Abstract
The first total synthesis for the (Z)-17-methyl-13-octadecenoic acid was accomplished in seven steps and in a 45% overall yield. The use of (trimethylsilyl)acetylene was key in the synthesis. Based on a previous developed strategy in our laboratory the best synthetic route towards the title compound was first acetylide coupling of (trimethylsilyl)acetylene to the long-chain protected 12-bromo-1-dodecanol followed by a second acetylide coupling to the short-chain 3-methyl-1-bromobutane, which resulted in higher yields. Complete spectral data is also presented for the first time for this recently discovered fatty acid. The title compound displayed antiprotozoal activity against Leishmania donovani (EC50 = 19.8 μg/ml) and inhibited the leishmania DNA topoisomerase IB at concentrations of 50 μM.
Keywords: Fatty acids, leishmaniasis, (Z)-17-methyl-13-octadecenoic acid, Polymastia penicillus, sponges, synthesis, topoisomerase IB
1. Introduction
Marine sponges have provided some rather interesting phospholipid fatty acids with no counterpart in terrestrial organisms. One rather poorly studied class of fatty acids has been the iso methyl-branched nonadecenoic acids, from which only a handful number of compounds are known. Among these fatty acids it is worthwhile to mention here the (Z)-17-methyl-6-octadecenoic acid and the (Z)-17-methyl-7-octadecenoic acid, which were isolated from the mollusk Siphonaria denticulata and synthesized for the first time by our group (Carballeira et al., 2001). The isomeric (Z)-17-methyl-11-octadecenoic acid was also identified in the Caribbean sponge Calyx podatypa (Carballeira et al., 1998), but it is probably arising from a marine bacterium since it was first identified in the sulfate-reducing bacteria Desulfobacter and Desulfobulbus (Taylor and Parkes, 1983). Just recently, G. Barnathan and coworkers identified the unprecedented fatty acid (Z)-17-methyl-13-octadecenoic acid (1) in the phospholipids (0.8% relative abundance) of the North-East Atlantic sponge Polymastia penicillus, collected from the Gulf of Morbihan in Brittany, France (Denis et al., 2009). This rather unusual fatty acid is the iso methyl-branched nonadecenoic acid with the closest double bond to the ω end of the chain, i.e., a (n-5) fatty acid. However, acid 1 was identified in P. penicillus by means of mass spectrometry on the corresponding methyl ester and pyrrolidide derivative, only. The identification by GC-MS relied on minute amounts in a complex fatty acid mixture. It is likely that acid 1 was synthesized from a symbiotic bacterium of P. penicillus, thus implying that having the complete mass spectral data of 1 at hand will be useful to microbiologists seeking to characterize 1 in bacterial samples. The latter statement is backed up by the fact that acid 1 seems to be a two-carbon chain extension of the bacterial acid (Z)-15-methyl-11-hexadecenoic acid, an acid that has been identified in several sources but most interesting in Myxococcus xanthus (Boon et al., 1977; Reyes and Carballeira, 1996). M. xanthus is a myxobacterium whose fatty acids have been reported to sensitize Gram-negative and Gram-positive bacterial cells to the action of bacteriolytic enzymes (Boon et al., 1996). Therefore, it would also be of interest to explore the biological activity of the new acid 1, but it will remain unexplored until enough material becomes available for such studies. However, we believed that by applying our previous developed synthetic methodology for unsaturated iso-methyl branched fatty acids (Carballeira et al., 2007) to the synthesis of 1 enough material for such studies could be obtained. Therefore, in order to provide the complete characterization of 1 as well as to supply enough material for future biological studies we have developed the first total synthesis for the (Z)-17-methyl-13-octadecenoic acid (1). This synthesis was accomplished following a synthetic sequence consisting of seven steps and using (trimethylsilyl)acetylene as the key reagent in the synthesis (Scheme 1). It was also found that acid 1 displays antiprotozoal activity towards Leishmania donovani by a mechanism that probably involves inhibition of the leishmania DNA topoisomerase I.
Scheme 1.
Synthesis of (Z)-17-methyl-13-octadecenoic acid (1).
2. Materials and methods
2.1. Instrumentation
1H NMR (300 or 500 MHz) and 13C NMR (75 or 125 MHz) were either recorded on a Bruker DPX-300 or a Bruker DRX-500 spectrometer. 1H NMR chemical shifts are reported with respect to internal (CH3)4Si, 13C NMR chemical shifts are reported in parts per million relative to CDCl3 (77.0 ppm). GC/MS analyses were recorded at 70 eV using either a Hewlett Packard 5972A MS ChemStation or an Agilent 5975C MS ChemStation coupled to an Agilent 7890A GC where both instruments were equipped with a 30 m × 0.25 mm special performance capillary column (HP-5MS) of polymethyl siloxane crosslinked with 5% phenyl methylpolysiloxane. IR spectra were recorded on a Nicolet Magna 750 FT-IR spectrophotometer (Thermo-Nicolet, Madison, WI, USA). High resolution mass spectral data was performed at the Emory University Mass Spectrometry Center on a thermo LTQ-FTMS using APCI as the probe.
2.2. 12-bromo-1-[(tetrahydropyran-2-yl)oxy]dodecane (3)
To 2.60 g of 12-bromo-1-dodecanol in 15 ml of chloroform (CHCl3) was added dropwise 19.6 mmol of 2,3-dihydro-2H-pyran and catalytic amounts of p-toluenesulfonic acid (PTSA). The reaction mixture was stirred for 24h at room temperature. The organic layer was washed with water (2 × 20 ml), NaHCO3 (2 × 20 ml), CHCl3 (1 × 20 ml), and dried over MgSO4, filtered, and evaporated in vacuo. The crude product was purified using silica gel column chromatography eluting with hexane/ether (9:1). The pure product 3 was obtained as a colorless oil 2.66 g (7.63 mmol) for a 78% yield. IR (neat) νmax 2924, 2853, 1464, 1440, 1352, 1259, 1200, 1134, 1120, 1078, 1031, 985, 905, 869, 814, 722, 646, 564 cm−1; 1H NMR (CDCl3, 300 MHz) δ 4.57 (1H, brt, J = 2.4Hz), 3.86 (1H, m), 3.73 (1H, m), 3.50 (1H, m), 3.39 (1H, m), 3.39 (2H, t, J = 7.0 Hz, H-12), 1.84 (4H, m), 1.56 (4H, m), 1.39 (2H, m), 1.27 (16H, m, -CH2-); 13C NMR (CDCl3, 75 MHz) δ98.81 (d), 67.66 (t, C-1), 62.33 (t), 34.07 (t, C-12), 32.80 (t), 30.75 (t), 29.71 (t), 29.53 (t), 29.51 (t), 28.48 (t), 29.45 (t), 29.40 (t), 28.73 (t), 28.14 (t), 26.20 (t), 25.46 (t), 19.68 (t); GC-MS (70 eV) m/z (relative intensity) 347 (M+-1, 1), 165 (1), 164 (4), 163 (1), 162 (5), 150 (8), 149 (2), 148 (9), 137 (4), 136 (1), 135 (4), 134 (1), 115 (2), 112 (1), 111 (6), 109 (3), 108 (2), 103 (1), 102 (1), 101 (8), 99 (1), 97 (22), 96 (3), 95 (3), 86 (6), 85 (100), 84 (25), 83 (32), 82 (10), 81 (6), 69 (41), 68 (13), 67 (12), 57 (21), 56 (30), 55 (75). HRMS (APCI) Calcd for C17H34O2Br [M+H]+ 349.1737, found 349.1743.
2.3. 14-(Trimethylsilyl)-1-[(tetrahydropyran-2-yl)oxy]tetradec-13-yne (4)
To a stirred solution of 3.14 ml (22.7 mmol) of (trimethylsilyl)acetylene in 12.6ml of dry THF and under argon was added 2.10 ml (22.7 mmol) of n-BuLi (2.5 M) in hexane at −78 °C. After 2 minutes, 3.10 ml of HMPA and 2.64 g (7.56mmol) of 3 was added to the reaction mixture, maintaining the temperature at −78 °C. After 24h the reaction mixture was quenched with water. The organic product was extracted with brine solution (2 × 15 ml), diethyl ether (2 × 15 ml), dried over MgSO4, filtered, and evaporated in vacuo. The product was purified using silica gel column chromatography eluting with hexane/ether (9:1). The silylated alkyne 4 was obtained as a colorless oil 2.63 g (7.18 mmol) for a 95% yield. IR (neat) νmax 2924, 2855, 2172, 1466,1458, 1441, 1352, 1248, 1200, 1136, 1121, 1078, 1032, 839, 760, 638 cm−1; 1H NMR (CDCl3,300 MHz) δ 4.57 (1H, brt, J = 2.4Hz), 3.86 (1H, m), 3.73 (1H, m), 3.50 (1H, m), 3.39 (1H, m), 2.20 (2H, t, J = 7.1Hz, H-12), 1.81 (2H, m), 1.55 (6H, m, -CH2-), 1.28 (16H, m, -CH2-), 0.12 (9H, s, -Si(CH3)3); 13C NMR (CDCl3, 75 MHz) δ 107.68 (s, C-14), 98.82 (d), 84.18 (s, C-13), 67.68 (t), 62.33 (t), 30.76 (t), 29.73 (t), 29.57 (t), 29.47 (t), 29.55 (t), 29.06 (t), 28.78 (t), 28.61 (t), 26.22 (t), 25.48 (t), 19.83 (t), 19.68 (t), 0.16 (q, -Si(CH3)3); GC-MS (70 eV) m/z (relative intensity) 366 (M+, 1), 351 (1), 293 (1), 129 (1), 115 (1), 111 (3), 103 (14), 101 (8), 97 (5), 96 (4), 85 (100), 75 (20), 73 (51), 67 (12), 59 (17), 57 (12), 56 (14), 55 (17). HRMS (APCI) Calcd for C22H43O2 [M+H]+ 367.3027, found 367.3030.
2.4. 1-[(Tetrahydropyran-2-yl)oxy]tetradec-13-yne (5)
To a mixture of 2.58 g (9.67mmol) of 4 and 16.6 ml of dry THF was added dropwise 9.67 mmol of tetrabutylammonium fluoride (1M) at 0 °C. After 24h at room temperature the reaction was quenched with HCl (2M), and the organic layer was washed with brine (1 × 20 ml), ether (1 × 20 ml), dried over MgSO4, filtered, an evaporated in vacuo. The crude product was purified using silica gel column chromatography eluting with hexane/ether (9:1). The alkyne 5 was obtained as a colorless oil 2.07 g (7.03 mmol) for a 100% yield. IR (neat) νmax 3312, 2924, 2853, 1465, 1441, 1352, 1323, 1260, 1200, 1135, 1120, 1078, 1023, 986, 905, 869, 814, 722 cm−1; 1H NMR (CDCl3, 300 MHz) δ 4.57 (1H, t, J = 2.4 Hz), 3.86 (1H, m), 3.73 (1H, m), 3.50 (1H, m), 3.39 (1H, m), 2.17 (2H, dt, J = 6.8, 2.3 Hz, H-14), 1.93 (1H, t, J = 2.5 Hz, H-12), 1.81 (2H, m), 1.55 (6H, m, -CH2-), 1.28 (16H, m, -CH2-); 13C NMR (CDCl3, 75 MHz) δ 98.81 (d), 84.80 (s, C-13), 68.01 (d, C-14), 67.68 (t, C-1), 62.32 (t), 32.77 (t), 30.75 (t), 29.72 (t), 29.55 (t), 29.46 (t), 29.08 (t), 28.74 (t), 28.46 (t), 26.21 (t), 25.42 (t), 19.68 (t), 18.37 (t); GC-MS (70 eV) m/z (relative intensity) 293 (M+-1, 1), 115 (1), 109 (3), 101 (36), 85 (100), 81 (15), 69 (10), 67 (20), 57 (13), 56 (19), 55 (31). HRMS (APCI) Calcd for C19H35O2 [M+H]+ 295.2632, found 295.2634.
2.5. 17-Methyl-1-[(tetrahydropyran-2-yl)oxy]octadec-13-yne (6)
To a solution of 2.07 g (7.03 mmol) of 5 and 13.6 ml of dry THF at 0 °C, 2.28 ml (24.6 mmol) of n-BuLi (2.5 M) in hexane was added while stirring under an argon atmosphere. After 45 minutes, 3.38 ml of HMPA was added. After additional 15 minutes, 2.64 ml (21.1 mmol) of 1-bromo-3-methylbutane was added to the reaction mixture and the reaction was left stirring for 24h at room temperature. After this time the reaction mixture was quenched with water and the organic product was extracted with ether (2 × 30 ml), dried over MgSO4, filtered, and evaporated in vacuo. The crude product was purified using silica gel column chromatography eluting with hexane/ether (9:1). The product 6 was obtained as colorless oil 1.95 g (5.36 mmol) for a 76% yield. IR (neat) νmax 2924, 2853, 1466, 1445, 1383, 1366, 1352, 1260, 1200, 1136, 1121, 1078, 1032, 905, 870, 816 cm−1; 1H NMR (CDCl3, 300 MHz) δ 4.57 (1H, brt, J = 2.4Hz), 3.86 (1H, m), 3.73 (1H, m), 3.50 (1H, m), 3.39 (1H, m), 2.14 (4H, m, H-12, H-15), 1.83-1.45 (13H, m, -CH2-), 1.27 (16H, m, -CH2-), 0.87 (6H, d, J = 6.6 Hz, -CH(CH3)2); 13C NMR (CDCl3, 75 MHz) δ 98.82 (d), 80.19 (s), 80.09 (s), 67.69 (t, C-1), 62.33 (t), 38.13 (t), 30.76 (t), 29.14 (t, C-2), 29.59 (t), 29.53 (t), 29.49 (t), 29.15 (t), 28.84 (t), 27.14 (d, C-17), 26.23 (t, C-3), 25.48 (t), 22.18 (q, C-18, C-19), 19.68 (t), 18.73 (t, C-12), 16.73 (t, C-15); GC-MS (70 eV) m/z (relative intensity) 364 (M+, 2), 307 (1), 293 (4), 235 (1), 207 (1), 193 (1), 179 (1), 165 (1), 151 (1), 137 (2), 123 (3), 121 (3), 115 (1), 110 (5), 109 (9), 101 (32), 95 (24), 85 (100), 71 (2), 69 (15), 67 (30), 57 (18), 56 (21), 55 (36). HRMS (APCI) Calcd for C24H45O2 [M+H]+ 365.3414, found 365.3419.
2.6. 17-Methyloctadec-13-yn-1-ol (7)
To a mixture of methanol (30.0 ml) and 1.95 g (5.36 mmol) of 6 was added catalytic amounts of PTSA at the reaction mixture was stirred at 35 °C for 48h. After this time the organic extract was washed with a saturated solution of sodium bicarbonate (2 × 50 ml), ether (2 × 20 ml), dried over MgSO4, filtered, and evaporated in vacuo. The product was purified using silica gel column chromatography eluting with hexane/ether (9:1). The 17-methyloctadec-13-yn-1-ol (7) was obtained as a colorless oil 1.43 g (5.10 mmol) for a 95% yield. IR (neat) νmax 3315 (OH, broad), 2924, 2853, 1466, 1383, 1367, 1329, 1055 cm−1; 1H NMR (CDCl3, 500 MHz) δ 3.63 (2H, t, J = 6.4Hz, H-1), 2.13 (4H, m, H-12, H-15), 1.63 (1H, m. H-17), 1.55 (2H, m), 1.45 (1H, m), 1.39 (3H, m), 1.26 (16H, m, -CH2-), 0.87 (6H, d, J = 6.6 Hz, -CH(CH3)2); 13C NMR (CDCl3, 125 MHz) δ 80.20 (s), 80.09 (s), 63.07 (t, C-1), 38.14 (t, C-16), 32.78 (t, C-2), 29.59 (t), 29.57 (t), 29.52 (t), 29.42 (t), 29.14 (t), 28.83 (t), 27.15 (d, C-17), 25.72 (t, C-3), 22.18 (q, C-18, C-19), 18.73 (t, C-12), 16.73 (t, C-15); GC-MS (70 eV) m/z (relative intensity) 280 (M+, 1), 265 (2), 224 (1), 163 (1), 151 (1), 137 (2), 135 (5), 124 (5), 123 (5), 121 (7), 111 (7), 110 (57), 109 (25), 96 (26), 95 (100), 93 (18), 83 (15), 82 (52), 81 (82), 80 (14), 79 (28), 77 (10), 69 (37), 68 (25), 67 (63), 57 (18), 56 (20), 55 (68). HRMS (APCI) Calcd for C19H37O [M+H]+ 281.2839, found 281.2840.
2.7. (Z)-17-Methyloctadec-13-en-1-ol (8)
Into a 25-ml two-necked round-bottomed flask were placed dry hexane, 0.576 g (2.05 mmol) of alkyne 7, quinoline (1.6 ml), and palladium in activated carbon (Lindlar’s catalyst). One of the two necks was capped with a rubber septum and the other was connected via tygon tubing to a 25-ml graduated pipet ending in a 150 ml beaker with distilled water. While stirring at room temperature a 20 ml syringe with needle was used to withdraw air from the system and draw water up into the graduated pipet to the 0.0 ml mark. Hydrogen was then introduced into the system using a balloon filled with hydrogen attached to the hose barb-to-luer lock adapter with stopcock and a needle. The reaction mixture consumed 50.3 ml of hydrogen during 1h. The mixture was filtered and the solvent removed in vacuo. The product was purified under vacuum distillation (Kugelrohr) by removing impurities at 110 °C/3 mmHg. The (Z)-17-methyloctadec-13-en-1-ol (8) was obtained as a colorless oil 0.54 g (1.93 mmol) for a 94% yield. IR (neat) νmax 3335 (OH, broad), 3005, 2923, 2853, 1655, 1465, 1383, 1366, 1120, 1057, 719 cm−1; 1H NMR (CDCl3, 500 MHz) 5.33 (2H, m, H-13, H-14, J13,14 = 10.4 Hz), 3.63 (2H, t, J = 6.6Hz, H-1), 2.02 (4H, m, H-12, H-15), 1.66 (1H, m, H-17), 1.56 (2H, m, H-2), 1.27 (20H, m, -CH2-), 0.88 (6H, d, J = 6.6 Hz, -CH(CH3)2); 13C NMR (CDCl3, 125 MHz) 129.98 (d), 129.74 (d), 63.08 (t, C-1), 38.99 (t, C-16), 32.79 (t, C-2), 29.75 (t), 29.63 (t), 29.60 (t), 29.59 (t), 29.53 (t), 29.42 (t), 29.29 (t), 27.61 (d, C-17), 27.17 (t, C-12), 25.72 (t, C-15), 25.09 (t), 22.53 (q, C-18, C-19); GC-MS (70 eV) m/z (relative intensity) 282 (M+, 1), 265 (1), 264 (5), 236 (1), 221 (1), 194 (1), 180 (1), 166 (1), 165 (1), 139 (1), 138 (4), 125 (3), 124 (7), 123 (10), 111 (8), 110 (13), 109 (19), 97 (20), 96 (37), 95 (37), 83 (32), 82 (56), 81 (40), 71 (7), 70 (15), 69 (68), 68 (25), 67 (43), 57 (39), 56 (50), 55 (100). HRMS (APCI) Calcd for C19H39O [M+H]+ 283.2995, found 283.2996.
2.8. (Z)-17-Methyl-13-octadecenoic acid (1)
To a solution of 7.43 mmol of pyridinium dichromate and 5.0 ml of dimethylformamide (DMF) was added under argon a solution of 0.42 g (1.49 mmol) of alcohol 8 and 10.0 ml of DMF, and the reaction mixture was left stirring at room temperature for 48h. After this time, the reaction mixture was washed with water (3 × 25 ml), ethyl acetate (2 × 20 ml), dried over MgSO4, filtered, and the solvent evaporated in vacuo. The crude product was purified using florisil column chromatography eluting with ether. The (Z)-17-methyloctadec-13-enoic acid (1) was obtained as viscous oil 0.39 g (1.32 mmol) for an 89% yield. IR (neat) νmax 3500-2500, 3005, 2923, 2853, 1709 (C=O), 1464, 1412, 1383, 1366, 1119, 1078, 1037, 721 cm-1; 1H NMR (CDCl3, 500 MHz) δ 5.34 (2H, m, H-13, H-14, J13,14 = 10.3 Hz), 2.34 (2H, t, J = 7.5 Hz, H-2), 2.02 (4H, m, H-12, H-15), 1.63 (1H, m, H-17), 1.55 (2H, m, H-3), 1.28 (18H, m, -CH2-), 0.88 (6H, d, J = 6.6 Hz, -CH(CH3)2); 13C NMR (CDCl3, 125 MHz) δ 179.82 (s, C-1), 129.98 (d), 129.73 (d), 39.00 (t, C-16), 34.03 (t, C-2), 29.75 (t, C-11), 29.59 (t), 29.57 (t), 29.52 (t), 29.42 (t), 29.28 (t), 29.23 (t), 29.06 (t), 27.61 (d, C-17), 27.17 (t, C-12), 25.09 (t, C-15), 24.68 (t, C-3), 22.53 (q, C-18, C19); HRMS (APCI) Calcd for C19H37O2 [M+H]+ 297.2788, found 297.2789.
Methyl (Z)-17-methyl-13-octadecenoate
GC-MS (70 eV) m/z (relative intensity) 310 (M+, 7) 279 (16), 278 (31), 255 (12), 236 (8), 235 (6), 223 (9), 222 (5), 209 (1), 194 (5), 181 (2), 180 (3), 179 (2), 167 (2), 166 (2), 165 (2), 153 (3), 152 (4), 139 (5), 138 (5), 137 (5), 125 (8), 124 (6), 123 (9), 111 (15), 110 (10), 109 (11), 101 (4), 98 (18), 97 (31), 96 (21), 95 (18), 87 (28), 85 (6), 84 (23), 83 (40), 82 (15), 81 (22), 79 (7), 74 (48), 71 (9), 70 (18), 69 (84), 68 (15), 67 (28), 65 (3), 59 (22), 57 (37), 56 (55), 55 (100).
2.9 Cell Cultures
L. donovani (MHOM/ET67/L82 strain) promastigotes were propagated in a completely defined medium 199, supplemented with 10% heat inactivated fetal calf serum (FCS) and penicillin/streptomycin cocktail (containing 50 U/ml penicillin, 50 μg/ml streptomycin). The IC50 value was determined with different concentrations of compound 1 dissolved in DMSO (ranging from 0.6 to 25 μg/ml) added to cultures and cell population assessed by Coulter.
2.10 Purification of recombinant leishmanial TopIB
Expression of a recombinant topoisomerase IB from Leishmania donovani (LdTopIB) in a topoisomerase IB-deficient Saccharomyces cerevisiae strain has been described elsewhere (Villa et al., 2003). Purification of recombinant LdTopIB was done according to Diaz-González and coworkers (Diaz-González et al., 2008). Briefly, LdTopIB overexpressing yeasts were disrupted with one freeze/thaw cycle at −80 °C, with the purpose of weakening the yeast wall; after lysis with 425–600 μm acid-washed glass beads, the extracts were cleared by centrifugation at 15000 × g for 30 min at 4 °C. The protein suspension was loaded onto a phosphocellulose (P-11) column, previously equilibrated as manufacturer indications. LdTopIB was eluted at 4 °C with a discontinuous gradient of KCl (0.2, 0.4, 0.6, 0.8 and 1 M) in TEEG buffer, supplemented with 0.1 mg/ml sodium bisulphite, 0.8 mg/ml NaF and the protease inhibitors cocktail. Active fractions were further loaded onto a phenyl-sepharose column (Sigma-Aldrich, St Louis, US), eluted with a discontinuous inverse gradient of ammonium sulphate (1, 0.8, 0.6, 0.4 and 0.2 M) and then concentrated by Microcon YM-30 (Millipore) before use.
2.11 DNA relaxation assays
DNA topoisomerase I activity was assayed by the relaxation of negatively supercoiled plasmid DNA. The reaction mixture in a total volume of 20 μl contained 0.2 μg of supercoiled pHOT plasmid, 10 mM Tris-HCl buffer pH 7.5, 5 mM MgCl2, 0.1 mM EDTA, 15 μg/ml bovine serum albumin, 50 mM KCl and various extracts containing altered proteins or wild type enzyme, starting with 1 unit LdTopIB. The reaction mixtures were incubated for 30 min at 37 °C. The enzyme reactions were stopped by the addition of up to 1% SDS - final concentration – and digested by 2 mg/mL proteinase K with 1 h incubation to remove protein bonded to the DNA fragment. The extent of plasmid DNA relaxation was assessed by electrophoresis in a 1% agarose gel in 0.1 M Tris acetate EDTA (TAE) buffer pH 8.0 at 2 V/cm for 14 h. The gels were visualized under UV illumination after being stained with ethidium bromide (0.5 mg/ml) and a posterior electrophoresis in the presence of 0.1 mg/ml ethidium bromide, in order to separate the nicked DNA from the relaxed topoisomers. One unit of LdTopIB is defined as the amount of purified protein able to relax 0.2 μg of pHOT supercoiled DNA per 30 min at 37 °C.
3. Results and discussion
The synthesis of 1 started with commercially available (Aldrich) 12-bromo-1-dodecanol (2), which was protected with 3,4-dihydro-2H-pyran (DHP) in the presence of p-toluenesulfonic acid (PTSA) affording the corresponding dihydropyranyl protected alcohol 3 in a 78% isolated yield (Scheme 1). Compound 3 was then submitted to the first acetylide coupling reaction with the versatile reagent (trimethylsilyl)acetylene using n-BuLi in THF-HMPA at −78°C affording the trimethylsilylacetylenic derivative 4 in a 95% yield. Deprotection of the terminal trimethylsilyl group with tetrabutylammonium fluoride (TBAF) afforded the terminal alkyne 5 in an almost 100% yield. A second acetylide coupling with 3-methyl-1-bromobutane using n-Buli, THF-HMPA at 0 °C resulted in the iso-branched alkyne 6 in a 76% isolated yield (Scheme 1). Deprotection of the dihydropyranyl group in 6 with PTSA in methanol at 35 °C for 48 h afforded the 17-methyl-13-octadecyn-1-ol (7) in a 95% isolated yield. Catalytic hydrogenation under Lindlar’s conditions of 7 resulted in the (Z)-17-methyl-13-octadecen-1-ol (8) in a 94% yield with a 100% cis stereochemistry for the double bond. Final oxidation of the alcohol with pyridinium dichromate (PDC) in dimethylformamide (DMF) resulted in the formation of the desired acid 1 in an 89% yield. The overall total yield for the seven steps was 45%, which afforded enough material for its complete spectral characterization.
Acid 1 presented spectral data that confirmed its synthesis. The iso methyl branching was easily identified by both 1H NMR and 13C NMR spectrometry as well as by infrared spectroscopy (IR). In the 1H NMR spectra both terminal isopropyl methyl groups appeared as a doublet at δ 0.88 ppm, while in the 13C NMR both methyl groups resonated at δ 22.5 ppm. The IR spectrum of 1 was also very characteristic since the terminal isopropyl group was observed as a doublet at 1383 and 1366 cm−1. The presence of the olefinic hydrogens in 1 was easily detected in the 1H NMR spectra by the olefinic signals at δ 5.34 ppm. The 13C NMR of the olefinic region was most interesting, in particular the resonance of the allylic methylene carbons that confirmed the cis double bond stereochemistry. The C-12 allylic carbon resonated at δ 27.2 ppm, while the C-15 allylic carbon was observed more upfield at δ 25.1 ppm, confirming the presence of the cis double bond (Gunstone et al., 1977). It is well established that the allylic methylene carbons from cis double bonds in fatty acids resonate in 13C NMR at around 27 ppm, while the allylic methylene carbons from trans double bonds resonate at around 32 ppm (Gunstone et al., 1977). The cis double bond stereochemistry was also further confirmed by IR since a strong absorption at 721 cm−1 was observed for 1. As expected, the carboxylic acid carbonyl was observed in 13C NMR at δ 179.8 ppm and in IR at 1709 cm−1.
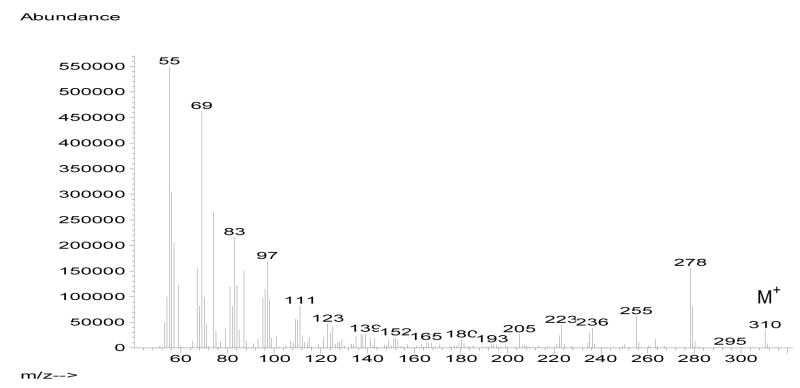
The methyl ester of the synthetic acid 1 was also prepared (HCl in MeOH) and its mass spectrum (Fig. 1) and GC retention time (ECL value) were compared to the ones reported for the natural methyl ester of 1 (Denis et al., 2009). The mass spectra and GC retention times of both synthetic and natural methyl (Z)-17-methyl-13-octadecenoates were comparable. For example, in the mass spectrum of the methyl ester of synthetic 1 both the M+-32 and M+-31 fragmentations peaks at m/z 278 (31%) and at m/z 279 (16%) can be easily seen. In addition, a strong M+-55 peak at m/z 255 (12%) as well as the typical McLafferty rearrangement at m/z 74 (48%) were also observed (Denis et al., 2009). However, the GC retention times (ECL values) for both methyl esters were just slightly different but within the range of where these iso methyl-branched nonadecenoic acid methyl esters normally elute (Carballeira et al., 2001). For example, the natural methyl ester of 1 was reported to have an ECL value of 18.21, while the synthetic methyl ester of 1 was determined to have an ECL value of 18.51. This small difference in ECL values could be ascribed to the slightly different GC columns (HP-1 vs. HP-5) employed in both cases.
Fig. 1.
Mass spectrum (70 eV) of methyl (Z)-17-methyl-13-octadecenoate.
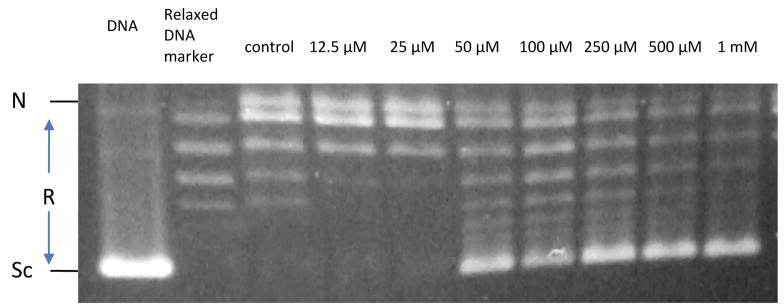
Aimed at exploring the biological activity of acid 1 we studied its antiprotozoal activity towards Leishmania donovani (the causative agent of leishmaniasis) and the inhibition of acid 1 of the leishmania DNA topoisomerase IB as a possible mechanism of action. We found that acid 1 is cytotoxic to L. donovani at an EC50 of 19.8 ± 7.0 μg/ml. Moreover, we also found that acid 1 completely inhibits the leishmania DNA topoisomerase IB at concentrations of 50 μM (Fig. 2). These results tend to indicate that the toxicity of 1 towards L. donovani could be due to the inhibition of its DNA topoisomerase IB. This finding is interesting since it has recently been demonstrated that there are substantial differences between the leismania DNA topoisomerase IB and the human DNA topoisomerase I (Balaña-Fouce et al., 2006). This means that one might be able to interfere with the protozoan DNA topoisomerase IB without harming the mammalian DNA topoisomerase I. In fact, we have shown that the acid (Z)-14-methyl-9-pentadecenoic acid, also a monounsaturated iso methyl-branched fatty acid, inhibits the human DNA topoisomerase I but at the higher concentrations of 500 μM (Carballeira et al., 2007). Therefore, these monounsaturated iso methyl-branched fatty acids seem to be more effective towards the leishmania DNA topoisomerase IB than the human DNA topoisomerase I.
Fig. 2.
Inhibition of relaxation activity of recombinant LdTopIB by compound 1. One unit of recombinant LdTop1 was assayed in a plasmid DNA relaxation assay for 30 min at 37 °C (as described under “Material and Methods”) in the presence of 12.5 to 1000 μM compound 1. Reaction products were resolved in agarose gel and subsequently visualized by ethidium bromide staining. The relative position of the negatively supercoiled DNA substrate is indicated by Sc, N is the nicked DNA, whereas the ladder of relaxed DNA topoisomer bands is labeled R. Reactions were stopped with a mixture of 1 % SDS and 6.1 μg of proteinase K. Lane 1 contains 0.2 mg of pHOT plasmid DNA and lane 2 is a relaxed marker.
In summary, we can conclude that the first synthesis for the (Z)-17-methyl-13-octadecenoic acid (1) was accomplished in seven steps and in a 45% overall yield. Certainly, the synthetic strategy of first coupling the (trimethylsilyl) acetylene to the long-chain bromoalcohol (78% yield) followed by a second acetylide coupling to the short-chain iso bromo alkane (76% yield) afforded good overall yields. Complete spectral data is also provided for the first time for acid 1. The synthetic route presented furnished enough material to study the antiprotozoal activity of 1 and these preliminary biological studies tend to indicate that fatty acids such as 1 might be good antiprotozoal compounds.
Acknowledgments
The project described was supported by Award Number SC1GM084708 from the National Institutes of General Medical Sciences of the NIH. We thank Dr. Fred Strobel (Emory University) for the high resolution mass spectral data. This research was also partially supported by a grant (Gr238) from Junta de Castilla y León and the Tropical Diseases Network (RICET) from Ministerio de Salud y Consumo (SPAIN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boon JJ, de Leeuw JW, vd Hoek GJ, Vosjan JH. Significance and taxonomic value of iso and anteiso monoenoic fatty acids and branched β-hydroxy acids in Desulfovibrio desulfuricans. J Bacteriol. 1977;129:1183–1191. doi: 10.1128/jb.129.3.1183-1191.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon PI, Virtue P, Nichols PD. Microbial consortia in wetland sediments: a biomarker analysis of the effects of hydrological regime, vegetation and season on benthic microbes. Marine and Freshwater Research. 1996;47:27–41. [Google Scholar]
- Carballeira NM, Pagán M, Rodríguez AD. Identification and total synthesis of novel fatty acids from the Caribbean sponge Calyx podatypa. J Nat Prod. 1998;61:1049–1052. doi: 10.1021/np9801413. [DOI] [PubMed] [Google Scholar]
- Carballeira NM, Cruz H, Hill CA, De Voss JJ, Garson M. Identification and total synthesis of novel fatty acids from the siphonarid limpet Siphonaria denticulata. J Nat Prod. 2001;64:1426–1429. doi: 10.1021/np010307r. [DOI] [PubMed] [Google Scholar]
- Carballeira NM, Montano N, Padilla LF. First total synthesis of (Z)-15-methyl-10-hexadecenoic acid and the (Z)-13-methyl-8-tetradecenoic acid. Chem Phys Lipids. 2007;145:37–44. doi: 10.1016/j.chemphyslip.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballeira NM, Sanabria D, Oyola D. An improved synthesis for the (Z)-14-methyl-9-pentadecenoic acid and its topoisomerase I inhibitory activity. ARKIVOC. 2007;viii:49–57. [PMC free article] [PubMed] [Google Scholar]
- Denis C, Wielgosz-Collin G, Bretéché A, Ruiz N, Rabesaotra V, Boury-Esnault N, Kornprobst JM, Barnathan G. New 17-methyl-13-octadecenoic and 3,16-docosadienoic acids from the sponge Polymastia penicillus. Lipids. 2009 doi: 10.1007/s11745-009-3291-9. in press. [DOI] [PubMed] [Google Scholar]
- Díaz-González R, Pérez-Pertejo Y, Pommier Y, Balaña-Fouce R, Reguera RM. Mutational study of the “catalytic tetrad” of DNA topoisomerase IB from the hemoflagellate Leishmania donovani: Role of Asp-353 and Asn-221 in camptothecin resistance. Biochem Pharmacol. 2008;76:608–619. doi: 10.1016/j.bcp.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunstone FD, Pollard MR, Scrimgeour CM, Vedanayagam HS. 13C-Nuclear magnetic resonance studies of olefinic fatty acids and esters. Chem Phys Lipids. 1977;18:115–129. doi: 10.1016/0009-3084(77)90031-7. [DOI] [PubMed] [Google Scholar]
- Balaña-Fouce R, Redondo CM, Pérez-Pertejo Y, Díaz-González R, Reguera RM. Targeting atypical trypanosomatid DNA topoisomerase I. Drug Discov Today. 2006;11:733–740. doi: 10.1016/j.drudis.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Reyes ED, Carballeira NM. A short synthesis of (Z)-15-methylhexadec-11-enoic acid. Synthesis. 1996:693–694. [Google Scholar]
- Taylor J, Parkes JR. The cellular fatty acids of the sulfate-reducing bacteria, Desulfobacter species, Desulfobulbus species and Desulfovibrio desulfuricans. J Gen Microbiol. 1983;129:3303–3309. [Google Scholar]
- Villa H, Otero Marcos AR, Reguera RM, Balaña-Fouce R, García-Estrada C, Pérez-Pertejo Y, Tekwani BL, Tyler PJ, Stuart KD, Bjornsti MA, Ordóñez D. A novel active DNA topoisomerase I in Leishmania donovani. J Biol Chem. 2003;278:3521–3526. doi: 10.1074/jbc.M203991200. [DOI] [PubMed] [Google Scholar]