Scholar]
- 64.Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clinical Chemistry. 2002;48:1437–1444. [PubMed] [Google Scholar]
- 65.Bradwell AR, Carr-Smith HD, Mead GP, Harvey TC, Drayson MT. Serum test for assessment of patients with Bence Jones myeloma. Lancet. 2003;361:489–491. doi: 10.1016/S0140-6736(03)12457-9. [DOI] [PubMed] [Google Scholar]
- 66.Drayson M, Tang LX, Drew R, Mead GP, Carr-Smith H, Bradwell AR. Serum free light-chain measurements for identifying and monitoring patients with nonsecretory multiple myeloma. Blood. 2001;97:2900–2902. doi: 10.1182/blood.v97.9.2900. [DOI] [PubMed] [Google Scholar]
- 67.Lachmann HJ, Gallimore R, Gillmore JD, et al. Outcome in systemic AL amyloidosis in relation to changes in concentration of circulating free immunoglobulin light chains following chemotherapy. Br J Haematol. 2003;122:78–84. doi: 10.1046/j.1365-2141.2003.04433.x. [DOI] [PubMed] [Google Scholar]
- 68.Abraham RS, Clark RJ, Bryant SC, et al. Correlation of serum immunoglobulin free light chain quantification with urinary Bence Jones protein in light chain myeloma. Clin Chem. 2002;48:655–657. [PubMed] [Google Scholar]
- 69.Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842–854. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 70.Kyle RA. Prognostic factors in multiple myeloma. Stem Cells. 1995;2:56–63. [PubMed] [Google Scholar]
- 71.Rapoport BL, Falkson HC, Falkson G. Prognostic factors affecting the survival of patients with multiple myeloma. A retrospective analysis of 86 patients. S-Afr-Med-J. 1990;79:65–67. [PubMed] [Google Scholar]
- 72.Greipp PR, San Miguel JF, Durie BG, et al. International Staging System for Multiple Myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 73.Rajkumar SV, Greipp PR. Prognostic factors in multiple myeloma. Hematology Oncology Clinics of North America. 1999;13:1295–1314. doi: 10.1016/s0889-8588(05)70128-3. [DOI] [PubMed] [Google Scholar]
- 74.Tricot G, Sawyer JR, Jagannath S, et al. Unique role of cytogenetics in the prognosis of patients with myeloma receiving high-dose therapy and autotransplants. J Clin Oncol. 1997;15:2659–2666. doi: 10.1200/JCO.1997.15.7.2659. [DOI] [PubMed] [Google Scholar]
- 75.Greipp PR. Prognosis in myeloma. Mayo Clinic Proceedings. 1994;69:895–902. doi: 10.1016/s0025-6196(12)61797-2. [Review] [75 refs] [DOI] [PubMed] [Google Scholar]
- 76.Facon T, Avet-Loiseau H, Guillerm G, et al. Chromosome 13 abnormalities identified by FISH analysis and serum beta2-microglobulin produce a powerful myeloma staging system for patients receiving high-dose therapy. Blood. 2001;97:1566–1571. doi: 10.1182/blood.v97.6.1566. [DOI] [PubMed] [Google Scholar]
- 77.Greipp PR, Leong T, Bennett JM, et al. Plasmablastic morphology--an independent prognostic factor with clinical and laboratory correlates: Eastern Cooperative Oncology Group (ECOG) myeloma trial E9486 report by the ECOG Myeloma Laboratory Group. Blood. 1998;91:2501–2507. [PubMed] [Google Scholar]
- 78.Greipp PR, Lust JA, WM OF, Katzmann JA, Witzig TE, Kyle RA. Plasma cell labeling index and beta 2-microglobulin predict survival independent of thymidine kinase and C-reactive protein in multiple myeloma. Blood. 1993;81:3382–3387. see comments. [PubMed] [Google Scholar]
- 79.Dispenzieri A, Rajkumar SV, Gertz MA, et al. Treatment of newly diagnosed multiple myeloma based on Mayo stratification of myeloma and risk-adapted therapy (mSMART): Consensus Statement. Mayo Clin Proc. 2007;82:323–341. doi: 10.4065/82.3.323. [DOI] [PubMed] [Google Scholar]
- 80.Blade J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 81.Durie BGM, Harousseau J-L, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 82.Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. see comments. [DOI] [PubMed] [Google Scholar]
- 83.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 84.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. see comment. [DOI] [PubMed] [Google Scholar]
- 85.Rajkumar SV, Hayman SR, Lacy MQ, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106:4050–4053. doi: 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458–3464. doi: 10.1182/blood-2006-04-015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lenz W. A short history of thalidomide embryopathy. Teratology. 1988;38:203–215. doi: 10.1002/tera.1420380303. [DOI] [PubMed] [Google Scholar]
- 88.Lenz W. A personal perspective on the thalidomide tragedy. Teratology. 1992;46:417–418. doi: 10.1002/tera.1420460505. [DOI] [PubMed] [Google Scholar]
- 89.Stephens T, Brynner R. Dark Remedy. The impact of thalidomide and its revival as a vital medicine. Cambrigde, MA: Perseus Publishing; 2001. [Google Scholar]
- 90.Lenz W. Thalidomide and congenital abnormalities. Lancet. 1962;1:45. doi: 10.1016/s0140-6736(62)91943-8. [DOI] [PubMed] [Google Scholar]
- 91.McBride WG. Thalidomide and congenital abnormalities. Lancet. 1961;2:1358. [Google Scholar]
- 92.Geraghty K. Profile of a role model: Francis Oldham Kelsey, MD, PhD. Vol. 2003. American Medical Association; 2001. http://www.ama-assn.org/ama/pub/printcat/5335.html) [Google Scholar]
- 93.Rogerson G. Thalidomide and congenital abnormalities. Lancet. 1962;1:691. [Google Scholar]
- 94.Woodyatt PB. Thalidomide. Lancet. 1962;1:750. [Google Scholar]
- 95.Grabstad H, Golbey R. Clinical experience with thalidomide in patients with cancer. Clin Pharmacol Therap. 1965;6:298–302. doi: 10.1002/cpt196563298. [DOI] [PubMed] [Google Scholar]
- 96.Olson KB, Hall TC, Horton J, Khung CL, Hosley HF. Thalidomide (N-phthaloylglutamimide) in the treatment of advanced cancer. Clin Pharmacol Ther. 1965;6:292–297. doi: 10.1002/cpt196563292. [DOI] [PubMed] [Google Scholar]
- 97.Sheskin J. Thalidomide in the treatment of lepra reactions. Clin Pharmacol Ther. 1965;6:303–306. doi: 10.1002/cpt196563303. [DOI] [PubMed] [Google Scholar]
- 98.Iyer CG, Languillon J, Ramanujam K, et al. WHO co-ordinated short-term double-blind trial with thalidomide in the treatment of acute lepra reactions in male lepromatous patients. Bulletin of the World Health Organization. 1971;45:719–732. [PMC free article] [PubMed] [Google Scholar]
- 99.Mascaro JM, Lecha M, Torras H. Thalidomide in the treatment of recurrent, necrotic, and giant mucocutaneous aphthae and aphthosis. Archives of Dermatology. 1979;115:636–637. [PubMed] [Google Scholar]
- 100.Lim SH, McWhannell A, Vora AJ, Boughton BJ. Successful treatment with thalidomide of acute graft-versus-host disease after bone-marrow transplantation. Lancet. 1988;1:117. doi: 10.1016/s0140-6736(88)90312-1. [DOI] [PubMed] [Google Scholar]
- 101.Saurat JH, Camenzind M, Helg C, Chapuis B. Thalidomide for graft-versus-host disease after bone marrow transplantation. Lancet. 1988;1:359. doi: 10.1016/s0140-6736(88)91151-8. [DOI] [PubMed] [Google Scholar]
- 102.Vogelsang GB, Santos GW, Colvin OM, Chen T. Thalidomide for graft-versus-host disease. Lancet. 1988;1:827. doi: 10.1016/s0140-6736(88)91690-x. [DOI] [PubMed] [Google Scholar]
- 103.Vogelsang GB, Wells MC, Santos GW, Chen TL, Hess AD. Combination low-dose thalidomide and cyclosporine prophylaxis for acute graft-versus-host disease in a rat mismatched model. Transplantation Proceedings. 1988;20:226–228. [PubMed] [Google Scholar]
- 104.Ringden O, Aschan J, Westerberg L. Thalidomide for severe acute graft-versus-host disease. Lancet. 1988;2:568. doi: 10.1016/s0140-6736(88)92688-8. [DOI] [PubMed] [Google Scholar]
- 105.Youle M, Clarbour J, Farthing C, et al. Treatment of resistant aphthous ulceration with thalidomide in patients positive for HIV antibody. BMJ. 1989;298:432. doi: 10.1136/bmj.298.6671.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reyes-Teran G, Sierra-Madero JG, Martinez del Cerro V, et al. Effects of thalidomide on HIV-associated wasting syndrome: a randomized, double-blind, placebo-controlled clinical trial. Aids. 1996;10:1501–1507. doi: 10.1097/00002030-199611000-00007. [DOI] [PubMed] [Google Scholar]
- 107.Zeldis JB, Williams BA, Thomas SD, Elsayed ME. S.T.E.P.S.: a comprehensive program for controlling and monitoring access to thalidomide. Clinical Therapeutics. 1999;21:319–330. doi: 10.1016/s0149-2918(00)88289-2. [DOI] [PubMed] [Google Scholar]
- 108.Lary JM, Daniel KL, Erickson JD, Roberts HE, Moore CA. The return of thalidomide: can birth defects be prevented? Drug Safety. 1999;21:161–169. doi: 10.2165/00002018-199921030-00002. [DOI] [PubMed] [Google Scholar]
- 109.Eriksson T, Bjorkman S, Hoglund P. Clinical pharmacology of thalidomide. European Journal of Clinical Pharmacology. 2001;57:365–376. doi: 10.1007/s002280100320. [DOI] [PubMed] [Google Scholar]
- 110.Stirling DI. Pharmacology of thalidomide. Semin Hematol. 2000;37:5–14. [Google Scholar]
- 111.Eriksson T, Bjorkman S, Roth B, Fyge A, Hoglund P. Stereospecific determination, chiral inversion in vitro and pharmacokinetics in humans of the enantiomers of thalidomide. Chirality. 1995;7:44–52. doi: 10.1002/chir.530070109. [DOI] [PubMed] [Google Scholar]
- 112.D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kenyon BM, Browne F, D’Amato RJ. Effects of thalidomide and related metabolites in a mouse corneal model of neovascularization. Experimental Eye Research. 1997;64:971–978. doi: 10.1006/exer.1997.0292. [DOI] [PubMed] [Google Scholar]
- 114.Barlogie B, Spencer T, Tricot G, et al. Long term follow up of 169 patients receiving a phase II trial of single agent thalidomide for advanced and refractory multiple myeloma (MM) Blood. 2000;96:514a. doi: 10.1182/blood.v98.2.492. A2213. [DOI] [PubMed] [Google Scholar]
- 115.Rajkumar SV, Fonseca R, Dispenzieri A, et al. Thalidomide in the treatment of relapsed multiple myeloma. Mayo Clin Proc. 2000;75:897–902. doi: 10.4065/75.9.897. [DOI] [PubMed] [Google Scholar]
- 116.Kumar S, Gertz MA, Dispenzieri A, et al. Response rate, durability of response, and survival after thalidomide therapy for relapsed multiple myeloma. Mayo Clinic Proceedings. 2003;78:34–39. doi: 10.4065/78.1.34. comment. [DOI] [PubMed] [Google Scholar]
- 117.Rajkumar SV, Witzig TE. A review of angiogenesis and anti-angiogenic therapy with thalidomide in multiple myeloma. Cancer Treat Rev. 2000;26:351–362. doi: 10.1053/ctrv.2000.0188. [DOI] [PubMed] [Google Scholar]
- 118.Rajkumar SV, Hayman S, Gertz MA, et al. Combination therapy with thalidomide plus dexamethasone for newly diagnosed myeloma. J Clin Oncol. 2002;20:4319–4323. doi: 10.1200/JCO.2002.02.116. [DOI] [PubMed] [Google Scholar]
- 119.Rajkumar SV, Dispenzieri A, Fonseca R, et al. Thalidomide for previously untreated indolent or smoldering multiple myeloma. Leukemia. 2001;15:1274–1276. doi: 10.1038/sj.leu.2402183. [DOI] [PubMed] [Google Scholar]
- 120.Rajkumar SV, Gertz MA, Lacy MQ, et al. Thalidomide as initial therapy for early-stage myeloma. Leukemia. 2003;17:775–779. doi: 10.1038/sj.leu.2402866. [DOI] [PubMed] [Google Scholar]
- 121.Weber D, Rankin K, Gavino M, Delasalle K, Alexanian R. Thalidomide alone or with dexamethasone for previously untreated multiple myeloma. J Clin Oncol. 2003;21:16–19. doi: 10.1200/JCO.2003.03.139. [DOI] [PubMed] [Google Scholar]
- 122.Rajkumar SV, Gertz MA, Kyle RA, Greipp PR. Current therapy for multiple myeloma. Mayo Clin Proc. 2002;77:813–822. doi: 10.4065/77.8.813. [DOI] [PubMed] [Google Scholar]
- 123.Anagnostopoulos A, Weber D, Rankin K, Delasalle K, Alexanian R. Thalidomide and dexamethasone for resistant multiple myeloma. Br J Haematol. 2003;121:768–771. doi: 10.1046/j.1365-2141.2003.04345.x. [DOI] [PubMed] [Google Scholar]
- 124.Dimopoulos MA, Hamilos G, Zomas A, et al. Pulsed cyclophosphamide, thalidomide and dexamethasone: an oral regimen for previously treated patients with multiple myeloma. Hematology Journal. 2004;5:112–117. doi: 10.1038/sj.thj.6200326. [DOI] [PubMed] [Google Scholar]
- 125.Garcia-Sanz R, Gonzalez-Fraile MI, Sierra M, Lopez C, Gonzalez M, San Miguel JF. The combination of thalidomide, cyclophosphamide and dexamethasone (ThaCyDex) is feasible and can be an option for relapsed/refractory multiple myeloma. Hematology Journal. 2002;3:43–48. doi: 10.1038/sj.thj.6200150. [DOI] [PubMed] [Google Scholar]
- 126.Kropff MH, Lang N, Bisping G, et al. Hyperfractionated cyclophosphamide in combination with pulsed dexamethasone and thalidomide (HyperCDT) in primary refractory or relapsed multiple myeloma. Br J Haematol. 2003;122:607–616. doi: 10.1046/j.1365-2141.2003.04473.x. [DOI] [PubMed] [Google Scholar]
- 127.Coleman M, Leonard J, Lyons L, et al. BLT-D (clarithromycin [Biaxin], low-dose thalidomide, and dexamethasone) for the treatment of myeloma and Waldenstrom’s macroglobulinemia. Leukemia & Lymphoma. 2002;43:1777–1782. doi: 10.1080/1042819021000006303. [DOI] [PubMed] [Google Scholar]
- 128.Ghobrial IM, Rajkumar SV. Management of thalidomide toxicity. J Support Oncol. 2003;1:194–205. [PMC free article] [PubMed] [Google Scholar]
- 129.Osman K, Comenzo R, Rajkumar SV. Deep vein thrombosis and thalidomide therapy for multiple myeloma. N Engl J Med. 2001;344:1951–1952. doi: 10.1056/NEJM200106213442516. [DOI] [PubMed] [Google Scholar]
- 130.Zangari M, Anaissie E, Barlogie B, et al. Increased risk of deep-vein thrombosis in patients with multiple myeloma receiving thalidomide and chemotherapy. Blood. 2001;98:1614–1615. doi: 10.1182/blood.v98.5.1614. [DOI] [PubMed] [Google Scholar]
- 131.Zangari M, Siegel E, Barlogie B, et al. Thrombogenic activity of doxorubicin in myeloma patients receiving thalidomide: implications for therapy. Blood. 2002;100:1168–1171. doi: 10.1182/blood-2002-01-0335. [DOI] [PubMed] [Google Scholar]
- 132.Zangari M, Saghafifar F, Anaissie E, et al. Activated protein C resistance in the absence of factor V Leiden mutation is a common finding in multiple myeloma and is associated with an increased risk of thrombotic complications. Blood Coagulation & Fibrinolysis. 2002;13:187–192. doi: 10.1097/00001721-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 133.Rajkumar SV, Gertz MA, Witzig TE. Life-threatening toxic epidermal necrolysis with thalidomide therapy for myeloma. N Engl J Med. 2000;343:972–973. doi: 10.1056/NEJM200009283431315. [DOI] [PubMed] [Google Scholar]
- 134.Badros AZ, Siegel E, Bodenner D, et al. Hypothyroidism in patients with multiple myeloma following treatment with thalidomide. American Journal of Medicine. 2002;112:412–413. doi: 10.1016/s0002-9343(01)01137-8. [DOI] [PubMed] [Google Scholar]
- 135.Barlogie B, Desikan R, Eddlemon P, et al. Extended survival in advanced and refractory multiple myeloma after single-agent thalidomide: identification of prognostic factors in a phase 2 study of 169 patients. Blood. 2001;98:492–494. doi: 10.1182/blood.v98.2.492. [DOI] [PubMed] [Google Scholar]
- 136.Dimopoulos MA, Anagnostopoulos A, Weber D. Treatment of plasma cell dyscrasias with thalidomide and its derivatives. J Clin Oncol. 2003;21:4444–4454. doi: 10.1200/JCO.2003.07.200. [DOI] [PubMed] [Google Scholar]
- 137.Adams J. Development of the proteasome inhibitor PS-341. Oncologist. 2002;7:9–16. doi: 10.1634/theoncologist.7-1-9. [DOI] [PubMed] [Google Scholar]
- 138.Adams J. Proteasome inhibition in cancer: development of PS-341. Semin Oncol. 2001;28:613–619. doi: 10.1016/s0093-7754(01)90034-x. [DOI] [PubMed] [Google Scholar]
- 139.Adams J. Proteasome inhibitors as new anticancer drugs. Curr Opin Oncol. 2002;14:628–634. doi: 10.1097/00001622-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 140.Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 141.Adams J, Palombella VJ, Elliott PJ. Proteasome inhibition: a new strategy in cancer treatment. Invest New Drugs. 2000;18:109–121. doi: 10.1023/a:1006321828515. [DOI] [PubMed] [Google Scholar]
- 142.Adams J. Preclinical and clinical evaluation of proteasome inhibitor PS-341 for the treatment of cancer. Curr Op Chem Biol. 2002;6:493–500. doi: 10.1016/s1367-5931(02)00343-5. [DOI] [PubMed] [Google Scholar]
- 143.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 144.Varshavsky A. The ubiquitin system. Trend Biochem Sci. 1997;22:383–387. doi: 10.1016/s0968-0004(97)01122-5. [DOI] [PubMed] [Google Scholar]
- 145.Craiu A, Gaczynska M, Akopian T, et al. Lactacystin and clasto-lactacystin beta-lactone modify multiple proteasome beta-subunits and inhibit intracellular protein degradation and major histocompatibility complex class I antigen presentation. J Biol Chem. 1997;272:13437–13445. doi: 10.1074/jbc.272.20.13437. [DOI] [PubMed] [Google Scholar]
- 146.Orlowski M. The multicatalytic proteinase complex, a major extralysosomal proteolytic system. Biochemistry. 1990;29:10289–10297. doi: 10.1021/bi00497a001. [DOI] [PubMed] [Google Scholar]
- 147.Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 148.Almond JB, Cohen GM. The proteasome: a novel target for cancer chemotherapy. Leukemia. 2002;16:433–443. doi: 10.1038/sj.leu.2402417. [DOI] [PubMed] [Google Scholar]
- 149.Braun BC, Glickman M, Kraft R, et al. The base of the proteasome regulatory particle exhibits chaperone-like activity. Nature Cell Biology. 1999;1:221–226. doi: 10.1038/12043. [DOI] [PubMed] [Google Scholar]
- 150.Kloetzel PM. Antigen processing by the proteasome. Nature Rev Mol Cell Biol. 2001;2:179–187. doi: 10.1038/35056572. [DOI] [PubMed] [Google Scholar]
- 151.Pickart CM, VanDemark AP. Opening doors into the proteasome. Nature Struct Biol. 2000;7:999–1001. doi: 10.1038/81018. comment. [DOI] [PubMed] [Google Scholar]
- 152.Groll M, Bajorek M, Kohler A, et al. A gated channel into the proteasome core particle. Nature Struct Biol. 2000;7:1062–1067. doi: 10.1038/80992. comment. [DOI] [PubMed] [Google Scholar]
- 153.Lowe J, Stock D, Jap B, Zwickl P, Baumeister W, Huber R. Crystal structure of the 20S proteasome from the archaeon T. acidophilum at 3.4 A resolution. Science. 1995;268:533–539. doi: 10.1126/science.7725097. comment. [DOI] [PubMed] [Google Scholar]
- 154.Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. [PubMed] [Google Scholar]
- 155.Orlowski RZ, Eswara JR, Lafond-Walker A, Grever MR, Orlowski M, Dang CV. Tumor growth inhibition induced in a murine model of human Burkitt’s lymphoma by a proteasome inhibitor. Cancer Res. 1998;58:4342–4348. [PubMed] [Google Scholar]
- 156.Drexler HC. Activation of the cell death program by inhibition of proteasome function. Proc Natl Acad Sci USA. 1997;94:855–860. doi: 10.1073/pnas.94.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Delic J, Masdehors P, Omura S, et al. The proteasome inhibitor lactacystin induces apoptosis and sensitizes chemo- and radioresistant human chronic lymphocytic leukaemia lymphocytes to TNF-alpha-initiated apoptosis. Br J Cancer. 1998;77:1103–1107. doi: 10.1038/bjc.1998.183. comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.An B, Goldfarb RH, Siman R, Dou QP. Novel dipeptidyl proteasome inhibitors overcome Bcl-2 protective function and selectively accumulate the cyclin-dependent kinase inhibitor p27 and induce apoptosis in transformed, but not normal, human fibroblasts. Cell Death Diff. 1998;5:1062–1075. doi: 10.1038/sj.cdd.4400436. [DOI] [PubMed] [Google Scholar]
- 159.Traenckner EB, Wilk S, Baeuerle PA. A proteasome inhibitor prevents activation of NF-kappa B and stabilizes a newly phosphorylated form of I kappa B-alpha that is still bound to NF-kappa B. EMBO J. 1994;13:5433–5441. doi: 10.1002/j.1460-2075.1994.tb06878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mori S, Tanaka K, Omura S, Saito Y. Degradation process of ligand-stimulated platelet-derived growth factor beta-receptor involves ubiquitin-proteasome proteolytic pathway. J Biol Chem. 1995;270:29447–29452. doi: 10.1074/jbc.270.49.29447. [DOI] [PubMed] [Google Scholar]
- 161.Cusack JC, Jr, Liu R, Houston M, et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer Res. 2001;61:3535–3540. [PubMed] [Google Scholar]
- 162.Russo SM, Tepper JE, Baldwin AS, Jr, et al. Enhancement of radiosensitivity by proteasome inhibition: implications for a role of NF-kappaB. Int J Rad Oncol Biol Phys. 2001;50:183–193. doi: 10.1016/s0360-3016(01)01446-8. [DOI] [PubMed] [Google Scholar]
- 163.Shah SA, Potter MW, McDade TP, et al. 26S proteasome inhibition induces apoptosis and limits growth of human pancreatic cancer. J Cell Biochem. 2001;82:110–122. doi: 10.1002/jcb.1150. [DOI] [PubMed] [Google Scholar]
- 164.Teicher BA, Ara G, Herbst R, Palombella VJ, Adams J. The proteasome inhibitor PS-341 in cancer therapy. Clin Cancer Res. 1999;5:2638–2645. [PubMed] [Google Scholar]
- 165.Frankel A, Man S, Elliott P, Adams J, Kerbel RS. Lack of multicellular drug resistance observed in human ovarian and prostate carcinoma treated with the proteasome inhibitor PS-341. Clin Cancer Res. 2000;6:3719–3728. [PubMed] [Google Scholar]
- 166.Bold RJ, Virudachalam S, McConkey DJ. Chemosensitization of pancreatic cancer by inhibition of the 26S proteasome. J Surg Res. 2001;100:11–17. doi: 10.1006/jsre.2001.6194. [DOI] [PubMed] [Google Scholar]
- 167.Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- 168.Mitsiades N, Mitsiades CS, Poulaki V, et al. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci USA. 2002;99:14374–14379. doi: 10.1073/pnas.202445099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mitsiades N, Mitsiades CS, Richardson PG, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101:2377–2380. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 170.LeBlanc R, Catley LP, Hideshima T, et al. Proteasome inhibitor PS-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine model. Cancer Res. 2002;62:4996–5000. [PubMed] [Google Scholar]
- 171.Berenson JR, Jagannath S, Barlogie B, et al. Experience with long-term therapy using the proteasome inhibitor, bortezomib, in advanced multiple myeloma (MM) Proc Am Soc Clin Oncol. 2003;22:581. [Google Scholar]
- 172.Richardson PG, Schlossman RL, Weller E, et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002;100:3063–3067. doi: 10.1182/blood-2002-03-0996. [DOI] [PubMed] [Google Scholar]
- 173.Zangari M, Tricot G, Zeldis J, Eddlemon P, Saghafifar F, Barlogie B. Results of Phase I Study of CC-5013 for the Treatment of Multiple Myeloma (MM) Patients Who Relapse after High Dose Chemotherapy (HDCT) Blood. 2001:775a. A3226. [Google Scholar]
- 174.Dimopoulos MA, Spencer A, Attal M, et al. Study of Lenalidomide Plus Dexamethasone Versus Dexamethasone Alone in Relapsed or Refractory Multiple Myeloma (MM): Results of a Phase 3 Study (MM-010) Blood. 2005;106:6. [Google Scholar]
- 175.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus high-dose dexamethasone provides improved overall survival compared to high-dose dexamethasone alone for relapsed or refractory multiple myeloma (MM): Results of a North American phase III study (MM-009) Proc Am Soc Clin Oncol. 2006;24:A7521. [Google Scholar]
- 176.Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized Phase III Study of Pegylated Liposomal Doxorubicin Plus Bortezomib Compared With Bortezomib Alone in Relapsed or Refractory Multiple Myeloma: Combination Therapy Improves Time to Progression. J Clin Oncol. 2007;25:3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 177.Streetly M, Jones RW, Knight R, et al. An Update of the Use and Outcomes of the New Immunomodulatory Agent CC-4047 (Actimid) in Patients with Relapsed / Refractory Myeloma. Blood. 2003;102:236a. [Google Scholar]
- 178.Lacy MQ, Hayman SR, Gertz MA, et al. Pomalidomide (CC4047) Plus Low-Dose Dexamethasone (Pom/dex) Is Highly Effective Therapy in Relapsed Multiple Myeloma. Blood (ASH Annual Meeting Abstracts) 2008;112:866. [Google Scholar]
- 179.Jagannath S, Vij R, Stewart AK, et al. Initial Results of PX-171-003, An Open-Label, Single-Arm, Phase II Studyof Carfilzomib (CFZ) in Patients with Relapsed and Refractory Multiple Myeloma (MM) Blood (ASH Annual Meeting Abstracts) 2008;112:864. [Google Scholar]
- 180.Vij R, Wang M, Orlowski R, et al. Initial Results of PX-171-004, An Open-Label, Single-Arm, Phase II Study of Carfilzomib (CFZ) in Patients with Relapsed Myeloma (MM) Blood (ASH Annual Meeting Abstracts) 2008;112:865. [Google Scholar]
- 181.Hideshima T, Chauhan D, Podar K, Schlossman RL, Richardson P, Anderson KC. Novel therapies targeting the myeloma cell and its bone marrow microenvironment. Semin Oncol. 2001;28:607–612. doi: 10.1016/s0093-7754(01)90033-8. [DOI] [PubMed] [Google Scholar]
- 182.Musto P, Petrucci MT, Bringhen S, et al. A multicenter, randomized clinical trial comparing zoledronic acid versus observation in patients with asymptomatic myeloma. Cancer. 2008;113:1588–1595. doi: 10.1002/cncr.23783. [DOI] [PubMed] [Google Scholar]
- 183.Cavo M, Zamagni E, Tosi P, et al. Superiority of thalidomide and dexamethasone over vincristine-doxorubicindexamethasone (VAD) as primary therapy in preparation for autologous transplantation for multiple myeloma. Blood. 2005;106:35–39. doi: 10.1182/blood-2005-02-0522. [DOI] [PubMed] [Google Scholar]
- 184.Fermand J-P, Jaccard A, Macro M, et al. A Randomized Comparison of Dexamethasone + Thalidomide (Dex/Thal) vs Dex + Placebo (Dex/P) in Patients (pts) with Relapsing Multiple Myeloma (MM) Blood. 2006;108:3563. [Google Scholar]
- 185.Rajkumar SV, Blood E, Vesole DH, Fonseca R, Greipp PR. Phase III Clinical Trial of Thalidomide Plus Dexamethasone Compared With Dexamethasone Alone in Newly Diagnosed Multiple Myeloma: A Clinical Trial Coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24:431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- 186.Weber DM, Gavino M, Delasalle K, Rankin K, Giralt S, Alexanian R. Thalidomide alone or with dexamethasone for multiple myeloma. Blood. 1999;94(Suppl 1):604a. A 2686. [Google Scholar]
- 187.Cavo M, Zamagni E, Tosi P, et al. First-line therapy with thalidomide and dexamethasone in preparation for autologous stem cell transplantation for multiple myeloma. Haematologica. 2004;89:826–831. [PubMed] [Google Scholar]
- 188.Rajkumar SV, Rosiñol L, Hussein M, et al. A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study of Thalidomide plus Dexamethasone Versus Dexamethasone as Initial Therapy for Newly Diagnosed Multiple Myeloma. J Clin Oncol. 2008;26:2171–2177. doi: 10.1200/JCO.2007.14.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lokhorst H, van der Holt B, Zweegman S, et al. Final Analysis of HOVON-50 Randomized Phase III Study on the Effect of Thalidomide Combined with Adriamycine, Dexamethasone (AD) and High Dose Melphalan (HDM) in Patients with Multiple Myeloma (MM) ASH Annual Meeting Abstracts. 2008;112:157. [Google Scholar]
- 190.Lacy M, Gertz M, Dispenzieri A, et al. Lenalidomide Plus Dexamethasone (Rev/Dex) in Newly Diagnosed Myeloma: Response to Therapy, Time to Progression, and Survival. Blood. 2006;108:798. [Google Scholar]
- 191.Rajkumar SV, Jacobus S, Callander N, Fonseca R, Vesole D, Greipp P. A Randomized Phase III Trial of Lenalidomide Plus High-Dose Dexamethasone Versus Lenalidomide Plus Low-Dose Dexamethasone in Newly Diagnosed Multiple Myeloma (E4A03): A Trial Coordinated by the Eastern Cooperative Oncology Group. Blood. 2006;108:799. [Google Scholar]
- 192.Richardson PG, Chanan-Khan A, Schlossman RL, et al. Phase II Trial of Single Agent Bortezomib (VELCADE®) in Patients with Previously Untreated Multiple Myeloma (MM) Blood. 2004;104:100a. A336. [Google Scholar]
- 193.Jagannath S, Durie BG, Wolf J, et al. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol. 2005;129:776–783. doi: 10.1111/j.1365-2141.2005.05540.x. [DOI] [PubMed] [Google Scholar]
- 194.Harousseau J, Attal M, Leleu X, et al. Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of an IFM phase II study. Haematologica. 2006;91:1498–1505. [PubMed] [Google Scholar]
- 195.Harousseau J-L, Marit G, Caillot D, et al. VELCADE/Dexamethasone (Vel/Dex) Versus VAD as Induction Treatment Prior to Autologous Stem Cell Transplantation (ASCT) in Newly Diagnosed Multiple Myeloma (MM): An Interim Analysis of the IFM 2005-01 Randomized Multicenter Phase III Trial. Blood. 2006;108:56. [Google Scholar]
- 196.Barlogie B, Anaissie E, van Rhee F, et al. Incorporating bortezomib into upfront treatment for multiple myeloma: early results of total therapy 3. Br J Haematol. 2007;138:176–185. doi: 10.1111/j.1365-2141.2007.06639.x. [DOI] [PubMed] [Google Scholar]
- 197.Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006;367:825–831. doi: 10.1016/S0140-6736(06)68338-4. [DOI] [PubMed] [Google Scholar]
- 198.Facon T, Mary JY, Hulin C, et al. Major Superiority of Melphalan - Prednisone (MP) + Thalidomide (THAL) over MP and Autologous Stem Cell Transplantation in the Treatment of Newly Diagnosed Elderly Patients with Multiple Myeloma. Blood. 2005;106:230a. A780. [Google Scholar]
- 199.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370:1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 200.Hulin C, Virion J, Leleu X, et al. Comparison of melphalan-prednisone-thalidomide (MP-T) to melphalan-prednisone (MP) in patients 75 years of age or older with untreated multiple myeloma (MM). Preliminary results of the randomized, double-blind, placebo controlled IFM 01-01 trial. J Clin Oncol (Meeting Abstracts) 2007;25:8001. [Google Scholar]
- 201.Wijermans P, Schaafsma M, van Norden Y, et al. Melphalan + Prednisone Versus Melphalan + Prednisone + Thalidomide in Induction Therapy for Multiple Myeloma in Elderly Patients: Final Analysis of the Dutch Cooperative Group HOVON 49 Study. Blood (ASH Annual Meeting Abstracts) 2008;112:649. [Google Scholar]
- 202.Waage A, Gimsing P, Juliusson G, Turesson I, Fayers P. Melphalan-Prednisone-Thalidomide to Newly Diagnosed Patients with Multiple Myeloma: A Placebo Controlled Randomised Phase 3 Trial. ASH Annual Meeting Abstracts. 2007;110:78. [Google Scholar]
- 203.Palumbo A, Bringhen S, Liberati AM, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood. 2008;112:3107–3114. doi: 10.1182/blood-2008-04-149427. [DOI] [PubMed] [Google Scholar]
- 204.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus Melphalan and Prednisone for Initial Treatment of Multiple Myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 205.Palumbo A, Falco P, Corradini P, et al. Melphalan, Prednisone, and Lenalidomide Treatment for Newly Diagnosed Myeloma: A Report From the GIMEMA Italian Multiple Myeloma Network. J Clin Oncol. 2007;25:4459–4465. doi: 10.1200/JCO.2007.12.3463. [DOI] [PubMed] [Google Scholar]
- 206.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 207.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 208.Blade J, Vesole DH, Gertz M. Transplantation for multiple myeloma: who, when, how often? Blood. 2003;102:3469–3477. [Google Scholar]
- 209.Kumar A, Loughran T, Alsina M, Durie BG, Djulbegovic B. Management of multiple myeloma: a systematic review and critical appraisal of published studies. Lancet Oncology. 2003;4:293–304. doi: 10.1016/s1470-2045(03)01077-5. [DOI] [PubMed] [Google Scholar]
- 210.Moreau P, Facon T, Attal M, et al. Comparison of 200 mg/m2 melphalan and 8 Gy total body irradiation plus 140 mg/m2 melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myélome 9502 randomized trial. Blood. 2002;99:731–735. doi: 10.1182/blood.v99.3.731. [DOI] [PubMed] [Google Scholar]
- 211.Bensinger W, Giralt S, Holmberg L, et al. 166H0-DOTMP and high-dose melphalan before autologous peripheral blood stem cell transplantation in patients with multiple myeloma. Hematol J. 2003;4(Suppl 1):S215–216. [Google Scholar]
- 212.Dispenzieri A, Wiseman GA, Lacy MQ, et al. A Phase II Study of High Dose 153-Samarium EDTMP (153-Sm EDMTP) and Melphalan for Peripheral Stem Cell Transplantation (PBSCT) in Multiple Myeloma (MM) Blood. 2003;102:982a. [Google Scholar]
- 213.Fermand JP, Ravaud P, Chevret S, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92:3131–3136. [PubMed] [Google Scholar]
- 214.Facon T, Mary JY, Harousseau JL, et al. Front-line or rescue autologous bone marrow transplantation (ABMT) following a first course of high dose melphalan (HDM) in multiple myeloma (MM). Preliminary results of a prospective randomized trial (CIAM) protocol. Blood. 1996;88(Suppl1):685a. [Google Scholar]
- 215.Barlogie B, Kyle R, Anderson K, et al. Comparable Survival in Multiple Myeloma (MM) with High Dose Therapy (HDT) Employing MEL 140 mg/m2 + TBI 12 Gy Autotransplants Versus Standard Dose Therapy with VBMCP and No Benefit from Interferon (IFN) Maintenance: Results of Intergroup Trial S9321. Blood. 2003;102:42a. [Google Scholar]
- 216.Blade J, Sureda A, Ribera JM, et al. High-Dose Therapy Autotransplantation/Intensification Versus Continued Conventional Chemotherapy in Multiple Myeloma Patients Responding to Initial Chemotherapy. Definitive Results from PETHEMA after a Median Follow-Up of 66 Months. Blood. 2003;102:42a. [Google Scholar]
- 217.Rajkumar SV, Fonseca R, Lacy MQ, et al. Autologous stem cell transplantation for relapsed and primary refractory myeloma. Bone Marrow Transplantation. 1999;23:1267–1272. doi: 10.1038/sj.bmt.1701805. [DOI] [PubMed] [Google Scholar]
- 218.Blade J, Esteve J. Treatment approaches for relapsing and refractory multiple myeloma. Acta Oncologica. 2000;39:843–847. doi: 10.1080/028418600750063604. [Review] [44 refs] [DOI] [PubMed] [Google Scholar]
- 219.Barlogie B, Jagannath S, Vesole DH, et al. Superiority of tandem autologous transplantation over standard therapy for previously untreated multiple myeloma. Blood. 1997;89:789–793. [PubMed] [Google Scholar]
- 220.Barlogie B, Jagannath S, Desikan KR, et al. Total therapy with tandem transplants for newly diagnosed multiple myeloma. Blood. 1999;93:55–65. [PubMed] [Google Scholar]
- 221.Attal M, Harousseau JL, Facon T, et al. Double Autologous Transplantation Improves Survival of Multiple Myeloma Patients: Final Analysis of a Prospective Randomized Study of the “Intergroupe Francophone du Myelome” (IFM 94) Blood. 2002;100:5a. [Google Scholar]
- 222.Cavo M, Cellini C, Zamagni E, et al. Superiority of Double over Single Autologous Stem Cell Transplantation as First-Line Therapy for Multiple Myeloma. Blood. 2004;104:155a. A536. [Google Scholar]
- 223.Fermand JP, Alberti C, Marolleau JP. Single versus tandem high dose therapy (HDT) supported with autologous blood stem cell (ABSC) transplantation using unselected or CD34-enriched ABSC: results of a two by two designed randomized trial in 230 young patients with multiple myeloma (MM) Hematol J. 2003;4(Suppl 1):S59. [Google Scholar]
- 224.Goldschmidt H. Single vs. tandem autolgous transplantation in multiple myeloma: the GMMG experience. Hematol J. 2003;4(Suppl1):S61. [Google Scholar]
- 225.Sirohi B, Powles R, Singhal S, et al. High-Dose Melphalan and Second Autografts for Myeloma Relapsing after One Autograft: Results Equivalent to Tandem Autotransplantation. Blood. 2001;98:402a. A1690. [Google Scholar]
- 226.Mehta J, Singhal S. Graft-versus-myeloma. Bone Marrow Transplantation. 1998;22:835–843. doi: 10.1038/sj.bmt.1701459. [DOI] [PubMed] [Google Scholar]
- 227.Gahrton G, Svensson H, Cavo M, et al. Progress in allogenic bone marrow and peripheral blood stem cell transplantation for multiple myeloma: a comparison between transplants performed 1983--93 and 1994--8 at European Group for Blood and Marrow Transplantation centres. Br J Haematol. 2001;113:209–216. doi: 10.1046/j.1365-2141.2001.02726.x. [DOI] [PubMed] [Google Scholar]
- 228.Einsele H, Schafer HJ, Hebart H, et al. Follow-up of patients with progressive multiple myeloma undergoing allografts after reduced-intensity conditioning. Br J Haematol. 2003;121:411–418. doi: 10.1046/j.1365-2141.2003.04299.x. [DOI] [PubMed] [Google Scholar]
- 229.Crawley C, Lalancette M, Szydlo R, et al. Outcomes for reduced-intensity allogeneic transplantation for multiple myeloma: an analysis of prognostic factors from the Chronic Leukaemia Working Party of the EBMT. Blood. 2005;105:4532–4539. doi: 10.1182/blood-2004-06-2387. [DOI] [PubMed] [Google Scholar]
- 230.Maloney DG, Molina AJ, Sahebi F, et al. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood. 2003;102:3447–3454. doi: 10.1182/blood-2002-09-2955. [DOI] [PubMed] [Google Scholar]
- 231.Garban F, Attal M, Michallet M, et al. Prospective comparison of autologous stem cell transplantation followed by dose-reduced allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04 trial) in high-risk de novo multiple myeloma. Blood. 2006;107:3474–3480. doi: 10.1182/blood-2005-09-3869. [DOI] [PubMed] [Google Scholar]
- 232.Bruno B, Rotta M, Patriarca F, et al. A Comparison of Allografting with Autografting for Newly Diagnosed Myeloma. N Engl J Med. 2007;356:1110–1120. doi: 10.1056/NEJMoa065464. [DOI] [PubMed] [Google Scholar]
- 233.Rosinol L, Perez-Simon JA, Sureda A, et al. A prospective PETHEMA study of tandem autologous transplantation versus autograft followed by reduced-intensity conditioning allogeneic transplantation in newly diagnosed multiple myeloma. Blood. 2008;112:3591–3593. doi: 10.1182/blood-2008-02-141598. [DOI] [PubMed] [Google Scholar]
- 234.Mateos M-V, Hernandez J-M, Hernandez M-T, et al. Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: results of a multicenter phase 1/2 study. Blood. 2006;108:2165–2172. doi: 10.1182/blood-2006-04-019778. [DOI] [PubMed] [Google Scholar]
- 235.Jagannath S, Richardson PG, Sonneveld P, et al. Bortezomib appears to overcome the poor prognosis conferred by chromosome 13 deletion in phase 2 and 3 trials. Leukemia. 2006;21:151–157. doi: 10.1038/sj.leu.2404442. [DOI] [PubMed] [Google Scholar]
- 236.Sagaster V, Ludwig H, Kaufmann H, et al. Bortezomib in relapsed multiple myeloma: response rates and duration of response are independent of a chromosome 13q-deletion. Leukemia. 2006;21:164–168. doi: 10.1038/sj.leu.2404459. [DOI] [PubMed] [Google Scholar]
- 237.Myeloma Trialists’ Collaborative G. Interferon as therapy for multiple myeloma: an individual patient data overview of 24 randomized trials and 4012 patients. Br J Haematol. 2001;113:1020–1034. doi: 10.1046/j.1365-2141.2001.02857.x. [DOI] [PubMed] [Google Scholar]
- 238.Berenson JR, Crowley JJ, Grogan TM, et al. Maintenance therapy with alternate-day prednisone improves survival in multiple myeloma patients. Blood. 2002;99:3163–3168. doi: 10.1182/blood.v99.9.3163. [DOI] [PubMed] [Google Scholar]
- 239.Attal M, Harousseau J-L, Leyvraz S, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108:3289–3294. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 240.Spencer A, Prince M, Roberts AW, Bradstock KF, Prosser IW. First Analysis of the Australasian Leukaemia and Lymphoma Group (ALLG) Trial of Thalidomide and Alternate Day Prednisolone Following Autologous Stem Cell Transplantation (ASCT) for Patients with Multiple Myeloma (ALLG MM6) ASH Annual Meeting Abstracts. 2006;108:58. [Google Scholar]
- 241.Offidani M, Polloni C, Corvatta L, et al. Thalidomide-Dexamethasone vs Interferon-{alpha}-Dexamethasone as Maintenance Treatment after ThaDD Induction for Multiple Myeloma (MM): Final Analysis of a Prospective, Randomized Study. ASH Annual Meeting Abstracts. 2007;110:532. [Google Scholar]
- 242.Abdelkefi A, Ladeb S, Torjman L, et al. Single autologous stem-cell transplantation followed by maintenance therapy with thalidomide is superior to double autologous transplantation in multiple myeloma: results of a multicenter randomized clinical trial. Blood. 2008;111:1805–1810. doi: 10.1182/blood-2007-07-101212. [DOI] [PubMed] [Google Scholar]
- 243.Maiolino A, Hungria VT, Oliveira-Duarte G, et al. Thalidomide + Dexamethasone as Maintenance after Single Autologous Stem Cell Transplantation Improves Progression-Free Survival (PFS) in Advanced Multiple Myeloma. A Prospective Brazilian Randomized Trial. ASH Annual Meeting Abstracts. 2008;112:3703. [Google Scholar]
- 244.Morgan GJ, Jackson GH, Davies FE, et al. Maintenance Thalidomide May Improve Progression Free but Not Overall Survival; Results from the Myeloma IX Maintenance Randomisation. Blood (ASH Annual Meeting Abstracts) 2008;112:656. [Google Scholar]
- 245.Barlogie B, Tricot G, Anaissie E, et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N Engl J Med. 2006;354:1021–1030. doi: 10.1056/NEJMoa053583. see comment. [DOI] [PubMed] [Google Scholar]
- 246.Gertz MA, Lacy MQ, Inwards DJ, et al. Early harvest and late transplantation as an effective therapeutic strategy in multiple myeloma. Bone Marrow Transplantation. 1999;23:221–226. doi: 10.1038/sj.bmt.1701559. [DOI] [PubMed] [Google Scholar]
- 247.Alexanian R, Barlogie B, Dixon D. High-dose glucocorticoid treatment of resistant myeloma. Annals of Internal Medicine. 1986;105:8–11. doi: 10.7326/0003-4819-105-1-8. [DOI] [PubMed] [Google Scholar]
- 248.Gertz MA, Garton JP, Greipp PR, Witzig TE, Kyle RA. A phase II study of high-dose methylprednisolone in refractory or relapsed multiple myeloma. Leukemia. 1995;9:2115–2118. [PubMed] [Google Scholar]
- 249.Gucalp R, Theriault R, Gill I, et al. Treatment of cancer-associated hypercalcemia. Double-blind comparison of rapid and slow intravenous infusion regimens of pamidronate disodium and saline alone. Arch Intern Med. 1994;154:1935–1944. doi: 10.1001/archinte.154.17.1935. [DOI] [PubMed] [Google Scholar]
- 250.Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19:558–567. doi: 10.1200/JCO.2001.19.2.558. [DOI] [PubMed] [Google Scholar]
- 251.Berenson JR, Lichtenstein A, Porter L, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334:488–493. doi: 10.1056/NEJM199602223340802. see comments. [DOI] [PubMed] [Google Scholar]
- 252.Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer. 2001;91:1191–1200. doi: 10.1002/1097-0142(20010401)91:7<1191::aid-cncr1119>3.0.co;2-0. erratum appears in Cancer 2001 May 15;91(10):1956. [DOI] [PubMed] [Google Scholar]
- 253.Rosen LS, Gordon D, Kaminski M, et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastatses in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase II, double blind, comparative trial. Cancer J. 2001;7:377–387. [PubMed] [Google Scholar]
- 254.Berenson JR, Hillner BE, Kyle RA, et al. American Society of Clinical Oncology Clinical Practice Guidelines: The role of bisphosphonates in multiple myeloma. J Clin Oncol. 2002;20:3719–3736. doi: 10.1200/JCO.2002.06.037. [DOI] [PubMed] [Google Scholar]
- 255.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. Journal of Oral & Maxillofacial Surgery. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. see comment. [DOI] [PubMed] [Google Scholar]
- 256.Kademani D, Koka S, Lacy MQ, Rajkumar SV. Primary surgical therapy for osteonecrosis of the jaw secondary to bisphosphonate therapy. Mayo Clinic Proceedings. 2006;81:1100–1103. doi: 10.4065/81.8.1100. [DOI] [PubMed] [Google Scholar]
- 257.Badros A, Weikel D, Salama A, et al. Osteonecrosis of the Jaw in Multiple Myeloma Patients: Clinical Features and Risk Factors. J Clin Oncol. 2006;24:945–952. doi: 10.1200/JCO.2005.04.2465. [DOI] [PubMed] [Google Scholar]
- 258.Durie BG, Katz M, Crowley J. Osteonecrosis of the jaw and bisphosphonates. N Engl J Med. 2005;353:99–102. doi: 10.1056/NEJM200507073530120. discussion 199-102. [DOI] [PubMed] [Google Scholar]
- 259.Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. Journal of Oral & Maxillofacial Surgery. 2004;62:527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 260.Lacy MQ, Dispenzieri A, Gertz MA, et al. Mayo clinic consensus statement for the use of bisphosphonates in multiple myeloma. Mayo Clinic Proceedings. 2006;81:1047–1053. doi: 10.4065/81.8.1047. [DOI] [PubMed] [Google Scholar]
- 261.Dimopoulos MA, Kastritis E, Anagnostopoulos A, et al. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: evidence of increased risk after treatment with zoledronic acid. Haematologica. 2006;91:968–971. [PubMed] [Google Scholar]
- 262.Melton LJ, 3rd, Kyle RA, Achenbach SJ, Oberg AL, Rajkumar SV. Fracture risk with multiple myeloma: a population-based study. Journal of Bone & Mineral Research. 2005;20:487–493. doi: 10.1359/JBMR.041131. [DOI] [PubMed] [Google Scholar]
- 263.Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. Journal of Neurosurgery. 2003;98:21–30. doi: 10.3171/spi.2003.98.1.0021. [DOI] [PubMed] [Google Scholar]
- 264.Yussim E, Schwartz E, Sidi Y, Ehrenfeld M. Acute renal failure precipitated by non-steroidal anti-inflammatory drugs (NSAIDs) in multiple myeloma. Am J Hematol. 1998;58:142–144. doi: 10.1002/(sici)1096-8652(199806)58:2<142::aid-ajh10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 265.Wu MJ, Kumar KS, Kulkarni G, Kaiser H. Multiple myeloma in naproxen-induced acute renal failure. N Engl J Med. 1987;317:170–171. [PubMed] [Google Scholar]
- 266.Johnson WJ, Kyle RA, Pineda AA, O’Brien PC, Holley KE. Treatment of renal failure associated with multiple myeloma. Plasmapheresis, hemodialysis, and chemotherapy. Arch Intern Med. 1990;150:863–869. [PubMed] [Google Scholar]
- 267.Garton JP, Gertz MA, Witzig TE, et al. Epoetin alfa for the treatment of the anemia of multiple myeloma. A prospective, randomized, placebo-controlled, double-blind trial. Arch Intern Med. 1995;155:2069–2074. [PubMed] [Google Scholar]
- 268.Dammacco F, Castoldi G, Rodjer S. Efficacy of epoetin alfa in the treatment of anaemia of multiple myeloma. Br J Haematol. 2001;113:172–179. doi: 10.1046/j.1365-2141.2001.02715.x. erratum appears in Br J Haematol 2001 Sep;114(3):738. [DOI] [PubMed] [Google Scholar]
- 269.Oken MM, Pomeroy C, Weisdorf D, Bennett JM. Prophylactic antibiotics for the prevention of early infection in multiple myeloma. American Journal of Medicine. 1996;100:624–628. doi: 10.1016/s0002-9343(95)00043-7. [DOI] [PubMed] [Google Scholar]
- 270.Gertz MA, Kyle RA. Hyperviscosity syndrome. Journal of Intensive Care Medicine. 1995;10:128–141. doi: 10.1177/088506669501000304. [DOI] [PubMed] [Google Scholar]
- 271.Rajkumar SV, Kyle RA. Multiple myeloma: diagnosis and treatment. Mayo Clinic Proceedings. 2005;80:1371–1382. doi: 10.4065/80.10.1371. see comment. [DOI] [PubMed] [Google Scholar]
- 272.Kyle RA, Therneau TM, Rajkumar SV, et al. Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Blood. 2003;102:3759–3764. doi: 10.1182/blood-2003-03-0801. [DOI] [PubMed] [Google Scholar]
- 273.Gobbi PG, Baldini L, Broglia C, et al. Prognostic validation of the international classification of immunoglobulin M gammopathies: a survival advantage for patients with immunoglobulin M monoclonal gammopathy of undetermined significance? Clin Cancer Res. 2005;11:1786–1790. doi: 10.1158/1078-0432.CCR-04-1899. [DOI] [PubMed] [Google Scholar]
- 274.Kyle RA, Therneau TM, Rajkumar SV, et al. Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Semin Oncol. 2003;30:169–171. doi: 10.1053/sonc.2003.50062. [DOI] [PubMed] [Google Scholar]
- 275.Baldini L, Goldaniga M, Guffanti A, et al. Immunoglobulin M monoclonal gammopathies of undetermined significance and indolent Waldenstrom’s macroglobulinemia recognize the same determinants of evolution into symptomatic lymphoid disorders: proposal for a common prognostic scoring system. J Clin Oncol. 2005;23:4662–4668. doi: 10.1200/JCO.2005.06.147. [DOI] [PubMed] [Google Scholar]
- 276.Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol. 2003;30:110–115. doi: 10.1053/sonc.2003.50082. [DOI] [PubMed] [Google Scholar]
- 277.Dimopoulos MA, Moulopoulos LA, Maniatis A, Alexanian R. Solitary plasmacytoma of bone and asymptomatic multiple myeloma. Blood. 2000;96:2037–2044. [PubMed] [Google Scholar]
- 278.Dimopoulos MA, Kiamouris C, Moulopoulos LA. Solitary plasmacytoma of bone and extramedullary plasmacytoma. Hematol Oncol Clin North Am. 1999;13:1249–1257. doi: 10.1016/s0889-8588(05)70124-6. [DOI] [PubMed] [Google Scholar]
- 279.Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood. 2003;101:2496–2506. doi: 10.1182/blood-2002-07-2299. [DOI] [PubMed] [Google Scholar]
- 280.Browman GP, Bergsagel D, Sicheri D, et al. Randomized trial of interferon maintenance in multiple myeloma: a study of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1995;13:2354–2360. doi: 10.1200/JCO.1995.13.9.2354. [DOI] [PubMed] [Google Scholar]
- 281.Fassas AB, Spencer T, Sawyer J, et al. Both hypodiploidy and deletion of chromosome 13 independently confer poor prognosis in multiple myeloma. Br J Haematol. 2002;118:1041–1047. doi: 10.1046/j.1365-2141.2002.03757.x. [DOI] [PubMed] [Google Scholar]
- 282.Barlogie B, Kyle RA, Anderson KC, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol. 2006;24:929–936. doi: 10.1200/JCO.2005.04.5807. [DOI] [PubMed] [Google Scholar]
- 283.Attal M, Harousseau JL, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–2502. doi: 10.1056/NEJMoa032290. see comment. [DOI] [PubMed] [Google Scholar]
- 284.Blade J, Rosinol L, Sureda A, et al. High-dose therapy intensification compared with continued standard chemotherapy in multiple myeloma patients responding to the initial chemotherapy: long-term results from a prospective randomized trial from the Spanish cooperative group PETHEMA. Blood. 2005;106:3755–3759. doi: 10.1182/blood-2005-03-1301. [DOI] [PubMed] [Google Scholar]
- 285.Rajkumar SV, Jacobus S, Callander N, et al. A Randomized Trial of Lenalidomide Plus High-Dose Dexamethasone (RD) Versus Lenalidomide Plus Low-Dose Dexamethasone (Rd) in Newly Diagnosed Multiple Myeloma (E4A03): A Trial Coordinated by the Eastern Cooperative Oncology Group. ASH Annual Meeting Abstracts. 2007;110:74. [Google Scholar]
- 286.Harousseau JL, Mathiot C, Attal M, et al. VELCADE/Dexamethasone (Vel/D) Versus VAD as Induction Treatment Prior to Autologous Stem Cell Transplantion (ASCT) in Newly Diagnosed Multiple Myeloma (MM): Updated Results of the IFM 2005/01 Trial. ASH Annual Meeting Abstracts. 2007;110:450. [Google Scholar]
- 287.Cavo M, Patriarca F, Tacchetti P, et al. Bortezomib (Velcade(R))-Thalidomide-Dexamethasone (VTD) vs Thalidomide-Dexamethasone (TD) in Preparation for Autologous Stem-Cell (SC) Transplantation (ASCT) in Newly Diagnosed Multiple Myeloma (MM) ASH Annual Meeting Abstracts. 2007;110:73. [Google Scholar]
- 288.Harousseau JL, Attal M. The role of stem cell transplantation in multiple myeloma. Blood Reviews. 2002;16:245–253. doi: 10.1016/s0268-960x(02)00043-7. [DOI] [PubMed] [Google Scholar]
- 289.Fermand JP, Katsahian S, Divine M, et al. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: long-term results of a randomized control trial from the Group Myelome-Autogreffe. J Clin Oncol. 2005;23:9227–9233. doi: 10.1200/JCO.2005.03.0551. [DOI] [PubMed] [Google Scholar]
- 290.Palumbo A, Bringhen S, Petrucci MT, et al. Intermediate-dose melphalan improves survival of myeloma patients aged 50 to 70: results of a randomized controlled trial. Blood. 2004;104:3052–3057. doi: 10.1182/blood-2004-02-0408. [DOI] [PubMed] [Google Scholar]
- 291.Sonneveld P, van der Holt B, Vellenga E, et al. Intensive Versus Double Intensive Therapy in Untreated Multiple Myeloma: Final Analysis of the HOVON 24 Trial. ASH Annual Meeting Abstracts. 2005;106:2545. [Google Scholar]