Abstract
Objective
Intracranial arteriovenous malformation (AVM) associated with aneurysm has been infrequently encountered and the treatment for this malady is challenging. We report here on our clinical experience with AVMs associated with arterial aneurysms that were managed by multimodality treatments, including clipping of the aneurysm, microsurgery, Gamma-knife radiosurgery (GKS) and Guglielmi detachable coil (GDC) embolization.
Methods
We reviewed the treatment plans, radiological findings and clinical courses of 21 patients who were treated with GKS for AVM associated with aneurysm.
Results
Twenty-seven aneurysms in 21 patients with AVMs were enrolled in this study. Hemorrhage was the most frequent presenting symptom (17 patients : 80.9%). Bleeding was caused by an AVM nidus in 11 cases, aneurysm rupture in 5 and an undetermined origin in 1. Five patients were treated for associated aneurysm with clipping followed by GKS for the AVM and 11 patients were treated with GDC embolization combined with GKS for an AVM. Although 11 associated aneurysms remained untreated after GKS, none of them ruptured and 4 aneurysms regressed during the follow up period. Two aneurysms increased in size despite the disappearance of the AVM nidus after GKS and then these aneurysms were treated with GDC embolization.
Conclusion
If combined treatment using microsurgery, GKS and endovascular treatment can be adequately used for these patients, a better prognosis can be obtained. In particular, GKS and GDC embolization are considered to have significant roles to minimize neurologic injury.
Keywords: Arteriovenous malformation, Aneurysm, Radiosurgery, Endovascular treatment, GDC embolization
INTRODUCTION
The coexistence of intracranial aneurysms and arteriovenous malformations (AVMs) are rare complex vascular lesions and intracranial aneurysms coexist with approximately 15% of all AVMs1,3,31). The risk of hemorrhage in patients with AVMs and associated aneurysms was reported to be 7% per year, which is higher than the 3% risk of hemorrhage for patients with only AVMs3). Moreover, the risk for hemorrhage in patients with AVMs with intranidal aneurysms has been reported to be 9.8%-per-year31).
Management of a patient suffering AVMs and associated aneurysms is challenging because of the complex AVM-aneurysm hemodynamic relationship. It has been recommended that associated aneurysms should be treated initially or simultaneously with the AVMs1,4,35). Although one stage operation for treating both lesions is ideal and the recently advanced microsurgical techniques facilitate successful surgery, the complications related to this surgery can be fatal. The recent consensus for the management of complex cerebrovascular lesions emphasizes that safety is more important than efficacy when surgery is used to treat these combined lesions. Following the recent developments, AVMs and aneurysms have been successfully treated not only by craniotomy, but also by non-surgical treatment modalities including radiosurgery and embolization with Guglielmi detachable coil (GDC). Therefore, a proper combination of multiple treatment modalities in accordance with the clinical presentation and the angio-architectural relationship is helpful to achieve reliable clinical outcomes. This report represents the authors' experiences with 21 patients who had AVMs and associated arterial aneurysms, and we suggest that radiosurgery and GDC embolization can be an effective option to minimize the complications.
MATERIALS AND METHODS
The authors retrospectively reviewed the medical records and the angiographic findings of 21 patients who were diagnosed with AVMs and accompanying intracranial arterial aneurysms between March 2001 and December 2005. The patients who presented with AVM associated with pseudoaneurysms or venous aneurysms were excluded from this analysis. All the patients were treated by Gamma-knife radiosurgery (GKS) for their AVM, and the accompanying aneurysms were managed by multi-disciplinary treatment methods.
Radiosurgery for an AVM
After attaching a Leksell stereotactic instrument to a skull, cerebral angiography and magnetic resonance imaging were done in all cases. A GKS (Leksell Gamma Knife Type 2,3004B) was used for the treatment of the nidus of the AVM. The mean volume of the nidus was 4,029 mm3 (165-25,308 mm3) and the mean marginal radiation dose was 22.6 Gy (16-25 Gy) and the mean maximum dose was 37.1 Gy (28.6-41.7 Gy). In case of a feeding pedicle aneurysm, we didn't include this type of aneurysm in the radiosurgical target. Coexistence of arterial aneurysms did not influence the radiosurgical planning of AVMs. So, radiosurgery for AVMs with arterial aneurysms was identical to AVMs without aneurysms.
Classification of the associated arterial aneurysms
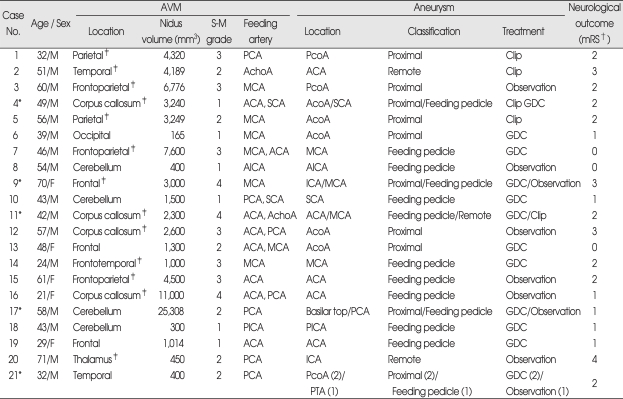
Because of the complex aneurysm-AVM blood flow relationship, classifying the associated aneurysms according to the angio-architectural relation between the feeding artery to the AVMs and the aneurysm location is mandatory for planning treatment. We defined the type of the associated arterial aneurysms according to the location of the artery harboring the aneurysm relative to the feeding artery of the AVM according to the categories established by Perata et al.27) (Table 1). The type of aneurysm was defined as "a feeding pedicle" if the aneurysm was located on a direct feeding vessel to the AVM, as "proximal in location" if it had arisen from the circle of Willis origin of an artery supplying to the AVM and as "remote" if the artery harboring the aneurysm was not involved in the AVM supply.
Table 1.
Angiographic findings and treatment of aneurysm
*Case No. 4, 9, 11, 17, 21 patients had multiple arterial aneurysms, †The modified Rankin Scale (mRS) is a measure of function, grades are defined as follows : 0-no symptoms; 1-no significant disability despite symptoms, able to carry out all usual duties and activities; 2-slight disability, unable to carry out all previous activities but able to look after own affairs without assistance; 3-moderate disability, requiring some help but able to walk without assistance; 4-moderately severe disability, unable to walk without assistance and unable to attend to own bodily needs without assistance; 5-severe disability, bedridden, incontinent, and requiring constant nursing care and attention; 6 represents death, ‡AVMs located on eloquent area (total 13). ACA : anterior cerebral artery, AcoA : anterior communicating artery, AchoA : anterior choroidal artery, AICA : anterior inferior cerebellar artery, GDC : intraaneurysmal embolization with Guglielmi detachable coil, ICA : internal cerebral artery, MCA : middle cerebral artery, PCA : posterior cerebral artery, PcoA : posterior communicating artery, PICA : posterior inferior cerebellar artery, PTA : posterior temporal artery, SCA : superior cerebellar artery, S-M Grade : Spetzler-Martin Grade
The majority of associated aneurysms in this analysis were incidentally recognized during angiography. Yet, 4 patients presented with spontaneous subarachnoid hemorrhage or a ruptured aneurysmal sac that was identified on the operative field. For these patients, the initial treatment was concentrated on the symptomatic aneurysms. We discriminated between the ruptured and unruptured aneurysms among multiple aneurysms in the cases of aneurysmal rupture at the time of the initial clinical presentation based on the location of the hematoma as related to the conventional angiographic findings and the operative findings.
Follow up
Follow-up lasted for a mean of 48 months (range : 24-84 months). All patients were monitored by means of conventional angiography at 24-36 months after the last treatment to identify the obliteration of the nidus or the size change of the associated aneurysms. Neurological outcomes were based on the modified Rankin Scale (mRS) at the last follow up date.
RESULTS
Clinical presentation and the associated arterial aneurysms
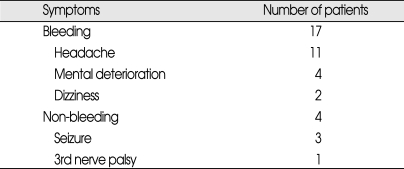
The hemorrhage was the most frequently presented finding (17 of the 21 patients) treated with GKS (Table 2). The bleeding focus was determined by the location and distribution of the hematoma on the computed tomography (CT) and this was correlated with the angiography and the operative findings. Bleeding was caused by an AVM nidus in 11 cases, by aneurysm rupture in 5 cases and by an undetermined origin in 1. Interestingly, one patient presented with simultaneous bleeding at both an aneurysm and an AVM. Four patients had no history of hemorrhage, and three of these suffered seizure and the other complained of third nerve palsy due to compression from a posterior communicating artery aneurysm, which was then treated by clipping.
Table 2.
Initial clinical presentation of 21 patients
There were a total of 27 aneurysms in 21 patients and five patients had AVMs with multiple arterial aneurysms. The associated aneurysms were the feeding pedicle type for 13 aneurysms (48.2%), the proximal in location type for 11 (40.7%) and the remote type for 3 (11.1%).
Radiosurgical outcome of the AVMs
All 21 patients underwent GKS, either as the initial treatment modality (14 patients) or as an adjuvant therapy following the treatment of aneurysms (7 patients). On the follow-up angiography, sixteen irradiated AVMs (76.2%) showed complete obliteration, 3 (14.3%) partial obliteration and 2 (9.5%) subtotal obliteration. In these case series, two patients (case No. 16, 17) had large AVMs (10 cm3 or more in volume). Our strategy of radiosurgery for large AVMs were as follows : 1) marginal dose lower than 20 Gy, 2) optimal treatment planning for minimizing superfluous irradiation (If enlarged draining veins often occupying nidus on angiography are precisely excluded, pure volume of nidus may be smaller than the volume simply estimated based on imaging studies, and 3) in case of AVM which is larger than 14 cm3 in volume and has several hemodynamically remote feeding arteries, the staged volumetric radiosurgery is considered to be the option. Therefore, in these two patients, the marginal dose was each 19 Gy and 20 Gy. Although case No. 17 was 25308 mm3 in volume, he underwent the single session radiosurgery because the nidus was supplied by single vessel (PCA). In the present study, despite the mean 22.6 Gy-marginal dose and the mean 37.1 Gy-maximum dose, there was no radiosurgical complication, such as adverse radiation effects and post-treatment hemorrhage. We think that these results may be attributed to precise measurement of the target volume and the optimal planning is no less critical than the low-dose radiation to protect normal surrounding structures. However, the number of these cases is too small to discuss which is more potent factor to avoid the radiosurgical complications between the optimal planning and low radiation dose.
Outcome of the associated arterial aneurysms
Of all 27 aneurysms in this study, 5 aneurysms were treated with clipping and 11 were treated with GDC embolization. Nine associated aneurysms remained untreated because of their broad neck or the small sized sac of the aneurysms. Two cases inevitably remained untreated because of unsuccessful GDC embolization. The changes in the size of these eleven aneurysms in 11 patients that were treated conservatively after the GKS for the AVMs were analyzed with a follow-up angiography. Equivocal change was seen in 5 patients and regression was seen in 4 (disappeared 3, decreased 1). The all spontaneous regressed aneurysms (4 of 11) were the feeding pedicle type. In two patients, even though their aneurysms were located in feeding pedicular artery and their AVMs were complete obliterated after GKS, the size of the aneurysmal sac was increased, and both patients were treated with GDC embolization without delay. There were no hemorrhagic events during the follow up periods.
DISCUSSION
The mechanisms of the AVM associated with aneurysm can be explained with several hypotheses2,11,24,26,36). At first, the aneurysm may occur due to hemodynamic stress related to the AVM. Second, both lesions are congenital malformations of vascular development. Third, these associations can accidentally exist togeather19). Among these hypothesis, the hemodynamic stress secondary to the increased blood flow of AVMs plays a significant role to provoke this coexistence of lesions1,5,7,10-13,15,17,18,21,23,25). This hypothesis is supported by a report showing that the associated aneurysms tend to be concentrated in feeding arteries which supply AVMs25) and the associated aneurysms may shrink in proportion to the occlusion of the AVMs6,14,17,18,22,31,35,37).
In spite of an incomplete understanding of the hemodynamic relationship between AVMs and their associated aneurysms, the hypothesis that decreased blood flow through the feeding artery following an AVM's obliteration might induce shrinkage or complete obliteration of the associated aneurysms has permitted treating the AVMs first, except for a patient whose initial clinical presentation is SAH. Even though there are opinions that elimination of AVMs may increase the intra-aneurysm pressure and the associated aneurysm may be prone to impending rupture1,10), as the AVMs are gradually occluded, the risk for aneurysmal rupture decreases because the blood flow and shear stress through the feeding artery are more critical hemodynamic factors than the arterial pressure12).
The previous published studies have suggested that the simultaneous surgical approach for treating both lesions in a single operation is the best option1,4,35). If a ruptured aneurysm is close to the nidus, then a one stage operation can be performed with a high success rate because the parenchymal hemorrhage from the aneurysm contributes to easy accessibility. The close anatomical relationship between the lesions facilitates eliminating both the nidus and the aneurysm simultaneously. Other reports have suggested that the associated aneurysm must be preventively eliminated because the mortality and morbidity related to aneurysmal rupture that is higher than for AVM's rupture6,20,28). Moreover, there is a low chance of rebleeding from an AVM compared to that from an aneurysm. However, the abrupt change of the hemodynamics due to the resection of an AVM may cause massive hemorrhage from the associated aneurysms1), and the intraoperative hemorrhage from a ruptured aneurysm may be fatal if proximal control of the artery harboring the aneurysm is not feasible.
In this case series, 16 of all 21 patients (76.2%) were managed by non-surgical treatment modalities such as GKS and GDC embolization with a relatively low complication rate. The efficacy and safety of radiosurgery for AVMs have been verified9,16,30,33,38) and GDC embolization is considered to be a useful treatment for aneurysms associated with the AVMs treated by radiosurgery8). For patients presenting with SAH and incidental AVMs, the symptomatic aneurysms were treated first and the AVMs were next treated with radiosurgery. We tried to treat the incidentally encountered aneurysms by GDC embolization or surgical clipping when possible. Nevertheless, in nine cases, the aneurysms were too small and broad necked that the best course was simply to observe them on follow-up. In 2 cases, GDC embolization failed because the location of the associated aneurysm was too distal. Therefore, 11 aneurysms were kept under clinical observation after GKS. On the follow up angiography, we found that 9 cases of aneurysm were decreased or stable after the complete obliteration of the nidus of the AVM. These clinical courses of the unsecured aneurysms can be attributed to the radiosurgical effect on the aneurysm, which is secondary to the decreased blood flow into the nidus.
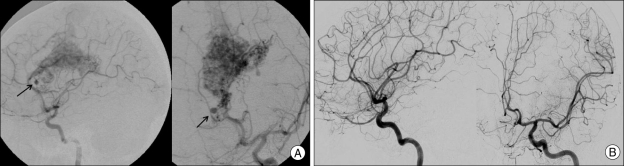
The rate for spontaneous regression of untreated feeding pedicular aneurysm after GKS for AVMs was 50% (4 of 8) and these regressed aneurysms were mainly located on the distal portion of the feeder to the nidus. Redekop et al.31) reported on the effect of AVM treatment on the aneurysm and they estimated the spontaneous regression rate of the feeding artery aneurysms that were between the proximal and distal pedicular artery. They revealed that the associated aneurysms on the distal pedicular feeder are easier to regress than those on the proximal pedicular feeder. This result suggests that associated aneurysms are more susceptible to regression in response to decreased blood flow into the nidus by the radiosurgical effect in the case that a distal branch of the artery harboring the associated aneurysm supplies only the nidus (Fig. 1).
Fig. 1.
Pre-radiosurgical and post-radiosurgical angiography show spontaneous regression of untreated feeding pedicular aneurysm after GKS. A : On angiographic examination of GKS, an aneurysm is detected at the distal feeding pedicualr artery. B : Fifty months after irradiation, angiography demonstrating obliterated nidus of AVM and related aneurysm. AVM : arteriovenous malformation, GKS : Gamma-knife radiosurgery.
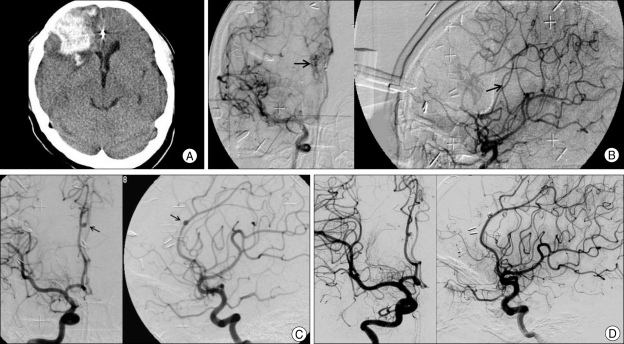
Yet, in this case series, two untreated feeding pedicular aneurysms were rather enlarged in size despite complete obliteration of the AVMs and both of these aneurysms were occluded by GDC embolization without delay. One of these two cases is illustrated in Fig. 2. This case implies that if the stepwise obliteration of the nidus induces the diversion of blood flow from the nidus to the neighboring brain tissue, then the blood flow through the branching arteries of the direct feeder supplying the nidus may increase and the blood flow through the proximal portion of the direct feeder supplying the nidus may paradoxically increase. Consequently, the flow-related aneurysm located on the proximal portion of the feeding artery has a chance to increase in size in spite of AVM obliteration.
Fig. 2.
Case of AVM with newly developed aneurysm after radiosurgery. A 21-year-old female patient with previous microsurgery for AVM nidus presented with intracranial hemorrhage on right frontal lobe on CT scan (A). On angiographic examination (B). remained nidus is detected and treated by Gamma knife (marginal dose 22 Gy). On follow-up angiographic examination, nidus of AVM is obliterated but de novo aneurysm (arrow) is detected at the previous feeding artery (C). This aneurysm was treated with GDC embolization and demonstrating of complete obliteration of nidus and coiled aneurysm after 2 years follow-up (D). AVM : arteriovenous malformation, GDC : Guglielmi detachable coil.
For the cases of remote aneurysms, their coexistence with AVMs can be explained by coincidental theory rather than by hemodynamic stress theory, and managing remote aneurysms in a fashion similar to general aneurysms that are without AVMs may be reasonable25,32). The fate of unsecured aneurysms with partial or complete obliterated AVMs remains unpredictable and the regression, enlargement and de novo aneurysm formation after substantial AVM therapy have been illustrated17,29,31,34). Therefore, close follow up of an unsecured aneurysm is mandatory, and complementary treatment should be provided for the associated aneurysm with their increased size after radiosurgery for the AVMs.
CONCLUSION
AVMs associated with aneurysm have the characteristics of a low incidence, a high bleeding tendency and they are difficult to manage. Treatment with GKS and GDC embolization is considered to have a significant role to minimize the neurologic injury. Furthermore, radiosurgery may be valuable as an initial single treatment modality for the AVMs associated with flow-related aneurysms, except for aneurysmal rupture. However, there is a chance that flow-related aneurysms will increase in size according to the unpredictable change of hemodynamics after curative treatment for an AVM, and so angiographic follow up and complementary treatments are important.
References
- 1.Batjer H, Suss RA, Samson D. Intracranial arteriovenous malformations associated with aneurysms. Neurosurgery. 1986;18:29–35. doi: 10.1227/00006123-198601000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Boyd-Wilson JS. The association of cerebral angiomas with intracranial aneurysms. J Neurol Neurosurg Psychiatry. 1959;22:218–223. doi: 10.1136/jnnp.22.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown RD, Jr, Wiebers DO, Forbes GS. Unruptured intracranial aneurysms and arteriovenous malformations : frequency of intracranial hemorrhage and relationship of lesions. J Neurosurg. 1990;73:859–863. doi: 10.3171/jns.1990.73.6.0859. [DOI] [PubMed] [Google Scholar]
- 4.Cockroft KM, Thompson RC, Steinberg GK. Aneurysms and arteriovenous malformations. Neurosurg Clin N Am. 1998;9:565–576. [PubMed] [Google Scholar]
- 5.Cronqvist S, Troupp H. Intracranial arteriovenous malformation and arterial aneurysm in the same patient. Acta Neurol Scand. 1966;42:307–316. doi: 10.1111/j.1600-0404.1966.tb01185.x. [DOI] [PubMed] [Google Scholar]
- 6.Cunha e Sa MJ, Stein BM, Solomon RA, McCormick PC. The treatment of associated intracranial aneurysms and arteriovenous malformations. J Neurosurg. 1992;77:853–859. doi: 10.3171/jns.1992.77.6.0853. [DOI] [PubMed] [Google Scholar]
- 7.Deruty R, Mottolese C, Soustiel JF, Pelissou-Guyotat I. Association of cerebral arteriovenous malformation and cerebral aneurysm. Diagnosis and management. Acta Neurochir (Wien) 1990;107:133–139. doi: 10.1007/BF01405792. [DOI] [PubMed] [Google Scholar]
- 8.Ezura M, Takahashi A, Jokura H, Shirane R, Yoshimoto T. Endovascular treatment of aneurysms associated with cerebral arteriovenous malformations : experiences after the introduction of Guglielmi detachable coils. 7 Suppl. J Clin Neurosci. 2000;7(Suppl 1):14–18. doi: 10.1054/jocn.2000.0703. [DOI] [PubMed] [Google Scholar]
- 9.Friedman WA, Bova FJ, Mendenhall WM. Linear accelerator radiosurgery for arteriovenous malformations: the relationship of size to outcome. J Neurosurg. 1995;82:180–189. doi: 10.3171/jns.1995.82.2.0180. [DOI] [PubMed] [Google Scholar]
- 10.Fuwa I, Matsukado Y, Kaku M, Nonaka S. Enlargement of a cerebral aneurysm associated with ruptured arteriovenous malformation. Acta Neurochir (Wien) 1986;80:65–68. doi: 10.1007/BF01809560. [DOI] [PubMed] [Google Scholar]
- 11.Gács G, Vinuela F, Fox AJ, Drake CG. Peripheral aneurysms of the cerebellar arteries. Review of 16 cases. J Neurosurg. 1983;58:63–68. doi: 10.3171/jns.1983.58.1.0063. [DOI] [PubMed] [Google Scholar]
- 12.Gao E, Young WL, Pile-Spellman J, Joshi S, Duong H, Stieg PE, et al. Cerebral arteriovenous malformation feeding artery aneurysms : a theoretical model of intravascular pressure changes after treatment. Neurosurgery. 1997;41:1345–1356. doi: 10.1097/00006123-199712000-00020. discussion 1356-1358. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi S, Arimoto T, Itakura T, Fujii T, Nishiguchi T, Komai N. The association of intracranial aneurysms and arteriovenous malformation of the brain. Case report. J Neurosurg. 1981;55:971–975. doi: 10.3171/jns.1981.55.6.0971. [DOI] [PubMed] [Google Scholar]
- 14.Hodozuka A, Sako K, Yonemasu Y, Suzuki N, Fujita T, Ohgami S. [Spontaneous disappearance of aneurysm after total removal of accompanying intracranial arteriovenous malformation Case report] . Neurol Med Chir (Tokyo) 1991;31:966–971. doi: 10.2176/nmc.31.966. [DOI] [PubMed] [Google Scholar]
- 15.Kader A, Young WL, Pile-Spellman J, Mast H, Sciacca RR, Mohr JP, et al. The influence of hemodynamic and anatomic factors on hemorrhage from cerebral arteriovenous malformations. Neurosurgery. 1994;34:801–807. doi: 10.1227/00006123-199405000-00003. discussion 807-808. [DOI] [PubMed] [Google Scholar]
- 16.Kjellberg RN, Hanamura T, Davis KR, Lyons SL, Adams RD. Bragg-peak proton-beam therapy for arteriovenous malformations of the brain. N Engl J Med. 1983;309:269–274. doi: 10.1056/NEJM198308043090503. [DOI] [PubMed] [Google Scholar]
- 17.Kondziolka D, Nixon BJ, Lasjaunias P, Tucker WS, TerBrugge K, Spiegel SM. Cerebral arteriovenous malformations with associated arterial aneurysms : hemodynamic and therapeutic considerations. Can J Neurol Sci. 1988;15:130–134. doi: 10.1017/s0317167100027487. [DOI] [PubMed] [Google Scholar]
- 18.Lasjaunias P, Piske R, Terbrugge K, Willinsky R. Cerebral arteriovenous malformations (C. AVM) and associated arterial aneurysms (AA). Analysis of 101 C. AVM cases, with 37 AA in 23 patients. Acta Neurochir (Wien) 1988;91:29–36. doi: 10.1007/BF01400524. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Zhu S, Jiao L, Wang H, Li X, Li G. Cerebral arteriovenous malformations associated with aneurysms--a report of 10 cases and literature review. J Clin Neurosci. 2000;7:254–256. doi: 10.1054/jocn.1999.0206. [DOI] [PubMed] [Google Scholar]
- 20.Locksley H. Natural history of subarachnoid hemorrhage, intracranial aneurysms and arteriovenous malformations. Based on 6368 cases in the cooperative study. J Neurosurg. 1966;25:219–239. doi: 10.3171/jns.1966.25.2.0219. [DOI] [PubMed] [Google Scholar]
- 21.Mckissock W, Paterson JH. A clinical survey of intracranial angiomas with special reference to their mode of progression and surgical treatment : a report of 110 cases. Brain. 1956;79:233–266. doi: 10.1093/brain/79.2.233. [DOI] [PubMed] [Google Scholar]
- 22.Meisel HJ, Mansmann U, Alvarez H, Rodesch G, Brock M, Lasjaunias P. Cerebral arteriovenous malformations and associated aneurysms : analysis of 305 cases from a series of 662 patients. Neurosurgery. 2000;46:793–800. doi: 10.1097/00006123-200004000-00004. discussion 800-802. [DOI] [PubMed] [Google Scholar]
- 23.Miyasaka K, Wolpert SM, Prager RJ. The association of cerebral aneurysms, infundibula, and intracranial arteriovenous malformations. Stroke. 1982;13:196–203. doi: 10.1161/01.str.13.2.196. [DOI] [PubMed] [Google Scholar]
- 24.Noterman J, Georges P, Brotchi J. Arteriovenous malformation associated with multiple aneurysms in the posterior fossa : a case report with a review of the literature. Neurosurgery. 1987;21:387–391. doi: 10.1227/00006123-198709000-00019. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto S, Handa H, Hashimoto N. Location of intracranial aneurysms associated with cerebral arteriovenous malformation : statistical analysis. Surg Neurol. 1984;22:335–340. doi: 10.1016/0090-3019(84)90135-6. [DOI] [PubMed] [Google Scholar]
- 26.Ostergaard JR. Association of intracranial aneurysm and arteriovenous malformation in childhood. Neurosurgery. 1984;14:358–362. doi: 10.1227/00006123-198403000-00020. [DOI] [PubMed] [Google Scholar]
- 27.Perata HJ, Tomsick TA, Tew JM., Jr Feeding artery pedicle aneurysms : association with parenchymal hemorrhage and arteriovenous malformation in the brain. J Neurosurg. 1994;80:631–634. doi: 10.3171/jns.1994.80.4.0631. [DOI] [PubMed] [Google Scholar]
- 28.Perret G, Nishioka H. Report on the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. Section VI. Arteriovenous malformations. An analysis of 545 cases of cranio-cerebral arteriovenous malformations and fistulae reported to the cooperative study. J Neurosurg. 1966;25:467–490. doi: 10.3171/jns.1966.25.4.0467. [DOI] [PubMed] [Google Scholar]
- 29.Pollock BE, Flickinger JC, Lunsford LD, Bissonette DJ, Kondziolka D. Hemorrhage risk after stereotactic radiosurgery of cerebral arteriovenous malformations. Neurosurgery. 1996;38:652–659. discussion 659-661. [PubMed] [Google Scholar]
- 30.Pollock BE, Flickinger JC, Lunsford LD, Maitz A, Kondziolka D. Factors associated with successful arteriovenous malformation radiosurgery. Neurosurgery. 1998;42:1239–1244. doi: 10.1097/00006123-199806000-00020. discussion 1244-1247. [DOI] [PubMed] [Google Scholar]
- 31.Redekop G, TerBrugge K, Montanera W, Willinsky R. Arterial aneurysms associated with cerebral arteriovenous malformations : classification, incidence, and risk of hemorrhage. J Neurosurg. 1998;89:539–546. doi: 10.3171/jns.1998.89.4.0539. [DOI] [PubMed] [Google Scholar]
- 32.Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms : a systematic review. Stroke. 1998;29:251–256. doi: 10.1161/01.str.29.1.251. [DOI] [PubMed] [Google Scholar]
- 33.Steiner L, Lindquist C, Adler JR, Torner JC, Alves W, Steiner M. Clinical outcome of radiosurgery for cerebral arteriovenous malformations. J Neurosurg. 1992;77:1–8. doi: 10.3171/jns.1992.77.1.0001. [DOI] [PubMed] [Google Scholar]
- 34.Stiefel MF, Al-Okaili R, Weigele JB, Hurst RW. De novo aneurysm formation and regression after brain arteriovenous malformation embolization : case report. Surg Neurol. 2007;67:99–101. doi: 10.1016/j.surneu.2006.02.046. discussion 101. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki J, Onuma T. Intracranial aneurysms associated with arteriovenous malformations. J Neurosurg. 1979;50:742–746. doi: 10.3171/jns.1979.50.6.0742. [DOI] [PubMed] [Google Scholar]
- 36.Tran-Dinh H, Williams LM, Jayasinghe LS. Association of intracranial aneurysm and arteriovenous malformation. Med J Aust. 1983;1:521–523. doi: 10.5694/j.1326-5377.1983.tb136196.x. [DOI] [PubMed] [Google Scholar]
- 37.Vymazal J, Liscàk R, Novotny J, Jr, Janousková L, Vladyka V. The role of Gamma Knife radiosurgery in arteriovenous malformation with aneurysms. Stereotact Funct Neurosurg. 1999;72(Suppl 1):175–184. doi: 10.1159/000056454. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto M, Barfod BE. Gamma knife radiosurgery for arteriovenous malformations : past hope and present reality. J Korean Neurosurg Soc. 2006;39:1–10. [Google Scholar]