Abstract
AIM: To determine whether Nigella sativa prevents hepatic ischemia-reperfusion injury to the liver.
METHODS: Thirty rats were divided into three groups as sham (Group 1), control (Group 2), and Nigella sativa (NS) treatment group (Group 3). All rats underwent hepatic ischemia for 45 min followed by 60 min period of reperfusion. Rats were intraperitoneally infused with only 0.9% saline solution in group 2. Rats in group 3 received NS (0.2 mL/kg) intraperitoneally, before ischemia and before reperfusion. Blood samples and liver tissues were harvested from the rats, and then the rats were sacrificed. Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) levels were determined. Total antioxidant capacity (TAC), catalase (CAT), total oxidative status (TOS), oxidative stress index (OSI) and myeloperoxidase (MPO) in hepatic tissue were measured. Also liver tissue histopathology was evaluated by light microscopy.
RESULTS: The levels of liver enzymes in group 3 were significantly lower than those in the group 2. TAC in liver tissue was significantly higher in group 3 than in group 2. TOS, OSI and MPO in hepatic tissue were significantly lower in group 3 than the group 2. Histological tissue damage was milder in the NS treatment group than that in the control group.
CONCLUSION: Our results suggest that Nigella sativa treatment protects the rat liver against to hepatic ischemia-reperfusion injury.
Keywords: Nigella sativa, Ischemia reperfusion injury, Liver
INTRODUCTION
Ischemia followed by reperfusion (I/R) may cause metabolic and structural hepatic damage, and may be due to trauma, sepsis, liver transplantation[1] or hepatic pedicle clamping during liver surgery[2]. This remains a significant problem for surgical procedures, and also remains limitation of liver transplantation[3].
Oxygen free radicals, produced on reperfusion, play a critical role in the injury caused by ischemia-reperfusion[4]. Reactive oxygen radicals lead to an inflammatory response and tissue damage by activating some mediators. It can also directly damage cell components[5]. Several attempts to reduce these mechanisms have been reported in the literature. Protection against reperfusion injury can be induced by assorted treatments including administration of antioxidants and anti-inflammatory drugs[4,6-8].
Various therapeutic effects, such as antioxidant, anti-inflammatory, anticancer[9], antihistaminic[10], antibacterial effects[11] have been described for Nigella sativa. Additionally, it has been shown that Nigella sativa has protective effect against ischemia reperfusion injury to various organs[12-14]. Thymoquinone, the active constituent of Nigella sativa seeds, is a pharmacologically active quinone, which possesses several properties including analgesic and anti-inflammatory actions[15]. It has been reported that thymoquinone prevents oxidative injury in various in vitro and in vivo studies in rats[16,17]. It has been suggested that thymoquinone may act as an antioxidant agent and prevents membrane lipid peroxidation in tissues[18]. The mechanism of action is still largely unknown. But, it seems these effects may be related to inhibition of eicosanoid generation, namely thromboxane B2 and leucotrienes B4 (by inhibiting cyclooxygenase and 5-lipooxygenase, respectively), and membrane lipid peroxidation[13].
Moreover, it has been demonstrated that Nigella sativa can significantly prevent hepatotoxicity[19] and might have protective effects against nephrotoxicity induced by either disease or chemicals[13]. But, the exact mechanism is not clear. There are also several clinical studies. In one study, the prophylactic effect of boiled extract of N. sativa on asthmatic disease was examined[20]. Similarly, black seed oil was shown to be an effective adjuvant for the treatment of patients with allergic diseases[21]. In another clinical study, significant benefits of Nigella sativa extract in the treatment of acute tonsillopharyngitis was shown[22]. Also, it was shown that Nigella sativa has anti-epileptic effects in children with refractory seizures[23].
Therefore, it seems possible that the administration of Nigella sativa might protect the liver against the ischemia reperfusion injury; therefore, our aim was to confirm this hypothesis. We investigated alterations in the oxidant- antioxidant balance by measuring oxidant parameters such as total oxidative status (TOS), oxidative stress index (OSI) and myeloperoxidase (MPO), and antioxidant parameters, such as total antioxidant capacity (TAC) and catalase (CAT) in the liver tissue. Also we examined histopathological changes in the liver parenchyma.
MATERIALS AND METHODS
Thirty male Wistar-albino rats weighting 190 g to 250 g were used in this experimental study. All animals were maintained under standard conditions, and were fed water and rodent chow ad libitum and treated in compliance with the National Institutes of Health guidelines. Rats were deprived of food, but not water, for 24 h before surgery.
Animals were divided into three groups, sham group (Group 1), control group (Group 2), and Nigella sativa treatment group (Group 3). All rats were anesthetized with 0.2 mL/100 g of ketamine hydrochloride intraperitoneally. After the abdomen was shaved and disinfected, a midline incision was made and rats underwent either sham surgery or ischemia-reperfusion. Ischemia was carried out by exposing the afferent and efferent blood vessels and then clamping for 45 min with a microvascular “bulldog” clamp. Forty five minutes later, the ischemic liver was reperfused by opening the clamp, and reperfusion was achieved for 60 min. Nigella sativa was given to the rats in treatment group, before ischemia and before reperfusion at a dose of 0.2 mL/kg by intraperitoneal route. We chose the dose of this agent according to reported studies about I/R and Nigella sativa, as this dose has been shown to be effective in previous studies[24,25]. Rats in the control group were infused only with saline. At the end of the procedures, the rats were killed and blood and liver tissue samples were obtained. A portion of liver was stored at -80°C for future analyses. Plasma alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) activities were measured for evaluating the liver functions. To assess oxidative injury, TAC, TOS and OSI levels were determined.
OSI and MPO levels
The enzyme analyses of liver tissue were performed on the supernatant fractions of the tissue homogenates. The tissues were homogenized in 150 mmol/L ice-cold KCl to make a 10% homogenate, using a glass Teflon homogenizer. Then, the samples were sonicated on ice ten times for 5 s. The homogenates were centrifuged at 12 500 g for 30 min at 3°C, and the supernatant fractions were obtained.
Tissue samples for histological staining were obtained and fixed in 10% formalin-phosphate-buffered saline at 4°C overnight. The samples were dehydrated and embedded in paraffin. Sections (7 μm) were cut and stained with hematoxylin and eosin. A pathologist evaluated the slides in a blinded manner.
Biochemical analyses
Plasma was used to measure AST, ALT and LDH as indicative parameters of hepatic function. The plasma activities of AST, ALT and LDH were estimated by commercially available kits using an autoanalyser (aeroset® Abbott Laboratories, Chicago, IL ).
Measurement of the total antioxidant capacity
TAC of supernatant fractions was determined using a novel automated measurement method developed by Erel[26]. In this method, hydroxyl radical, which is the most potent biological radical, is produced. In the assay, ferrous ion solution, which is present in Reagent 1, is mixed with hydrogen peroxide, which is present in Reagent 2. The sequential produced radicals such as brown colored dianisidinyl radical cation, produced by the hydroxyl radical, are also potent radicals. Using this method, antioxidative effect of the sample against the potent-free radical reactions, which is initiated by the produced hydroxyl radical, is measured. The assay has excellent precision values, lower than 3%. The results are expressed as nmol Trolox Equiv./mg protein.
Measurement of total oxidant status
TOS of supernatant fractions was determined using a novel automated measurement method, developed by Erel[27]. Oxidants present in the sample oxidize the ferrous ion-o-dianisidine complex to ferric ion. The oxidation reaction is enhanced by glycerol molecules, which are abundantly present in the reaction medium. The ferric ion makes a colored complex with xylenol orange in an acidic medium. The color intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. The assay is calibrated with hydrogen peroxide and the results are expressed in terms of nmol H2O2 Equiv./mg protein.
Oxidative stress index
Percent ratio of TOS level to TAC level was accepted as OSI. OSI value was calculated according to the following formula[28]: OSI (Arbitrary Unit) = TOS (nmol H2O2 Equiv./mg protein)/TAC (nmol Trolox Equiv./mg protein).
Determination of myeloperoxidase activity
The MPO (EC 1.11.1.7) activity was determined, using a 4-aminoantipyrine/phenol solution as the substrate for MPO-mediated oxidation by H2O2 and changes in absorbance at 510 nm were recorded[29]. One unit of MPO activity was defined as that which degraded 1 mol H2O2/min at 25°C. The results were expressed as mU/g protein.
Determination of catalase activity
Liver catalase activity was determined by Goth’s colorimetric method, in which supernatant was incubated in H2O2 substrate, and the enzymatic reaction stopped by the addition of ammonium molybdate. The intensity of the yellow complex formed by molybdate and H2O2 was measured at 405 nm[30].
Histopathologic evaluation
Liver tissues were embedded in paraffin, cut into 3 to 5-μm sections, and mounted. After deparaffinization, the tissues were stained with hematoxylin and eosin (HE) for histological examination. Histological examination was performed by a pathologist.
Statistical analysis
For statistical analyses, nonparametric independent group comparisons were made. For multiple comparisons, the Kruskal-Wallis was used for comparisons between groups and the Mann-Whitney test used if any statistical significance was found. A level of 5% (P < 0.05) was considered statistically significant. Data were expressed as median and range.
RESULTS
Plasma ALT, AST, and LDH levels in the Nigella sativa treatment group were significantly lower than those in the control and sham groups (P < 0.01, P < 0.01 and P < 0.05, respectively, and P < 0.01 for all). They were significantly higher in the control group than those in the sham group (P < 0.01 for all). The results are summarized in Table 1.
Table 1.
Clinical parameters, oxidative and antioxidative parameters in sham, I/R and I/R + NS rats (n = 10, mean ± SD)
| Sham | I/R | I/R + NS | P | |
| Clinical parameters | ||||
| AST (U/L) | 132 ± 22 | 952 ± 251b | 571 ± 137df | 0.001 |
| ALT (U/L) | 86 ± 17 | 695 ± 206b | 321 ± 128df | 0.001 |
| LDH (U/L) | 534 ± 181 | 4334 ± 760b | 3113 ± 729de | 0.001 |
| TAC (nmol Trolox Eqv./mg protein) | 2.96 ± 0.4 | 2.17 ± 0.6a | 3.07 ± 0.2e1 | 0.029 |
| TOS (nmol H2O2 Eqv./mg protein) | 10.4 ± 2.2 | 15.9 ± 2.0b | 12.8 ± 3.112 | 0.003 |
| OSI (Arbitrary Unite) | 3.54 ± 0.7 | 7.76 ± 1.9b | 3.76 ± 0.612 | 0.002 |
| MPO (U/g protein) | 9.4 ± 1.8 | 13.2 ± 1.7b | 11.2 ± 2.2f1 | 0.004 |
| CAT (U/mg protein) | 18.4 ± 3.9 | 10.1 ± 1.9b | 18.3 ± 2.212 | 0.004 |
AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; LDH: Lactate dehydrogenase; TAC: Total antioxidant capacity; TOS: Total oxidative status; OSI: Oxidative stress index; MPO: Myeloperoxidase; CAT: Catalase.
P < 0.05,
P < 0.01, Sham groups vs I/R groups;
P < 0.01, Sham groups vs I/R + NS groups;
P < 0.05,
P < 0.01, I/R groups vs I/R + NS groups.
P > 0.05, Sham groups vs I/R + NS groups;
P < 0.001, I/R groups vs I/R + NS groups.
TAC and CAT activities in liver tissue were significantly higher in Group 3 than those in Group 2 (P < 0.05 and P < 0.001, respectively). However, TAC and CAT activities in liver tissue were significantly lower in Group 2 than those in Group 1 (P < 0.05 and P < 0.01, respectively). TOS and OSI in hepatic tissue were significantly lower in Group 3 than those in Group 2 (P < 0.001 for both). Also MPO levels in hepatic tissue were significantly lower in Group 3 than those in Group 2 (P < 0.01). However, TAS, OSI and MPO levels in hepatic tissue were significantly higher in Group 2 than those in Group 1 (P < 0.01 for all). There were not statistically significant differences between the Nigella sativa treatment group and the sham group regarding to the oxidant and antioxidant parameters (P > 0.05). The results are summarized in Table 1.
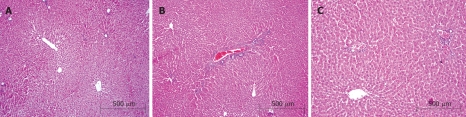
In histopathological evaluation, there were no pathological changes in liver tissue of the sham group (Figure 1A). Liver specimens from rats after ischemia-reperfusion exhibited focal necrosis and infiltration of leukocytes (Figure 1B). Nigella sativa treatment significantly decreased these pathological changes (Figure 1C). Histological tissue damage was milder in the Nigella sativa treatment group than that in the control group.
Figure 1.
A: Normal liver tissue; B: Histopathological findings 60 min after reperfusion in the control group; C: Histopathological findings 60 min after reperfusion in the Nigella sativa treatment group.
Nigella sativa did not produce any adverse side effects in the doses tested in our study.
DISCUSSION
An excessive production of oxygen free radicals has been reported in ischemic reperfused liver, leading to tissue damage, and this is an unavoidable process in liver transplantation and in the surgical procedures in which the Pringle maneuver is used[4]. It has been shown in many studies that supplementation of free radical scavengers is helpful in reducing hepatic ischemia reperfusion induced tissue damage[4,6,8]. Nigella sativa has been identified as a potent antioxidant acting as a free radical scavenger[9]. Therefore, it should not be surprising that Nigella sativa pretreatment has a protective effect on hepatic ischemia reperfusion injury in rats.
In some studies, to reduce the intestinal ischemia reperfusion injury, agents are administrated only before ischemia or reperfusion, and in some other studies both before ischemia and reperfusion. We preferred to administer both before ischemia and reperfusion as reported previously[31].
In the present study Nigella sativa treatment markedly attenuated ALT, AST and LDH activities which are associated with hepatic parenchymal injury. The increase of AST, ALT and LDH activities observed in control groups can be elucidated by lipid peroxidation leading to cytolysis, which is caused by the oxygen free radical formed during the reperfusion phase[32]. The decrease of AST, ALT, and LDH activities observed in the rats treated with Nigella sativa, when compared to the rats in control group, suggests a possible protective effect of Nigella sativa treatment in the hepatic ischemia/reperfusion condition.
Despite determination of either oxidants or antioxidant components alone may give information about oxidative stress, determination of oxidants along with antioxidants is more useful in this context[33]. So, we preferred to measure oxidants and antioxidant capacity simultaneously to assess oxidative stress more exactly. In the present study, we measured oxidative stress with OSI which was detected using both oxidative and antioxidative parameters. We evaluated TAC which reflects the antioxidative status and TOS to investigate oxidative status using a more recently developed measurement methods by Erel[27,28]. Nigella sativa treatment significantly reduced OSI and TOS levels, which show oxidative stress, and increased TAC levels, which show antioxidant capacity, in liver tissue. Oxidative stress activates mechanisms that lead to the synthesis of proinflammatory cytokines and cell adhesion molecules. Therefore, oxidative stress may contribute to an inflammatory response induced by endotoxemia after hepatic ischemia reperfusion. Our data confirm that liver ischemia reperfusion increases oxidative stress, an effect that not only produces direct tissue damage, but also modulates production of toxic cytokines leading to inflammation and leukocyte infiltration, consistent with previous studies. In addition to this, Nigella sativa treatment alleviated pathological structural changes.
Infiltration of neutrophils into tissues is commonly assessed by changes in activity of MPO, which is an enzyme found primarily in neutrophils. Increased MPO activity in the liver of rats after I/R suggests activation of an inflammatory response. In our study, we observed increased MPO activity in the liver tissue, and this may indicate that neutrophil accumulation and lipid peroxidation contributes to ischemia reperfusion-induced liver injury. Previously, it has been reported that the activated neutrophils located in the inflammatory foci and secreting MPO into the extracellular space can convert hydroperoxides into free radicals, triggering lipid peroxidation[34]. This is consistent with the results of our present study.
Catalase is an oxidoreductase enzyme, which transforms H2O2 into H2O and O2. It can protect cells from damage induced by ischemia reperfusion through scavenging reactive oxygen species[8]. The results of the present study showed that treatment with Nigella sativa can increase catalase activity, and this is consistent with its protective effect.
Although preliminary, our data indicate that Nigella sativa exhibits protective effects on liver tissue against ischemia reperfusion injury. The results of this present study may have clinical applications to the liver surgery associated with the Pringle maneuver and hepatic transplantation. However, more investigations are required to evaluate the protective effects of Nigella sativa on liver tissue damage in clinical and experimental models to verify this conclusion.
COMMENTS
Background
Hepatic ischemia-reperfusion (I/R) injury may occur in a variety of clinical settings such as trauma, sepsis, liver transplantation or hepatic pedicle clamping during a liver surgery and this remains a significant problem. Oxygen free radicals, produced on reperfusion have been shown to play a major role in hepatic I/R injury. Various therapeutic effects have been described for Nigella sativa. Additionally, it has been presented that Nigella sativa has protective effect against ischemia reperfusion injury to various organs. Therefore, it seems possible that the administration of Nigella sativa might protect the liver against the ischemia reperfusion injury and thus, we aimed to confirm this hypothesis.
Research frontiers
Tissue ischemia initiates a series of events that can ultimately lead to cellular dysfunction and necrosis. But, resumption of blood flow can paradoxically create more tissue injury, possibly because of the production of oxygen-derived cytotoxic products. It is important to reduce the oxidative stress mechanism and protect the liver tissue against the ischemia and reperfusion injury. This study demonstrated that Nigella sativa exhibits protective effects on liver tissue against ischemia reperfusion injury.
Innovations and breakthroughs
In previous studies, it has been demonstrated that Nigella sativa has protective effects against ischemia reperfusion injury on various organs. However, its protective effects on liver tissue against ischemia reperfusion injury are unclear.
Applications
It seems possible that the administration of Nigella sativa might protect the liver against the ischemia reperfusion injury which can occur due to a liver surgery, trauma or sepsis. Future studies will be required to verify the effectiveness of this substance.
Terminology
Reperfusion injury refers to damage to tissue caused when blood supply returns to the tissue after a period of ischemia. The absence of oxygen and nutrients from blood creates a condition in which the restoration of circulation results in inflammation and oxidative damage through the induction of oxidative stress rather than restoration of normal function.
Peer review
In the present study authors investigated the effects of Nigella sativa, an annual flowering plant, on hepatic ischemia-reperfusion injury. The late effects of Nigella sativa should be evaluated and survival experiments would be helpful.
Footnotes
Peer reviewers: Valentin Fuhrmann, MD, Department of Internal Medicine 4, Intensive Care Unit, Medical University Vienna, Waehringer Guertel 18-20, A-1090 Vienna, Austria; Andrej Khandoga, MD, Institute for Surgical Research Ludwig-Maximilians-University of Munich, Marchioninistr. 27, Munich 81377, Germany
S- Editor Li DL L- Editor Rippe RA E- Editor Ma WH
References
- 1.Shin T, Kuboki S, Huber N, Eismann T, Galloway E, Schuster R, Blanchard J, Pritts TA, Lentsch AB. Activation of peroxisome proliferator-activated receptor-gamma during hepatic ischemia is age-dependent. J Surg Res. 2008;147:200–205. doi: 10.1016/j.jss.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Gulik TM, de Graaf W, Dinant S, Busch OR, Gouma DJ. Vascular occlusion techniques during liver resection. Dig Surg. 2007;24:274–281. doi: 10.1159/000103658. [DOI] [PubMed] [Google Scholar]
- 3.He XS, Ma Y, Wu LW, Wu JL, Hu RD, Chen GH, Huang JF. Dynamical changing patterns of glycogen and enzyme histochemical activities in rat liver graft undergoing warm ischemia injury. World J Gastroenterol. 2005;11:2662–2665. doi: 10.3748/wjg.v11.i17.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hassan-Khabbar S, Cottart CH, Wendum D, Vibert F, Clot JP, Savouret JF, Conti M, Nivet-Antoine V. Postischemic treatment by trans-resveratrol in rat liver ischemia-reperfusion: a possible strategy in liver surgery. Liver Transpl. 2008;14:451–459. doi: 10.1002/lt.21405. [DOI] [PubMed] [Google Scholar]
- 5.Montalvo-Jave EE, Escalante-Tattersfield T, Ortega-Salgado JA, Pina E, Geller DA. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J Surg Res. 2008;147:153–159. doi: 10.1016/j.jss.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polat KY, Aydinli B, Polat O, Aydin U, Yazici P, Ozturk G, Gundogdu C, Kiziltunc A. The protective effect of aprotinin and alpha-tocopherol on ischemia-reperfusion injury of the rat liver. Transplant Proc. 2008;40:63–68. doi: 10.1016/j.transproceed.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 7.Pulitano C, Aldrighetti L. The protective role of steroids in ischemia-reperfusion injury of the liver. Curr Pharm Des. 2008;14:496–503. doi: 10.2174/138161208783597353. [DOI] [PubMed] [Google Scholar]
- 8.Shen SQ, Zhang Y, Xiang JJ, Xiong CL. Protective effect of curcumin against liver warm ischemia/reperfusion injury in rat model is associated with regulation of heat shock protein and antioxidant enzymes. World J Gastroenterol. 2007;13:1953–1961. doi: 10.3748/wjg.v13.i13.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khalife KH, Lupidi G. Nonenzymatic reduction of thymoquinone in physiological conditions. Free Radic Res. 2007;41:153–161. doi: 10.1080/10715760600978815. [DOI] [PubMed] [Google Scholar]
- 10.Kanter M, Coskun O, Uysal H. The antioxidative and antihistaminic effect of Nigella sativa and its major constituent, thymoquinone on ethanol-induced gastric mucosal damage. Arch Toxicol. 2006;80:217–224. doi: 10.1007/s00204-005-0037-1. [DOI] [PubMed] [Google Scholar]
- 11.Morsi NM. Antimicrobial effect of crude extracts of Nigella sativa on multiple antibiotics-resistant bacteria. Acta Microbiol Pol. 2000;49:63–74. [PubMed] [Google Scholar]
- 12.Bayrak O, Bavbek N, Karatas OF, Bayrak R, Catal F, Cimentepe E, Akbas A, Yildirim E, Unal D, Akcay A. Nigella sativa protects against ischaemia/reperfusion injury in rat kidneys. Nephrol Dial Transplant. 2008;23:2206–2212. doi: 10.1093/ndt/gfm953. [DOI] [PubMed] [Google Scholar]
- 13.Hosseinzadeh H, Parvardeh S, Asl MN, Sadeghnia HR, Ziaee T. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia-reperfusion injury in rat hippocampus. Phytomedicine. 2007;14:621–627. doi: 10.1016/j.phymed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 14.El-Abhar HS, Abdallah DM, Saleh S. Gastroprotective activity of Nigella sativa oil and its constituent, thymo-quinone, against gastric mucosal injury induced by ischaemia/reperfusion in rats. J Ethnopharmacol. 2003;84:251–258. doi: 10.1016/s0378-8741(02)00324-0. [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Fattah AM, Matsumoto K, Watanabe H. Antinociceptive effects of Nigella sativa oil and its major component, thymoquinone, in mice. Eur J Pharmacol. 2000;400:89–97. doi: 10.1016/s0014-2999(00)00340-x. [DOI] [PubMed] [Google Scholar]
- 16.Daba MH, Abdel-Rahman MS. Hepatoprotective activity of thymoquinone in isolated rat hepatocytes. Toxicol Lett. 1998;95:23–29. doi: 10.1016/s0378-4274(98)00012-5. [DOI] [PubMed] [Google Scholar]
- 17.Mansour MA, Ginawi OT, El-Hadiyah T, El-Khatib AS, Al-Shabanah OA, Al-Sawaf HA. Effects of volatile oil constituents of Nigella sativa on carbon tetrachloride-induced hepatotoxicity in mice: evidence for antioxidant effects of thymoquinone. Res Commun Mol Pathol Pharmacol. 2001;110:239–251. [PubMed] [Google Scholar]
- 18.Mansour MA, Nagi MN, El-Khatib AS, Al-Bekairi AM. Effects of thymoquinone on antioxidant enzyme activities, lipid peroxidation and DT-diaphorase in different tissues of mice: a possible mechanism of action. Cell Biochem Funct. 2002;20:143–151. doi: 10.1002/cbf.968. [DOI] [PubMed] [Google Scholar]
- 19.Turkdogan MK, Ozbek H, Yener Z, Tuncer I, Uygan I, Ceylan E. The role of Urtica dioica and Nigella sativa in the prevention of carbon tetrachloride-induced hepatotoxicity in rats. Phytother Res. 2003;17:942–946. doi: 10.1002/ptr.1266. [DOI] [PubMed] [Google Scholar]
- 20.Boskabady MH, Javan H, Sajady M, Rakhshandeh H. The possible prophylactic effect of Nigella sativa seed extract in asthmatic patients. Fundam Clin Pharmacol. 2007;21:559–566. doi: 10.1111/j.1472-8206.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- 21.Kalus U, Pruss A, Bystron J, Jurecka M, Smekalova A, Lichius JJ, Kiesewetter H. Effect of Nigella sativa (black seed) on subjective feeling in patients with allergic diseases. Phytother Res. 2003;17:1209–1214. doi: 10.1002/ptr.1356. [DOI] [PubMed] [Google Scholar]
- 22.Dirjomuljono M, Kristyono I, Tjandrawinata RR, Nofiarny D. Symptomatic treatment of acute tonsillo-pharyngitis patients with a combination of Nigella sativa and Phyllanthus niruri extract. Int J Clin Pharmacol Ther. 2008;46:295–306. doi: 10.5414/cpp46295. [DOI] [PubMed] [Google Scholar]
- 23.Akhondian J, Parsa A, Rakhshande H. The effect of Nigella sativa L. (black cumin seed) on intractable pediatric seizures. Med Sci Monit. 2007;13:CR555–CR559. [PubMed] [Google Scholar]
- 24.Kanter M, Coskun O, Budancamanak M. Hepatoprotective effects of Nigella sativa L and Urtica dioica L on lipid peroxidation, antioxidant enzyme systems and liver enzymes in carbon tetrachloride-treated rats. World J Gastroenterol. 2005;11:6684–6688. doi: 10.3748/wjg.v11.i42.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Shenawy NS, Soliman MF, Reyad SI. The effect of antioxidant properties of aqueous garlic extract and Nigella sativa as anti-schistosomiasis agents in mice. Rev Inst Med Trop Sao Paulo. 2008;50:29–36. doi: 10.1590/s0036-46652008000100007. [DOI] [PubMed] [Google Scholar]
- 26.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–119. doi: 10.1016/j.clinbiochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Bolukbas C, Bolukbas FF, Horoz M, Aslan M, Celik H, Erel O. Increased oxidative stress associated with the severity of the liver disease in various forms of hepatitis B virus infection. BMC Infect Dis. 2005;5:95. doi: 10.1186/1471-2334-5-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei H, Frenkel K. Relationship of oxidative events and DNA oxidation in SENCAR mice to in vivo promoting activity of phorbol ester-type tumor promoters. Carcinogenesis. 1993;14:1195–1201. doi: 10.1093/carcin/14.6.1195. [DOI] [PubMed] [Google Scholar]
- 30.Goth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991;196:143–151. doi: 10.1016/0009-8981(91)90067-m. [DOI] [PubMed] [Google Scholar]
- 31.Sener G, Tosun O, Sehirli AO, Kacmaz A, Arbak S, Ersoy Y, Ayanoglu-Dulger G. Melatonin and N-acetylcysteine have beneficial effects during hepatic ischemia and reperfusion. Life Sci. 2003;72:2707–2718. doi: 10.1016/s0024-3205(03)00187-5. [DOI] [PubMed] [Google Scholar]
- 32.Chouker A, Martignoni A, Schauer RJ, Dugas M, Schachtner T, Kaufmann I, Setzer F, Rau HG, Lohe F, Jauch KW, et al. Alpha-gluthathione S-transferase as an early marker of hepatic ischemia/reperfusion injury after liver resection. World J Surg. 2005;29:528–534. doi: 10.1007/s00268-004-7431-3. [DOI] [PubMed] [Google Scholar]
- 33.Tarpey MM, Wink DA, Grisham MB. Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. Am J Physiol Regul Integr Comp Physiol. 2004;286:R431–R444. doi: 10.1152/ajpregu.00361.2003. [DOI] [PubMed] [Google Scholar]
- 34.Cetinkaya A, Bulbuloglu E, Kurutas EB, Kantarceken B. N-acetylcysteine ameliorates methotrexate-induced oxidative liver damage in rats. Med Sci Monit. 2006;12:BR274–BR278. [PubMed] [Google Scholar]