Abstract
AIM: To examine the ability of cyclin-dependent kinase inhibitor (CDKI) roscovitine (Rosco) to enhance the antitumor effects of conventional chemotherapeutic agents acting by different mechanisms against human colorectal cancer.
METHODS: Human colorectal cancer cells were treated, individually and in combination, with Rosco, taxol, 5-Fluorouracil (5-FU), doxorubicin or vinblastine. The antiproliferative effects and the type of interaction of Rosco with tested chemotherapeutic drugs were determined. Cell cycle alterations were investigated by fluorescence-activated cell sorter FACS analysis. Apoptosis was determined by DNA fragmentation assay.
RESULTS: Rosco inhibited the proliferation of tumor cells in a time- and dose-dependent manner. The efficacies of all tested chemotherapeutic drugs were markedly enhanced 3.0-8.42 × 103 and 130-5.28 × 103 fold in combination with 5 and 10 μg/mL Rosco, respectively. The combination of Rosco and chemotherapeutic drugs inhibited the growth of human colorectal cancer cells in an additive or synergistic fashion, and in a time and dose dependent manner. Rosco induced apoptosis and synergized with tested chemotherapeutic drugs to induce efficient apoptosis in human colorectal cancer cells. Sequential, inverted sequential and simultaneous treatment of cancer cells with combinations of chemotherapeutic drugs and Rosco arrested the growth of human colorectal cancer cells at various phases of the cell cycle as follows: Taxol/Rosco (G2/M- and S-phases), 5-FU/Rosco (S-phase), Dox/Rosco (S-phase) and Vinb/Rosco (G2/M- and S-phases).
CONCLUSION: Since the efficacy of many anticancer drugs depends on their ability to induce apoptotic cell death, modulation of this parameter by cell cycle inhibitors may provide a novel chemo-preventive and chemotherapeutic strategy for human colorectal cancer.
Keywords: Human colorectal cancer cell lines, Cyclin dependent kinase inhibition, Chemosensitization, Synergy, Apoptosis, Cell cycle
INTRODUCTION
Colon cancer is the second leading cause of cancer death in the United States and is one of the most common cancers in Western countries[1]. Lack of improvement in overall survival and failure of the current systemic therapies have mandated that new approaches to this disease be explored.
A hallmark of neoplastic evolution and progression is deregulation of cell cycle control mechanisms. The key regulators of transition from one cell cycle phase to the next are the cyclin-dependent kinases (CDKs). CDKs are serine/threonine kinases that regulate cell cycle progression in a highly coordinated manner[2]. A CDK enzyme complex becomes fully active after binding of its proper cyclin. Progression through cell cycle is mediated by the orchestrated activation and breakdown of CDK complexes[2].
A basis for selectivity of CDK-directed therapies against neoplastic cells might arise from the fact that alteration of CDK structure and function plays a key role in the pathogenesis of neoplasia[3]. At least one of the following changes is almost ubiquitously evident in human neoplasms: overexpression of cyclin D; amplification or structural alteration of CDK4; deletion or mutation of p16INK4A; mutation of the CDK4 or 6/cyclin D substrate pRb; and loss of p21WAF1/CIP1A function through deletion or mutation of its transactivator P53. In relation to colon cancer, p27KIP1 loss has been found to occur not by gene deletion or mutation, but by increased proteolysis of the CDK inhibitor (CDKI)[4]. Therefore, replacement of at least some of the missing capacity to inhibit cell cycle progression may restore some measure of cell cycle control. In contrast to their normal counterparts, transformed cells proliferate very rapidly due to the enhanced activity of the CDK[2]. Thus, inhibition of CDK/cyclin complexes offers a promising therapeutic strategy in the defense against cancer[5].
Many types of potential CDK modulators are conceivable. These include molecules that directly inhibit ATP or protein substrate binding; alter regulatory phosphorylations of the catalytic subunit; inhibit CDK catalytic subunit binding with its respective cyclin or other accessory proteins; mimic the action or increase the expression of endogenous CDK inhibitors, p16INK4A (or its homologues, p15INK4B, p18INK4C, and p19INK4D), p21WAF1, and p27KIP1; interfere with the proper appearance and disappearance of cyclins; and finally alter normal signals for import of CDKs into the nucleus or localization to appropriate subcellular structures[6].
Pharmacological inhibitors of CDKs display selective anti-proliferative effects on cycling cells, especially malignant ones[7]. Depending on the selectivity profile of these novel drugs, growth inhibition in different phases of the cell cycle is observed[8]. Compounds targeting the activity of CDK4/6 block cells in early G1, whereas selective inhibitors of CDK1/2 arrest cell cycle in G1/S and G2/M[8]. Interestingly, some inhibitors, especially those targeting the activity of CDK2, are able to selectively induce apoptosis in cancer cells[9,10].
CDKIs, representing a well-defined group of biologically active compounds, are structurally related to adenosine-5-triphosphate, ATP[7,8,11]. They antagonize binding of kinases to ATP. Differentially substituted adenines yielded a group of inhibitors such as roscovitine (Rosco), olomoucine and purvalanol[7]. These close analogs, characterized by increasing potency, differ in selectivity. Due to their selectivity and relative low direct cytotoxicity, CDKIs clearly provide useful anticancer drugs and offer an alternative to classic chemotherapeutics. In the present study, we have investigated whether Rosco could inhibit the growth of human colorectal cancer cells and increase their sensitivity to conventional chemotherapies.
MATERIALS AND METHODS
Cell culture and reagents
Human colorectal cancer cell lines (SW48, SW1116 and SW 837) were obtained from the American Type Culture Collection (ATCC, Rockville, Md., USA). Cells were cultured in Leibovitz’s L-15 medium supplemented with 10% inactivated fetal bovine serum and 2 mmol/L glutamine. The L-15 medium formulation was devised for use in a free gas exchange with atmospheric air. CO2 is detrimental to cells when using this medium for cultivation. All the other chemicals were purchased from Sigma Chemical Co.
Time and dose dependency of the antiproliferative effects induced in human colorectal cancer cells by treatment with Rosco
Human colorectal cancer cell lines (SW48, SW1116 and SW837) were plated (27 × 103 cells/well) into 96-well plates and incubated at 37°C in a non-CO2 incubator. Cells were treated with various concentrations of Rosco (0-40 μg/mL) or DMSO (0.3% final concentration) for various time periods beginning at 24 h after seeding the cells in culture. Control cells were untreated. Cell proliferation was determined at various time intervals (24-168 h) by 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as previously described[12].
In vitro efficacy of single and combined treatment of Rosco and chemotherapeutic drugs on the growth of human colorectal cancer cells
Human colorectal cancer cell lines (SW48, SW1116 and SW837) were treated with taxol (10-11-10-6 mol/L), doxorubicin (10-11-10-6 mol/L), 5-fluorouracil (5-FU) (10-9-10-4 mol/L), vinblastine (10-12-10-7 mol/L) or combinations of the tested chemotherapeutic drug and Rosco (5 and 10 μg/mL) for 96 h. At the end of treatment, control and drug treated cells were scored for proliferation using the MTT assay.
Analysis of the type of interaction between Rosco and chemotherapeutic drugs in human colorectal cancer cells
Human colorectal cancer cell lines (SW48, SW1116 and SW837) were treated with Rosco (1, 10, 15, 20, 25 μg/mL) and chemotherapeutic drugs (0.5 IC50-4 IC50) individually and in combination. The effect of the combinations of Rosco and chemotherapeutic drugs on cell growth was determined as previously described[12,13] using the following formulae: SFA+B > (SFA) × (SFB), antagonistic; SFA+B = (SFA) × (SFB); additive; SFA+B < (SFA) × (SFB), synergistic, where SF is the surviving fraction, and A and B indicate the agents used alone, while A + B refers to the agents used in combination.
Cell cycle analysis
Cell cycle phase distribution of human colorectal cancer cells treated with Rosco, chemotherapeutic drugs and their combinations was determined by flow cytometry as previously described[12]. Human colorectal cancer cells (SW837, 5 × 105 cells/well in 24 well plates) were treated with taxol (12 × 10-8 mol/L, 72 h), doxorubicin (8 × 10-7 mol/L, 72 h), 5-FU (4.8 × 10-5 mol/L, 72 h), vinblastine (2.6 × 10-7 mol/L, 72 h), Rosco (15 μg/mL) and combinations of Rosco and chemotherapeutic drugs. The combinations were added in a sequential manner, drug (24 h) followed by Rosco (48 h); inverted sequential manner, Rosco (24 h) followed by drug (48 h) and simultaneous manner (72 h). The tested cells were collected by trypsinization, and then washed with cold phosphate-buffered saline, and counted using a cell counter. A sample of 3 × 106 cells/mL was processed using DNA-Prep kit (Beckman & Coulter, Fa., USA) and a DNA-Prep Epics workstation (Beckman & Coulter). During this process, the cell sample was treated with a cell membrane-permeabilizing agent and then with propidium and RNase enzyme. The sample was then incubated at room temperature for at least 15 min before analysis by aligned flow cytometry (Epics XL, Beckman & Coulter). The percentage of cells in different cell cycle phases was evaluated using the Phoenix statistical software package, advanced DNA cell cycle software (Phoenix Flow System, San Diego, Calif., USA).
DNA fragmentation analysis
DNA fragmentation assay was performed as previously described[14]. Briefly, colorectal cancer cells (5 × 105 cells/well, SW1116 and SW837) were treated with taxol (1.2 × 10-7 mol/L), doxorubicin (8 × 10-7 mol/L), 5-FU (4.8 × 10-5 mol/L), vinblastine (2.6 × 10-7 mol/L), Rosco (15 μg/mL) and the combinations of Rosco, and tested drugs for 72 h. The cell pellets were lysed with 100 μmol/L of hypotonic buffer (10 mmol/L Tris (pH 8.0), 20 mmol/L EDTA containing 0.5 % Triton X-100) for 30 min at 4°C. Following cell lysis, the intact chromatin (pellet) was separated from DNA fragments (supernatant) by centrifugation for 15 min at 12 000 g. The supernatants containing DNA were precipitated overnight with 0.5 mol/L NaCl and 50% isopropyl alcohol at -20°C. Pellets were recovered by centrifugation at 12 000 g for 10 min, air dried, resuspended in 30 μL of TE buffer supplemented with 1 mg/mL RNase I at 37°C for 30 min, and then with 2 mg/mL of proteinase K for another 1 h. DNA samples were supplemented with 3 μL of sample buffer (0.25% bromophenol blue, 30% glyceric acid), and electrophorectically separated on a 1.5% agrose gel containing 0.1 μg/mL ethidium bromide at 80 V for 2 h. DNA fragments were visualized by ultraviolet transillumination.
Statistical analysis
Results are representative of two to three individual experiments. Errors are expressed as standard errors of the percentage of the means. Where appropriate, data were analyzed using ANOVA.
RESULTS
Inhibition of the proliferation of human colorectal cancer cells by Rosco
In this study, we initially investigated the effect of Rosco on the proliferation of human colorectal cancer cells using three human colorectal cancer cell lines (SW48, SW1116 and SW837). The results, shown in Figure 1, indicated that all cell lines tested were sensitive to micromolar range of Rosco, in a dose and time dependent manner. Rosco inhibited the growth of SW48 after 24-144 h of drug treatment. It affected the growth of the colorectal cancer cell line SW1116 slightly after 48 h of treatment. However, a dramatic inhibition of cell growth was observed after 72-144 h of treatment. Rosco slightly affected the growth of SW837 after 24 h of treatment. However, a marked inhibition was observed after 48-144 h of treatment with Rosco.
Figure 1.
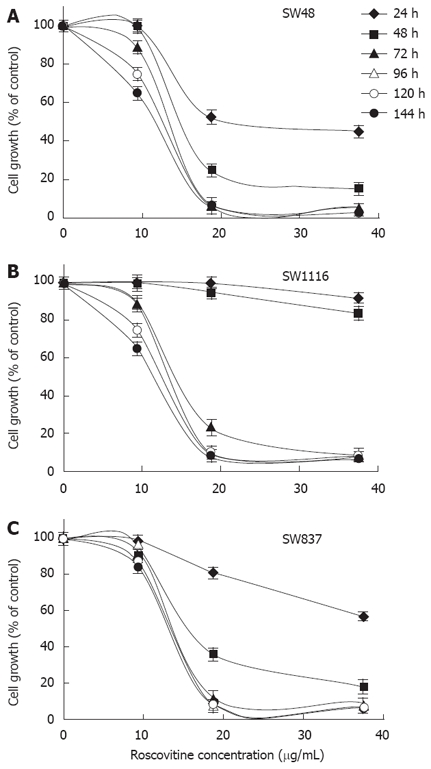
Time and dose dependent effect of roscovitine on the proliferation of human colorectal cancer cell lines. Human colorectal cancer cell lines SW48 (A), SW1116 (B) and SW837 (C) were plated (27 × 103 cells/well) into 96-well plates and incubated at 37°C in a non-CO2 incubator. Twenty four hours after starting the culture, the cells were treated with various concentrations of Rosco (0-40 μg /mL) or DMSO (0.3%, final concentration) for various time periods (24-144 h). Control (0.3% DMSO treated) and Rosco treated colorectal cancer cells were scored for proliferation using an MTT assay. Roscovitine concentrations 5 µg/mL and 10 μg/mL were used in the subsequent studies.
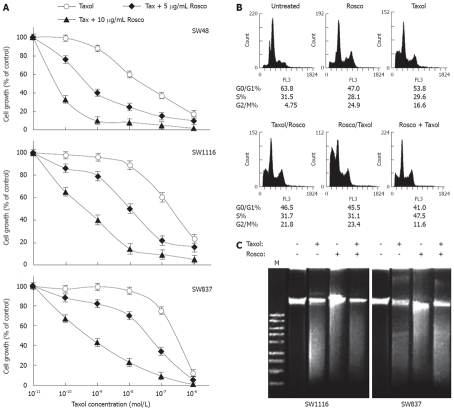
Modulation of taxol cytotoxicity on human colorectal cancer cells by combination with CDKI Rosco
The ability of Rosco to enhance the sensitivity of human colorectal cancer cells to taxol was assessed by treating human colorectal cancer cell lines SW48, SW1116 and SW837 with taxol (10-11-10-6 mol/L) or taxol (10-11-10-6 mol/L) and Rosco (5 or 10 μg/mL) for 96 h. The results summarized in Figure 2A and Table 1 clearly indicated that the combination of taxol and 5 μg/mL Rosco enhanced (65-fold) the anticancer activity of taxol on human colorectal cancer cell line SW48 (Table 1). The difference between SW48 growth inhibition produced by treatment with taxol alone [IC50 (taxol) = 4.8 × 10-8 mol/L] and that produced by treatment with the combination of taxol and 5 μg/mL Rosco [IC50 (taxol + 5 μg/mL Rosco) = 7.4 × 10-10 mol/L] (Table 1) was statistically non-significant (P = 0.127). The combination of taxol and 10 μg/mL Rosco greatly increased the sensitivity of human colorectal cancer cells to taxol (640-fold) compared to treatment with taxol alone (Table 1). The difference in SW48 growth inhibition produced by treatment with combination of taxol and 10 μg/mL Rosco (IC50 = 7.5 × 10-11 mol/L) and that produced by treatment with taxol alone (IC50 = 4.8 × 10-8 mol/L) (Table 1) was significant (P = 0.012).
Figure 2.
Potentiation of anticancer effect of taxol on human colorectal cancer cell lines by combination with cyclin dependent kinase inhibitor roscovitine. A: Human colorectal cancer cell lines were treated with taxol (10-11-10-6 mol/L), or the combination of taxol (10-11-10-6 mol/L) and Rosco (5 μg/mL or 10 μg/mL) for 96 h. At the end of treatment, control and drug treated cells were scored for proliferation using the MTT assay; B: Cell cycle phase distribution of human colorectal cancer cells (SW837, 5 × 105 cells/well) treated with taxol (12 × 10-8 mol/L, 72 h); Rosco (15 μg/mL, 72 h); sequential combination: taxol (12 × 10-8 mol/L, 24 h) followed by Rosco (15 μg/mL, 48 h); inverted sequential combination: Rosco (15 μg/mL, 24 h) followed by taxol (12 × 10-8 mol/L, 48 h) and simultaneous combination: taxol plus Rosco (12 × 10-8 mol/L, 15 μg/mL, 72 h) was evaluated by flow cytometry. The percentage of cells in different phases of the cycle was calculated by cell cycle analysis software, Multicycle (Phoenix Flow System, San Diego CA, USA); C: Human colorectal cancer cells, SW1116 and SW837 (5 × 105 cells/well), were treated with taxol (12 × 10-8 mol/L), Rosco (15 μg/mL), and the combination of taxol plus Rosco (12 × 10-8 mol/L + 15 μg/mL) for 72 h. DNA fragments were extracted and analyzed on 1% agrose gel.
Table 1.
IC50 and sensitization ratio of taxol and its combinations with Rosco towards human colorectal cancer cell lines
| Treatment with taxol and combinations with Rosco |
IC50 (mol/L) |
Sensitization ratio1 |
||||
| SW48 | SW1116 | SW837 | SW48 | SW1116 | SW837 | |
| Taxol | 4.8 × 10-8 | 1.2 × 10-7 | 3.8 × 10-7 | - | - | - |
| Taxol + 5 μg/mL Rosco | 7.4 × 10-10 | 1.0 × 10-8 | 5.4 × 10-8 | 65 | 12 | 7 |
| Taxol + 10 μg/mL Rosco | 7.5 × 10-11 | 8.1 × 10-10 | 6.7 × 10-10 | 6.4 × 102 | 1.5 × 102 | 5.7 × 102 |
Sensitization ratio = IC50 (taxol)/IC50 (taxol + Rosco).
The combination of taxol and 5 μg/mL Rosco had a higher growth inhibitory effect on SW1116 (12-fold) compared to treatment with taxol alone (Table 1). The difference in SW1116 growth inhibition produced by treatment with the combination of taxol and 5 μg/mL Rosco (IC50 = 1 × 10-8 mol/L), and that produced by treatment with taxol alone (1.2 × 10-7 mol/L) was non-significant (P = 0.256). On the other hand, the combination of taxol and 10 μg/mL Rosco markedly increased SW1116 growth inhibition (150-fold) compared to treatment with taxol alone (Table 1). The difference in SW1116 growth inhibition produced by the combination of taxol and 10 μg/mL Rosco (IC50 = 8.1 × 10-10 mol/L) and that produced by taxol alone (1.2 × 10-7 mol/L) was statistically significant (P = 0.03).
Treatment of SW837 cells with various concentrations of taxol inhibited their growth in a dose dependent manner with IC50 = 3.8 × 10-7 mol/L. The combination of taxol and 5 μg/mL Rosco produced higher SW837 growth inhibition (IC50 = 5.4 × 10-8 mol/L, sensitization ratio = 7-fold) than that produced by treatment with taxol alone (Table 1). This difference in SW837 growth inhibition was non-significant (P = 0.365). Treatment of SW837 with the combination of taxol and 10 μg/mL Rosco resulted in a more significant growth inhibition (IC50 = 6.7 × 10-10 mol/L, sensitization ratio = 570 and P = 0.045) compared to that produced by treatment with taxol alone (Figure 2, Table 1). The combinations of taxol and Rosco produced additive and/or synergistic effects depending upon the type of cell line used, relative concentrations of the mixed drugs and exposure time (Table 2).
Table 2.
Analysis of the combined effects of taxol and Rosco on human colorectal cancer cell lines
| Combined treatment of taxol and Rosco |
Combination interaction at various durations of treatment in human colorectal cancer cells1 |
||||||||
| SW48 | SW1116 | SW837 | |||||||
| 2 d | 4 d | 6 d | 2 d | 4 d | 6 d | 2 d | 4 d | 6 d | |
| Taxol 0.5 IC50 + Rosco 1.0 μg/mL | ant | ant | ant | ant | ant | syn | ant | ant | syn |
| Taxol 1.0 IC50 + Rosco 10 μg/mL | ant | ant | add | ant | ant | ant | ant | ant | syn |
| Taxol 2.0 IC50 + Rosco 15 μg/mL | ant | ant | ant | syn | ant | ant | ant | ant | ant |
| Taxol 3.0 IC50 + Rosco 20 μg/mL | ant | ant | ant | ant | ant | ant | ant | ant | ant |
| Taxol 4.0 IC50 + Rosco 25 μg/mL | ant | add | add | syn | ant | add | ant | ant | ant |
The data are based on the mean of absorbance measurements of 3 independent experiments. ant: Antagonistic; add: Additive; syn: Synergistic.
Cell cycle analysis of human colorectal cancer cells treated with taxol, Rosco or their combinations added in sequential (taxol followed by Rosco), inverted sequential or simultaneous manner was determined by flow cytometry as described in Materials and Methods. Treatment of colorectal cancer cells with taxol or Rosco resulted in growth arrest at G2/M phase, 24.9% and 16.6%, respectively, compared to 4.75% for untreated cells (Figure 1B). The combination of taxol and Rosco added in sequential manner growth arrested colorectal cancer cell in G2/M (21.8%). Also, the same combination added in an inverted sequential manner growth arrested colorectal cancer cells in G2/M (23.4%), meanwhile when the same combination was used in a simultaneous manner, colorectal cancer cells were growth arrested in G2/M (11.6%) and S (47.5%) phases, respectively, (Figure 2B). The effect of taxol, Rosco and their combination on inducing programmed cell death in human colorectal cancer cell lines SW1116 and SW837 was studied using DNA fragmentation analysis. The combination of taxol and Rosco induced apoptosis more efficiently compared to single treatment with taxol or Rosco (Figure 2C).
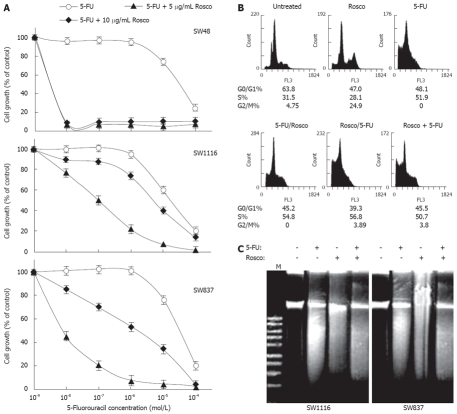
Modulation of 5-FU cytotoxicity on human colorectal cancer cells by combination with the CDKI Rosco
The anti-proliferative activities of 5-FU, Rosco and their combinations against human colorectal cancer cells are summarized in Figure 3. The combination of 5-FU (10-9-10-4 mol/L) and Rosco (5 μg/mL) exerted a very potent anticancer effect (P < 0.0001) on SW48 with IC50 = 5.7 × 10-9 mol/L compared to IC50 = 4.8 × 10-5 mol/L when SW48 cells were treated with 5-FU alone (Figure 3A). These results indicate that Rosco (5 μg/mL) increased the sensitivity of SW48 to 5-FU by about 8.42 × 103-fold (Table 3). The same combination inhibited the growth of colorectal cancer cell lines SW1116 and SW837 with IC50 values equal to 6.7 × 10-6 mol/L and 1.8 × 10-6 mol/L compared to IC50 values equal to 2.5 × 10-5 mol/L and 4.8 × 10-5 mol/L obtained when these cells were treated with Rosco alone, respectively, (Figure 3A, Table 3). Rosco (5 μg/mL) increased the sensitivity of SW1116 and SW837 to 5-FU by about 3.73 and 26.7 fold, respectively. The increase in SW1116 and SW837 growth inhibition after treatment with this combination was found to be statistically non-significant P = 0.519 and P = 0.122, respectively, compared to single treatment with 5-FU.
Figure 3.
Potentiation of 5-FU anticancer effect on human colorectal cancer cell lines by combination with cyclin dependent kinase inhibitor roscovitine. A: Human colorectal cancer cell lines were treated with 5-FU (10-9-10-4 mol/L), and the combination of 5-FU (10-9-10-4 mol/L) and Rosco (5 μg/mL or 10 μg/mL) for 96 h. At the end of treatment, control and drug treated cells were scored for proliferation using the MTT assay; B: Cell cycle phase distribution of human colorectal cancer cells (SW837, 5 × 105 cells/well) treated with 5-FU (4.8 × 10-5 mol/L, 72 h); Rosco (15 μg/mL, 72 h); sequential combination: 5-FU (4.8 × 10-5 mol/L, 24 h) followed by Rosco (15 μg/mL, 48 h); inverted sequential combination: Rosco (15 μg/mL, 24 h) followed by 5-FU (4.8 × 10-5 mol/L, 48 h) and simultaneous combination: 5-FU plus Rosco (4.8 × 10-5 mol/L, 15 μg/mL, 72 h) was evaluated by flow cytometry. The percentage of cells in different phases of the cycle was calculated as described above; C: Human colorectal cancer cells, SW1116 and SW837 (5 × 105 cells/well), were treated with 5-FU (4.8 × 10-5 mol/L), Rosco (15 μg/L), and the combination of 5-FU plus Rosco (4.8 × 10-5 mol/L + 15 μg/mL) for 72 h. DNA fragments were extracted and analyzed on 1% agrose gel.
Table 3.
IC50 and sensitization ratio of 5-FU and its combinations with Rosco towards human colorectal cancer cell lines
| Treatment with 5-FU and combinations with Rosco |
IC50 (mol/L) |
Sensitization ratio1 |
||||
| SW48 | SW1116 | SW837 | SW48 | SW1116 | SW837 | |
| 5-FU | 4.8 × 10-5 | 2.5 × 10-5 | 4.8 × 10-5 | - | - | - |
| 5-FU + 5 μg/mL Rosco | 5.7 × 10-9 | 6.7 × 10-6 | 1.8 × 10-6 | 8.42 × 103 | 3.73 | 26.7 |
| 5-FU + 10 μg/mL Rosco | 5.7 × 10-9 | 1.0 × 10-7 | 9.1 × 10-9 | 8.42 × 103 | 250 | 5.28 × 103 |
Sensitization ratio = IC50 (5-FU)/IC50 (5-FU + Rosco).
The combination of 5-FU (10-9-10-4 mol/L) and Rosco (10 μg/mL) exerted a marked growth inhibition on all the tested colorectal cancer cells SW48 (P < 0.0001), SW1116 (P = 0.05) and SW837 (P = 0.005) with IC50 values equal to 5.7 × 10-9 mol/L, 1.0 × 10-7 mol/L and 9.1 × 10-9 mol/L, respectively, compared to IC50 values of 4.8 × 10-5 mol/L, 2.5 × 10-5 mol/L, and 4.8 × 10-5 mol/L exerted on SW 48, SW1116 and SW837, respectively, when treated with 5-FU alone (Figure 3A, Table 3). The combination of 5-FU (10-9-10-4 mol/L) and Rosco (10 μg/mL) increased the sensitivity of human colorectal cancer cell lines SW48, SW1116 and SW837 by 8.42 × 103, 250 and 5.28 × 103 fold, respectively (Table 3). The combination of 5-FU and Rosco had synergistic effects on SW1116, additive effects on SW837 and antagonistic/additive effects on SW48 (Table 4). The type of interaction between the mixed drugs depends upon their relative concentrations, exposure time and the tested cell line.
Table 4.
Analysis of the combined effects of 5-FU and Rosco on human colorectal cancer cell lines
| Combined treatment of 5-FU and Rosco |
Combination interaction at various durations of treatment in human colorectal cancer cells1 |
||||||||
| SW48 | SW1116 | SW837 | |||||||
| 2 d | 4 d | 6 d | 2 d | 4 d | 6 d | 2 d | 4 d | 6 d | |
| 5-FU 0.5 IC50 + Rosco 1.0 μg/mL | ant | ant | ant | syn | syn | syn | ant | ant | add |
| 5-FU 1.0 IC50 + Rosco 15 μg/mL | ant | ant | ant | syn | syn | syn | ant | syn | add |
| 5-FU 2.0 IC50 + Rosco 20 μg/mL | ant | ant | ant | syn | add | add | add | ant | ant |
| 5-FU 3.0 IC50 + Rosco 25 μg/mL | ant | ant | add | ant | ant | ant | add | ant | ant |
The data are based on the mean of absorbance measurements of 3 independent experiments; ant: Antagonistic; add: Additive; syn: Synergistic.
The effect of this combination on human colorectal caner cell cycle was also evaluated. Treatment with Rosco alone growth inhibited colorectal cancer cells in G2/M phase (24.9% vs 4.75% for untreated), while treatment with 5-FU alone growth arrested cancer cells in S-phase (51.9% vs 31.5% for untreated). However the combination of 5-FU and Rosco added in sequential, inverted sequential and simultaneous manners growth arrested human colorectal cancer cells in S-phase: 54.8%, 56.8% and 50.7%, respectively, compared to 51.5% in the S-phase for untreated (Figure 3B). The combination of 5-FU (4.8 × 10-5 mol/L) and Rosco (15 μg/mL) had synergistic or additive apoptotic effects on SW1116 and SW837 compared to single treatment with 5-FU or Rosco (Figure 3C).
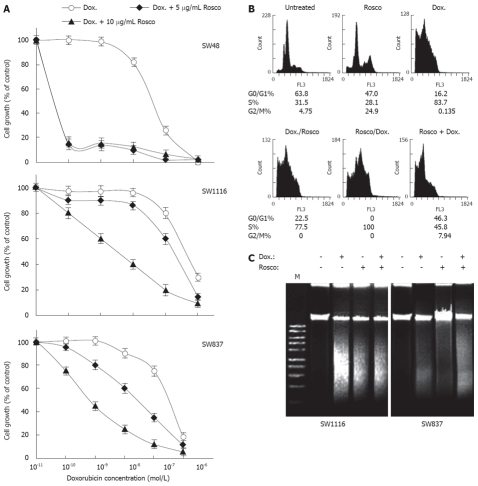
Modulation of doxorubicin cytotoxicity on human colorectal cancer cells by combination with CDKI Rosco
Human colorectal cancer cells were treated with doxorubicin (10-11-10-6 mol/L) or combinations of doxorubicin (10-11-10-6 mol/L) and Rosco (5 or 10 μg/mL) for 96 h. The combination of doxorubicin and Rosco had a very potent anti-proliferative effect on the colorectal cancer cell line SW48 (IC50 = 5.8 × 10-11 mol/L, P = 0.009) compared to the effect of doxorubicin alone (IC50 = 5.4 × 10-8 mol/L) (Figure 4A, Table 5). The combination of doxorubicin and 5 μg/mL Rosco slightly increased the growth inhibition exerted on SW1116 and SW837 with IC50 values equal to 2 × 10-7 mol/L and 4.1 × 10-8 mol/L, respectively, compared to IC50 values equal to 6 × 10-7 mol/L and 4.1 × 10-7 mol/L obtained when SW1116 and SW837 were treated with doxorubicin alone, respectively, (Figure 3A, Table 5). The combination of doxorubicin and 5 μg/mL Rosco increased the sensitivity of SW1116 (3 fold) and SW837 (10 fold) to doxorubicin (Table 5). The difference in growth inhibition produced by treatment of SW1116 (P = 0.543) and SW837 (P = 0.33) with doxorubicin plus 5 μg/mL was statistically non-significant. The combination of doxorubicin and Rosco (10 μg/mL) produced very potent anti-proliferative effects on SW48 (IC50 = 5.8 × 10-11 mol/L, P = 0.012), SW1116 (IC50 = 4.5 × 10-9 mol/L, P = 0.068), and SW837 (IC50 = 8.2 × 10-10 mol/L, P = 0.049) compared to treatment with Rosco alone (Figure 4A, Table 5). The combination of doxorubicin and Rosco exhibited an additive effect on SW48, synergistic and additive effects on SW1116 and synergistic effect on SW837 in a time and dose dependent manner (Table 6).
Figure 4.
Potentiation of doxorubicin anticancer effect on human colorectal cancer cell lines by combination with cyclin dependent kinase inhibitor roscovitine. A: Human colorectal cancer cell lines were treated with doxorubicin (10-11-10-6 mol/L), and the combination of doxorubicin (10-11-10-6 mol/L) plus Rosco (5 μg/mL or 10 μg/mL) for 96 h. At the end of treatment, control and drug treated cells were scored for proliferation using an MTT assay; B: Cell cycle phase distribution of human colorectal cancer cells (SW837, 5 × 105 cells/well) treated with doxorubicin (8 × 10-7 mol/L, 72 h); Rosco (15 μg/mL, 72 h); sequential combination: doxorubicin (8 × 10-7 mol/L, 24 h) followed by Rosco (15 μg/mL, 48 h); inverted sequential combination: Rosco (15 μg/mL, 24 h) followed by doxorubicin (8 × 10-7 mol/L, 48 h) and simultaneous combination: doxorubicin plus Rosco (8 × 10-7 mol/L + 15 μg/mL, 72 h) was evaluated by flow cytometry. The percentage of cells in different phases of the cycle was calculated as previously described; C: Human colorectal cancer cells, SW1116 and SW837, (5 × 105 cells/well) were treated with doxorubicin (8 × 10-7 mol/L), Rosco (15 μg/mL), and the combination of doxorubicin plus Rosco (8 × 10-7 mol/L + 15 μg/mL) for 72 h. DNA fragments were extracted and analyzed on 1% agrose gel.
Table 5.
IC50 and sensitization ratio of doxorubicin and combinations with Rosco towards human colorectal cancer cell lines
| Treatment with doxorubicin and its combinations with Rosco |
IC50 (mol/L) |
Sensitization ratio1 |
||||
| SW48 | SW1116 | SW837 | SW48 | SW1116 | SW837 | |
| Dox. | 5.4 × 10-8 | 6 × 10-7 | 4.1 × 10-7 | - | - | - |
| Dox. + 5 μg/mL Rosco | 5.8 × 10-11 | 2 × 10-7 | 4.1 × 10-8 | 9.3 × 102 | 3 | 10 |
| Dox. + 10 μg/mL Rosco | 5.8 × 10-11 | 4.5 × 10-9 | 8.2 × 10-10 | 9.3 × 102 | 1.3 × 102 | 5.0 × 102 |
Sensitization ratio = IC50 (Dox.)/IC50 (Dox. + Rosco).
Table 6.
Analysis of the combined effects of doxorubicin and Rosco on human colorectal cancer cell lines
| Combined treatment of doxorubicin and Rosco |
Combination interaction at various durations of treatment in human colorectal cancer cells1 |
||||||||
| SW48 | SW1116 | SW837 | |||||||
| 2 d | 4 d | 6 d | 2 d | 4 d | 6 d | 2 d | 4 d | 6 d | |
| Dox 0.5 IC50 + Rosco 1.0 μg/mL | ant | ant | ant | syn | syn | syn | syn | syn | syn |
| Dox 1.0 IC50 + Rosco 10 μg/mL | ant | ant | ant | syn | syn | syn | syn | syn | syn |
| Dox 2.0 IC50 + Rosco 15 μg/mL | ant | ant | ant | syn | syn | syn | syn | syn | syn |
| Dox 3.0 IC50 + Rosco 20 μg/mL | ant | add | add | syn | syn | add | syn | syn | add |
| Dox 4.0 IC50 + Rosco 25 μg/mL | ant | add | add | syn | ant | add | syn | ant | ant |
The data are based on the mean of absorbance measurements of 3 independent experiments; ant: Antagonistic; add: Additive; syn: Synergistic.
The effect of the combination on colorectal cancer cell cycle was also investigated. Treatment with Rosco growth arrested cancer cells in G2/M (24.9% vs 4.75% for untreated), while doxorubicin markedly growth arrested colorectal cancer cells in S-phase (83.7% vs 51.5% for untreated). The combination of doxorubicin and Rosco added to the culture in a sequential manner, i.e., doxorubicin followed by Rosco, growth arrested cancer cells in S-phase (77.5% vs 31.5% for untreated). The same combination added in an inverted sequential manner growth arrested cancer cells completely in S-phase (100% vs 31.5% for untreated). However, when the same combination was added in a simultaneous manner, the colorectal cancer cells were arrested in both S-phase (45.8% vs 31.5% for untreated) and G2/M phase (7.94% vs 4.75% for untreated) (Figure 4B). The apoptosis inducing effect of the combination of doxorubicin (8 × 10-7 mol/L) and Rosco (15 μg/mL) was also tested. This combination enhanced apoptosis in SW1116 and SW837 compared to single treatment of doxorubicin and Rosco (Figure 4C).
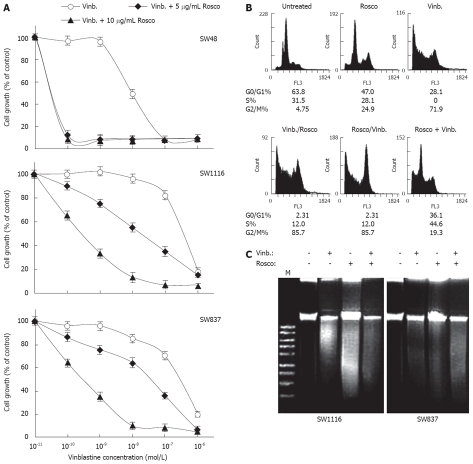
Modulation of vinblastine cytotoxicity on human colorectal cancer cells by combination with CDKI Rosco
The ability of cyclin dependent kinase inhibitor Rosco to sensitize human colorectal cancer cells to vinblastine was evaluated. Treatment of cancer cells with a combination of vinblastine (10-12-10-7 mol/L) and Rosco (5 or 10 μg/mL) dramatically growth inhibited SW48 cells (P = 0.023 or P = 0.019) with IC50 = 5.4 × 10-11 mol/L compared to IC50 = 1.0 × 10-8 mol/L for the effect of vinblastine alone (Figure 5). These results clearly indicate that Rosco (5 or 10 μg/mL) sensitized SW48 cells to vinblastine anticancer activity by 190 fold (Table 7). Treatment of SW1116 with the combination of vinblastine and Rosco (5 μg/mL) produced a higher growth inhibition of SW1116 (IC50 = 7.5 × 10-8 mol/L, sensitization ratio = 8.4 fold) (Figure 5A, Table 7) compared to treatment with vinblastine alone (IC50 = 6.3 × 10-7 mol/L). The increase in the growth inhibition induced by mixing vinblastine with 5 μg/mL of Rosco was found to be statistically non-significant (P = 0.216). On the other hand, treatment of SW1116 with vinblastine and 10 μg/mL Rosco markedly enhanced the cytotoxicity of vinblastine (IC50 = 7.9 × 10-10 mol/L, P = 0.013) towards SW1116 demonstrating a great increase in the chemo-sensitization of SW1116 (8 × 102-fold) to vinblastine (Table 7). Similar results were obtained with the colorectal cancer cell line SW837. The combination of vinblastine and Rosco (5 μg/mL) slightly increased the growth inhibition of SW837 (IC50 = 5 × 10-8 mol/L, sensitization ratio = 8.4) compared to single treatment with vinblastine (IC50 = 4.2 × 10-7 mol/L) (Table 7). However, the combination of vinblastine and 10 μg/mL Rosco exerted significant growth inhibition of SW837 cells (IC50 = 4.6 × 10-10 mol/L, P = 0.023) compared to treatment with vinblastine alone (IC50 = 4.2 × 10-7 mol/L) demonstrating a marked increase in the sensitivity of SW837 (9.1 × 102-fold) to vinblastine.
Figure 5.
Potentiation of vinblastine anticancer effect on human colorectal cancer cell lines by combination with cyclin dependent kinase inhibitor roscovitine. A: Human colorectal cancer cell lines were treated with vinblastine (10-12-10-7 mol/L) and the combination of vinblastine (10-12-10-7 mol/L) plus Rosco (5 μg/mL or 10 μg/mL) for 96 h. At the end of treatment, control and drug-treated cells were scored for proliferation using an MTT assay; B: Cell cycle phase distribution of human colorectal cancer cells (SW837, 5 × 105 cells/well) treated with vinblastine (2.6 × 10-7 mol/L, 72 h); Rosco (15 μg/mL, 72 h); sequential combination: vinblastine (2.6 × 10-7 mol/L, 24 h) followed by Rosco (15 μg/mL, 48 h); inverted sequential combination: Rosco (15 μg/mL, 24 h) followed by vinblastine (2.6 × 10-7 mol/L, 48 h) and simultaneous combination: vinblastine plus Rosco (2.6 × 10-7 mol/L + 15 μg/mL, 72 h) was evaluated by flow cytometry. The percentage of cells in different phases of the cycle was calculated as described above; C: Human colorectal cancer cells, SW1116 and SW837, (5 × 105 cells/well) were treated with vinblastine (2.6 × 10-7 mol/L), Rosco (15 μg/mL) and the combination of vinblastine plus Rosco (2.6 × 10-7 mol/L + 15 μg/mL) for 72 h. DNA fragments were extracted and analyzed on 1% agrose gel.
Table 7.
IC50 and sensitization ratio of vinblastine and its combinations with Rosco towards human colorectal cancer cell lines
| Treatment with vinblastine and combinations with Rosco |
IC50 (mol/L) |
Sensitization ratio1 |
||||
| SW48 | SW1116 | SW837 | SW48 | SW1116 | SW837 | |
| Vinb. | 1 × 10-8 | 6.3 × 10-7 | 4.2 × 10-7 | - | - | - |
| Vinb. + 5 μg/mL Rosco | 5.4 × 10-11 | 7.5 × 10-8 | 5.0 × 10-8 | 1.9 × 102 | 8.4 | 8.4 |
| Vinb. + 10 μg/mL Rosco | 5.4 × 10-11 | 7.9 × 10-10 | 4.6 × 10-10 | 1.9 × 102 | 8.0 × 102 | 9.1 × 102 |
Sensitization ratio = IC50 (Vinb.)/IC50 (Vinb. + Rosco).
The combination of vinblastine (2IC50) and Rosco (15 μg/mL) had synergistic effect on SW48 after 4 d of combination treatment (Table 8). All the tested combinations had additive or synergistic effects on SW1116 after 2 d of treatment. The combination of vinblastine (IC50) and Rosco (10 μg/mL) had additive and synergistic effects on SW1116 and SW837 after 4 and 6 d of treatment, respectively (Table 8).
Table 8.
Analysis of the combined effects of vinblastine and Rosco on human colorectal cancer cell lines
| Combined treatment of vinblastine and Rosco |
Combination interaction at various durations of treatment in human colorectal cancer cells1 |
||||||||
| SW48 | SW1116 | SW837 | |||||||
| 2 d | 4 d | 6 d | 2 d | 4 d | 6 d | 2 d | 4 d | 6 d | |
| Vinb 1.0 IC50 + Rosco 10 μg/mL | ant | ant | ant | add | add | add | ant | syn | syn |
| Vinb 2.0 IC50 + Rosco 15 μg/mL | ant | syn | ant | syn | ant | ant | ant | ant | ant |
| Vinb 3.0 IC50 + Rosco 20 μg/mL | ant | ant | ant | syn | ant | ant | ant | ant | ant |
| Vinb 4.0 IC50 + Rosco 25 μg/mL | ant | ant | ant | syn | ant | ant | ant | ant | ant |
The data are based on the mean of absorbance measurements of 3 independent experiments; ant: Antagonistic; add: Additive; syn: Synergistic.
The effects of single and combined treatment with vinblastine (2.6 × 10-7 mol/L) and Rosco (15 μg/mL) on colorectal cancer cell cycle distribution indicated that treatment of SW837 with Rosco growth inhibited colorectal cancer cells in G2/M (24.9% vs 4.75% for untreated), while, vinblastine treatment markedly growth arrest these cells in G2/M (71.9% vs 4.75% for untreated). The combination of vinblastine (2.6 × 10-7 mol/L) and Rosco (15 μg/mL) added in sequential manner (vinblastine followed by Rosco) greatly growth arrested SW837 cells in G2/M (76.6% vs 4.75% for untreated). The same combination added in an inverted sequential manner markedly growth arrested the cells in G2/M (85.7% vs 4.75% for untreated). The same combination added simultaneously to SW837 cells growth arrested these cells both in S- (44.6% vs 31.6% for untreated) and G2/M-(19.3% vs 4.75% for untreated) phases (Figure 5B). The combination of vinblastine (2.6 × 10-7 mol/L) and Rosco (15 μg/mL) had a marked apoptotic effect on colorectal cancer cell lines SW1116 and SW837 compared to single treatments with vinblastine (2.6 × 10-7 mol/L) or Rosco (15 μg/mL) (Figure 5C).
DISCUSSION
The central finding of the present study is that the cyclin dependent kinase inhibitor Rosco improved the therapeutic activity of several conventional chemotherapeutic drugs namely taxol, doxorubicin, 5-FU, and vinblastine that act by different mechanisms in human colorectal cancer cells. This finding is significant because chemotherapeutic drugs cause high toxicity to normal tissues during treatment of colorectal cancer as well as other cancers.
The adverse health effects of the conventional chemotherapeutic drugs, such as immunosuppression and cardiomyopathy, which severely increase in a dose-dependent manner, as well as development of primary or secondary drug resistance in tumor cells, limit their clinical success in cancer chemotherapy[15]. The increase in systemic toxicity and drug resistance, the major drawbacks of anticancer chemotherapeutic agents, has led to a new challenge in the field of cancer research. To overcome such problems, extensive research has been directed towards reducing systemic toxicity and increasing drug activity in cancer therapy[15,16]. In this regard, combination chemotherapy has received increasing attention in the search for compounds that could increase the therapeutic index of clinical anticancer drugs[17].
Deregulation of the cell cycle and oncogenic overexpression of several cell cycle related gene products in many human cancers provide new opportunities for anticancer drug discovery. Efforts to exploit these targets are progressing quite well, with inhibition of cyclin dependent kinase activity emerging as the most productive approach at present. This has resulted in the development of several small molecules, with specific and potent CDK inhibitory effects, which are now undergoing clinical trials in phasesIand II, and the results awaited with expectation.
CDKs are essential players in the intracellular control of the cell cycle. Since CDKs and their regulatory partners are frequently deregulated and exhibit enhanced activity in human cancers, their inhibition by selectively acting drugs offers a new concept in the therapeutic strategy[5,18]. Recently, a number of pharmacological inhibitors of CDKs were developed, one efficient group of such compounds is based on the substitution of purines and pyrimidines. Substituted purines represent CDK inhibitors that are structurally most similar to ATP[7]. Among a series of C2, N6, N9-substituted adenines, Rosco displays high efficiency and selectivity towards some CDKs. Out of 25 kinases investigated, only a few were significantly inhibited by Rosco with IC50 values lower than 1 μmol/L. CDK2/cyclin B and CDK2/cyclin A were identified as the best targets[8]. Through its high selectivity, Rosco is predestinated to be a potent anti-mitotic drug. It acts not only as a cell cycle blocker[19-22], but seems also to induce apoptosis[11,22-24].
There is still a lack of systematic knowledge about the cytotoxic effects of Rosco on normal and malignant cells. The exact discrimination between inhibition of cell proliferation and impairment of cell viability is necessary. Moreover, the pro- or anti-apoptotic action of Rosco on different cells is until now contradictory and has to be conscientiously examined. The consequences of Rosco-induced cell cycle arrest and apoptosis in colorectal cancer cells upon chemo-sensitization of such cells to conventional therapeutic drugs have not yet been investigated. In this study, we addressed the ability of Rosco to synergize with conventional chemotherapeutic drugs, acting by different mechanisms, to induce efficient apoptosis in human colorectal cancer cell lines.
In the present study, human colorectal cancer cells were shown to be sensitive to the antiproliferative and cytotoxic effects of Rosco with IC50 values: (11.56-25 μg/mL), (11.62-14.78 μg/mL) and (13.44-16.25 μg/mL) for SW48, SW1116 and SW837, respectively, after 24-144 h, 72-144 h and 48-144 h of Rosco treatment, respectively. These results are consistent with those reported for other cell types[8,25]. To explore whether inhibition of cell growth observed in the Rosco treated human colorectal cancer cells synergize with the conventional chemotherapeutic drugs acting by different mechanisms to induce efficient apoptosis of human colorectal cancer cells, we tested the efficacy of single and combined treatments with Rosco and taxol, doxorubicin, 5-FU or vinblastine on the growth of human colorectal cancer cells. Our results indicated that Rosco (5 and 10 μg/mL) markedly sensitized the tested human colorectal cancer cells to taxol (sensitization ratio = 7.0-6.4 × 102), doxorubicin (sensitization ratio = 3.0-9.3 × 102), 5-FU (sensitization ratio = 3.73-8.42 × 103) and vinblastine (sensitization ratio = 9-8 × 103) (Tables 1, 3, 5 and 7). The combination of Rosco and chemotherapeutic drugs inhibited the growth of human colorectal cancer cells in an additive or synergistic fashion, and in a time- and dose-dependent manner (Tables 2, 4, 6 and 8). Treatment of human colorectal cancer cells with Rosco, conventional chemotherapeutic drugs or the combination of Rosco and conventional chemotherapeutic drugs added in a sequential or inverted sequential manner growth arrested colorectal cancer cells in G2/M- or S- phase of the cell cycle. While, simultaneous addition of Rosco and conventional chemotherapeutic drugs double blocked colorectal cancer cells in G2/M- and S- phases of the cell cycle for all the tested drugs except for 5-FU, where, its combination with Rosco growth arrested colorectal cancer cells in S-phase. The growth arrest of colorectal cancer cells in G2/M following Rosco treatment may facilitate the induction of apoptosis and sensitize the cells to conventional chemotherapeutic drugs.
Another notable observation from our morphological analysis was the extensive detachment of cells from the cell culture substratum after exposure to Rosco (data not shown). Recent evidence suggests that cellular attachment to the substratum is mediated by the interaction of integrins with ECM components such as fibronectin, collagen, and vitronectin[26]. Binding of integrins to these adhesion molecules results in the activation of focal adhesion kinase[27] accompanied by phosphorylation and recruitment of a number of related cytoskeletal and signaling molecules, thereby transducing anchorage and survival messages to the nucleus[28,29]. Conversely, the uncoupling of integrins from ECM proteins leads to disruption of integrin-mediated signal transduction, inactivation of focal adhesion kinase, detachment of cells from the ECM, and apoptotic cell death[30]. Our data suggest that following Rosco treatment, human colorectal cancer cells detach from cell culture substratum, and die via apoptosis as indicated by the DNA fragmentation assay. This notion is consistent with previous reports demonstrating that cells deprived of matrix attachment underwent apoptosis[31]. Thus, the extensive detachment of cells from the cell culture substratum, and the apoptotic cell death observed in our experimental system may be due to the uncoupling of integrin-mediated signaling and/or disruption of cell-matrix interactions induced by Rosco. In addition to facilitating apoptosis, which will have its impact on chemosensitization of human colorectal cancer cells to conventional chemotherapeutic drugs, the loss of adhesion induced by this CDK inhibitor may deny cell anchorage and traction necessary for growth and migration and thus prevent colorectal cancer invasion and metastasis, the major cause of death in colorectal cancer patients. Because adhesion and invasion are crucial to the initiation of metastatic growth[32], additional studies on the effect of Rosco on cell adhesion to extracellular matrix components as well as the anti-invasive potential of the CDK inhibitor could be extremely rewarding. These studies are currently ongoing in our laboratory.
Rosco may prevent the assembly of actin fibers by modulating the expression and/or activity of Rho GTPases, which have been reported to be involved in the regulation of actin microfilament organization and other associated activities[33]. Disruption of actin microfilament architecture by Rosco has some biological implications. In view of the role played by actin microfilaments in various aspects of cellular physiology such as cell-cell interaction, proliferation, and secretion[33], it can be argued that all of these cellular activities could be affected in colorectal tumors following Rosco treatment.
Rosco strongly up-regulates wt p53 protein in cancer cells[19,20,22,34]. Since p53 protein plays a pivotal role in the regulation of cell cycle, the biological effect of Rosco cannot be restricted to the direct inhibition of distinct kinases. Considering the multiple p53 targets and functions, it is obvious that the Rosco-induced upregulation of p53 in cancer cells may essentially contribute to the cell cycle arrest, chromatin silencing and initiation as well as execution of apoptosis.
The role of CKDs in chemosensitization, and the potential downstream effectors of CDKs inhibition have been investigated by Crescenzi et al[5]. They showed that lung adenocarcinoma cell line H1299 treated with a nontoxic concentration of Rosco renders H1299 cells significantly more susceptible to doxorubicin or etoposide. In these cells, Rosco does not modulate senescence, but markedly reduces the capacity of H1299 cells to repair damage and resume proliferation after treatment. Combined treatment with Rosco and doxorubicin, or etoposide was found to enhance G2-M accumulation, to increase the amount of γ-H2AX foci and to inhibit DNA repair. Two main repair pathways, homologous recombination and NHEJ, cooperate to repair DNA DSBs[35]. Crescenzi et al[5] investigated the ability of Rosco to modulate those two processes in doxorubicin-treated cells. They reported the ability of Rosco to negatively modulate DNA-PK activity in H1299 cells[5,36] and showed that Rosco significantly reduces the efficiency of recombination repair identifying a novel mechanism of action by which Rosco affects tumor cells that is inhibition of DNA DSBs repair.
The role of CDK2 and CDK1 kinases as targets for Rosco in tumor chemosensitization has also been investigated by Crescenzi et al[5]. In this study, experiments with inducible dn-K2 clones indicated that loss of Cdk2 and Cdk1 activity was responsible for the chemosensitizing effect of Rosco Overexpression of dn-K2 in H1299 cells potentiates doxorubicin-induced G2-M arrest and inhibited recovery of the cells after treatment. It is worth noting that overexpression of dn-K2 results in both Cdk2 and Cdk1 inhibition[37]. Furthermore, analyses of homologous recombination in Hela cells transiently overexpressing either dn-K2 or dn-K1 or Cdk2 confirmed a role for Cdks in modulation of DNA repair processes[5]. A role for CDK in the control of DNA repair pathways has also been studied in the yeast cells[38,39]. Combined treatment of Rosco and DNA-damaging agents not only enhances drug-induced apoptosis, but also effectively hampers the recovery of mildly damaged tumor cells after treatment. Rosco, by hindering both homologous recombination and NHEJ repair processes, has the potential to inhibit recovery of mildly damaged tumor cells after chemotherapeutic drug treatment, and to increase the susceptibility of tumor cells to chemotherapy. Our results clearly indicated that Rosco synergizes with chemotherapeutic drugs to induce efficient apoptosis of human colorectal cancer cells. Important issues that need to be addressed in order to advance these agents to the clinical arena include the best drug administration schedule, testing various combinations with standard chemotherapeutic agents, the best tumor types to be targeted, and demonstration of CDK modulation of tumor samples from cancer patients.
COMMENTS
Background
Cyclin-dependent kinases (CDKs) are serine/threonine kinases that play a key role in regulating cell cycle progression. Aberrant expression or altered activity of distinct CDK complexes results in escape of cells from the cell cycle control and leads to malignant transformation. Therefore, inhibition of CDKs in malignant cells provides a new strategy in the fight against cancer. The present study examined the ability of roscovitine (Rosco), a CDK inhibitor (CDKI), to enhance the anticancer effects of chemotherapeutic drugs that act by different mechanisms on human colorectal cancer cells. The authors have also investigated whether Rosco differentially affects the cell cycle distribution of drug-treated human colorectal cancer cells.
Research frontiers
Extensive research has been directed towards reducing systemic toxicity and increasing drug activity in cancer therapy. Combination chemotherapy has received increasing attention in the search for compounds that could increase the therapeutic index of clinical anticancer drugs. This study indicated that combinations of agents directed at different pathways or different steps of pathways involved in apoptosis can cause the cells to reach an apoptosis threshold resulting in synergistic apoptosis and increased therapeutic index of the anticancer drugs.
Innovations and breakthroughs
The central finding of this study is that the cyclin dependent kinase inhibitor Rosco improved the therapeutic activity of several conventional drugs namely taxol, 5-fluorouracil (5-FU), doxorubicin and vinblastine that act by different mechanisms. Also, Rosco differentially affected the cell cycle distribution of drug-treated colorectal cancer cells.
Applications
Chemotherapeutic drugs are highly toxic to normal tissues during treatment of colorectal cancer as well as other cancers. Rosco increases the sensitivity of several conventional chemotherapeutic drugs namely taxol, doxorubicin, 5-FU, and vinblastine. This finding is significant because increasing drug activity may reduce systemic toxicity in cancer therapy.
Terminology
CDKIs are a heterogeneous group of compounds that are able to inhibit CDKs involved in the cell cycle, transcription or neuronal functions. CDKIs are a chemically diverse, flat, hydrophobic heterocycles that compete with ATP. Rosco (CDKI) is structurally related to ATP, it blocks the cell cycle and induces apoptosis.
Peer review
A tremendous amount of in vitro work has been done looking at the anti colorectal cancer cell effect of Rosco, a novel CDK inhibitor, to enhance the antitumor effects of conventional chemotherapy agents. The approach is very well designed and the presentation of data is very detailed. It looks promising and may yield clinical benefits in the future.
Footnotes
Supported by Kuwait University, Research Project No. SL01/05
Peer reviewers: Francis Seow-Choen, Professor, Seow-Choen Colorectal Centre, Mt Elizabeth Medical Centre, Singapore, 3 Mt Elizabeth Medical Centre #09-10, 228510, Singapore; Alessandro Fichera, MD, FACS, FASCRS, Assistant Professor, Department of Surgery, University of Chicago, 5841 S. Maryland Ave, MC 5031, Chicago IL 60637, United States
S- Editor Li DL L- Editor Alpini GD E- Editor Ma WH
References
- 1.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Nurse P. Cyclin dependent kinases and cell cycle control (nobel lecture) Chembiochem. 2002;3:596–603. doi: 10.1002/1439-7633(20020703)3:7<596::AID-CBIC596>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 3.Hall M, Peters G. Genetic alterations of cyclins, cyclin-dependent kinases, and Cdk inhibitors in human cancer. Adv Cancer Res. 1996;68:67–108. doi: 10.1016/s0065-230x(08)60352-8. [DOI] [PubMed] [Google Scholar]
- 4.Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM, Pagano M. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- 5.Crescenzi E, Palumbo G, Brady HJ. Roscovitine modulates DNA repair and senescence: implications for combination chemotherapy. Clin Cancer Res. 2005;11:8158–8171. doi: 10.1158/1078-0432.CCR-05-1042. [DOI] [PubMed] [Google Scholar]
- 6.Meijer L. Chemical inhibitors of cyclin-dependent kinases. Prog Cell Cycle Res. 1995;1:351–363. doi: 10.1007/978-1-4615-1809-9_29. [DOI] [PubMed] [Google Scholar]
- 7.Gray N, Detivaud L, Doerig C, Meijer L. ATP-site directed inhibitors of cyclin-dependent kinases. Curr Med Chem. 1999;6:859–875. [PubMed] [Google Scholar]
- 8.Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux JP. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 9.Edamatsu H, Gau CL, Nemoto T, Guo L, Tamanoi F. Cdk inhibitors, roscovitine and olomoucine, synergize with farnesyltransferase inhibitor (FTI) to induce efficient apoptosis of human cancer cell lines. Oncogene. 2000;19:3059–3068. doi: 10.1038/sj.onc.1203625. [DOI] [PubMed] [Google Scholar]
- 10.Mgbonyebi OP, Russo J, Russo IH. Roscovitine induces cell death and morphological changes indicative of apoptosis in MDA-MB-231 breast cancer cells. Cancer Res. 1999;59:1903–1910. [PubMed] [Google Scholar]
- 11.De Azevedo WF, Leclerc S, Meijer L, Havlicek L, Strnad M, Kim SH. Inhibition of cyclin-dependent kinases by purine analogues: crystal structure of human cdk2 complexed with roscovitine. Eur J Biochem. 1997;243:518–526. doi: 10.1111/j.1432-1033.1997.0518a.x. [DOI] [PubMed] [Google Scholar]
- 12.Abaza MS, Al-Attiyah RJ, Al-Saffar AM, Al-Sawan SM, Moussa NM. Antisense oligodeoxynucleotide directed against c-myb has anticancer activity and potentiates the antiproliferative effect of conventional anticancer drugs acting by different mechanisms in human colorectal cancer cells. Tumour Biol. 2003;24:241–257. doi: 10.1159/000076139. [DOI] [PubMed] [Google Scholar]
- 13.Lam PK, To EW, Chan ES, Liew CT, Lung IW, King WK. In vitro inhibition of head and neck cancer-cell growth by human recombinant interferon-alpha and 13-cis retinoic acid. Br J Biomed Sci. 2001;58:226–229. [PubMed] [Google Scholar]
- 14.Fan XM, Wong BC, Wang WP, Zhou XM, Cho CH, Yuen ST, Leung SY, Lin MC, Kung HF, Lam SK. Inhibition of proteasome function induced apoptosis in gastric cancer. Int J Cancer. 2001;93:481–488. doi: 10.1002/ijc.1373. [DOI] [PubMed] [Google Scholar]
- 15.Raghavan D, Koczwara B, Javle M. Evolving strategies of cytotoxic chemotherapy for advanced prostate cancer. Eur J Cancer. 1997;33:566–574. doi: 10.1016/s0959-8049(96)00510-2. [DOI] [PubMed] [Google Scholar]
- 16.Figg WD, Arlen P, Gulley J, Fernandez P, Noone M, Fedenko K, Hamilton M, Parker C, Kruger EA, Pluda J, et al. A randomized phase II trial of docetaxel (taxotere) plus thalidomide in androgen-independent prostate cancer. Semin Oncol. 2001;28:62–66. doi: 10.1016/s0093-7754(01)90157-5. [DOI] [PubMed] [Google Scholar]
- 17.Millikan R, Baez L, Banerjee T, Wade J, Edwards K, Winn R, Smith TL, Logothetis C. Randomized phase 2 trial of ketoconazole and ketoconazole/doxorubicin in androgen independent prostate cancer. Urol Oncol. 2001;6:111–115. doi: 10.1016/s1078-1439(00)00123-x. [DOI] [PubMed] [Google Scholar]
- 18.Blagosklonny MV, Darzynkiewicz Z, Figg WD. Flavopiridol inversely affects p21(WAF1/CIP1) and p53 and protects p21-sensitive cells from paclitaxel. Cancer Biol Ther. 2002;1:420–425. doi: 10.4161/cbt.1.4.21. [DOI] [PubMed] [Google Scholar]
- 19.David-Pfeuty T. Potent inhibitors of cyclin-dependent kinase 2 induce nuclear accumulation of wild-type p53 and nucleolar fragmentation in human untransformed and tumor-derived cells. Oncogene. 1999;18:7409–7422. doi: 10.1038/sj.onc.1203103. [DOI] [PubMed] [Google Scholar]
- 20.David-Pfeuty T, Nouvian-Dooghe Y, Sirri V, Roussel P, Hernandez-Verdun D. Common and reversible regulation of wild-type p53 function and of ribosomal biogenesis by protein kinases in human cells. Oncogene. 2001;20:5951–5963. doi: 10.1038/sj.onc.1204741. [DOI] [PubMed] [Google Scholar]
- 21.Vitali L, Yakisich JS, Vita MF, Fernandez A, Settembrini L, Siden A, Cruz M, Carminatti H, Casas O, Idoyaga Vargas V. Roscovitine inhibits ongoing DNA synthesis in human cervical cancer. Cancer Lett. 2002;180:7–12. doi: 10.1016/s0304-3835(01)00827-8. [DOI] [PubMed] [Google Scholar]
- 22.Wojciechowski J, Horky M, Gueorguieva M, Wesierska-Gadek J. Rapid onset of nucleolar disintegration preceding cell cycle arrest in roscovitine-induced apoptosis of human MCF-7 breast cancer cells. Int J Cancer. 2003;106:486–495. doi: 10.1002/ijc.11290. [DOI] [PubMed] [Google Scholar]
- 23.Mgbonyebi OP, Russo J, Russo IH. Roscovitine induces cell death and morphological changes indicative of apoptosis in MDA-MB-231 breast cancer cells. Cancer Res. 1999;59:1903–1910. [PubMed] [Google Scholar]
- 24.Yakisich JS, Boethius J, Lindblom IO, Wallstedt L, Vargas VI, Siden A, Cruz MH. Inhibition of DNA synthesis in human gliomas by roscovitine. Neuroreport. 1999;10:2563–2567. doi: 10.1097/00001756-199908200-00023. [DOI] [PubMed] [Google Scholar]
- 25.Mihara M, Shintani S, Kiyota A, Matsumura T, Wong DT. Cyclin-dependent kinase inhibitor (roscovitine) suppresses growth and induces apoptosis by regulating Bcl-x in head and neck squamous cell carcinoma cells. Int J Oncol. 2002;21:95–101. [PubMed] [Google Scholar]
- 26.Wen LP, Fahrni JA, Troie S, Guan JL, Orth K, Rosen GD. Cleavage of focal adhesion kinase by caspases during apoptosis. J Biol Chem. 1997;272:26056–26061. doi: 10.1074/jbc.272.41.26056. [DOI] [PubMed] [Google Scholar]
- 27.Haimovich B, Lipfert L, Brugge JS, Shattil SJ. Tyrosine phosphorylation and cytoskeletal reorganization in platelets are triggered by interaction of integrin receptors with their immobilized ligands. J Biol Chem. 1993;268:15868–15877. [PubMed] [Google Scholar]
- 28.Leventhal PS, Shelden EA, Kim B, Feldman EL. Tyrosine phosphorylation of paxillin and focal adhesion kinase during insulin-like growth factor-I-stimulated lamellipodial advance. J Biol Chem. 1997;272:5214–5218. doi: 10.1074/jbc.272.8.5214. [DOI] [PubMed] [Google Scholar]
- 29.Marushige Y, Marushige K. Alterations in focal adhesion and cytoskeletal proteins during apoptosis. Anticancer Res. 1998;18:301–307. [PubMed] [Google Scholar]
- 30.Fukai F, Mashimo M, Akiyama K, Goto T, Tanuma S, Katayama T. Modulation of apoptotic cell death by extracellular matrix proteins and a fibronectin-derived antiadhesive peptide. Exp Cell Res. 1998;242:92–99. doi: 10.1006/excr.1998.4076. [DOI] [PubMed] [Google Scholar]
- 31.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong NC, Mueller BM, Barbas CF, Ruminski P, Quaranta V, Lin EC, Smith JW. Alphav integrins mediate adhesion and migration of breast carcinoma cell lines. Clin Exp Metastasis. 1998;16:50–61. doi: 10.1023/a:1006512018609. [DOI] [PubMed] [Google Scholar]
- 33.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 34.Wesierska-Gadek J, Schmid G. Dual action of the inhibitors of cyclin-dependent kinases: targeting of the cell-cycle progression and activation of wild-type p53 protein. Expert Opin Investig Drugs. 2006;15:23–38. doi: 10.1517/13543784.15.1.23. [DOI] [PubMed] [Google Scholar]
- 35.Jackson SP. Sensing and repairing DNA double-strand breaks. Carcinogenesis. 2002;23:687–696. doi: 10.1093/carcin/23.5.687. [DOI] [PubMed] [Google Scholar]
- 36.Maggiorella L, Deutsch E, Frascogna V, Chavaudra N, Jeanson L, Milliat F, Eschwege F, Bourhis J. Enhancement of radiation response by roscovitine in human breast carcinoma in vitro and in vivo. Cancer Res. 2003;63:2513–2517. [PubMed] [Google Scholar]
- 37.Hu B, Mitra J, van den Heuvel S, Enders GH. S and G2 phase roles for Cdk2 revealed by inducible expression of a dominant-negative mutant in human cells. Mol Cell Biol. 2001;21:2755–2766. doi: 10.1128/MCB.21.8.2755-2766.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ira G, Pellicioli A, Balijja A, Wang X, Fiorani S, Carotenuto W, Liberi G, Bressan D, Wan L, Hollingsworth NM, et al. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature. 2004;431:1011–1017. doi: 10.1038/nature02964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aylon Y, Liefshitz B, Kupiec M. The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. EMBO J. 2004;23:4868–4875. doi: 10.1038/sj.emboj.7600469. [DOI] [PMC free article] [PubMed] [Google Scholar]