Abstract
Objective
To compare two testing protocols for evaluating range of motion (ROM) changes in the preloaded cadaveric spines implanted with a mobile core type Charité™ lumbar artificial disc.
Methods
Using five human cadaveric lumbosacral spines (L2-S2), baseline ROMs were measured with a bending moment of 8 Nm for all motion modes (flexion/extension, lateral bending, and axial rotation) in intact spine. The ROM was tracked using a video-based motion-capturing system. After the Charité™ disc was implanted at the L4-L5 level, the measurement was repeated using two different methods : 1) loading up to 8 Nm with the compressive follower preload as in testing the intact spine (Load control protocol), 2) loading in displacement control until the total ROM of L2-S2 matches that when the intact spine was loaded under load control (Hybrid protocol). The comparison between the data of each protocol was performed.
Results
The ROMs of the L4-L5 arthroplasty level were increased in all test modalities (p < 0.05 in bending and rotation) under both load and hybrid protocols. At the adjacent segments, the ROMs were increased in all modes except flexion under load control protocol. Under hybrid protocol, the adjacent segments demonstrated decreased ROMs in all modalities except extension at the inferior segment. Statistical significance between load and hybrid protocols was observed during bending and rotation at the operative and adjacent levels (p < 0.05).
Conclusion
In hybrid protocol, the Charité™ disc provided a relatively better restoration of ROM, than in the load control protocol, reproducing clinical observations in terms of motion following surgery.
Keywords: Range of motion, Lumbar spinal arthroplasty, Charité™, Follower preload, Load control protocol, Hybrid protocol
INTRODUCTION
Maintaining the motion of joints rather than obliterating movement has been widely accepted in the appendicular skeleton where total hip and knee replacement have became two of the most successful surgical procedures performed today. Intervertebral disc arthroplasty has been a subject of great interest and debate since the concept of replacing a portion, or all, of the intervertebral disc was introduced over 45 years ago. The surgical community is beginning to accept the concept of arthroplasty over arthrodesis in spinal applications. The goals of spine arthroplasty are to reduce and/or eliminate the fusion problems, namely; prolonged recuperation time, pseudoarthrosis, donor site morbidity and potentials for adjacent disc degeneration. Similar to fusion, replacing the degenerated disc with a prosthetic implant removes the proinflammatory tissues and potentially the primary pain generator and restores alignment while preserving functional motion. Motion preservation may decrease the incidence of symptomatic disc degeneration at the adjacent levels which is linked to fusion9,14,15,21). In the United States, only the Charité™ (DePuy Spine, Raynham, MA, USA) and the ProDisc (Synthes Spine, Paoli, PA, USA) are currently commercially available among the numerous lumbar artificial discs (ADs) in varying developmental stages.
In vitro testing of the AD in cadaveric spines helps to predict the range of motion (ROM) of the implant and adjacent spinal segments. The load-carrying capacity of the lumbar spine can be increased under a compressive follower load, as long as the load path remains within a small range around the estimated centers of rotation of the lumbar segments33). The follower load path provides an explanation of how the whole lumbar spine can be lordotic and yet resist large compressive loads20). The method for applying a compressive follower preload to the multisegmented spine specimen (L2-S2) was adapted from a published method so that its path approximated the tangent of the curve of the lumbar spine20,33).
To our knowledge, there have been few reports on the biomechanical study of the AD under a physiologic compressive preload. The classic flexibility testing protocol was reported not to be appropriate for the understanding of the biomechanics of the construct at the adjacent levels, and hybrid testing protocol was advocated recently16). The authors evaluated the biomechanical performance of the preloaded human cadaveric spine implanted with the Charité™ AD, compared with the intact spine, using two different methods : load control protocol and hybrid protocol.
MATERIALS AND METHODS
Cadaveric specimen preparation and fixation
Nine human cadaveric lumbosacral spines (L2-S2) were obtained from Science Care Anatomical (Phoenix, AZ, USA), and International Biological, Inc. (Grosse Pointe Farms, MI, USA). After specimens containing osseous abnormalities were excluded based upon anteroposterior and lateral radiographs, five (two males and three females) cadaveric spines were selected for the study. Bone mineral density (BMD) was measured by using dual-energy X-ray absorptiometry with a bone densitometer (Hologic QDR 4500A; Hologic, Inc., Waltham, MA, USA). For biomechanical testing, en bloc specimens were stored at -20℃ until thawed at room temperature prior to manipulation, and were kept moist during all procedures. The attached paravertebral musculature was adequately removed to expose the facet surfaces of the vertebrae, avoiding disruption to the joint capsules, ligaments, discs, and bone structures.
Each lumbosacral spine was fixed by drilling and fixing screws to the highest and the lowest segments. The end segments and screws were cast into two potting fixtures with polymethylmethacrylate (PMMA, COE tray plastic, GC America, Alsip, IL, USA), and the PMMA-covered ends were potted in polyester resin (Bondo, Atlanta, GA, USA)20).
Discectomy and artificial disc implantation
Load-testing was performed with the spine in the intact state prior to any surgical procedure. Throughout the testing cycle, specimens were kept moist with lukewarm saline.
The Charité™ AD (Fig. 1) was placed in a 36℃ saline bath for 72 hours prior to implantation, so the discs were near biophysiological condition. Anterior discectomy was performed at the L4-L5 level with appropriate ring and cup curettes. Posterior osteophytes and the posterior longitudinal ligament were excised while maintaining the integrity of the lateral annulus.
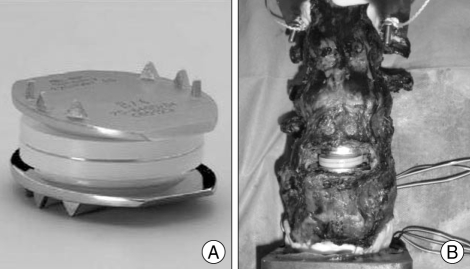
Fig. 1.
Photographs showing the Charité™(DePuy Spine, Raynham, MA, USA) alone (A) and implanted in the human cadaveric lumbosacral spine (B).
The device was implanted in the discectomy defect per manufacturer's specifications, and according to the previously reported surgical technique30,32). The midline of the spinal column was marked by placing a screw in the vertebral body above the index disc. This was confirmed radiographically by anteroposterior fluoroscopy before discectomy. A complete discectomy was made while preserving the lateral circumferential attachments of the annulus fibrosus. Parallel distraction and restoration of the normal intervertebral disc height was accomplished with the use of the central spreader and twisting distracting chisels of graded widths from 7.5 to 9.5 mm. Good coverage of the cross-sectional area of the vertebral endplates was optimized by trying different sizing templates and checking the fit intraoperatively with fluoroscopy. The trials for the artificial disc were available in four sizes (size 2, 3, 4 and 5) depending on the size of the cadaveric specimen, and size 3 and 4 were adequate for most of the specimens. The endplates of the Charité™ AD were available in four angles (0, 5, 7.5 and 10 degrees) and the sliding core was available in five different heights (7.5, 8.5, 9.5, 10.5 and 11.5 mm).
The optimal position in the frontal plane was in the midline, but on the lateral image, it was 2 mm posterior to the midline. This position is known to reproduce the physiologic instantaneous axis of rotation (IAR), throughout the flexion-extension arc, of the normal disc13). The fluoroscopy was used throughout the procedure to verify the correct position of the AD.
Biomechanical testing
The potting fixtures for L2 and S2 were attached to the upper and lower spine-loading fixtures of a biomechanical loading frame (MTS 858; Materials Testing Systems, Mini Bionix®, Eden Prairie, MN, USA), respectively.
The ROM is the maximum displacement under the maximum-applied-load. Three infrared reflective markers were placed on the superior and inferior vertebrae of the surgically treated levels, and the vertebral motion was tracked using a video-based motion-capturing system (MacReflex; Qualisys Medical AB, Gottenburg, Sweden)20).
The method for applying a compressive follower preload to the multisegmented spine specimen (L2-S2) was adapted from a published method so that its path approximated the tangent of the curve of the lumbar spine (Fig. 2)20,33). The load was applied bilaterally by cables and dead weights. The loading cables were firmly anchored to the cup holding the L2 vertebral body and passed freely through cable guides attached to the bodies of L3-L5. The cable path approximated the tangent to the curve of the lumbar spine. Because the cable guides move with the vertebrae, the cable arrangement assures that the load path approximately passes through the center of rotation of each vertebra regardless of its motion33). Thus, the compressive load was applied along a follower load path rather than vertical load path.
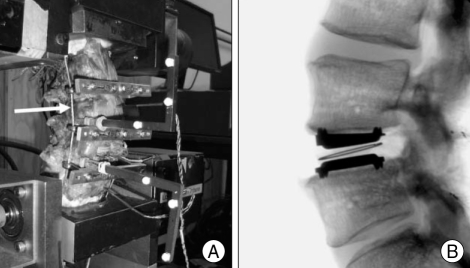
Fig. 2.
Photographs showing the biomechanical testing of ROM in the preloaded human cadaveric spine implanted with a Charité™ at L4-L5 (A) and lateral radiograph of the implant (B). The arrow indicates the loading cable for follower preload. The compressive follower preload was applied so that its path approximated the tangent of the curve of the lumbar spine (With permission from Ha SK, Kim SH, Kim DH, Park JY, Lim DJ, Lee SK. Biomechanical study of lumbar spinal arthroplasty with a semi-constrained artificial disc (Activ L) in the human cadaveric spine. J Korean Neurosurg Soc 45 :169-175, 2009.). ROM : range of motion.
The moments were applied to both L2 and S2 up to 8 Nm, with a loading rate of 0.3 Nm/second, and a constant 400 N axial follower preload was applied throughout the loading. These moments were selected as safe loads on the human cadaveric lumbar spine based on published data of biomechanical testing17,20,24). Axial rotation was determined by the upper spine fixator, whereas flexion, extension, and lateral bending were determined by the rotation of both spine fixators in the respective coronal and sagittal planes.
Baseline measurements of the ROM were performed for each intact spine in six modes of motion i.e. flexion, extension, right/left lateral bending and right/left axial rotation with the physiologic compressive follower preloaded condition. To stabilize the viscoelastic effect for each mode of testing, the loading was applied three times with only the result of the third loading being used.
After the Charité™ was implanted at the L4-L5 disc, the measurements of the ROM were repeated in the same manner under the same preload. The ROMs at the operative level, the level above and below the operative level were determined for each specimen after Charité™ implantation with two different methods : 1) Load control protocol : the specimens were loaded up to 8 Nm with the 400 N follower preload, essentially the same as the testing for the intact spine, 2) Hybrid protocol : the specimens were loaded in displacement control with the 400 N follower preload until the global ROM of L2-S2 reached that of the intact spine, regardless of the load attained. The data were compared to those of the intact spine, and then comparison between the data of each protocol was performed.
Statistical analysis
The mean ROM was determined and normalized according to individual levels, i.e. to the operative level and to the levels above and below the operative level. The values of right/left lateral bending were summed up into lateral bending, and those of right/left axial rotation were summed up into axial rotation, thus making four biomechanical modes of motion : flexion, extension, lateral bending, and axial rotation.
Because the number of specimens was small and the data could not be assumed to be normally distributed, nonparametric statistical methods were used to ascertain the statistical significance of intergroup differences, and were compared to the intact spines. Paired comparisons between different treatment groups were made by using Wilcoxon paired tests, and statistical significance was established at a probability value of 0.05. Values are presented as the mean ± SE.
RESULTS
The mean age (± SD) of the two male and three female specimens at the time of death was 59.8 ± 18.4 years (range 33-75 years). The mean BMD value (± SD) of the spines was 0.81 ± 0.09 g/cm2.
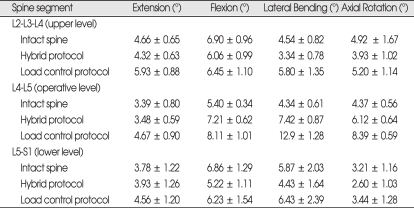
The values of ROM (mean ± SE) at the operative level (L4-L5), the levels above (L2-L3-L4) and below (L5-S1) the operative level for all specimens are shown in Table 1. The ROM values at the operative level, the levels above and below for each specimen were normalized with respect to that of the intact spine as shown in Fig. 3. Under load control, the ROMs across entire L2-S2 segments for the instrumented spine were increased in all modes of motion, for 16% in flexion/extension, 70% in lateral bending and 36% in axial rotation.
Table 1.
The mean ROM values* at the upper, operative, and lower segments before and after arthroplasty with Charité™ at L4-L5 under physiologic compressive follower preload of 400 N
*Values are presented as the mean ± SE. ROM : range of motion
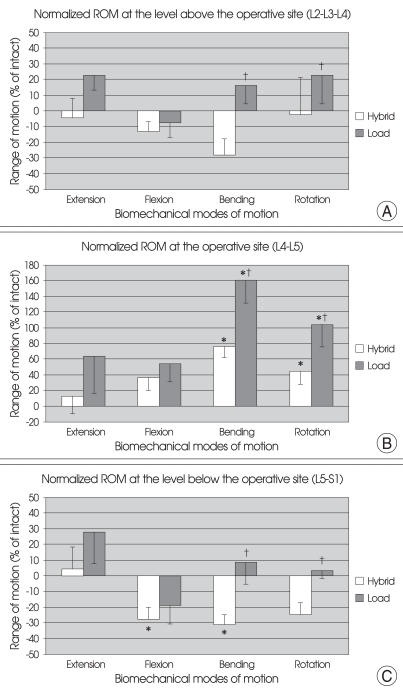
Fig. 3.
Graphs showing the mean with SE of normalized ROM values at the superior (A), operative (B), and inferior (C) segments after arthroplasty with Charité™ at L4-L5 under physiologic compressive follower preload (bending = right/left lateral bending; rotation = right/left axial rotation.) *p < 0.05 versus intact spine state. †p < 0.05 between hybrid protocol and load control protocol. ROM : range of motion.
At the level above the operative disc (L2-L3-L4)
As compared to the intact spine, the ROM of the superior segments (L2-L3-L4) was decreased in all four modes of motion under hybrid testing (-4.44 ± 12.31% in extension; -13.23 ± 6.51% in flexion; -28.56 ± 10.60% in bending and -2.13 ± 23.36% in rotation). Under load control testing, ROM in flexion was decreased (-7.57 ± 9.34%) but all the other modes of motion had increased ROM (22.86 ± 9.63% in extension; 16.15 ± 11.77% in bending and 22.71 ± 18.91% in rotation). Using both testing methods, however, the ROM had no significant difference from that of the intact spine. In both bending and rotation, the ROM for hybrid testing and load control testing differed significantly (p < 0.05) (Fig. 3A).
At the operative level (L4-L5)
As compared to the intact spine, the ROM at the operative level (L4-L5) was increased in all four modes of motion in both methods of testing : For hybrid protocol, 13.43 ± 23.15% in extension; 36.75 ± 16.19% in flexion; 76.96 ± 14.94% in bending and 45.04 ± 16.37% in rotation, and for load control protocol, 63.60 ± 46.58% in extension; 54.18 ± 22.73% in flexion; 160.79 ± 29.44% in bending and 104.30 ± 28.78% in rotation. For both bending and rotation, both protocols showed a significant difference to that of the intact spine (p < 0.05) and the ROM for both methods of testing showed significant difference from each other too (p < 0.05) (Fig. 3B).
At the level below the operative disc (L5-S1)
As compared to the intact spine, under hybrid protocol, the ROM of the inferior segment (L5-S1) was decreased for all the modes of motion except extension (4.30 ± 14.0% in extension; -27.83 ± 7.53% in flexion; -31.29 ± 6.48% in bending and -24.53 ± 7.30% in rotation. Significant difference from intact spine was observed in both flexion and bending (p < 0.05). Under load control testing, except for flexion, the ROM in all modes of motion were increased (27.69 ± 19.97% in extension; -19.22 ± 11.43% in flexion; 8.46 ± 13.87% in bending and 3.27 ± 4.94% in rotation), however, there was no statistically significant difference from that of the intact spine. For both bending and rotation, significant difference was observed between the two testing protocols (p < 0.05) (Fig. 3C).
DISCUSSION
Disc degeneration, a major contributor to chronic low back pain, is thought to occur with the loss of nuclear hydrostatic pressure and the release of inflammatory products34,39). Further degeneration results in so-called degenerative disc disease (DDD) associated with spinal instability, loss of the disc height and the resultant disc collapse, herniation, foraminal and central stenosis, facet arthropathy, and intractable back pain often requiring surgical treatment. When conservative treatments are failed, treatments for lumbar DDD generally consist of disc excision with or without interbody fusion. Unfortunately, fusion procedures are not always assured of success. Furthermore, though the incidence of pseudoarthrosis is estimated to be less than 10%, this is by no means negligible7,24,35). Moreover, fusion procedures are associated with risks of dural or neural injuries, chronic back pain, and stiffness. In addition, the fusion of vertebral segments increases the strain at the adjacent levels. With increasing strain, fusion leads to an increased adjacent-level disease and the patients may eventually suffer from symptomatic degenerative disease rostral or caudal to the fused segments. This accelerated process of degeneration adjacent to fused segments has been reported in the cervical and lumbar spine23,27,36,41) and may warrant the use of ADs.
Regardless of the type of AD, the goal of arthroplasty is to allow for neural decompression, while replicating the biomechanical performance of the intact healthy disc. Thus, in order for an intervertebral disc prosthesis to mimic a natural disc, it has to establish stability, maintain the caliber of the neural foramina and disc height, and provide normal spinal kinematics in all degrees of range of motion, while avoiding the development of adjacent degenerative changes. For these goals, many AD implants and prosthetic nuclei have been developed over the years.
Implants are widely varied and include spherical implants10), spring-loaded ADs22,26), rubberized implants located between metal plates8,38), nucleus pulposus replacements25,35), the polyethylene slip-core center joints (SB Charité™; DePuy Spine, Inc., Raynham, MA, USA)2,3,18,28,42), the polyethylene core ball-and-socket design (ProDisc; Synthes Spine, Paoli, PA, USA)1,29), or the metal-on-metal design (Maverick; Medtronic Sofamor Danek, Inc., Memphis, TN, USA and FlexiCore; Stryker Spine, Allendale, NJ, USA)19,24). The devices can also be classified based on the number of components (two versus three versus four), the interfacing materials (metal-on-polymer versus metal-on-metal), and/or the hypothesized kinematic constraints of their articulations (less constrained versus more constrained)12). The advantages and disadvantages of each of these devices with respect to ease of implantation, device longevity, generation of wear debris, and biomechanics remain theoretical, but will no doubt be emphasized in subsequent marketing efforts. Many of the devices have had a fairly large amount of follow-up data in Europe, and some have undergone assessment in formal clinical trials in the United States and are pending for approval here1,3,18,24,25,28,29). Some of these implants, such as the Charité™ and the ProDisc have been the subject of 10 to 15 years of clinical follow-up review in Europe1,2,18,28,29,42). In the United States, the Charité™ and the ProDisc II were Food & Drug Association (FDA) approved for general distribution and are currently commercially available, and two others (the Maverick and the FlexiCore) are in investigational device exemption (IDE) trials12,19,24).
The Charité™ AD consists of three components : two cobalt-chrome-molybdenum (CoCrMo) endplates with a concave articulating surface and external spikes and a free-floating, biconvex, ultra-high molecular weight polyethylene (UHMWPE) core with a circumferential metal ring as radiographic marker (Fig. 1). The primary attachment of the plates is made possible by three anterior and posterior spikes, which are forcefully implanted into the cranial and caudal vertebral endplates. The external spikes provide for immediate engagement of the CoCrMo endplates into the vertebral endplates. The UHMWPE insert snap fits between the endplates and provides a biconvex-bearing-surface for the concave articulating surfaces of the endplates12). The coated layers of plasma-sprayed porous titanium and calcium phosphate provide for potential osseous ingrowth and long-term stability of the plates after implantation11,31). The plates are currently available in five footprint geometrical configurations adaptable to the size of the vertebral endplates, each with four available angles (0, 5, 7.5, and 10 degrees). This allows for built-in lordosis with variations of 0 to 20 degrees. The unconstrained design allows the core to translate dynamically within the disc space during normal spinal motion, moving posteriorly in flexion and anteriorly in lumbar extension. The device is designed to provide 14 degrees of total flexion-extension.
The Charité™ is known to provide not only unloading of the posterior facet structures during this normal replication of motion but also allowing forgiveness for slight off-center positioning of the implant11). It was also reported to restore motion to the level of the intact segment in flexion-extension and lateral bending and increase motion in axial rotation at the operative level5). Those studies, however, were under a standard biomechanical conditions, not in a physiologic condition.
Patwardhan et al.33) reported that the load-carrying capacity of the lumbar spine can be significantly increased under a compressive follower load, as long as the load path remained within a small range around the estimated centers of rotation of the lumbar segments. In their study, the lumbar spines, loaded in compression along the follower load path, supported a much larger compressive load of up to 1,200 N if it was applied along a path that approximated the tangent to the curve of the lumbar spine33). Others have reported that the lumbar spine became unstable in the frontal plane under a vertical load of less than 100 N, which was far below the physiologic loads estimated in vivo4). This follower load path provides an explanation of how the whole lumbar spine can be lordotic and yet resist large compressive loads.
In the current study of the authors, we evaluated the changes in ROM of the human cadaveric spines implanted with the Charité™ prosthesis under a physiologic compressive follower preload, compared with the spine's intact state. Theoretically, to reduce the incidence of adjacent segment disease, the compensatory decrease of the adjacent segment motion should be made by the use of an AD.
The ROMs of the L4-L5 arthroplasty level were increased in all motion modes under both load control and hybrid protocols, and bending and rotation showed a significant difference to that of the intact spine (p < 0.05) and a significant difference between the two testing protocols (p < 0.05).
At the superior and inferior segments, the ROM under hybrid protocol demonstrated decrease of ROM during all the motion modes, except for a small increase during extension at the inferior segment, as compared to the intact spine. Using load control, however, the ROM of those segments showed increases during all the motion modes, except for decreases during flexion at the superior and inferior levels. A statistical significance between the hybrid and load control protocols was observed during bending and rotation at the arthroplasty and adjacent levels (p < 0.05).
In terms of motion, the hybrid protocol demonstrated a relatively better restoration of ROM at the operative and the adjacent segments after arthroplasty than did the load control protocol. In all segments tested under hybrid protocol, lateral bending showed the largest ROM difference and extension showed a relatively smaller difference compared to the intact spine among the four modes of motion. The largest increase in motion in the lateral bending may be caused by the unconstrained design of the prosthesis and the weakened lateral annulus after operation.
The classic flexibility testing protocol was reported not to be appropriate for the understanding of the biomechanics of the construct at the adjacent levels, and new testing protocol was advocated16). In previous biomechanical studies of an AD implant without follower preload, Hitchon et al.24), reported that no changes were observed in ROM values at the L3-L4, irrespective of the manipulations at the L4-L5 level. This observation underscores the fact that in load-controlled conditions involving the application of pure moments, any manipulation at the operative level does not result in compensation in the motion at the adjacent levels. Displacement controlled studies in cadaveric in vitro calf and human lumbar spines, however, have shown an increase in motion and intradiscal pressure at intact spinal levels adjacent to instrumented segments6,37,40). It is suspected that this increase in motion and intradiscal pressure accompanying fusion may contribute to the development of expedited DDD at levels adjacent to a fused spinal segment27,36,41). The implantation of the AD in place of instrumentation and fusion may contribute to reducing the incidence of adjacent segment degenerative disease that often accompanies the latter24).
Though this study has many limitations, such as in vitro experiment, a small sample size, cadaver specimens without muscle, and no consideration of wear and tear of the implant, the results are implying future investigations including study in other loading modes, effects of surgical variables, comparisons with fusion model, other AD designs and motion preservation systems, and more.
CONCLUSION
Analysis of our results indicates that in an in vitro compressive follower preload setting of Charité™ arthroplasty, the hybrid protocol provided a relatively better restoration of ROM at the operative level than did the load control setting. With the hybrid protocol, the ROM at the adjacent levels was decreased during all the motion modes, except for a small increase during extension at the inferior segment. The hybrid testing protocol is thus advocated as it better reproduces clinical observations in terms of motion following surgery than the load control.
References
- 1.Bertagnoli R, Kumar S. Indications for full prosthetic disc arthroplasty : a correlation of clinical outcome against a variety of indications. Eur Spine J. 2002;11(Suppl 2):S131–S136. doi: 10.1007/s00586-002-0428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Büttner-Janz K, Schellnack K, Zippel H. Biomechanics of the SB Charité lumbar intervertebral disc endoprosthesis. Int Orthop. 1989;13:173–176. doi: 10.1007/BF00268042. [DOI] [PubMed] [Google Scholar]
- 3.Cinotti G, David T, Postacchini F. Results of disc prosthesis after a minimum follow-up period of 2 years. Spine (Phila Pa 1976) 1996;21:995–1000. doi: 10.1097/00007632-199604150-00015. [DOI] [PubMed] [Google Scholar]
- 4.Crisco JJ, Panjabi MM, Yamamoto I, Oxland TR. Euler stability of the human ligamentous lumbar spine. Part II : Experiment. Clin Biomech. 1992;7:27–32. doi: 10.1016/0268-0033(92)90004-N. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham BW, Gordon JD, Dmitriev AE, Hu N, McAfee PC. Biomechanical evaluation of total disc replacement arthroplasty : an in vitro human cadaveric model. Spine (Phila Pa 1976) 2003;21:S110–S117. doi: 10.1097/01.BRS.0000092209.27573.90. [DOI] [PubMed] [Google Scholar]
- 6.Dekutoski MB, Schendel MJ, Ogilvie JW, Olsewski JM, Wallace LJ, Lewis JL. Comparison of in vivo and in vitro adjacent segment motion after lumbar fusion. Spine (Phila Pa 1976) 1994;19:1745–1751. doi: 10.1097/00007632-199408000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Dickman CA, Yahiro MA, Lu HT, Melkerson MN. Surgical treatment alternatives for fixation of unstable fractures of the thoracic and lumbar spine. A meta-analysis. Spine (Phila Pa 1976) 1994;19(20) Suppl:2266S–2273S. doi: 10.1097/00007632-199410151-00003. [DOI] [PubMed] [Google Scholar]
- 8.Enker P, Steffee A, McMillin C, Keppler L, Biscup R, Miller S. Artificial disc replacement. Preliminary report with a 3-year minimum follow-up. Spine (Phila Pa 1976) 1993;18:1061–1070. [PubMed] [Google Scholar]
- 9.Etebar S, Cahill DW. Risk factors for adjacent-segment failure following lumbar fixation with rigid instrumentation for degenerative instability. J Neurosurg. 1999;90:163–169. doi: 10.3171/spi.1999.90.2.0163. [DOI] [PubMed] [Google Scholar]
- 10.Fernström U. Arthroplasty with intercorporal endoprosthesis in herniated disc and in painful disc. Acta Chir Scand Suppl. 1966;357:154–159. [PubMed] [Google Scholar]
- 11.Geisler FH, Blumenthal SL, Guyer RD, McAfee PC, Regan JJ, Johnson JP, et al. Neurological complications of lumbar artificial disc replacement and comparison of clinical results with those related to lumbar arthrodesis in the literature : results of a multicenter, prospective, randomized investigational device exemption study of Charité intervertebral disc. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1:143–154. doi: 10.3171/spi.2004.1.2.0143. [DOI] [PubMed] [Google Scholar]
- 12.German JW, Foley KT. Disc arthroplasty in the management of the painful lumbar motion segment. Spine (Phila Pa 1976) 2005;30:S60–S67. doi: 10.1097/01.brs.0000174511.66830.e9. [DOI] [PubMed] [Google Scholar]
- 13.Gertzbein SD, Seligman J, Holtby R, Chan KW, Ogston N, Kapasouri A, et al. Centrode characteristics of the lumbar spine as a function of segmental instability. Clin Orthop Relat Res. 1986:48–51. [PubMed] [Google Scholar]
- 14.Ghiselli G, Wang JC, Bhatia NN, Hsu WK, Dawson EG. Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg Am. 2004;86A:1497–1503. doi: 10.2106/00004623-200407000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Ghiselli G, Wang JC, Hsu WK, Dawson EG. L5-S1 segment survivorship and clinical outcome analysis after L4-L5 isolated fusion. Spine. 2003;28:1275–1280. doi: 10.1097/01.BRS.0000065566.24152.D3. discussion 1280. [DOI] [PubMed] [Google Scholar]
- 16.Goel VK, Grauer JN, Patel TC, Biyani A, Sairyo K, Vishnubhotla S, et al. Effects of charité artificial disc on the implanted and adjacent spinal segments mechanics using a hybrid testing protocol. Spine (Phila Pa 1976) 2005;30:2755–2764. doi: 10.1097/01.brs.0000195897.17277.67. [DOI] [PubMed] [Google Scholar]
- 17.Goel VK, Weinstein JN, Patwardhan AG. Biomechanics of intact ligamentous spine. In: Goel VK, Weinstein JN, editors. Biomechanics of the Spine : Clinical and Surgical Perspectives. FL: CRC Press; 1990. pp. 97–156. [Google Scholar]
- 18.Griffith SL, Shelokov AP, Büttner-Janz K, LeMaire JP, Zeegers WS. A multicenter retrospective study of the clinical results of the LINK SB Charité intervertebral prosthesis. The initial European experience. Spine (Phila Pa 1976) 1994;19:1842–1849. doi: 10.1097/00007632-199408150-00009. [DOI] [PubMed] [Google Scholar]
- 19.Guyer RD, Ohnmeiss DD. Intervertebral disc prostheses. Spine (Phila Pa 1976) 2003;28:S15–S23. doi: 10.1097/01.BRS.0000076843.59883.E1. [DOI] [PubMed] [Google Scholar]
- 20.Ha SK, Kim SH, Kim DH, Park JY, Lim DJ, Lee SK. Biomechanical study of lumbar spinal arthroplasty with a semi-constrained artificial disc (activ L) in the human cadaveric spine. J Korean Neurosurg Soc. 2009;45:169–175. doi: 10.3340/jkns.2009.45.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hambly MF, Wiltse LL, Raghavan N, Schneiderman G, Koenig C. The transition zone above a lumbosacral fusion. Spine (Phila Pa 1976) 1998;23:1785–1792. doi: 10.1097/00007632-199808150-00012. [DOI] [PubMed] [Google Scholar]
- 22.Hedman TP, Kostuik JP, Fernie GR, Hellier WG. Design of an intervertebral disc prosthesis. Spine (Phila Pa 1976) 1991;16:S256–S260. doi: 10.1097/00007632-199106001-00016. [DOI] [PubMed] [Google Scholar]
- 23.Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519–528. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Hitchon PW, Eichholz K, Barry C, Rubenbauer P, Ingalhalikar A, Nakamura S, et al. Biomechanical studies of an artificial disc implant in the human cadaveric spine. J Neurosurg Spine. 2005;2:339–343. doi: 10.3171/spi.2005.2.3.0339. [DOI] [PubMed] [Google Scholar]
- 25.Klara PM, Ray CD. Artificial nucleus replacement : clinical experience. . Spine (Phila Pa 1976) 2002;27:1374–1377. doi: 10.1097/00007632-200206150-00022. [DOI] [PubMed] [Google Scholar]
- 26.Kostuik JP. The Kostuik artificial disc. In: Weinstein JN, editor. Clinical Efficacy and Outcome in the Diagnosis and Treatment of Low Back Pain. New York: Raven Press; 1992. pp. 259–270. [Google Scholar]
- 27.Lee CK. Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine (Phila Pa 1976) 1988;13:375–377. doi: 10.1097/00007632-198803000-00029. [DOI] [PubMed] [Google Scholar]
- 28.Lemaire JP, Skalli W, Lavaste F, Templier A, Mendes F, Diop A, et al. Intervertebral disc prosthesis. Results and prospects for the year 2000. Clin Orthop Relat Res. 1997:64–76. doi: 10.1097/00003086-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Mayer HM, Wiechert K, Korge A, Qose I. Minimally invasive total disc replacement : surgical technique and preliminary clinical results. Eur Spine J. 2002;11(Suppl 2):S124–S130. doi: 10.1007/s00586-002-0446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McAfee PC. Artificial disc prosthesis. In: Kaech DL, Jinkins JR, editors. Spinal Restabilization Procedures. Amsterdam: Elsevier Science BV; 2002. pp. 299–310. [Google Scholar]
- 31.McAfee PC, Cunningham BW, Orbegoso CM, Sefter JC, Dmitriev AE, Fedder IL. Analysis of porous ingrowth in intervertebral disc prostheses : a nonhuman primate model. Spine (Phila Pa 1976) 2003;28:332–340. doi: 10.1097/01.BRS.0000048504.08086.42. [DOI] [PubMed] [Google Scholar]
- 32.McAfee PC, Fedder IL, Saiedy S, Shucosky EM, Cunningham BW. Experimental design of total disk replacement-experience with a prospective randomized study of the SB Charité. Spine. 2003;28:S153–S162. doi: 10.1097/01.BRS.0000092217.34981.E1. [DOI] [PubMed] [Google Scholar]
- 33.Patwardhan AG, Havey RM, Meade KP, Lee B, Dunlap B. A follower load increases the load-carrying capacity of the lumbar spine in compression. Spine (Phila Pa 1976) 1999;24:1003–1009. doi: 10.1097/00007632-199905150-00014. [DOI] [PubMed] [Google Scholar]
- 34.Ray CD. The Raymedica prosthesis disc nucleus : an update. In: Keach DL, Jinkins JR, editors. Spinal Restabilization Procedures. Amsterdam: Elsevier Science BV; 2002. pp. 273–282. [Google Scholar]
- 35.Ray CD. Threaded titanium cages for lumbar interbody fusions. Spine (Phila Pa 1976) 1997;22:667–679. doi: 10.1097/00007632-199703150-00019. discussion 679-680. [DOI] [PubMed] [Google Scholar]
- 36.Schlegel JD, Smith JA, Schleusener RL. Lumbar motion segment pathology adjacent to thoracolumbar, lumbar, and lumbosacral fusions. Spine (Phila Pa 1976) 1996;21:970–981. doi: 10.1097/00007632-199604150-00013. [DOI] [PubMed] [Google Scholar]
- 37.Shono Y, Kaneda K, Abumi K, McAfee PC, Cunningham BW. Stability of posterior spinal instrumentation and its effects on adjacent motion segments in the lumbosacral spine. Spine (Phila Pa 1976) 1998;23:1550–1558. doi: 10.1097/00007632-199807150-00009. [DOI] [PubMed] [Google Scholar]
- 38.Steffee AD. The Steffee artificial disc. In: Weinstein JN, editor. Clinical Efficacy and Outcome in the Diagnosis and Treatment of Low Back Pain. New York: Raven Press; 1992. pp. 245–258. [Google Scholar]
- 39.Tropiano P, Huang RC, Girardi FP, Marnay T. Lumbar disc replacement : preliminary results with ProDisc II after a minimum follow-up period of 1 year. J Spinal Disord Tech. 2003;16:362–368. doi: 10.1097/00024720-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Weinhoffer SL, Guyer RD, Herbert M, Griffith SL. Intradiscal pressure measurements above an instrumented fusion. A cadaveric study. Spine (Phila Pa 1976) 1995;20:526–531. doi: 10.1097/00007632-199503010-00004. [DOI] [PubMed] [Google Scholar]
- 41.Whitecloud TS, 3rd, Davis JM, Olive PM. Operative treatment of the degenerated segment adjacent to a lumbar fusion. Spine (Phila Pa 1976) 1994;19:531–536. doi: 10.1097/00007632-199403000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Zeegers WS, Bohnen LM, Laaper M, Verhaegen MJ. Artificial disc replacement with the modular type SB Charité III : 2-year results in 50 prospectively studied patients. Eur Spine J. 1999;8:210–217. doi: 10.1007/s005860050160. [DOI] [PMC free article] [PubMed] [Google Scholar]