Abstract
A 43-year-old woman presented with dizziness, ataxia and right hearing difficulty. Her magnetic resonance images demonstrated an inhomogeneously contrast-enhanced large tumor growing into right cavernous sinus and Meckel's cave located totally within intradural retroclival region. She underwent retromastoid suboccipital craniotomy to resect the tumor mass and adjuvant gamma knife radiosurgery for remnant tumor at 1 month after operation. Adjuvant radiosurgery after surgical excision seems to be effective for the treatment of intradural extraosseous chordomas.
Keywords: Chordoma, Intradural, Retroclival
INTRODUCTION
Chordomas are rare tumors of central nervous system (about 0.2% of 6000 brain tumors)15) that arise from remnants of the primitive notochord5,7). They can arise elsewhere, but tend to cluster at the two ends of the primitive notochord, approximately 35% cranially in sphenooccipital region (clivus), and 50% in the spine at the sacrococcygeal region19). This neoplasm is thought to be essentially extradural in nature and generally associated with extradural extension and bone destruction. However, if allowed to grow for long periods, it may invade the dura and extend intradurally as well as extradurally13,16).
Intradural chordomas are very rare tumors that should be distinguished from neurinomas and classic chordomas, because of their different biological behaviors.
We describe the rare case of a patient with intradural chordoma located in retroclival and cerebellopontine angle region, which was near totally resected via retromastoid suboccipital approach, followed by adjuvant gamma-knife radiosurgery for remnant tumor.
CASE REPORT
This 43-year-old woman was healthy until she suffered an episode of dizziness, ataxia, mild right hearing difficulty and right facial numbness.
Examination
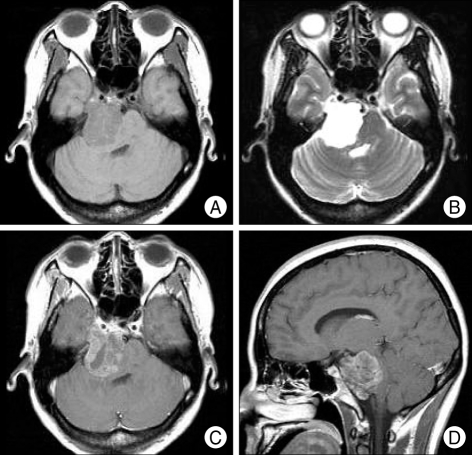
On initial evaluation, the patient had ataxic gait. Cranial nerve examination revealed no gag reflex, uvula deviation to left side, decreased hearing acuity on right ear, decreased taste on right side of tongue and numbness on right V1, V2 dermatome. The remainder of the neurological examination was within normal ranges. Magnetic resonance imaging demonstrated that the tumor located in the retroclival and right crerebellopontine angle, growing into right cavernous sinus and Meckel's cave and compressing the brainstem from medulla to midbrain (Fig. 1). The tumor appeared as a low-intensity area on the T1-weighted image and as a high-intensity area on the T2-weighted image. The tumor was enhanced inhomogeneously after administration of gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA).
Fig. 1.
Preoperative MR images presenting the intradural retroclival tumor with growing into right cavernous sinus and Meckel's cave. A : Axial T1-weigted imgae. B : Axial T-2 weighted image. C : Axial Gd-enhanced T1-weighted image. D : Sagittal Gd-enhanced T1-weighted image.
Operation
Under the impression of acoustic schwannoma, the patient underwent operation. We chose the retromastoid suboccipital approach. Crainotomy was made until transverse sinus and part of a sigmoid sinus were exposed. A soft, friable and grayish mass was seen. The tumor was clearly distinguished from the brain cortex and was easily removed by means of tumor forceps and suction tools. Also, this tumor contained a necrotic materials. Some areas of tumor was attached to right trigeminal nerve and located near the right facial and vestibulocochlear nerve complex. After near total resection of tumor (Fig. 2), these nerves (trigeminal, facial and vestibulocochlear nerves), superior cerebellar artery and posterior inferior cerebellar artery were well preserved. Rest of operation was uneventful.
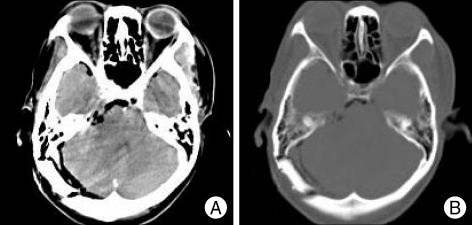
Fig. 2.
Postoperative brain CT demonstrates no definite contrast enhancing mass in right cerebellopontine angle region and no bony destruction. A : Axial contrast enhanced brain CT image. B : Bone-setting image.
Postoperative course
The patient complained dizziness, but other symptoms, such as ataxia, right facial numbness without paralysis, right hearing disturbance, were much more improved. Inparticular, the hearing disturbance was more improved after operation. Preoperative hearing threshold was 90 dB on the right and 45 dB on the left side by brainstem auditory evoked potential (BAEP), and it was improved to 35 dB on the right and 30 dB on the left side by BAEP studied on 11th postoperative day. Postoperative MR imaging indicated residual tumor in right cerebellopontine area (Fig. 3). Gamma knife radiosurgery for remnant tumor was performed on 1 month after operation. The remnant tumor volume was 8.3 cc and marginal dose of 15 Gy was administrated. MR imaging, performed 14months after gamma knife radiosurgery, revealed decreased size of remnant tumor compared with one before gamma knife radiosurgery (Fig. 4).
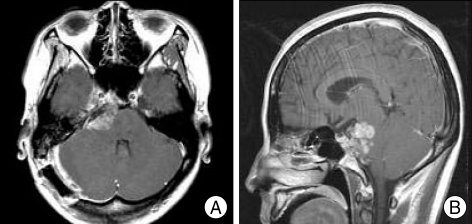
Fig. 3.
Follow-up MR images at 1 month after operation demonstrates a remnant tumor. A : Axial Gd-enhanced T1-weighted image. B : Sagittal Gd-enhanced T1-weighted image.
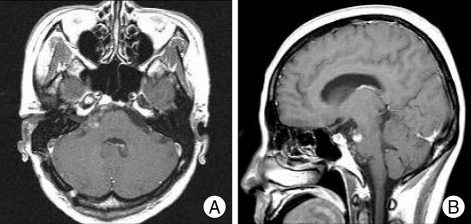
Fig. 4.
MR images, performed 14 months after gammaknife radiosurgery, representing decreased remnant tumor in size. A : Axial Gd-enhanced T1-weighted image. B : Sagittal Gd-enhanced T1-weighted image.
Histological findings
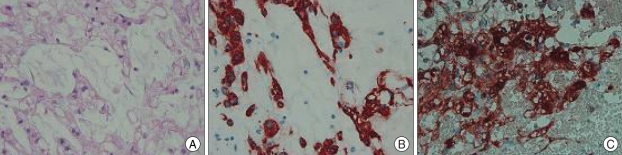
The tumor tissue was histologically characterized by lobules composed of typical physaliphorous cells with abundantly vacuolated cytoplasm. Immunohistochemical analysis showed the positive expression for cytokeratin, epithelial membrane antigen, vimentin and S-100 protein (Fig. 5). The histological features and antigen expression were consistent with the diagnosis of chordoma.
Fig. 5.
Photomicrograph of the intradural tumor showing typical physaliphorous cells in mucinous matrix. H & E stain ×200 (A). Immunohistochemical stains showing expression of cytokeratin (B), and S-100 protein (C).
DISCUSSION
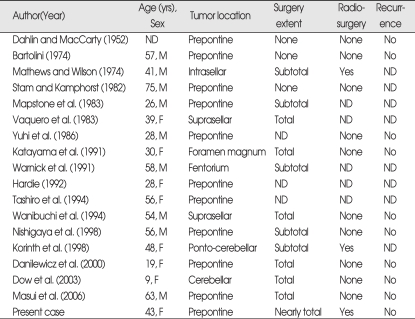
Including the this case, only 18 previous cases of primary intradural and intradural extraosseous chordoma have been reported (Table 1)1,4,6,8,13). It can be seen that many are from the pre-MRI era, and many do not provide histological or immuno-cytochemical evidence, raising the possibility that some may not be entirely extraosseous and some may be not actually be chordoma. Their locations included the prepontine region in ten cases, the suprasellar region in two cases and the intrasellar, foramen magnum, tentorium and ponto-cerebellar region in one case, respectively
Table 1.
Summary of cases of intracranial intradural chordomas
ND : not described
Ectopic notochordal tissue was found in an intradural location anterior to the pons in up to 2% at autopsy; these have been given name "ecchordosis physaliphora"2,3,20). The differential diagnosis of ecchordosis physaliphora and chordoma based on histological and radiological features is likely to be difficult. The MIB-1 proliferating cell index may be useful in the histological differential diagnosis of chordomas, which are neoplastic tumors, and ecchordoses, which are not true tumor13).
Intradural extraosseous chordoma has clearly different features from those of typical chordoma17). These entities show different biological behavior, with gradient evolution of growth and malignancy from the usually asymptomatic ecchordosis, to the slowly evolving intradural chordoma, to the highly malignant and invasive chordoma10). Classic chordomas in bone frequently have ill-defined margins, and complete resection is usually not feasible even with extensive surgery because a few tumor cells are often left behind in the bone or dura. In contrast, the intradural type of tumor characteristically has a slower growth pattern, sharply circumscribed margins that allow total excision, and it never metastasizes6,11,20).
Most patients with skull base chordomas have been treated with a combination of surgery and radiotherapy, although the efficacy of radiosurgery is controversial21). Some authors14) reported that the surgically total excision was goal of treatment because the intradural chordomas had slower growth pattern and sharply circumscribed margins compared with classic chordomas. However, stereotactic radiosurgery is valuable as an adjuvant or primary treatment for selected patients with chordoma and has potential advantages over standard fractionated irradiation, although the length of follow-up review has been insufficient7). For tumor control, conventional radiotherapy needs very high doses of radiation. This dose may carry a high risk of severe radiation injury to the normal brain structures9). In contrast, radiosurgery has the advantage of high dose single-session irradiation and efficacy for small sized tumor mass7,12). For this reason, we selected stereotactic radiosurgery for remnant tumor in our case. Also, proton-beam irradiation is reported to be efficacious for the treatment of chordomas18).
CONCLUSION
We achieved near total resection of intradural chordoma and adjuvant gamma knife radiosurgery was performed for remnant tumor at 1 month after surgery. Although there are no sufficient data for efficacy of an adjuvant gamma-knife radiosurgery, the tumor of our case gradually decreased in size based on follow-up MR imaging.
References
- 1.Bartolini G. [Cordoma del clivus quale causa di emorragia cerebrale : discussione anatomo-clinica su di una osservazione autoptica] Boll Soc Ital Biol Sper. 1974;50:912–918. [PubMed] [Google Scholar]
- 2.Congdon CC. Benign and malignant chordomas : a clinicoanatomical study of 22 cases. Am J Pathol. 1952;28:793–821. [PMC free article] [PubMed] [Google Scholar]
- 3.Dahlin DC, MacCarty CS. Chordoma. Cancer. 1952;5:1170–1178. doi: 10.1002/1097-0142(195211)5:6<1170::aid-cncr2820050613>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.Dow GR, Robson DK, Jaspan T, Punt JA. Intradural cerebellar chordoma in a child : a case report and review of the literature. Childs Nerv Syst. 2003;19:188–191. doi: 10.1007/s00381-002-0707-8. [DOI] [PubMed] [Google Scholar]
- 5.Inci S, Palaoglu S, Önol B, Erbengi A. Low cervical chordoma: case report. Spinal Cord. 1996;34:358–360. doi: 10.1038/sc.1996.65. [DOI] [PubMed] [Google Scholar]
- 6.Katayama Y, Tsubokawa T, Hirasawa T, Takahata T, Nemoto N. Intradural extraosseous chordoma in the foramen magnum region. Case report. J Neurosurg. 1991;75:976–979. doi: 10.3171/jns.1991.75.6.0976. [DOI] [PubMed] [Google Scholar]
- 7.Kondziolka D, Lunsford LD, Flickinger JC. The role of radiosurgery in the management of chordoma and chondrosarcoma of the cranial base. Neurosurgery. 1991;29:38–45. doi: 10.1097/00006123-199107000-00007. discussion 45-46. [DOI] [PubMed] [Google Scholar]
- 8.Korinth M, Schönrock L, Mayfrank L, Gilsbach JM. Primary intradural pontocerebellar chordoma metastasizing in the subarachnoid spinal canal. Zentralbl Neurochir. 1999;60:146–150. [PubMed] [Google Scholar]
- 9.Krayenbuhl H, Yasagil MG. Cranial chordomas. Prog Neurol Surg. 1975;6:380–434. [Google Scholar]
- 10.Macdonald RL, Cusimano MD, Deck JH, Gullane PJ, Dolan EJ. Cerebrospinal fluid fistula secondary to ecchordosis physaliphora. Neurosurgery. 1990;26:515–518. doi: 10.1097/00006123-199003000-00022. discussion 518-519. [DOI] [PubMed] [Google Scholar]
- 11.Mapstone TB, Kaufman B, Ratcheson RA. Intradural chordoma without bone involvement : nuclear magnetic resonance (NMR) appearance. J Neurosurg. 1983;59:535–537. doi: 10.3171/jns.1983.59.3.0535. [DOI] [PubMed] [Google Scholar]
- 12.Muthukumar N, Kondziolka D, Lunsford LD, Flickinger JC. Stereotactic radiosurgery for chordoma and chondrosarcoma : further experiences. Int J Radiat Oncol Biol Phys. 1998;41:387–392. doi: 10.1016/s0360-3016(98)00051-0. [DOI] [PubMed] [Google Scholar]
- 13.Nishigaya K, Kaneko M, Ohashi Y, Nukui H. Intradural retroclival chordoma without bone involvement : no tumor regrowth 5 years after operation. Case report. J Neurosurg. 1998;88:764–768. doi: 10.3171/jns.1998.88.4.0764. [DOI] [PubMed] [Google Scholar]
- 14.Roberti F, Sekhar LN, Jones RV, Wright DC. Intradural cranial chordoma: a rare presentation of an uncommon tumor. Surgical experience and review of the literature. J Neurosurg. 2007;106:270–274. doi: 10.3171/jns.2007.106.2.270. [DOI] [PubMed] [Google Scholar]
- 15.Russell DS, Rubinstein LJ. Pathology of Tumor of the Nervous System. London: Edward Arnold; 1989. pp. 664–765. [Google Scholar]
- 16.Samii M, Cheatham ML, Becker DP. Chordomas, chondromas, and chondrosarcomas. In: Samii M, Cheatham ML, Becker DP, editors. Atlas of Cranial Base Surgery. UCLA and Hannover Experience. Philadelphia: WB Saunders; 1995. pp. 186–187. [Google Scholar]
- 17.Steenberghs J, Kiekens C, Menten J, Monstrey J. Intradural chordoma without bone involvement. Case report and review of the literature. J Neurosurg. 2002;97:94–97. [PubMed] [Google Scholar]
- 18.Suit HD, Goitein M, Munzenrider J, Verhey L, Davis KR, Koehler A, et al. Definitive radiation therapy for chordoma and chondrosarcoma of base of skull and cervical spine. J Neurosurg. 1982;56:377–385. doi: 10.3171/jns.1982.56.3.0377. [DOI] [PubMed] [Google Scholar]
- 19.Tomlinson FH, Scheithauer BW, Miller GM, Onofrio BM. Extraosseous spinal chordoma. Case report. J Neurosurg. 1991;75:980–984. doi: 10.3171/jns.1991.75.6.0980. [DOI] [PubMed] [Google Scholar]
- 20.Wolfe JT, 3rd, Scheithauer BW. "Intradural chordoma" or "giant ecchordosis physaliphora"? Report of two cases. Clin Neuropathol. 1987;6:98–103. [PubMed] [Google Scholar]
- 21.Yasagil MG. Microneurosurgery. Vol IVB. Stuttgart, Germany: Thieme; 1996. Microneurosurgery of CNS Tumor; pp. 188–191. [Google Scholar]