Abstract
The gastrointestinal innate and adaptive immune system continuously faces the challenge of potent stimuli from the commensal microflora and food constituents. These local immune responses require a tight control, the outcome of which is in most cases the induction of tolerance. Local T cell immunity is an important compartment of the specific intestinal immune system. T cell reactivity is programmed during the initial stage of its activation by professional presenting cells. Mucosal dendritic cells (DCs) are assumed to play key roles in regulating immune responses in the antigen-rich gastrointestinal environment. Mucosal DCs are a heterogeneous population that can either initiate (innate and adaptive) immune responses, or control intestinal inflammation and maintain tolerance. Defects in this regulation are supposed to lead to the two major forms of inflammatory bowel disease (IBD), Crohn’s disease (CD) and ulcerative colitis (UC). This review will discuss the emerging role of mucosal DCs in regulating intestinal inflammation and immune responses.
Keywords: Dendritic cells, Commensal, Inflammatory bowel disease, Mucosal immunity, Host defence
INTRODUCTION
The intestinal innate and adaptive immune system has evolved in response to potent stimuli derived from constituents of the commensal microflora. In most cases these local immune responses achieve tolerance to the intestinal microflora and food antigens. Defects of the tightly regulated mucosal immune responses are assumed to result in inflammatory bowel disease (IBD), such as Crohn’s disease (CD) and ulcerative colitis (UC)[1,2]. Local T cell immunity is an important compartment of the specific intestinal immune system. T cell reactivity is programmed during the initial stage of its activation by dendritic cells (DCs) that can either initiate (innate and adaptive) immune responses, or control intestinal inflammation and maintain tolerance[3-5]. DCs reside in mucosal tissues or recirculate in the blood and lymphoid tissues[6]. The lamina propria of the small and large intestine are effector sites of mucosal tissues. Inductive sites are Peyer’s patches (PP), intestinal lymph follicles (iLFs), DC aggregates and mesenteric lymph nodes (MLNs) (Table 1). The local microenvironment influences the phenotype of DCs, a heterogeneous population that can be divided into conventional DCs (CD8α+CD11b-, CD4+CD11b+, CD4-CD11b+)[7,8] and plasmacytoid DCs (B220+CD11clow) (Table 2) and are characterized by a remarkable plasticity between DCs[9]. In the lamina propria of the small and large intestine, DCs are ideally situated to survey the constituents of the commensal microflora and monitor food antigens[10]. After antigen recognition at peripheral sites DCs migrate to regional draining lymph nodes to initiate (innate and adaptive) immune responses[11]. This review will discuss the emerging role of mucosal DCs in regulating intestinal inflammation and immune responses.
Table 1.
Anatomic compartments of GALT
|
Compartment |
Gut segment |
||||
| Inductive/effector sites | Structure | Abbreviation |
Small intestine |
Large intestine3 | |
| Upper1 | Lower2 | ||||
| Effector | Lamina propria | LP | +++ | +++ | +++ |
| Intraepithelial lymphocytes | IEL | +++ | +++ | +++ | |
| Inductive | Peyer’s patch | PP | + | +++ | - |
| Intestinal lymph follicle | iLF | + | +++ | - | |
| Intestinal lymph aggregate | iLA | + | ++ | ++ | |
| Mesenteric lymph node | MLN | - | - | - | |
Distribution of the described structures in the upper small intestine (duodenum and jejunum);
Distribution of the described structures in the upper small intestine (ileum);
Distribution of the described structures in the upper small intestine (ileum). “+++”: Very frequent; “++”: Frequent; “+”: Randomly; “-“: Not present.
Table 2.
Mucosal DCs and their proposed function
|
DC1 |
Intestinal compartment2 |
Comment3 | Ref. | |||||
| Lineage | Phenotype | MLN | siLP | cLP | PP | iLF | ||
| cDC | CD4-CD8- | +++ | +++ | +++ | +++ | +++ | Express CD103 or CX3CR1. | 12-14, 47, 86, 99 |
| CD11b+CD11c+ | Mediate intestinal antigen acquisition. | |||||||
| Involved in the RA dependent Treg conversation. | ||||||||
| Permitting homing of conventional T cells to intestinal tissues by inducing CCR9 and α4β7. | ||||||||
| CD4+CD8-CD11b+CD11c+ | +++ | - | - | ++ | + | Prime CD4 T cell responses. | 8, 28 | |
| CD4-CD8+CD11b-CD11c+ | ++ | - | - | ++ | ++ | Prime CD8 T cell responses. | 8, 28 | |
| pDC | B220+PDCA1+CD11c+ | + | +/- | +/- | + | + | Produce type I interferons | 10 |
cDC: Conventional DC; pDC: Plasmacytoid DC; MLN: Mesenteric lymph node; PP: Peyer´s patch; cLP: colonic lamina propria; siLP: small intestinal lamina propria; iLF: intestinal lymph follicle.
DC lineage;
Phenotype of the described DC lineage;
Distribution of the described DC lineage in distinct intestinal compartments.
DCs IN THE SMALL INTESTINE
The lamina propria of the small intestine is populated by CD4-CD8α- cDCs. Only 2%-5% plasmacytoid DCs reside in the small intestinal lamina propria[12]. Mucosal DCs can be discriminated into DCs that express CX3CR1 (the receptor for fractalkine/CX3CL1)[12] and into DCs that express the integrin αE chain CD103[13,14]. CD103+ DCs originate from Ly-6ChighCCR2high monocytes. Conversely Ly-6ClowCCR2low monocytes repopulate CX3CR1+CD11b+ mucosal DCs[15]. CX3CR1+CD11b+ DCs directly access the intestinal lumen by extending transepithelial dendrites in a CX3CR1-dependent manner to survey the intestinal lumen[12], whereas CD103+ DCs induce the expression of CCR9 and α4β7 integrin on cognate CD4 and CD8 T cells to facilitate homing of T cells to small intestinal tissues[13,16] and induce the differentiation of regulatory T cells (Tregs) in the absence of exogenous cytokines.
DCs IN THE LARGE INTESTINE
The cLP is populated by CD4-CD8- DCs which endocytose and process antigens and induce T cell proliferation. Compared to their splenic counterparts TLR-4, -5 and -9 expression by colonic CD4-CD8-C DCs is low[17]. Only few CD8+ and plasmacytoid DCs are found in the cLP[17]. In human biopsies from colonic tissues, CD3-CD14-CD16-CD19-CD34- DCs with an immature state with low TLR-2 and -4 expressions were observed[18]. In addition the presence of CD83+ and DC-SIGN+ DCs was described[19]. Human and mouse mucosal DCs are assumed to be less responsive to microbial-derived TLR-ligands compared to spleen or blood born DCs[17] Despite the functional subspecifications of DCs, DCs are characterized by a remarkable plasticity between DCs which is influenced by the local environment, the antigen itself or the activation state of the DC[9,20].
DCs IN PPs
PP, iLF, CP and DC clusters of the small and large intestine belong to the gut associated lymphoid tissues (GALTs)[6], secondary lymphoid structure, which lack in contrast to lymph nodes the afferent lymph and are located in close proximity to the intestinal epithelium. In PP the subepithelial dome regions beneath the follicle-associated epithelium can be discriminated from follicular and interfollicular regions, which serve as inductive sites where immune responses are primed. Specialized epithelial cells, the M cells, deliver luminal antigens to DCs located in the subepithelial dome regions beneath the follicle associated epithelium[21]. The subepithelial dome regions are populated with CD8α-CD11b+B220- DCs and with CD8α-CD11b-B220- DCs[22,23]. By expressing high concentrations of the chemokines CCL20, CXCL16 and CCL9, the follicle-associated epithelium creates a specific micromilleu that allows DCs to selectively migrate towards the follicle associated epithelium[24,25]. Upon pathogen challenge CCR6+ DCs which are located in the interfollicular regions are recruited towards the follicle-associated epithelium to process microbial antigens, and to facilitate rapid local adaptive immune responses[23]. In the absence of CCR6 in CCR6GFP/GFP mice CX3CR1+ DCs that lack CCR6 expression reside in the follicle-associated epithelium[23,26]. In the interfollicular regions CD11b-CD8α- and CD11b- CD8α+ DCs as well as plasmacytoid DCs are found that produce IL-12 and IL-10, and induce the differentiation of IFN-γ secreting TH1 cells, whereas the CD11b+ subsets produce low IL-12 and high IL-10 levels and prime IL-10 producing T cells[22,27]. CD8-33D1+ DCs, but not CD8α+DEC205+ DCs are specialized to present processed antigens in a MHC class II dependent manner to CD4 T cells[28]. Reoviral antigens are sampled and processed by CD11b-CD8α- DCs to initiate TH1 adaptive immune responses likely resulting in protective host defence to viral antigens[29].
DCs IN INTESTINAL LYMPHOID TISSUES
Intestinal lymphoid aggregates (iLAs) can be discriminated from PP, which comprises iLF and CP that are closely associated with the epithelial lining in the small and large intestine[30]. CP were defined as tiny aggregates of c-Kit+IL-7R+ cells[31], whereas iLFs are constituted by solitary B follicles that are localized in the anti-mesenteric regions of the intestinal wall, and contain small numbers of mature T lymphocytes, but also c-Kit+ and IL-7R+ cells[32-34]. By analyzing large numbers of such aggregates, the classification of iLA into CP and iLF was recently challenged showing that most of them display properties intermediate between CP and iLF and were termed small intestinal lymphoid tissues (siLT)[35]. In the large intestine, subepithelial DC aggregates locate in the regions of cLP where inflammation develops[36]. DC aggregates are formed by CD8α+, CD8α- and B220lo DCs. DCs with high MHC II expression, but low expression of the costimulatory molecules CD40, CD80 and CD86 extend transepithelial dendrites into the intestinal lumen[37]. During colitis induced by adoptive transfer of CD45RBhi CD4 T cells into immunocompromised RAG mice, FoxP3 regulatory T cells accumulate in colonic DC aggregates producing TGF-β suggesting that DC aggregates are important structures to regulate inflammation[37].
DCs IN MLN
Luminal antigens are sampled by lamina propria DCs and by DCs located in PP, CP, iLF and DC clusters at the base of intestinal villi. The DCs transport the sampled antigens via the lymph to MLN, where naïve T cells are primed and adaptive immune responses are induced[38] (Figure 1). In MLN, blood-born DCs and DCs that originate from PP or the lamina propria are present[6]. In MLN, blood-born DCs are assumed to express CD8αhiCD11b-αLintβ7int and CD8α-CD11bhiαLloβ7lo DCs, whereas DCs derived from mucosal sites are characterized by CD8αintCD11b-αLloβ7hi and CD8α-CD11bhiαLloβ7hi expression[39], allowing the discrimination of DCs of distinct origins. CCR7 expression guides lamina propria DCs to MLN[40]. Blood-born DCs enter the MLN via interactions with mucosal addressin cellular adhesion molecule (MAdCAM-1) and peripheral node adressin (PNAd) expressed by high endothelial venules (HEVs)[41]. Apoptotic cell bodies derived from intestinal epithelial cells (iECs) are phagocytosed and transported to MLN to induce tolerance against self-antigens[42]. The depletion of MLN results in defective oral tolerance. Lamina propria DCs are continuously carrying commensal bacteria to the MLN[43], which can be further potentate by stimulating DCs with TLR-7/8 agonists[44]. IgA production is induced that is supposed to limit the dissemination of commensal bacteria further than the MLN preventing effectively systemic infections[45].
Figure 1.
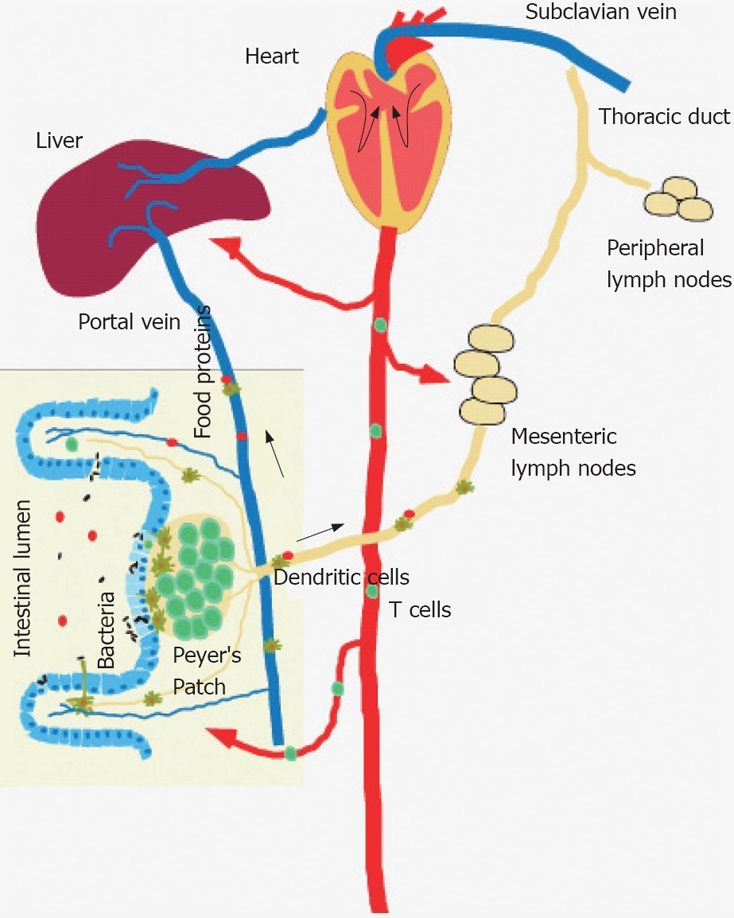
Bacterial and food antigens are continuously surveyed by the mucosal immune system, processed and transported via the lymph to mesenteric lymph nodes or via the portal vein to the liver. DCs present processed luminal antigens to naïve T cells to achieve tolerance or to initiate host defence to pathogens.
DCs IN INTESTINAL ANTIGEN ACQUISITION PATHWAYS
Intestinal DCs play a key role in monitoring the intestinal lumen by continuously sampling and processing luminal antigens[46,47]. There are different pathways by which luminal antigens can gain access in the lamina propria[48]. In all pathways, mucosal DCs are supposed to play key roles. Restricted to the ileum DCs extend transepithelial dendrites in a CX3CR1 mediated manner to survey the intestinal lumen to continuously survey the commensal microflora and to monitor food antigens[12,49]. M cells located in the follicle associated epithelium of PP are specialized epithelial cells which gained the ability to translocate luminal antigens via the intestinal barrier and deliver luminal antigens to DCs located in the subepithelial dome regions of PP[21]. Upon pathogen challenge, CCR6+ DCs are recruited to the dome regions to process pathogens, and to trigger rapid local adaptive immune responses[23]. As well CD11b-CD8α- CD11c+ interfollicular DCs process antigens derived from luminal reovirus[29]. M cells within the small intestinal villous epithelium are able to translocate pathogens via the epithelial barrier to DCs located in the lamina propria[50]. The intestinal epithelium by itself plays an important role in sensing the luminal microenvironment. The intestinal epithelium express Toll-like receptors, such as TLR2[51], TLR-4[52], TLR-5[53] and TLR-9[54] to recognize microbial derived pathogen associated molecular patterns (PAMPs) for the regulation of protective innate immune responses. As well upon LPS-stimulation iECs express the intracellular pathogen recognition pattern NOD1[55] and NOD2[56,57], in which mutations are associated with an increased susceptibility for CD[58,59]. IECs directly participate in intestinal antigen uptake pathways by delivering antigens or exosomes to lamina propria DCs. Secreted IgG binds to cognate luminal antigens to form IgG/antigen complexes that are recycled by neonatal FcRn receptors and delivered to lamina propria DCs[60,61]. IECs also express MHC II and may present luminal antigens to CD4 T cells. After luminal exposure with ovalbumin (OVA), OVA is taken up at apical and basolateral surfaces of the epithelial cells. Then, it enters the early endosomes and may be delivered to late endosomes where it is processed in the presence of MHC II, and presented in the context of class II to T cells[62]. It needs to be dissected in future work whether all or specific luminal antigen acquisition routes are linked to the induction and constant renewal of tolerance and to the development of protective immune responses. DCs are assumed to play key roles in all luminal antigen acquisition routes, and are supposed to represent a major factor for the processing and presentation of orally delivered antigens and the induction of tolerance.
DC MEDIATE INNATE AND ADAPTIVE IMMUNE RESPONSES TO THE COMMENSAL FLORA
The gastrointestinal immune system faces the tremendous challenge to deal with potent stimuli for the innate and adaptive immune system derived from the commensal microflora and food antigens. DCs are sentinels of the mucosal immune system to survey the constituents of the luminal commensals and to trigger host responses to pathogens[63]. It is thought that gastrointestinal diseases, such as IBDs are results of deregulated immune responses to the commensal microflora[1]. Although germ-free (GF) mice and rats generate alloreactive T cell responses[64] and cellular immunity to certain bacteria (after monocolonization)[65,66], the cellularity of their immune system is greatly reduced[67-69]. In this regard, the numbers of IL-17A producing TH17 cells is reduced in the lamina propria of GF animals[70]. Because commensal flora is a major driving force of the homeostatic proliferation of naïve T cells in the periphery, the reduced cellularity in the immune system of GF mice may be the result of the deficient peripheral expansion of recent thymic T cell emigrants[71,72]. When GF mice are fed bacterial carbohydrate polysaccharide A (PSA) CD4 T cells are activated resulting in a TH1 response imprinted by DCs[72]. After conventionalization of GF rats OX62+ DCs increased, whereas CD4+ DCs located in the follicle associated epithelium decreased indicating that CD4+ DCs phagocytose microbial derived antigens, relocate to the follicular regions, and elicit rapid immune responses to the microflora to achieve tolerance[68]. Furthermore, it has been described that the inducible isoform of nitric oxide synthase (iNOS) expressing DCs is markedly reduced in GF or MyD88-deficient animals indicating that the commensal flora is required for the accumulation of iNOS expressing DCs at mucosal sites[73]. In iNOS-/- animals serum IgA levels are reduced suggesting that iNOS expressing DCs play a role in IgA class switching required to prevent the uncontrolled dissemination of the commensal flora into the host.
HEMATOGENOUS DISSEMINATION OF INTESTINAL ANTIGENS
Although the priming of T cells mainly occurs in draining lymph nodes, antigen-rich blood from the small and large intestine is transported to the liver via the portal vein, and pressed through a network of sinusoids and scanned by antigen-presenting cells and lymphocytes in the liver[74,75] (Figure 1). One third of liver cells are constituted by non-parenchymal cells (NPCs), which include liver sinusoidal endothelial cells (LSECs), Kupffer cells, biliary cells, stellate cells (Ito or fat-storing) cells and lymphocytes[74,75]. Resident APCs in the liver are Kupffer cells, LSEC[76], Ito cells[77] and DCs[75]. Plasmacytoid B220+CD11c+ DCs as well as B220-CD11c+ DCs are found. The latter can be further divided into major immature (CD40lo CD80lo CD86lo MHC class IIlo) CD11cint and minor mature (CD40hi CD80hi CD86hi MHC class IIhi) CD11chi DCs[78]. Further, the presence of CD8α+CD11b- and CD8α-CD11b+ DCs has been reported[78]. The continuous exposure of resident APCs with the bacterial cell wall derived LPS promotes the induction of CD4 regulatory T cells and may explain the dominance of IL-10 in the liver[79,80]. If Kupffer cells are depleted by gadolonium chloride treatment, liver tolerance becomes impaired raising the possibility that the liver is increasingly recognized as an innate and adaptive immune organ[81].
DCs IN ORAL TOLERANCE INDUCTION
Mucosal DCs play a key role in the development of oral tolerance, a phenomenon, in which systemic immune responses to a defined peptide/protein are blunted, when the same protein has been orally fed before the rodents were systemically challenged[82]. The triggering of oral tolerance in patients with IBD is impaired[83]; however, the exact mechanism has not been determined. Mucosal DCs are critical for the induction of oral tolerance as shown by studies, in which flt-3 ligand was injected in mice leading to the expansion of DC subsets[84]. After the expansion of the intestinal DC pool, enhanced oral tolerance was observed. Because oral tolerance can be transferred by adoptive transfer of T cells into recipient mice, interactions between DCs and T cells seems to be essential for the development of oral tolerance, in which CD4 CD25 FoxP3 regulatory and IL-10 and TGF-β secreting TH3 cells that suppress systemic immune responses are primed[85]. When spleen DCs are compared to mucosal DCs, the mucosal DCs are more efficient in inducing FoxP3 expression than spleen DCs in the presence of TGF-β[86,87]. Further analyses demonstrated that CD103+ DCs are able to induce the differentiation of Treg cells via the production of the Vitamin A metabolite, retinoic acid, in presence of TGF-β, which in addition results in the recruitment of T cells and B cells to intestinal tissues[88-90]. Blocking of TGF-β abrogates the ability of CD103+ DCs to induce Treg cells[91,92]. However, parallel studies have indicated that exogenous TGF-β has not to been added to this system in order to obtain FoxP3 expressing Treg cells[86,87] raising the possibility that gut DCs activates latent TGF-β present in mucosal tissues. In this regard, the integrin αvβ8 expressed by DCs is required to activate TGF-β in vivo. Mice, in which DCs lack αvβ8, develop IBD and autoimmunity[93].
Oral tolerance is not impaired in PP-deficient mice demonstrating that lamina propria DCs play a key role in the development of oral tolerance by sampling the luminal content, and transporting antigens to the MLN[94]. After depletion of the MLN, the development of oral tolerance is reduced indicating that the MLN are a major site for priming tolerance[95]. Interestingly, it has been suggested, that intestinal self antigens can be presented by lymph node stroma cells beyond the MLNs[96]. It also may depend on the presence of certain pathogens within the lumen, because Heligmosomoides polygyus infection correlates with DC activation and IL-10 expression[97]. This provides evidence that the studies of intestinal microbial responses not only require the recognition of region- and compartment-specific immune responses, but the consideration of the interplay of different commensals and pathogens in modulating mucosal immune responses.
DC DEPENDENT Treg CELL CONVERSION
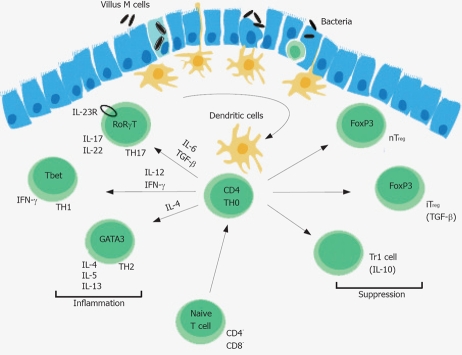
Treg cells play a key role for the development of oral tolerance, and the regulation of intestinal inflammation triggered by the intestinal microflora. CD103+ but not CD103- DCs purified from GALT-induced generation of FoxP3 cells in vitro[98]. Treg cells can be distinguished from other T cell populations by the surface expression of the α chain of the high affinity IL-2 receptor (CD25), and the transcription factor FoxP3 that is essential for Treg development. Deadly autoimmunity is prevented by Treg cells as exemplified in X-linked immunodeficiency, polyendocrinopathy, enteropathy (IPEX) syndrome in humans[98]. The conversion of Treg cells in the GALT can be prevented by inhibitors of retinal dehydrogenases, an enzyme that is highly expressed by CD103+ DCs, which converts retinol (vitamine A) into retinoic acid[86,87,91,99]. Retinoic acid binds to nuclear retinoic acid receptors that upon ligation can inhibit the activity of activating protein-1 (AP-1), a transcription factor, which can interfere with nuclear factor of activated T cells (NFAT)-FoxP3 complex[100,101]. However, the exact retinoic acid dependent signal transduction pathways required for the conversion of Treg cells remains to be elucidated. Retinoic acid production by CD103+ DCs also induce the up-regulation of the integrin α4β7, and the CC chemokine receptor CCR9 which binds thymus-expressed chemokine (TECK) permitting the accumulation of Treg cells intestinal tissues[13]. The retinoic acid dependent conversation of Treg cells depends on the presence of TGF-β, a cytokine that, in the presence of IL-6 and IL-23, can also induce the generation of TH17 cells[102] (Figure 2). A small subset of naïve T cells in the small intestine co-express the transcription factors FoxP3 and RORγt[103]. High expression of TGF-β represses IL-23 receptor expression and favours the generation of FoxP3 Treg cells, whereas low TGF-β expression in concert with IL-6, IL-21 and IL-23 relieves FoxP3 mediated RORγT inhibition promoting TH17 cells[103]. TH17 cells accumulate in the lamina propria of patients with IBD indicating that TGF-β can induce regulatory and pro-inflammatory T cell subsets[104].
Figure 2.
DCs continuously survey the intestinal lumen, phagocytose and process luminal antigens and present them to T cells, which will differentiate in presence of IL-6 and TGF-β to IL-17A and IL-17F producing TH17 cells, in presence of IL-12 and IFN-γ into TH1 cells or in presence of IL-4 into TH2 cells. nTreg, natural occurring regulatory T cell; iTreg, inducible regulatory T cells; Tr1, regulating T cell.
DCs IN IBD
Various animal models have provided insights that mucosal DCs play a key role in IBD. However, the specific function of certain DCs are unknown and needs to be determined in future work, which will provide information on mechanisms leading to IBD and limiting intestinal inflammation to achieve protective mucosal immune responses. In agreement with animal models DCs accumulate at sites of inflammation in patients with IBD. It was found that the pathogen recognition receptors TLR-2 and -4 as well as the activation/maturation marker CD40 are upregulated by intestinal DCs derived from patients with CD[18]. Furthermore, increased numbers of TNF-α producing MDC8+ monocytes, which may be precursors of mucosal DC populations, were found in patients with IBD and, hence, the treatment CD patients with anti-TNF-α antibodies resulted in reduced DC activation[105,106]. In inflamed tissues DCs are matured and increased in numbers. The CD83-CD80+DCSIGN+ DC subsets produce the cytokine IL-12 and IL-18, which promote TH1 development[19]. Also, in the peripheral blood and in the lamina propria of patients with CD or UC the numbers of CD86+CD40+ DCs are increased. In addition, DCs generated ex vitro from peripheral blood monocytes of IBD patients show increased abilities to stimulate immune responses[107-109]. Mice studies indicated that DCs isolated from inflamed colonic tissues and DCs located in the terminal ileum, which continuously sample commensal bacteria produce IL-23, but little IL-12[110,111]. It was discovered that IL-10 KO mice that spontaneously develop colitis are protected from colitis when bred on IL12p19, but not on IL-12 p35-deficient mice. In similar studies, Helicobacter hepaticus infected immunocompromised RAG-/- bred on IL-12 p19 deficient mice, but not on IL-12 p35 deficient background mice were protected from colitis indicating that DC derived IL-23 plays a major role in intestinal pathology[112,113]. The formation of granulomas, histological characteristics of CD, depends on the release of IL-23 by DC-like cell types that are characterized by CD11c and F4/80 expression[114]. Genome-wide association studies showed associations between CD and UC patients, and a gene encoding a subunit for the IL-23 receptor suggested a major role of IL-23 in the pathogenesis of IBD[115,116]. IL-23 seems to be essential for the expansion and maintenance, but not for the initial induction of IL-17 producing CD4 T cells (TH17) cells[117]. Studies in which TH1 and TH17 cells were generated co-cultures, in which naïve T cells were cultured with fecal extracts pulsed DCs, and in the presence of TH1 or TH17 promoting cytokines indicated that TH17 cells are more pathogenic than TH1 cells[118]. Recent published observations report that colitis induced by transfer of IFNγ-deficient T cells in RAG-/- mice is associated with elevated numbers of TH17 cells in the lamina propria[70]. The adoptive transfer of IL-17F, but not IL-17A deficient CD4 T cells ameliorated the IBD in the transfer model[119,120]. Interesting findings implicated that the IL-1 and IL-23 dependent priming of TH17 effector cells required a NOD2 dependent pathway, and that monocyte derived DCs from CD patients with mutated NOD2 failed to efficiently activate TH17 effector cells[121]. In this regard the TH17 cytokine IL-22 mediates mucosal defence to bacterial pathogens, and ameliorates chronic colitis in the TCRαKO model by stimulating mucus production and globlet cell restitution under inflammatory conditions[122]. In addition IL-22, which is released by T and DCs, act together with IL-17 to clear bacterial infections at mucosal sites[123,124]. When conventional DCs are depleted in a CD11c DTR transgenic animal system by diphtheria toxin applications, the severity of colitis is suppressed in the dextran sodium sulfate (DSS) colitis model[125]. Furthermore, in mice with an iEC specific deletion of IKKβ failed to clear Trichuris muris infection characterized by severe intestinal pathology[126]. In these mice an increased accumulation of DCs at mucosal sites was observed that produce IL-12/23 p40 and TNF-α. In addition, an accumulation of IFN-γ producing TH1 and IL-17 producing TH17 cells in the MLN was found. Specific depletion of NEMO (IKKγ) or of both IKKα and IKKβ is essential for the activation of NF-κB activation induce IBD[127]. Constitutive NF-κB activation in IECs by commensal flora may condition DCs to prevent tissue inflammation. Thymic stromal lymphopoietin (TSLP) produced by epithelial cells is involved in the conditioning of DCs to prime less harmful TH2 and Treg responses[126,128]. Together, these data suggest that DCs are conditioned by iECs to promote immunosuppressive T cell responses. However, DCs and their precursors are sensitive to proinflammatory activation signals, which could help to participate into the long persistence of local T cell activation patterns promoting IBD.
CONCLUSION
Mucosal DCs may have several functions in the mucosal immune system to accomplish tolerance and to maintain homeostasis. Tolerance to intestinal self antigens, oral antigens and the commensal flora is achieved by interactions of DCs with regulatory and effector T cells. DCs are also involved in triggering deleterious T cell responses to the endogenous microflora being the basis of IBD. Mucosal DCs express the integrin alpha E (CD103) or the receptor for fractalkine/CX3CR1. CX3CR1 expressing DCs are involved in luminal antigen recognition pathways, whereas CD103 DCs metabolize vitamin A to retinoic acid and are involved in the conversion of T cells to regulatory T cells. Genetically engineered mouse models, and cellular approaches will be increasingly available to study the biology of CD103 and CX3CR1 DCs in immune responses to the commensal flora, and their role in initiating and regulating intestinal inflammation. DCs maintain intestinal homeostasis allowing the peaceful coexistence with the endogenous microflora. The discovery of specific DCs associated with luminal antigen acquisition and oral tolerance will allow developing strategies for targeting defined antigen acquisition routes to design therapeutic treatments for patients with IBD.
Footnotes
Supported by The Deutsche Forschungsgemeinschaft, No. Ni 575/4-1
Peer reviewer: Bret Lashner, MD, Professor of Medicine, Department of Gastroenterology, Cleveland Clinic/A30, 9500 Euclid Avenue, Cleveland, OH 44195, United States
S- Editor Zhong XY E- Editor Zhang WB
References
- 1.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Strober W. Immunology. Unraveling gut inflammation. Science. 2006;313:1052–1054. doi: 10.1126/science.1131997. [DOI] [PubMed] [Google Scholar]
- 3.Nagler-Anderson C. Man the barrier! Strategic defences in the intestinal mucosa. Nat Rev Immunol. 2001;1:59–67. doi: 10.1038/35095573. [DOI] [PubMed] [Google Scholar]
- 4.Niess JH, Reinecker HC. Dendritic cells in the recognition of intestinal microbiota. Cell Microbiol. 2006;8:558–564. doi: 10.1111/j.1462-5822.2006.00694.x. [DOI] [PubMed] [Google Scholar]
- 5.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 7.Vremec D, Shortman K. Dendritic cell subtypes in mouse lymphoid organs: cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J Immunol. 1997;159:565–573. [PubMed] [Google Scholar]
- 8.Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O'Keeffe M. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J Immunol. 2001;166:5448–5455. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 9.Kelsall BL, Rescigno M. Mucosal dendritic cells in immunity and inflammation. Nat Immunol. 2004;5:1091–1095. doi: 10.1038/ni1104-1091. [DOI] [PubMed] [Google Scholar]
- 10.Bjorck P. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood. 2001;98:3520–3526. doi: 10.1182/blood.v98.13.3520. [DOI] [PubMed] [Google Scholar]
- 11.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 12.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 13.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, Agace WW. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, Agace WW, Parker CM, Powrie F. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jakubzick C, Tacke F, Ginhoux F, Wagers AJ, van Rooijen N, Mack M, Merad M, Randolph GJ. Blood monocyte subsets differentially give rise to CD103+ and CD103- pulmonary dendritic cell populations. J Immunol. 2008;180:3019–3027. doi: 10.4049/jimmunol.180.5.3019. [DOI] [PubMed] [Google Scholar]
- 16.Stenstad H, Ericsson A, Johansson-Lindbom B, Svensson M, Marsal J, Mack M, Picarella D, Soler D, Marquez G, Briskin M, et al. Gut-associated lymphoid tissue-primed CD4+ T cells display CCR9-dependent and -independent homing to the small intestine. Blood. 2006;107:3447–3454. doi: 10.1182/blood-2005-07-2860. [DOI] [PubMed] [Google Scholar]
- 17.Takenaka S, Safroneeva E, Xing Z, Gauldie J. Dendritic cells derived from murine colonic mucosa have unique functional and phenotypic characteristics. J Immunol. 2007;178:7984–7993. doi: 10.4049/jimmunol.178.12.7984. [DOI] [PubMed] [Google Scholar]
- 18.Hart AL, Al-Hassi HO, Rigby RJ, Bell SJ, Emmanuel AV, Knight SC, Kamm MA, Stagg AJ. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology. 2005;129:50–65. doi: 10.1053/j.gastro.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 19.te Velde AA, van Kooyk Y, Braat H, Hommes DW, Dellemijn TA, Slors JF, van Deventer SJ, Vyth-Dreese FA. Increased expression of DC-SIGN+IL-12+IL-18+ and CD83+IL-12-IL-18- dendritic cell populations in the colonic mucosa of patients with Crohn's disease. Eur J Immunol. 2003;33:143–151. doi: 10.1002/immu.200390017. [DOI] [PubMed] [Google Scholar]
- 20.Kelsall BL. Innate and adaptive mechanisms to control of pathological intestinal inflammation. J Pathol. 2008;214:242–259. doi: 10.1002/path.2286. [DOI] [PubMed] [Google Scholar]
- 21.Neutra MR, Mantis NJ, Kraehenbuhl JP. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat Immunol. 2001;2:1004–1009. doi: 10.1038/ni1101-1004. [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki A, Kelsall BL. Unique functions of CD11b+, CD8 alpha+, and double-negative Peyer's patch dendritic cells. J Immunol. 2001;166:4884–4890. doi: 10.4049/jimmunol.166.8.4884. [DOI] [PubMed] [Google Scholar]
- 23.Salazar-Gonzalez RM, Niess JH, Zammit DJ, Ravindran R, Srinivasan A, Maxwell JR, Stoklasek T, Yadav R, Williams IR, Gu X, et al. CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer's patches. Immunity. 2006;24:623–632. doi: 10.1016/j.immuni.2006.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwasaki A, Kelsall BL. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J Exp Med. 2000;191:1381–1394. doi: 10.1084/jem.191.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao X, Sato A, Dela Cruz CS, Linehan M, Luegering A, Kucharzik T, Shirakawa AK, Marquez G, Farber JM, Williams I, et al. CCL9 is secreted by the follicle-associated epithelium and recruits dome region Peyer's patch CD11b+ dendritic cells. J Immunol. 2003;171:2797–2803. doi: 10.4049/jimmunol.171.6.2797. [DOI] [PubMed] [Google Scholar]
- 26.Cook DN, Prosser DM, Forster R, Zhang J, Kuklin NA, Abbondanzo SJ, Niu XD, Chen SC, Manfra DJ, Wiekowski MT, et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 2000;12:495–503. doi: 10.1016/s1074-7613(00)80201-0. [DOI] [PubMed] [Google Scholar]
- 27.Iwasaki A, Kelsall BL. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med. 1999;190:229–239. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 29.Fleeton MN, Contractor N, Leon F, Wetzel JD, Dermody TS, Kelsall BL. Peyer's patch dendritic cells process viral antigen from apoptotic epithelial cells in the intestine of reovirus-infected mice. J Exp Med. 2004;200:235–245. doi: 10.1084/jem.20041132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blum KS, Pabst R. Keystones in lymph node development. J Anat. 2006;209:585–595. doi: 10.1111/j.1469-7580.2006.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanamori Y, Ishimaru K, Nanno M, Maki K, Ikuta K, Nariuchi H, Ishikawa H. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J Exp Med. 1996;184:1449–1459. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorenz RG, Chaplin DD, McDonald KG, McDonough JS, Newberry RD. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin beta receptor, and TNF receptor I function. J Immunol. 2003;170:5475–5482. doi: 10.4049/jimmunol.170.11.5475. [DOI] [PubMed] [Google Scholar]
- 33.Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, Hachimura S, Kaminogawa S, Takahashi-Iwanaga H, Iwanaga T, Kiyono H, et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol. 2002;168:57–64. doi: 10.4049/jimmunol.168.1.57. [DOI] [PubMed] [Google Scholar]
- 34.Pabst O, Herbrand H, Worbs T, Friedrichsen M, Yan S, Hoffmann MW, Korner H, Bernhardt G, Pabst R, Forster R. Cryptopatches and isolated lymphoid follicles: dynamic lymphoid tissues dispensable for the generation of intraepithelial lymphocytes. Eur J Immunol. 2005;35:98–107. doi: 10.1002/eji.200425432. [DOI] [PubMed] [Google Scholar]
- 35.McKenna DJ, Guan XM, Shulgin AT. 3,4-Methylenedio-xyamphetamine (MDA) analogues exhibit differential effects on synaptosomal release of 3H-dopamine and 3H-5-hydroxytryptamine. Pharmacol Biochem Behav. 1991;38:505–512. doi: 10.1016/0091-3057(91)90005-m. [DOI] [PubMed] [Google Scholar]
- 36.Leithauser F, Trobonjaca Z, Moller P, Reimann J. Clustering of colonic lamina propria CD4(+) T cells to subepithelial dendritic cell aggregates precedes the development of colitis in a murine adoptive transfer model. Lab Invest. 2001;81:1339–1349. doi: 10.1038/labinvest.3780348. [DOI] [PubMed] [Google Scholar]
- 37.Leithauser F, Meinhardt-Krajina T, Fink K, Wotschke B, Moller P, Reimann J. Foxp3-expressing CD103+ regulatory T cells accumulate in dendritic cell aggregates of the colonic mucosa in murine transfer colitis. Am J Pathol. 2006;168:1898–1909. doi: 10.2353/ajpath.2006.050228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 39.Jang MH, Sougawa N, Tanaka T, Hirata T, Hiroi T, Tohya K, Guo Z, Umemoto E, Ebisuno Y, Yang BG, et al. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J Immunol. 2006;176:803–810. doi: 10.4049/jimmunol.176.2.803. [DOI] [PubMed] [Google Scholar]
- 40.Sallusto F, Palermo B, Lenig D, Miettinen M, Matikainen S, Julkunen I, Forster R, Burgstahler R, Lipp M, Lanzavecchia A. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur J Immunol. 1999;29:1617–1625. doi: 10.1002/(SICI)1521-4141(199905)29:05<1617::AID-IMMU1617>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 41.Soderberg KA, Linehan MM, Ruddle NH, Iwasaki A. MAdCAM-1 expressing sacral lymph node in the lymphotoxin beta-deficient mouse provides a site for immune generation following vaginal herpes simplex virus-2 infection. J Immunol. 2004;173:1908–1913. doi: 10.4049/jimmunol.173.3.1908. [DOI] [PubMed] [Google Scholar]
- 42.Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, MacPherson GG. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 44.Yrlid U, Cerovic V, Milling S, Jenkins CD, Klavinskis LS, MacPherson GG. A distinct subset of intestinal dendritic cells responds selectively to oral TLR7/8 stimulation. Eur J Immunol. 2006;36:2639–2648. doi: 10.1002/eji.200636426. [DOI] [PubMed] [Google Scholar]
- 45.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 46.Niess JH, Reinecker HC. Dendritic cells: the commanders-in-chief of mucosal immune defenses. Curr Opin Gastroenterol. 2006;22:354–360. doi: 10.1097/01.mog.0000231807.03149.54. [DOI] [PubMed] [Google Scholar]
- 47.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 48.Corthesy B. Roundtrip ticket for secretory IgA: role in mucosal homeostasis? J Immunol. 2007;178:27–32. doi: 10.4049/jimmunol.178.1.27. [DOI] [PubMed] [Google Scholar]
- 49.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jang MH, Kweon MN, Iwatani K, Yamamoto M, Terahara K, Sasakawa C, Suzuki T, Nochi T, Yokota Y, Rennert PD, et al. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc Natl Acad Sci USA. 2004;101:6110–6115. doi: 10.1073/pnas.0400969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 52.Hornef MW, Frisan T, Vandewalle A, Normark S, Richter-Dahlfors A. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med. 2002;195:559–570. doi: 10.1084/jem.20011788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hershberg RM. The epithelial cell cytoskeleton and intracellular trafficking. V. Polarized compartmentalization of antigen processing and Toll-like receptor signaling in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G833–G839. doi: 10.1152/ajpgi.00208.2002. [DOI] [PubMed] [Google Scholar]
- 54.Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S, et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006;8:1327–1336. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- 55.Girardin SE, Tournebize R, Mavris M, Page AL, Li X, Stark GR, Bertin J, DiStefano PS, Yaniv M, Sansonetti PJ, et al. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2001;2:736–742. doi: 10.1093/embo-reports/kve155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barnich N, Aguirre JE, Reinecker HC, Xavier R, Podolsky DK. Membrane recruitment of NOD2 in intestinal epithelial cells is essential for nuclear factor-{kappa}B activation in muramyl dipeptide recognition. J Cell Biol. 2005;170:21–26. doi: 10.1083/jcb.200502153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hisamatsu T, Suzuki M, Reinecker HC, Nadeau WJ, McCormick BA, Podolsky DK. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124:993–1000. doi: 10.1053/gast.2003.50153. [DOI] [PubMed] [Google Scholar]
- 58.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 59.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 60.Yoshida M, Kobayashi K, Kuo TT, Bry L, Glickman JN, Claypool SM, Kaser A, Nagaishi T, Higgins DE, Mizoguchi E, et al. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Invest. 2006;116:2142–2151. doi: 10.1172/JCI27821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, Lencer WI, Blumberg RS. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–783. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 62.Buning J, Hundorfean G, Schmitz M, Zimmer KP, Strobel S, Gebert A, Ludwig D. Antigen targeting to MHC class II-enriched late endosomes in colonic epithelial cells: trafficking of luminal antigens studied in vivo in Crohn's colitis patients. FASEB J. 2006;20:359–361. doi: 10.1096/fj.05-4807fje. [DOI] [PubMed] [Google Scholar]
- 63.Hapfelmeier S, Muller AJ, Stecher B, Kaiser P, Barthel M, Endt K, Eberhard M, Robbiani R, Jacobi CA, Heikenwalder M, et al. Microbe sampling by mucosal dendritic cells is a discrete, MyD88-independent step in DeltainvG S. Typhimurium colitis. J Exp Med. 2008;205:437–450. doi: 10.1084/jem.20070633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nielsen HE. Reactivity of lymphocytes from germfree rats in mixed leukocyte culture and in graft-versus-host reaction. J Exp Med. 1972;136:417–425. doi: 10.1084/jem.136.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Butterton JR, Ryan ET, Shahin RA, Calderwood SB. Development of a germfree mouse model of Vibrio cholerae infection. Infect Immun. 1996;64:4373–4377. doi: 10.1128/iai.64.10.4373-4377.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yrios JW, Balish E. Immune response of athymic and euthymic germfree mice to Campylobacter spp. Infect Immun. 1986;54:339–346. doi: 10.1128/iai.54.2.339-346.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Helgeland L, Vaage JT, Rolstad B, Midtvedt T, Brandtzaeg P. Microbial colonization influences composition and T-cell receptor V beta repertoire of intraepithelial lymphocytes in rat intestine. Immunology. 1996;89:494–501. doi: 10.1046/j.1365-2567.1996.d01-783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamanaka T, Helgeland L, Farstad IN, Fukushima H, Midtvedt T, Brandtzaeg P. Microbial colonization drives lymphocyte accumulation and differentiation in the follicle-associated epithelium of Peyer's patches. J Immunol. 2003;170:816–822. doi: 10.4049/jimmunol.170.2.816. [DOI] [PubMed] [Google Scholar]
- 69.Helgeland L, Dissen E, Dai KZ, Midtvedt T, Brandtzaeg P, Vaage JT. Microbial colonization induces oligoclonal expansions of intraepithelial CD8 T cells in the gut. Eur J Immunol. 2004;34:3389–3400. doi: 10.1002/eji.200425122. [DOI] [PubMed] [Google Scholar]
- 70.Niess JH, Leithauser F, Adler G, Reimann J. Commensal gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J Immunol. 2008;180:559–568. doi: 10.4049/jimmunol.180.1.559. [DOI] [PubMed] [Google Scholar]
- 71.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 72.Wang Q, McLoughlin RM, Cobb BA, Charrel-Dennis M, Zaleski KJ, Golenbock D, Tzianabos AO, Kasper DL. A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J Exp Med. 2006;203:2853–2863. doi: 10.1084/jem.20062008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tezuka H, Abe Y, Iwata M, Takeuchi H, Ishikawa H, Matsushita M, Shiohara T, Akira S, Ohteki T. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–933. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- 74.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 75.Wick MJ, Leithauser F, Reimann J. The hepatic immune system. Crit Rev Immunol. 2002;22:47–103. [PubMed] [Google Scholar]
- 76.Rubinstein D, Roska AK, Lipsky PE. Antigen presentation by liver sinusoidal lining cells after antigen exposure in vivo. J Immunol. 1987;138:1377–1382. [PubMed] [Google Scholar]
- 77.Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, Modlin RL, Liblau RS, Gressner AM, Kaufmann SH. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 78.Jomantaite I, Dikopoulos N, Kroger A, Leithauser F, Hauser H, Schirmbeck R, Reimann J. Hepatic dendritic cell subsets in the mouse. Eur J Immunol. 2004;34:355–365. doi: 10.1002/eji.200324336. [DOI] [PubMed] [Google Scholar]
- 79.Knolle PA, Uhrig A, Hegenbarth S, Loser E, Schmitt E, Gerken G, Lohse AW. IL-10 down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells through decreased antigen uptake via the mannose receptor and lowered surface expression of accessory molecules. Clin Exp Immunol. 1998;114:427–433. doi: 10.1046/j.1365-2249.1998.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Knolle PA, Germann T, Treichel U, Uhrig A, Schmitt E, Hegenbarth S, Lohse AW, Gerken G. Endotoxin down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells. J Immunol. 1999;162:1401–1407. [PubMed] [Google Scholar]
- 81.Roland CR, Mangino MJ, Duffy BF, Flye MW. Lymphocyte suppression by Kupffer cells prevents portal venous tolerance induction: a study of macrophage function after intravenous gadolinium. Transplantation. 1993;55:1151–1158. doi: 10.1097/00007890-199305000-00041. [DOI] [PubMed] [Google Scholar]
- 82.Macpherson AJ, Smith K. Mesenteric lymph nodes at the center of immune anatomy. J Exp Med. 2006;203:497–500. doi: 10.1084/jem.20060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kraus TA, Toy L, Chan L, Childs J, Mayer L. Failure to induce oral tolerance to a soluble protein in patients with inflammatory bowel disease. Gastroenterology. 2004;126:1771–1778. doi: 10.1053/j.gastro.2004.03.076. [DOI] [PubMed] [Google Scholar]
- 84.Viney JL, Mowat AM, O'Malley JM, Williamson E, Fanger NA. Expanding dendritic cells in vivo enhances the induction of oral tolerance. J Immunol. 1998;160:5815–5825. [PubMed] [Google Scholar]
- 85.Nagatani K, Sagawa K, Komagata Y, Yamamoto K. Peyer's patch dendritic cells capturing oral antigen interact with antigen-specific T cells and induce gut-homing CD4(+)CD25(+) regulatory T cells in Peyer's patches. Ann N Y Acad Sci. 2004;1029:366–370. doi: 10.1196/annals.1309.020. [DOI] [PubMed] [Google Scholar]
- 86.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 88.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 89.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 90.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 91.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 93.Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kraus TA, Brimnes J, Muong C, Liu JH, Moran TM, Tappenden KA, Boros P, Mayer L. Induction of mucosal tolerance in Peyer's patch-deficient, ligated small bowel loops. J Clin Invest. 2005;115:2234–2243. doi: 10.1172/JCI19102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Forster R, Pabst O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee JW, Epardaud M, Sun J, Becker JE, Cheng AC, Yonekura AR, Heath JK, Turley SJ. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007;8:181–190. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- 97.Chen CC, Louie S, McCormick BA, Walker WA, Shi HN. Helminth-primed dendritic cells alter the host response to enteric bacterial infection. J Immunol. 2006;176:472–483. doi: 10.4049/jimmunol.176.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.von Boehmer H. Oral tolerance: is it all retinoic acid? J Exp Med. 2007;204:1737–1739. doi: 10.1084/jem.20071251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li Y, Hashimoto Y, Agadir A, Kagechika H, Zhang X. Identification of a novel class of retinoic acid receptor beta-selective retinoid antagonists and their inhibitory effects on AP-1 activity and retinoic acid-induced apoptosis in human breast cancer cells. J Biol Chem. 1999;274:15360–15366. doi: 10.1074/jbc.274.22.15360. [DOI] [PubMed] [Google Scholar]
- 101.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 102.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 103.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de Baey A, Mende I, Riethmueller G, Baeuerle PA. Phenotype and function of human dendritic cells derived from M-DC8(+) monocytes. Eur J Immunol. 2001;31:1646–1655. doi: 10.1002/1521-4141(200106)31:6<1646::aid-immu1646>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 106.de Baey A, Mende I, Baretton G, Greiner A, Hartl WH, Baeuerle PA, Diepolder HM. A subset of human dendritic cells in the T cell area of mucosa-associated lymphoid tissue with a high potential to produce TNF-alpha. J Immunol. 2003;170:5089–5094. doi: 10.4049/jimmunol.170.10.5089. [DOI] [PubMed] [Google Scholar]
- 107.Ikeda Y, Akbar F, Matsui H, Onji M. Characterization of antigen-presenting dendritic cells in the peripheral blood and colonic mucosa of patients with ulcerative colitis. Eur J Gastroenterol Hepatol. 2001;13:841–850. doi: 10.1097/00042737-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 108.Murakami H, Akbar SM, Matsui H, Horiike N, Onji M. Macrophage migration inhibitory factor activates antigen-presenting dendritic cells and induces inflammatory cytokines in ulcerative colitis. Clin Exp Immunol. 2002;128:504–510. doi: 10.1046/j.1365-2249.2002.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yeung MM, Melgar S, Baranov V, Oberg A, Danielsson A, Hammarstrom S, Hammarstrom ML. Characterisation of mucosal lymphoid aggregates in ulcerative colitis: immune cell phenotype and TcR-gammadelta expression. Gut. 2000;47:215–227. doi: 10.1136/gut.47.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Krajina T, Leithauser F, Moller P, Trobonjaca Z, Reimann J. Colonic lamina propria dendritic cells in mice with CD4+ T cell-induced colitis. Eur J Immunol. 2003;33:1073–1083. doi: 10.1002/eji.200323518. [DOI] [PubMed] [Google Scholar]
- 111.Becker C, Wirtz S, Blessing M, Pirhonen J, Strand D, Bechthold O, Frick J, Galle PR, Autenrieth I, Neurath MF. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J Clin Invest. 2003;112:693–706. doi: 10.1172/JCI17464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, Cua DJ, Powrie F, Cheever AW, Maloy KJ, et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mizoguchi A, Ogawa A, Takedatsu H, Sugimoto K, Shimomura Y, Shirane K, Nagahama K, Nagaishi T, Mizoguchi E, Blumberg RS, et al. Dependence of intestinal granuloma formation on unique myeloid DC-like cells. J Clin Invest. 2007;117:605–615. doi: 10.1172/JCI30150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Glas J, Seiderer J, Wetzke M, Konrad A, Torok HP, Schmechel S, Tonenchi L, Grassl C, Dambacher J, Pfennig S, et al. rs1004819 is the main disease-associated IL23R variant in German Crohn's disease patients: combined analysis of IL23R, CARD15, and OCTN1/2 variants. PLoS ONE. 2007;2:e819. doi: 10.1371/journal.pone.0000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 118.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 119.Izcue A, Hue S, Buonocore S, Arancibia-Carcamo CV, Ahern PP, Iwakura Y, Maloy KJ, Powrie F. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–570. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, Muller FJ, Hommes DW, Zaat SA, Kapsenberg ML, de Jong EC. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 122.Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 124.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Abe K, Nguyen KP, Fine SD, Mo JH, Shen C, Shenouda S, Corr M, Jung S, Lee J, Eckmann L, et al. Conventional dendritic cells regulate the outcome of colonic inflammation independently of T cells. Proc Natl Acad Sci USA. 2007;104:17022–17027. doi: 10.1073/pnas.0708469104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, Yost EA, Gruber AD, May MJ, Greten FR, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 127.Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 128.Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, Nespoli A, Viale G, Allavena P, Rescigno M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]