Abstract
INTRODUCTION
The hexose transmembrane transporters SGLT1 and GLUT2 are present in low quantities in ileum where little glucose absorption occurs normally; however, glucose uptake in ileum is highly adaptable after small bowel resection.
HYPOTHESIS
Ileal adaptability for glucose absorption after jejunal resection is mediated predominately by upregulation of GLUT2.
METHODS
Rats underwent 70%, proximal-based jejunoileal resection. Transporter-mediated glucose uptake was measured in proximal and distal remnant ileum 1 and 4 wk postoperatively (n=6 rats, each) and in corresponding ileal segments in control and 1 wk sham laparotomy rats (n=6, each) without and with selective inhibitors of SGLT1 and GLUT2. In separate groups of rats (n=6, each), protein (Western blots), mRNA (RT-PCR), and villus height (histomorphology) were measured.
RESULTS
After 70% proximal intestinal resection, there was no dramatic change in protein or mRNA expression per cell of either SGLT1 or GLUT2, but median glucose uptake (nmol/cm/min) increased markedly from 52 (range, 28-63) in controls to 118 (range, 80-171) at 1 wk, and 203 (range, 93-248) at 4 wk (p≤0.04 each) correlating with change in villus height (p≤0.03).
CONCLUSIONS
Ileal adaptation for glucose transport occurs through cellular proliferation (hyperplasia) and not through cellular upregulation of glucose transporters.
Introduction
Short bowel syndrome is a devastating clinical problem that usually results from operative resection of diseased intestine resulting in an inadequate length of residual bowel (1). The treatment options for these patients are limited, and outcomes with these therapies are often poor (2-4). The ability of the ileum to adapt after massive small bowel resection has created interest in studying the cellular mechanisms responsible for ileal adaptation to uncover novel therapies for short bowel patients (5-11). Additionally, current models of ileal adaptation have suggested a cellular upregulation of membrane expression of intestinal hexose transporters which makes models of ileal adaptation particularly interesting in understanding cellular mechanisms responsible for the regulation of intestinal hexose transporters (12-13).
The primary glucose transporter in the small intestine has been thought traditionally to be SGLT1, an active sodium-glucose co-transporter (14-17). With normal intestinal continuity, SGLT1 is expressed and functions at a very low level in the ileum where the presence of luminal glucose is also very low (18). Glucose absorption in the ileum increases after massive small bowel resection when luminal glucose loads to the distal gut are increased (9, 12-13, 19). It has been reported that the ileum adapts by increasing surface area through increased villus height and crypt depth, but most investigators believe that this adaptation is also due, in part, to upregulation of the primary intestinal glucose transporter SGLT1. Data from our laboratory and others suggested that GLUT2, a facilitated glucose transporter typically localized to the basolateral membrane, may also have a substantive role in apical glucose transport in the jejunum (20-24). Whether or not upregulation of apical GLUT2 plays a role in ileal adaptation is not known, and if so, to what extent. We hypothesized that after a massive, proximal-based small bowel resection, the ileum would adapt not only by hyperplasia but also by upregulating both the gene and protein expression and function of both SGLT1 and apical GLUT2 within the enterocyte.
Design
Rats underwent a 70%, proximal-based small bowel resection (see below). These rats were then survived and studied at 1 or 4 wk (n=12, each group). An additional group of 12 rats were studied 1 wk after sham celiotomy to control for anesthesia and other postoperative changes; a group of 12 naïve control rats (NC) were studied as a negative control. All rats were housed in a 12 h light-dark cycle (6AM lights on; 6PM lights off) and were allowed free access to standard rat chow (5001 Rodent Diet, PMI Nutrition International LLC, Brentwood, MO) and water.
Twelve rats were designated for study in each group at each time point; 6 rats were used for mRNA and protein analysis of SGLT1 and GLUT2, while the remaining 6 rats per groups were used to measure villus height and transporter-mediated glucose uptake without and with the SGLT1 inhibitor, phlorizin, and with the GLUT2 inhibitor, phloretin.
Small Intestinal Resection
After approval from the Mayo Clinic Institutional Animal Care and Use Committee, male Lewis rats (250-300 g) were anesthetized using inhaled 2% isoflurane induction followed by intraperitoneal injection of sodium thiopental (50 mg/kg). A short-celiotomy (1 cm) was performed, and the small bowel was extra-corporealized. The proximal 70% of the small intestine starting from the ligament of Treitz was resected after ligating the mesenteric blood supply leaving about 14 cm of distal ileum. An end-to-end, single layer anastomosis was then performed using running 7-0 polypropylene sutures. The intestine was then returned into the peritoneal cavity, and the abdominal wall was closed in two layers with running 5-0 polyglactin suture. Sham celiotomy was performed under similar anesthesia using a short-celiotomy with extra-corporealization of the entire small bowel. The intestine was manipulated manually for 5 min prior to reduction back into the abdomen. Abdominal closure was performed as above. Post-operatively, all animals were maintained on water containing acetaminophen for 48 h prior to having free access to chow.
Tissue Harvest
At the time of tissue harvest, rats were anesthetized with inhaled 2% isoflurane followed by intraperitoneal injection of sodium thiopental (50 mg/kg). All tissue was harvested at 9AM due to known diurnal patterns in expression and function of hexose transporters (15, 18, 25-26). The duodenum was cannulated just distal to the pylorus and was flushed with cold (4°C) mammalian Ringers solution (in mM: 128 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 KH2PO4, 1.2 MgSO4, 20 NaHCO3; pH 7.3-7.4; 290 mOsm). The remnant ileum was excised. In NC and shams, the distal 14 cm of ileum was harvested which corresponded to the same length of ileum left in the resection animals. For each group, 6 rats were designated randomly for mRNA and protein analysis. In these animals, the remnant ileum was opened, and in ice-cold, phosphate-buffered saline (PBS), the mucosa was scraped with a glass slide. The samples for mRNA analysis were placed in RNA stabilization buffer (RNALater, Qiagen, Valencia, CA), snap frozen in liquid nitrogen, and then stored at -80°C. The samples for protein analysis were collected separately from both the proximal and distal portions of the remnant ileum, placed in cold RIPA buffer containing protease inhibitors Halt (Pierce, Rockford, IL) and PMSF, snap frozen in liquid nitrogen, and stored at -80°C for later batch analysis. The other 6 rats in each group were designated randomly for measurements of glucose uptake using our modification of the everted sleeve technique and histologic analysis (18, 27). These animals were anesthetized in a similar fashion followed by flushing of the entire small bowel with 4°C mammalian Ringers solution. The proximal and distal portions of the remnant ileum after resection and the corresponding ileal region in the NC and sham animals were placed in 4°C mammalian Ringers solution oxygenated with 95% O2/5% CO2 until study. In addition, 0.5 cm of proximal and distal portions of ileal segments were pinned on a support and fixed in 10% buffered formalin for histomorphometry.
mRNA Measurement
Reverse transcription real-time polymerase chain reaction (PCR) was used to quantitate mRNA levels for SGLT1 and GLUT2 (18, 26). The mucosal samples stored in RNA stabilization buffer were thawed on ice and homogenized. Samples from the proximal remnant and the distal remnant were studied as distinct groups. RNA was isolated using the RNeasy Midi kit (Qiagen, Valencia, CA). RNA was then reverse transcribed into cDNA using the Super Script III kit (Invitrogen, Carlsbad, CA). The resultant cDNA was stored at -20°C. cDNA levels of SGLT1, GLUT2, and the stably expressed housekeeping gene, glyceraldehyde-6-phosphate dehydrogenase (GAPDH) were determined using real-time PCR. PCR was performed in a 7500 thermocycler (Applied Biosystems, San Francisco, CA) using Taqman® chemistries with primers and fluorescently-labeled probes in assay mixes purchased from Applied Biosystems. Standard curves from serial dilutions of known copy numbers were used to calculate the number of copies of cDNA for each sample. All samples were run as duplicates with 2 μl of sample cDNA (or known standard) added to 23 μl of master mix for a total sample volume of 25 μl. Real-time PCR was carried out at 95°C for 10 min followed by 40 cycles of 15 s at 95° and 1 min at 60°C after which fluorescence measurements were made. Transporter copy numbers were normalized to copy numbers of GAPDH from each sample.
Protein Measurement
Western blotting was used to measure semi-quantitatively the protein levels of SGLT1 and GLUT2 (26). Tissue samples stored in RIPA buffer containing protease inhibitors were thawed on ice and placed in RIPA lysis buffer containing protease inhibitors to prevent protein degradation (18). Samples from the proximal remnant ileum were studied separately from the distal samples. Samples were homogenized using a Kontes Pellet Pestle (Fischer Scientific, Pittsburg, PA). The protein-containing supernatant was then separated by centrifugation at 5000 × g for 15 min. Protein concentrations were measured by the bicinchoninic acid method (Pierce, Rockford, IL); 200 ug of protein was resolved on a 10% SDS-PAGE gel (Bio-Rad, Hercules, CA) and transferred electrically to a PVDF membrane (Millipore, Bedford, MA). Membranes were blocked using 5% milk in tris-buffered saline with Tween (TBS-T). To quantitate protein and GAPDH in the same sample, the membranes were cut between GAPDH and the specific proteins of interest. GAPDH was used as a stably expressed “housekeeping” protein against which SGLT1 and GLUT2 were compared (see below, Data Analysis). Cut membranes were then incubated overnight at 4°C with primary antibody SGLT1 (from Abcam, Cambridge, MA) and GLUT2 antibody from Chemicon International, Temecula, CA; GAPDH antibody from US Biological, Swampscott, MA). After incubation with primary antibody, membranes were rinsed three times with TBS-T and incubated with a secondary antibody in TBS-T containing 5% milk. Horseradish peroxidase-conjugated, goat anti-rabbit IgG was used for SGLT1 and GLUT2, (Sigma, St. Louis, MO and Upstate, Lake Placid, NY, respectfully) and horseradish peroxidase-conjugated, goat anti-mouse IgG was used for GAPDH (Sigma, St. Louis, MO). Protein bands were visualized with a colorimetric reaction using Opti-4CN Substrate kits (Bio-Rad). Amplified Opti-4CN substrate kit was used to enhance SGLT1 and GLUT2 bands. Membranes were scanned, and Scion Image (Scion Corp, MA) was used for semi-quantitative measurements of protein levels based on band densitometry. All transporter protein measurements were normalized to those of GAPDH in an attempt to estimate the amount of protein per enterocyte.
Transporter-Mediated Glucose Uptake
We measured transporter-mediated glucose absorption using a previously described modified, everted sleeve technique (18, 27). After tissue harvest, the targeted segment of intestine was everted so that the mucosal surface was exposed externally. Intestinal segments were then mounted on steel rods (diameter: 4 mm) and secured with two 5-0 silk ties. The redundant edges of the tissue were excised leaving a 1-cm everted segment. Due to intestinal dilation in the resection groups, larger caliber steel rods were necessary—5 mm diameter in the 1-wk group and 6 mm diameter in the 4-wk group. Sleeves were kept in chilled (4°C) mammalian Ringers solution bubbled with 95% O2/5% CO2 until ready for absorption experiments. Prior to measurements of absorption, tissues were transferred to a 38°C bath, preincubated in 8 ml of mammalian Ringers solution bubbled with 95% O2/5% CO2 for 5 min, and then placed in 8 ml of 38°C mammalian Ringers solution with iso-osmotic replacement of NaCl using 20 mM D-glucose. The solution was stirred at 1,200 rpm to mix the “unstirred layer.” Radiolabelled glucose probes (1 μCi of 14C-D-glucose and 2 μCi of 3H-L-glucose) were included in the test solution to measure the different pathways of glucose absorption. After 1 min incubation in the glucose solution, the tissues were removed, rinsed quickly in 30 ml of chilled mammalian Ringers solution stirred at 1,200 rpm for 20 s, and placed in glass scintillation vials. One ml of tissue solubilizer (Perkin-Elmer, Boston, MA) was added to the vials containing the tissue segments and kept in a 50°C water bath for 3 h. After complete solubilization, 15 ml of scintillation counting cocktail (Opti-Fluor, Perkin-Elmer, Shelton, CT) was added, and probe counts were determined using techniques of dual-marker liquid scintillation counting with a standard quench curve. When quench values were too high for the resection samples due to ileal hypertrophy, solubilized samples were separated into 2 or 3 aliquots to bring the counts into the window of validated quench and counted separately with an additional 15 ml of counting cocktail in each aliquot. The counts were then totaled, and a single uptake calculation was performed for each sample.
Phlorizin was used to inhibit SGLT1 activity at a dosage (0.2 mM) used previously (24, 28-31). The phlorizin was solubilized in ethanol, and 100 μl was then added to the 8 ml incubation bath to achieve a concentration of 0.2 mM. Phloretin was used to inhibit GLUT2 activity at a dosage (1 mM) used previously (24, 32-33) and was also solubilized in ethanol and added to the incubation bath in 100 μl aliquots to achieve a concentration of 1 mM. Vehicle experiments using 100 μl of ethanol in the glucose test solution had been conducted previously and shown to cause no effect on transporter-mediated glucose uptake (24). Three separate, 1-cm sleeves were obtained from the proximal remnant ileum for study either without inhibitors, with phlorizin, or with phloretin in the 20 mM glucose test solution. An additional 3 sleeves were obtained from the distal remnant ileum and studied similarly.
Villus Height, Intestinal Diameter, and Intestinal Length
The formalin-fixed tissues from all groups were embedded in paraffin and sectioned along the villus axis. A total of 18 sections were taken from each tissue sample, and hematoxylin and eosin staining was performed with three sections per each slide. Maximum villus height was measured from above the crypt to the tip of the villus at 10× magnification using an optical reticule with a micrometer. All 18 sections were reviewed per each segment with at least three measurements of villus height per slide such that at least 54 measurements were made for each segment (proximal and distal) per rat. At the time of tissue harvest, the diameter of the ileum was evaluated subjectively, and the length of the remnant ileum in the resection animals was measured from the ileocecal valve, proximally to the anastomosis prior to excision to assess for any changes in intestinal length.
Data Analysis
mRNA and Protein levels
To determine relative changes in gene expression of mRNA and protein levels, the measurements of SGLT1 and GLUT2 in the proximal and distal remnant ileum were normalized to levels of GAPDH, a stably expressed “housekeeping” gene. The relative expressions of mRNA and protein for SGLT1 or GLUT2 in the proximal and distal remnant ileum were compared on the same RT-PCR and Western blot to prevent potential errors in loading; all samples were also run in duplicate. Median values of protein were calculated for each rat in each group, and a grand median with inter-quartile range (IQR) was calculated per group.
Glucose uptake
To calculate transporter-mediated glucose uptake, total glucose uptake needed to be corrected for glucose adherent to the non-absorbed, extra-mucosal layer and for passive, non-carrier-mediated uptake. 3H-L-glucose is not absorbed by transporter-mediated uptake and was thus used to correct for this adherent glucose and passive uptake (18). Transporter-mediated glucose uptake is expressed as nmol/cm/min.
Statistical Analysis
Statistical analysis was performed using JMP software. Continuous variables were compared using Kruskal-Wallis analysis for non-parametric data sets when comparing more than two groups; Wilcoxon rank sums were used to compare directly the non-parametric datasets. P-values were corrected according to the Bonferroni method, and a corrected p value of ≤ 0.05 was considered significant. All data are reported as the median ± IQR or range; n values are number of rats.
Results
Operative Outcomes
At the time of tissue harvest, all rats that underwent either sham celiotomy or intestinal resection had gained weight over the study period (data not shown). None of the surviving animals required exclusion from the study due to post-operative complications.
mRNA
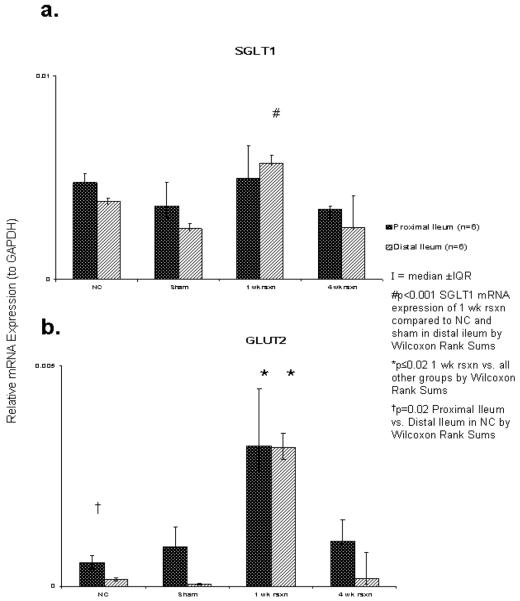
SGLT1 mRNA expression was no different among the groups in the proximal remnant ileum (p≥0.9). However, in the distal ileum SGLT1 mRNA expression in the 1 wk after resection group did increase (p<0.001), but the magnitude of change was small (from a median of 3×10-3 to 6×10-3) which was not present 4 wk after resection (p=1.0). There was no difference in SGLT1 mRNA expression between the proximal or distal remnant ileum (p≥0.06) (Figure 1a).
Figure 1.
Relative expressions (against GAPDH) of total cellular transporter mRNA by real-time RT-PCR: a) SGLT1; b) GLUT2.
The overall relative expression of GLUT2 mRNA was low among all 4 groups (range, 4×10-5 to 3×10-4). Similar to the SGLT1 mRNA expression in the distal ileum, the 1 wk after resection group did have a higher relative expression of GLUT2 mRNA, however this was found in both the proximal and distal segments (p≤0.02). The magnitude of this increase was small (from a median of 1×10-4 to 7×10-4) and was not present at 4 wk after resection (p≥0.2). Additionally, within the control group, relative expression of GLUT2 mRNA was greater in the proximal ileum compared to the distal ileum (p=0.02) (see Figure 1b).
Protein Expression
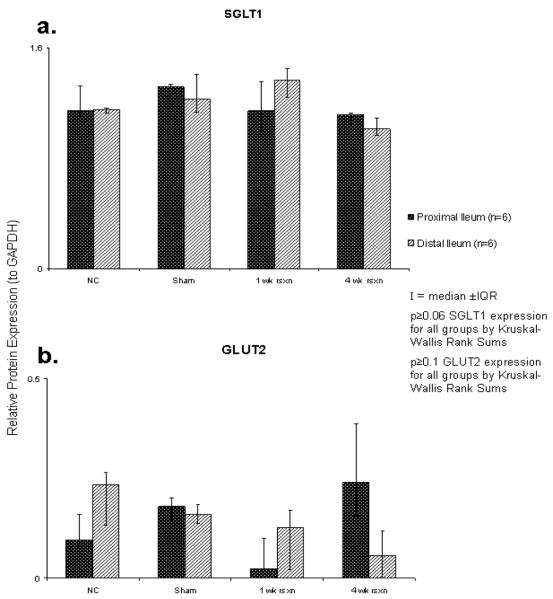
Relative expression of SGLT1 protein by Western blotting did not demonstrate any difference between the four groups (p≥0.06), specifically, after 70% proximal-based resection, there was no increase in SGLT1 expression in the remnant ileum compared to NC ileum. Additionally, there was no difference in relative expression of SGLT1 between distal or proximal ileal segments within any of the groups (p≥0.3) (Figure 2a). Relative expression of GLUT2 protein by Western blotting was very low among the 4 groups (range, 0.02 to 0.24). No difference in GLUT2 protein expression was found between the 4 groups (p≥0.1), nor was there a difference between proximal and distal segments of ileum (p≥0.2) (Figure 2b).
Figure 2.
Relative expressions (against GAPDH) of total cellular transporter protein by Western blot: a) SGLT1; b) GLUT2.
Transport Data
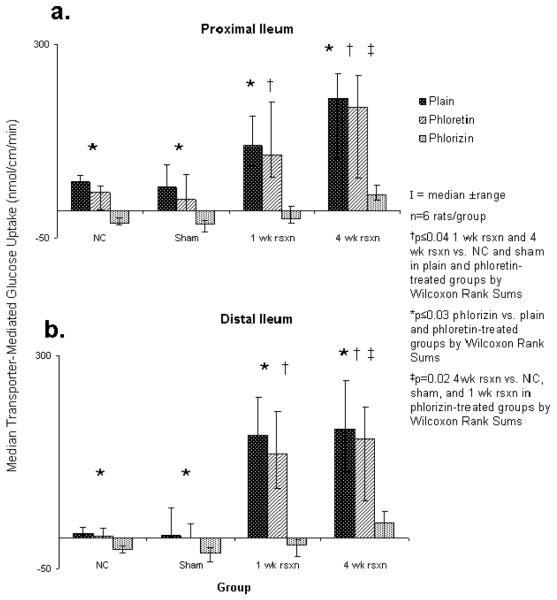
At 1 and 4 wk after small bowel resection, transporter-mediated glucose uptake within the proximal ileum increased markedly compared to the NC and sham groups (p≤0.04). There was no further statistical increase in transporter-mediated glucose uptake, however, from 1 wk to 4 wk after resection (p=0.4). Treatment with the GLUT2 inhibitor, phloretin, had no effect on transporter-mediated glucose uptake in the proximal ileum in any group (p≥0.08). In contrast, treatment with the SGLT1 inhibitor, phlorizin, led to a marked decrease in transporter-mediated glucose uptake in the proximal ileum in all 4 groups (p=0.003). At 4 wk after resection, the effect of phlorizin on the rats 4 wk after resection was marked but possibly not as profound compared to the other three groups (p=0.02) (Figure 3a).
Figure 3.
Transporter-mediated glucose uptake in both proximal and distal ileum in plain glucose, and with phloretin and with phlorizin.
In the distal ileum, there was a similar marked increase in transporter-mediated glucose uptake at 1 and 4 wk after resection compared to the NC and sham groups (p=0.02). The addition of phloretin also had no effect on transporter-mediated glucose uptake in the distal ileum in any group (p≥0.6), however, phlorizin decreased markedly transporter-mediated glucose uptake in this segment (p≤0.03). This inhibitory effect of phlorizin was also somewhat blunted at 4 wk after resection compared to the distal ileal segments in the other 3 groups (p=0.01) (Figure 3b).
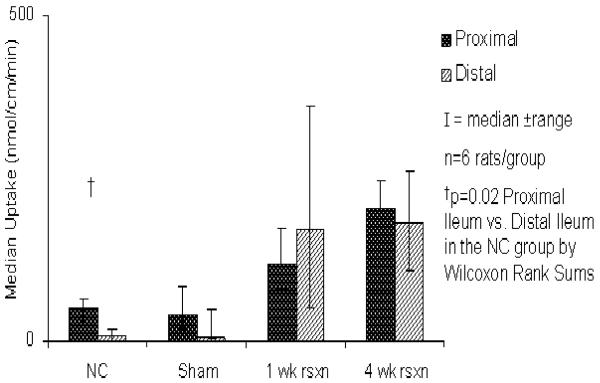
In the naïve control group, transporter-mediated glucose uptake was greater in the proximal ileum compared to the distal ileum (p=0.02) in the absence of any inhibitors. This difference in uptake, however, was lost in the sham controls as well as the rats both 1 and 4 wk after resection (Figure 4). There was no difference in transporter-mediated glucose uptake between the proximal and distal segments in any of the experimental groups in the presence of either phloretin or phlorizin (Figure 3).
Figure 4.
Transporter-mediated glucose uptake in the glucose test solution.
Villus Height and Intestinal Length
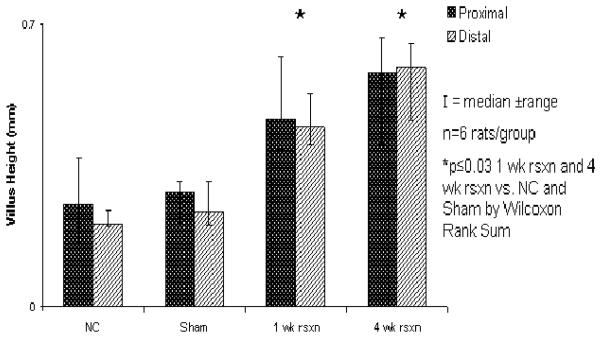
Within the proximal segment, median villus height was greater at 1 and 4 wk after resection compared to NC rats (0.47 and 0.58 vs 0.25 mm, resp; p≤0.03; Figure 5). Similarly, in the distal ileum, villus height was also greater at 1 and 4 wk after resection compared to NC (0.45 and 0.59 vs 0.21 mm; p≤0.01; Figure 5). There was no difference between NC and sham in either the proximal or distal ileum (p=0.9). Additionally, there was no difference in villus height in rats at 1 and 4 wk after resection in either the distal or proximal remnant ileum (p≥0.09). Villus height also did not differ between the distal or proximal ileum within any of the experimental groups.
Figure 5.
Changes in villus height after 70% proximal small intestinal resection.
All resected animals were left with a remnant ileum of 14 cm in length at the time of resection. One wk after resection, this length had shortened to a median 12 cm (range, 11-13 cm; p<0.01); after 4 wk, the intestinal length had increased to a median of 19 cm (range, 15-22 cm; p<0.01). In addition to intestinal length, gross luminal diameter also increased over time, and while actual measurements could not be measured reliably, the everted sleeve technique necessitated larger caliber steel rods for eversion (4 mm in NC and shams, 5 mm 1 wk after resection, and 6 mm 4 wk after resection).
Discussion
After proximal-based small bowel resections, the ileum is capable of adapting to the loss of absorptive surface area by effectively increasing absorption of water, electrolytes, and nutrients to maintain adequate hydration and nutrition. This inherent adaptability has made the ileum an area of interest, especially as it pertains to intestinal hexose transporters. Currently, the cellular mechanisms responsible for regulating the expression of the three primary hexose transporters (SGLT1, GLUT2, and GLUT5) are poorly understood. Because these transporters are expressed normally in such low levels in the ileum, we hoped that using a model of ileal adaptation would help us examine patterns in expression and function of these transporters before and after adaptation.
Our data confirm that the ileum, which in the normal, intact gut is not involved in much of the glucose absorption from the gut, is highly adaptable and can increase its glucose transport after a 70% proximal-based, small bowel resection in the rat. Both of our resection groups demonstrated a marked increase in transporter-mediated glucose uptake. This adaptation appears to occur quickly, because the effect was present at 1 wk after small bowel resection and, there was no difference in transporter-mediated glucose uptake at 1 wk compared to 4 wk after resection. When we examined the relative expressions of mRNA, we found very subtle changes at 1 wk after resection in SGLT1 mRNA expression in the distal remnant ileum and GLUT2 mRNA expression in both proximal and distal segments. These very small changes may reflect adaptive changes; however, we did not find any significant changes in comparing NC and shams to the resection animals in terms of SGLT1 and GLUT2 protein expression. These effects could be a result of post-translational regulation as we have previously described (18), but the most plausible explanation is that, because the magnitude of change in mRNA expression that we observed was so small, it is likely of no clinical significance. Furthermore, the fact that cellular protein expression remained stable across all four groups suggests that the ileal adaptation after a 70% proximal-based, small intestinal resection was not mediated by upregulation of hexose transporter expression within the enterocyte.
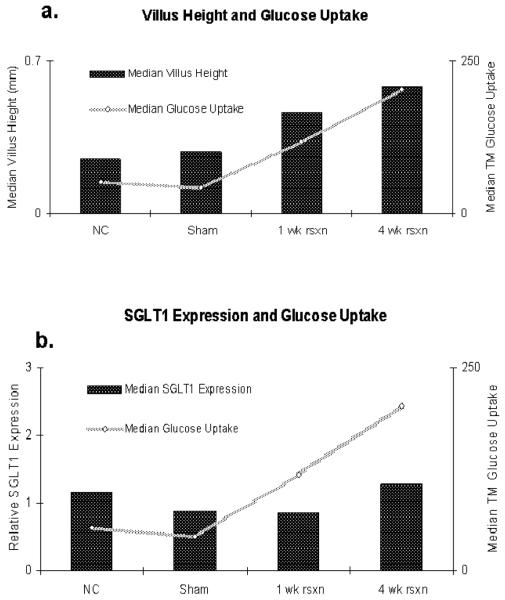
Histologically, we observed a marked increase in villus height between NC and sham rats compared to the resection groups, which indicates a cellular proliferation with lengthening of villus height in the resection group. We interpret this finding to represent hyperplastic changes in the ileal mucosa after the proximal resection that results in an adaptive response in glucose absorption secondary to more enterocytes rather than each individual enterocyte upregulating hexose transporter expression and function within the apical membrane of the ileal enterocytes. Indeed, changes in transporter-mediated glucose uptake correlated directly with changes in villus height across the four groups and not with protein (or mRNA) expression (Figure 6).
Figure 6.
Correlation of transporter-mediated (TM) glucose uptake plotted against a) villus height and b) SGLT1 protein expression by Western blot.
The intestinal lengths measured after resection further support the concept of intestinal hyperplasia. At 4 wk post-resection, the remnant ileum appears to have increased its length significantly from baseline length, although it is possible that this lengthening represented natural growth of the intestine as the rats grew 4 wk older as opposed to an adaptive response. Another interesting finding was that at 1 wk after resection, the ileum appeared to have shortened. We did not observe a further increase in transporter-mediated glucose uptake between 1 and 4 wk, because the everted sleeve technique measures transport over a 1-cm segment of intestine. It may be that, despite the lack of change in villus height and transporter-mediated glucose uptake per centimeter of tissue between 1 and 4 wk, the ileum's hyperplastic growth from week 1 to week 4 also includes lengthening, and, over time, this lengthening further increases the absolute absorptive capacity. Furthermore, the intestinal diameter also appeared increased; although we did not directly measure this parameter, the sleeves required larger bore rods for eversion after resection. This increase in diameter (without any further increase in villus height) did not affect the measured glucose uptake over the 1-cm of tissue that was studied from 1 to 4 wk, but over greater lengths of intestine, where an increase in diameter impacts overall surface area more significantly, intestinal dilation also contributes to a greater absorptive capacity.
Ultimately, these findings suggest that ileal adaptation may not be a good model for studying cellular mechanisms within the enterocyte responsible for regulating hexose transporter expression, at least after proximal resection. In contrast, understanding the mechanisms responsible for increased villus height and intestinal length will be beneficial in treating short bowel syndrome. Our data, however, do suggest that changes in luminal content in the distal ileum after proximal resection did not have a marked effect on enterocyte expression of SGLT1 and GLUT2. If this were the case, one would expect changes in gene expression of SGLT1 and/or GLUT2 protein in this model, because the luminal content of hexoses in the ileum would have increased markedly. The exact mechanism regulating expression cannot be elucidated clearly from our data but could include hormonal or neural mechanisms.
It is accepted widely that SGLT1 is the primary apical glucose transporter in the enterocyte and that GLUT2 functions primarily in the basolateral membrane to transport glucose out of the cell into the systemic circulation. Yet, data from our laboratory and others have suggested the presence of apical GLUT2 in jejunal glucose absorption, and we hypothesized that apical GLUT2 would therefore play an important role in ileal adaptation. When the GLUT2 inhibitor, phloretin, was administered, however, there was no effect on transporter-mediated glucose uptake in any of the groups. This observation suggests strongly that GLUT2 does not play an important role in intestinal apical glucose absorption in the ileum, consistent possibly with the small amount of GLUT2 protein expression we found as well. Moreover, treatment with the SGLT1 inhibitor, phlorizin, resulted in a dramatic, virtually complete inhibition of transporter-mediated glucose uptake, further supporting the belief that, in the ileum, SGLT1 is the predominant apical glucose transporter.
An additional observation was a rebound in transporter-mediated glucose uptake at 4 wk after proximal resection in the phlorizin-treated group. While uptake was still considerably low in the presence of phlorizin at 4 wk after resection, it was significantly greater than the other three groups under the same inhibitory conditions. Because phlorizin is a competitive inhibitor of SGLT1, one explanation might be that the hyperplastic changes at 4 wk after resection resulted in an excess of SGLT1 that was able to overcome the phlorizin inhibition; however, this was not the case at 1 wk after resection, where expression of total cellular SGLT1 protein and histologic data matched that of the 4 wk resection group. It remains possible that an additional transporter is upregulated or newly expressed in a delayed fashion in the ileum, such as GLUT7, a hexose transporter that has been described recently in the literature (34), however our data are inadequate to answer this question.
Initially when we studied the resection animals in a pilot study, we noticed a gross difference between the proximal and distal portion of the remnant ileum 4 wk after resection. The proximal segment appeared to be dilated more than the caliber of the ileum distally. For this reason, our studies were conducted in both proximal and distal segments of the remnant ileum. Indeed, we found that there was a significant decrease in transporter-mediated glucose uptake in this distal segment of ileum in the NC rats; the protein expression data for SGLT1 and GLUT2 did not, however, correlate with this finding.
Our study has several limitations. First, our technique for protein analysis cannot distinguish cytoplasmic from apical membranous transporters. Therefore, it is possible that the distal ileum expresses similar total cellular amounts of hexose transporter protein after resection, but more of this protein transporter is trafficked to the apical membrane in the proximal ileum. Our techniques can not differentiate this possibility. Expression of these proteins in a segment of intestine that is not normally exposed to large amounts of glucose is inefficient, and it may be that expression of these transporters is not regulated by the luminal milieu but by other mechanisms. Again, our data is insufficient to draw a definitive conclusion. Finally, the rats after 70% proximal resection did gain weight, although modest (x̄ =29 g), compared to a weight gain in control rats of about 62 g; results might have been different in a more radical proximal resection (>70%), but our interest was more in the regulation of SGLT1 and GLUT expression.
After small bowel resection, the difference in transporter-mediated glucose uptake between proximal and distal ileum was lost, and protein expression and histology were no different either. These data demonstrate that despite different baseline absorptive capacity between proximal and distal ileum with a normal intestinal length, in the setting of short bowel, both the proximal and distal regions of this remnant ileum appear to be equally capable of adaptation through dilation, lengthening, and increased transport of glucose per cm length.
Conclusion
The ileum appears to be highly adaptable in its ability to increase glucose absorption via SGLT1 after massive proximal-based, small bowel resection—but this increase in absorptive capacity is due to cellular proliferation by villus hyperplasia and intestinal lengthening as opposed to upregulation of glucose transporters within the enterocyte. Despite a lesser SGLT1 activity under normal conditions in the distal ileum compared to the proximal ileum, the distal ileum has a similar capacity to adapt as the proximal portion after 70% proximal-based, small bowel resection. There appears to be little or no role of GLUT2 in baseline apical glucose transport in the ileum or after adaptation.
Acknowledgments
Research supported by NIH grant DK39337 (MGS)
References
- 1.Vanderhoof JA, Langnas AN. Short-bowel syndrome in children and adults. Gastroenterology. 1997;113:1767–78. doi: 10.1053/gast.1997.v113.pm9352883. [DOI] [PubMed] [Google Scholar]
- 2.Georgeson KE, Breaux CW. Outcome and intestinal adaptation in neonatal short-bowel syndrome. J Pediatr Surg. 1992;27:344–48. doi: 10.1016/0022-3468(92)90859-6. [DOI] [PubMed] [Google Scholar]
- 3.Tavakkolizadeh A, Whang FE. Understanding and augmenting human intestinal adaptation: a call form ore clinical research. J Parenter Nutr. 2002;26:251–5. doi: 10.1177/0148607102026004251. [DOI] [PubMed] [Google Scholar]
- 4.Scolapio JS, Camilleri M, Fleming CR. Gastrointestinal motility considerations in patients with short-bowel syndrome. Dig Dis. 1997;15:253–62. doi: 10.1159/000171602. [DOI] [PubMed] [Google Scholar]
- 5.Dekaney CM, Fong JJ, Rigby RJ, Lund PK, Henning SJ, Helmrath MA. Expansion of intestinal stem cells associated with long-term adaptation following ileocecal resection in mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1013–G1022. doi: 10.1152/ajpgi.00218.2007. [DOI] [PubMed] [Google Scholar]
- 6.Haxhija EQ, Yang H, Spencer AU, Sun X, Teitelbaum DH. Intestinal epithelial cell proliferation is dependent on the site of massive small bowel resection. Pediatr Surg Int. 2007;23:379–90. doi: 10.1007/s00383-006-1855-9. [DOI] [PubMed] [Google Scholar]
- 7.Baksheev L, Fuller PJ. Gene expression in the adapting small bowel after massive small bowel resection. J Gastroenterol. 2006;41:1041–1052. doi: 10.1007/s00535-006-1896-9. [DOI] [PubMed] [Google Scholar]
- 8.Nelson DW, Liu X, Holst JJ, Raybould HE, Ney DM. Vagal afferents are essential for maximal resection-induced intestinal adaptive growth in orally fed rats. Am J Physiol Integr Comp Physiol. 2006;291:R1256–R1264. doi: 10.1152/ajpregu.00247.2006. [DOI] [PubMed] [Google Scholar]
- 9.Sigalet DL, Bawazir O, Martin GR, Wallace LE, Zaharko G, Miller A, Zubaidi A. Glucagon-like peptide-2 induces specific pattern of adaptation in remnant jejunum. Dig Dis Sci. 2006;51:1557–66. doi: 10.1007/s10620-006-9077-5. [DOI] [PubMed] [Google Scholar]
- 10.Helmrath MA, Fong JJ, Dekaney CM, Henning SJ. Rapid expansion of intestinal secretory lineages following a massive small bowel resection in mice. Am J Physiol Gastrointest Liver Physiol. 2007;292:G215–G222. doi: 10.1152/ajpgi.00188.2006. [DOI] [PubMed] [Google Scholar]
- 11.Ozturk H, Ozturk H, Yagmur Y, Uzunlar KM. Effects of melatonin on intestinal adaptive response after massive bowel resection in rats. Dig Dis Sci. 2006;51:333–37. doi: 10.1007/s10620-006-3134-y. [DOI] [PubMed] [Google Scholar]
- 12.Martin GR, Wallace LE, Sigalet DL. Glucagon-like peptide-2 upregulation of intestinal blood flow and glucose uptake is nitiric oxide dependent in TPN-fed piglets. Gastroenterology. 2003;125:136–47. doi: 10.1016/s0016-5085(03)00667-x. [DOI] [PubMed] [Google Scholar]
- 13.Martin GR, Wallce LE, Sigalet DL. Glucagon-like peptide-2 induces intestinal adaptation in parenterally fed rats with short bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2004;286:G964–G972. doi: 10.1152/ajpgi.00509.2003. [DOI] [PubMed] [Google Scholar]
- 14.Ferraris RP. Dietary and developmental regulation of intestinal sugar transport. Biochem J. 2001;360:265–76. doi: 10.1042/0264-6021:3600265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corpe CP, Burant CF. Hexose transporter expression in rat small intestine: effect of diet on diurnal variation. Am J Physiol Gastrointest Liver physiol. 1996;271:G211–G216. doi: 10.1152/ajpgi.1996.271.1.G211. [DOI] [PubMed] [Google Scholar]
- 16.Ferraris RP, Diamond JM. Specific regulation of intestinal nutrient transporters by their dietary substrates. Annual Rev of Physiol. 1989;51:125–41. doi: 10.1146/annurev.ph.51.030189.001013. [DOI] [PubMed] [Google Scholar]
- 17.Ferraris RP, Diamond JM. Regulation of intestinal sugar transport. Physiol Reviews. 1997;77:257–302. doi: 10.1152/physrev.1997.77.1.257. [DOI] [PubMed] [Google Scholar]
- 18.Houghton SG, Iqbal CW, Duenes JA, Fatima J, Kasparek MS, Sarr MG. Coordinated, diurnal hexose transporter expression in rat small bowel: implications for small bowel resection. Surgery. 2008;143:79–93. doi: 10.1016/j.surg.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fedorak RN, Cheeseman CI, Thomson AB, Porter VM. Altered glucose carrier expression: mechanism of intestinal adaptation during streptozocin-induce diabetes in rats. Am J Physiol Gastrointest Liver Physiol. 1991;261:G585–G591. doi: 10.1152/ajpgi.1991.261.4.G585. [DOI] [PubMed] [Google Scholar]
- 20.Helliwell PA, Richardson M, Kellett GL, et al. Stimulation of fructose transport across intestinal brush-border membrane by PMA is mediated by GLUT2 and dynamically regulated by protein kinase C. Biochem J. 2000;350:149–154. [PMC free article] [PubMed] [Google Scholar]
- 21.Helliwell PA, Richardson M, Kellett GL, et al. Regulation of GLUT5, GLUT2, and intestinal brush-border fructose absorption by the ERK, p38, and PI 3-kinase intracellular signaling pathways: implications for adaptation to diabetes. Biochem J. 2000;350:163–169. [PMC free article] [PubMed] [Google Scholar]
- 22.Au A, Gupta A, Cheeseman CI, et al. Rapid insertion of GLUT2 into the rat jejunal brush-border membrane promoted by glucagon-like peptide. Biochem J. 2002;367:247–254. doi: 10.1042/BJ20020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Affleck JA, Helliwell PA, Kellett GL. Immunocytochemical detection of GLUT2 at the rat intestinal brush-border membrane. J Histochem Cytochem. 2003;51(11):1567–1574. doi: 10.1177/002215540305101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iqbal CW, Fatima J, Duenes JA, Kasparek MS, Sarr MG. GLUT2 trafficking to the apical membrane of enterocytes via SGLT1 signalling through protein kinase C. Gastroenterology. 2007;132:A-891. Abstract M1764. [Google Scholar]
- 25.Tavakkolizadeh A, Berger UV, Levitsky LL, et al. Diurnal rhythmicity in intestinal SGLT1 function, Vmax, and mRNA expression topoghraphy. Am J Physiol Gastrointest Liver Physiol. 2001;280:G209–G215. doi: 10.1152/ajpgi.2001.280.2.G209. [DOI] [PubMed] [Google Scholar]
- 26.Houghton SG, Zarroug AE, Sarr MG, et al. The diurnal periodicity of hexose transporter mRNA and protein levels in the rat jejunum: role of vagal innervation. Surgery. 2006;139:542–549. doi: 10.1016/j.surg.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Karasov WH, Diamond JM. A simple method for measuring intestinal solute uptake in vitro. J Comp Physiol. 1983;152:105–116. [Google Scholar]
- 28.Iqbal CW, Sarr MG, Duenes JA, Fatima J, Kasparek MS, Houghton SG. Activity of hexose transporters after small intestinal denervation in the rat: role of intrinsic and extrinsic denervation (Abstract 23) J Surg Res. 2007;137:160. [Google Scholar]
- 29.Manome SH, Kuriaki K. Effects of insulin, phlorizin andsome metabolic inhibitors on the glucose absorption from the small intestine. Arch Int Pharm Therapie. 1961;130:187–94. [PubMed] [Google Scholar]
- 30.Oulianove N, Falk S, Berteloot A. Two-step mechanism of phlorizin binding to the SGLT1 protein in kidney. J Membrane Biol. 2001;179:223–242. doi: 10.1007/s002320010049. [DOI] [PubMed] [Google Scholar]
- 31.Loike JD, Hickman S, Fischbarg J, et al. Sodium-glucose co-transporters display sodium- and phlorizin-dependent water permeability. J Membrane Biol. 2001;179:223–242. [Google Scholar]
- 32.Forsling ML, Widdas WF. The effect of temperature on the competitive inhibition of glucose transfer in erythrocytes by phenolphthalein, phloretin, and stilboestrol. J Physiol. 1968;194:545–554. doi: 10.1113/jphysiol.1968.sp008423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boudry G, Cheeseman CI, Perdue MH. Psychological stress impairs Na+ -dependent glucose absorption and increases GLUT2 expression in the rat jejunal brush-border membrane. Am J Physiol Regul Integr Comp Physiol. 2007;292:R862–R867. doi: 10.1152/ajpregu.00655.2006. [DOI] [PubMed] [Google Scholar]
- 34.Qiang L, Manolescu A, Ritzel M, Yao S, Slugoski M, Young JD, Chen XZ, Cheeseman CI. Cloning and functional characterization of the human GLUT7 isoform SLC2A7 from the small intestine. Am J Physiol Gastrointest Liver Physiol. 2004;287:G236–G242. doi: 10.1152/ajpgi.00396.2003. [DOI] [PubMed] [Google Scholar]