Abstract
Solitary extramedullary plasmacytomas are isolated plasma cell tumors of soft tissue that typically do not metastasize. They are rare and account for 4% of all plasma cell tumors. To our knowledge, only 14 cases of solitary extramedullary plasmacytomas in the sphenoid sinus have been reported. A 32-year-old man presented to our department with complaint of ocular pain in the right eyeball and diplopia. Physical and neurological examinations revealed intact and prompt direct and indirect light reflexes in both pupils and limitation of extraocular muscle movement seen with the lateral gaze of the right eyeball. Magnetic resonance imaging suggested the presence of mucocele or mycetoma, therefore surgical resection was performed with endoscopic endonasal transsphenoidal approach. Histopathology was consistent with plasmacytoma. Systemic work-up did not show any evidence of metastasis and the sphenoid sinus was the sole tumor site, and therefore the diagnosis of solitary extramedullary plasmacytoma was confirmed. We report a rare case of solitary extramedullary plasmacytoma in the sphenoid sinus with successful treatment using the endoscopic endonasal transsphenoidal resection and adjuvant radiotherapy.
Keywords: Plasmacytoma, Sphenoid sinus, Endoscopic surgical resection
INTRODUCTION
Extramedullary plasmacytomas are rare tumors initially described by Schridde et al. in 190516). Most reported extramedullary plasmacytomas are solitary6,8,13). The solitary extramedullary plasmacytoma (SEP) is a rare tumor and comprises approximately 3% of all plasma cell neoplasms12,17). More than 90% of SEPs originate in the head, neck, and upper respiratory tract, and affected tissues include the nasal cavity, sinuses, oropharynx, salivary glands, and larynx3,7,14,19,20).
Alexious et al.1) identified 869 SEP cases in the medical literature between 1905 and 1997, and only 14 of these cases (1.6%) had primary sites in the sphenoid sinus. We report a case of SEP in the sphenoid sinus that resulted in skull base destruction, and discuss the clinical manifestations and therapeutic modalities in this case.
CASE REPORT
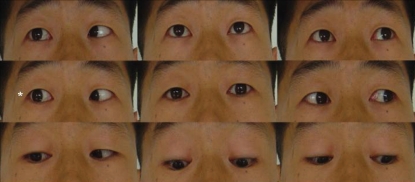
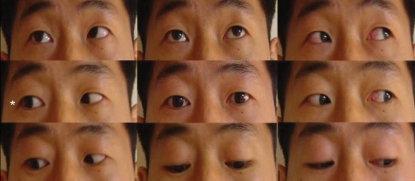
A 32-year-old man was referred to our department with complaint of ocular pain with diplopia on the right eyeball, which had worsened significantly 2 weeks prior to presentation. On physical and neurological examinations, direct and indirect light reflexes in both pupils were prompt; limitation of extraocular muscle movements was noted with lateral gaze movements of the right eyeball (Fig. 1). All other extraocular muscle movements were intact. Visual acuity was normal, and there were no abnormal findings in the hypothalamo-pituitary function test.
Fig. 1.
Limitation of lateral gaze of the right eyeball at the time of initial presentation.
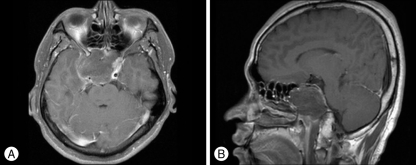
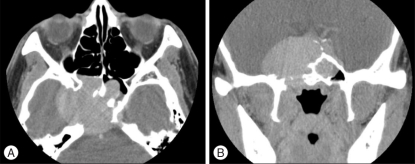
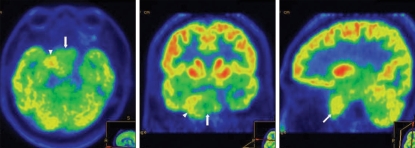
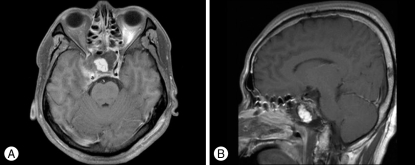
Magnetic resonance imaging (MRI) revealed a huge mass lesion with expansive signal in the sphenoid sinus (Fig. 2). The mass showed rim enhancement with intravenous gadolinium (Gd) injection, an upwardly displaced pituitary gland, and compression of the pituitary stalk and right cavernous sinus in the T1-weighted MR image. Computed tomography (CT) scan of the skull base demonstrated a lobulated mass-like lesion and large, diffuse, and irregular bony destruction of petrous tip portion and sphenoid ridge of right temporal bone (Fig. 3). Tumor staining was not visualized with conventional cerebral angiography. Positron emission tomography (PET) scan of the brain suggested the presence of hypermetabloic mass lesion in the sphenoid sinus which showed similar 18F-Fluorodeoxyglucose (FDG) uptake to that of gray matter in the brain, with a maximum standard uptake of volume (SUV) of 6.6 (Fig. 4).
Fig. 2.
Preoperative magnetic resonance image (MRI) with gadolinium (Gd) enhancement. A : A lobulated and hypointense mass-like lesion in the axial image which fills the sphenoid sinus. It shows peripheral enhancement in the T1-weighted MRI scan with intravenous Gd injection. The size of mass is 5 cm×4.4 cm×5 cm. B : The pituitary gland and cavernous portion of internal cerebral artery are displaced upward to just below optic chiasm in the sagittal image.
Fig. 3.
Computed tomography (CT) scan reveals diffuse and irregular bony destruction of the right tip of the petrous region of the temporal bone and the right parasellar area in the axial image (A) and the coronal image (B).
Fig. 4.
Positron emission tomography (PET) shows a similar uptake of 18F-fluorodeoxyglucose to that in brain parenchyma. This suggests the presence of a hypermetabolic mass lesion in the sphenoid sinus (arrow) and destruction of skull base (arrowhead).
The patient underwent surgery 3 days after admission. Surgical resection was performed with endoscopic endonasal transsphenoidal approach since the clinical suspicion of a mucocele, mycetoma, or a lymphoproliferative reaction was high. The tumor was of low vascularity and was fibrotic, and tumor cells of the capsule had infiltrated the surrounding bone. The mucosal layer of the sphenoid sinus was removed, but residual tumor tissue around the right cavernous sinus was not resected because of the difficulty of hemostasis control with endonasal endoscopic approach. An abdominal fat graft was applied to the sphenoid sinus, and the sphenoid sinus opening was covered with Tefron® (Boston Scientific Cooperation, Natick, MA, USA). Immediate postoperative MRI scanning with Gd enhancement revealed the presence of remnant mass tissue around the right cavernous sinus (Fig. 5).
Fig. 5.
Immediate postoperative magnetic resonance image illustrates the residual mass around right cavernous sinus area and the grafted fatty tissue in the sphenoid sinus in the axial image (A) and sagittal image (B).
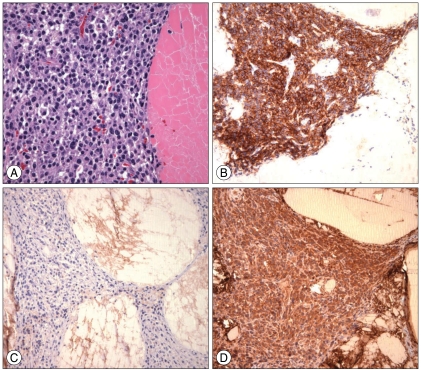
Tumor samples were composed of several pieces of whitish-yellow and friable soft tissue upon examination. These samples were resected with a piecemeal pattern during surgery. Fig. 6A showed abundant aggregates of bright eosinophilic materials which were intermingled with cellular components. The cellular components were primarily composed of plasma cells and occasional lymphocytes, histiocytes, and multinucleated giant cells. The plasma cells had eccentric cartwheel nuclei, perinuclear hof, and basophilic cytoplasm. These cells occasionally displayed enlarged nuclei with increased nuclear-cytoplasmic ratio. The cytoplasm showed negative immunoreactivity for the leukocyte common antigen and CD20, but positive immunoreactivity for CD68, epithelial membrane antigen (EMA), and CD38 (Fig. 6B). Additionally, lambda light chain dominance was identified (Fig. 6C, D). Plasmacytoma diagnosis was confirmed with several histopathologic findings including proliferation of plasma cells, positive immunoreactivity for CD38, and expression of the lambda restricted light chain. The Ki-67 labeling index was about 3%.
Fig. 6.
(A) The plasma cells have eccentric cartwheel nuclei, a perinuclear hof, and basophilic cytoplasm (hematoxylin and eosin staining, ×400). (B) Cytoplasm shows positive immunoreactivity during immunohistochemical staining in CD 38. The plasmacytoma reveals no kappa light chain crystalline deposits (C) but shows extensive lambda light chain crystalline deposits (D).
Systemic workup for finding dissemination of tumor cells including bone marrow aspiration, skeletal radiographic survey, complete blood count, serum biochemistry analysis, monoclonal immunoglobulin levels of serum and urine, and whole body PET-CT was performed. Normocellular marrow was detected on bone marrow aspirates with an adequate myeloid/erythroid ratio, and malignant plasma cells were not identified in stained bone marrow aspirate slides. No osteolytic lesions were identified in the skeletal survey, and anemia, hypercalcemia and renal impairment were not identified on the serum biochemistry profile. Serum and urinary monoclonal immunoglobulins were not detected. Immunoelectrophoresis of serum and urine protein detected no paraproteins or M-proteins. PET-CT scan did not reveal evidence of systemic spread, and the sphenoid sinus was the sole site of tumor. Therefore, SEP was confirmed in this clinical case.
The patient did well post-operatively and was free of ocular pain. The patient received radiotherapy one month after surgery (a total 4,000 cGy in 20 fractions) because of residual tumor around the right cavernous sinus. His diplopia completely resolved after radiotherapy (Fig. 7). The patient experienced no further tumor progression or symptom recurrence during 8 months of diagnostic follow-up exams, and follow-up MRIs revealed no tumor recurrence.
Fig. 7.
Significant improvement in the lateral gaze of the right eyeball after 12 weeks of surgery.
DISCUSSION
Plasmacytomas are immunoproliferative, monoclonal tumors of the B-cell line and are classified as non-Hodgkin's lymphomas. They originate as clones of malignant transformed plasma cells. The typical clinical presentations of pituitary region plasmacytomas include headache and ocular muscle paresis without higher or lower hormone production in the adenophysis21).
There are no international guidelines for the diagnosis of SEP. Recommended diagnostic criteria for SEP were set by the subgroup of the Guidelines Working Group of the UK Myeloma Forum (UKMF). Recommended diagnostic criteria for SEP are as follows : 1) a single extramedullary mass of clonal plasma cells, 2) histologically normal marrow aspirates and trephine biopsies, 3) normal results on skeletal surveys, including the radiology of long bones, 4) no anemia, hypercalcemia or renal impairment due to plasma dyscrasia, and 5) low or absent serum or urinary level of monoclonal immunoglobulins18). The presence of clonal plasma cells by modern phenotypic techniques has not usually been required when generalized disease was ruled out2,10,11). The diagnosis of SEP should require confirmation of CD38-expressing plasma cells and the dominance of either cytoplasmic kappa or lambda light chains. These concurrent features are usually seen in malignant plasma cells9). The tumor in this case report expressed positive immunoreactivity for the CD38 phenotype and lambda-restricted cytoplasm.
There is no universal consensus for the treatment of SEP. According to the "Guidelines for Management of SEP" by UKMF, SEP should be treated by radical radiotherapy of the primary tumor with a margin of at least 2 cm since it is a highly radiosensitive tumor18). However, Galieni et al.7) suggested that sole surgical removal of SEP could be performed for small masses and as a secondary therapy after failure of local irradiation in elimination of the mass. Chemotherapy has not been successful in SEP treatment, although disease dissemination can be successfully treated with alkylating agents23). In the case of intracranial solitary plasmacytomas which are very rare locations, surgical resection followed radiotherapy can treat the disease successfully4). Miller et al.15) reported a case of plasmacytoma which occurred in the sphenoid sinus among 20 patients with the diagnosis of plasmacytoma of the head and neck region at the Cleveland Clinic Foundation between 1976 and 1993. They performed the biopsy of tumor and treated the patient with radiation of 6,400 cGy. After 4 years of treatment, the patient was still alive and had no evidence of tumor progression. Wein et al.22) treated a patient with plasmacytoma in the sphenoclival region with systemic chemotherapy after endoscopic biopsy. Weber and Jaksche.21) reported a case of solitary plasmacytoma of the pituitary area. They performed transsphenoidal surgery and adjuvant radiotherapy.
The prognosis of patients with SEP is significantly better than that of patients with solitary bony plasmacytoma. The incidence of progression is less than 30% in SEP patients, and clinical progression is characterized by of bony lesions as in typical multiple myeloma, bone marrow plasmacytosis, and monoclonal proteins5). Most carefully staged SEP patients can be cured, and at least 70% of patients are disease-free 10 years post-treatment5).
CONCLUSION
We report a rare case of SEP in the sphenoid sinus with destruction of skull base. Surgical resection using endoscopic endonasal transsphenoidal approach and adjuvant radiotherapy were combined for the treatment. Systemic diagnostic work ups for SEP are significant in establishing therapeutic plans and in predicting disease prognosis. It is essential to closely monitor patients with this rare tumor to prevent recurrence.
References
- 1.Alexiou C, Kau RJ, Dietzfelbinger H, Kremer M, Spiess JC, Schratzenstaller B, et al. Extramedullary Plasmacytoma; tumor occurrence and therapeutic concepts. Cancer. 1999;85:2305–2314. [PubMed] [Google Scholar]
- 2.Bolek TW, Marcu RB, Mendenhall NP. Solitary plasmacytoma of bone and soft tissue. Int J Radiat Oncol Biol Phys. 1996;36:329–333. doi: 10.1016/s0360-3016(96)00334-3. [DOI] [PubMed] [Google Scholar]
- 3.Brinch L, Hannisdal E, Abrahamsen AF, Kvaløy S, Langholm R. Extramedullary plasmacytomas and solitary plasma cell tumours of bone. Eur J Haematol. 1990;44:132–135. doi: 10.1111/j.1600-0609.1990.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 4.Choi WJ, Yee GT, Choi CY, Whang CJ. Huge size intracranial plasmacytoma treated with surgery and fractionated stereotactic radiotherapy. J Korean Neurosurg Soc. 2006;40:110–113. [Google Scholar]
- 5.Dimopoulos MA, Kiamouris C, Moulopoulos LA. Solitary plasmacytoma of bone and extramedullary plasmacytoma. Hematol Oncol Clin North Am. 1999;13:1249–1257. doi: 10.1016/s0889-8588(05)70124-6. [DOI] [PubMed] [Google Scholar]
- 6.Ewing MR, Foote FW., Jr Plasma-cell tumors of the mouth and upper air passages. Cancer. 1952;5:499–513. doi: 10.1002/1097-0142(195205)5:3<499::aid-cncr2820050310>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 7.Galieni P, Cavo M, Pulsoni A, Avvisati G, Bigazzi C, Neri S, et al. Clinical outcome of extramedullary plasmacytoma. Haematologica. 2000;85:47–51. [PubMed] [Google Scholar]
- 8.Gromer RC, Duvall AJ., 3rd Plasmacytoma of the head and neck. J Laryngol Otol. 1973;87:861–872. doi: 10.1017/s0022215100077732. [DOI] [PubMed] [Google Scholar]
- 9.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, et al. A revised European-American classification of lymphoid neoplasms : a proposal from the international lymphoma study group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 10.Harwood AR, Knowling MA, Bergsagel DE. Radiotherapy of extramedullary plasmacytoma of the head and neck. Clin Radiol. 1981;32:31–36. doi: 10.1016/s0009-9260(81)80242-5. [DOI] [PubMed] [Google Scholar]
- 11.Kapadia SB, Desai U, Cheng VS. Extramedullary plasmacytoma of the head and neck. A clinicopthologic study of 20 cases. Medicine (Baltimore) 1982;61:317–329. doi: 10.1097/00005792-198209000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Knowling MA, Harwood AR, Bergsagel DE. Comparison of extramedullary plasmacytomas with solitary and multiple plasma cell tumors of bone. J Clin Oncol. 1983;1:255–262. doi: 10.1200/JCO.1983.1.4.255. [DOI] [PubMed] [Google Scholar]
- 13.Kotner LM, Wang CC. Plasmacytoma of the upper air and food passages. Cancer. 1972;30:414–418. doi: 10.1002/1097-0142(197208)30:2<414::aid-cncr2820300217>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Liebross RH, Ha CS, Cox JD, Weber D, Delasalle K, Alexanian R. Clinical course of solitary extramedullary plasmacytoma. Radiother Oncol. 1999;52:245–249. doi: 10.1016/s0167-8140(99)00114-0. [DOI] [PubMed] [Google Scholar]
- 15.Miller FR, Lavertu P, Wanamaker JR, Bonafede J, Wood BG. Plasmacytomas of the head and neck. Otolaryngol Head Neck Surg. 1998;119:614–618. doi: 10.1016/S0194-5998(98)70021-X. [DOI] [PubMed] [Google Scholar]
- 16.Schridde H. [Weitere Untersuchungern über die Koernelungen der Plasmazellen] Zentralbl Allg Pathol Anat. 1905;6:433–435. [Google Scholar]
- 17.Shih LY, Dunn P, Leung WM, Chen WJ, Wang PN. Localised plasmacytomas in Taiwan : comparison between extramedullary plasmacytoma and solitary plasmacytoma of bone. Br J Cancer. 1995;71:128–133. doi: 10.1038/bjc.1995.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soutar R, Lucraft H, Jackson G, Reece A, Bird J, Low E, et al. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Br J Heamatol. 2004;124:717–726. doi: 10.1111/j.1365-2141.2004.04834.x. [DOI] [PubMed] [Google Scholar]
- 19.Susnerwala SS, Shanks JH, Banerjee SS, Scarffe JH, Farrington WT, Slevin NJ. Extramedullary plasmacytoma of the head and neck region : clinicopathological correlation in 25 cases. Br J Cancer. 1997;75:921–927. doi: 10.1038/bjc.1997.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wax MK, Yun KJ, Omar RA. Extramedullary plasmacytomas of the head and neck. Otolaryngol Head Neck Surg. 1993;109:877–885. doi: 10.1177/019459989310900517. [DOI] [PubMed] [Google Scholar]
- 21.Weber J, Jaksche H. Solitary plasmacytoma of the pituitary area. Acta Neurochir (Wien) 1999;141:219–220. doi: 10.1007/s007010050291. [DOI] [PubMed] [Google Scholar]
- 22.Wein RO, Popat SR, Doerr TD, Dutcher PO. Plasma cell tumors of the skull base : four case reports and literature review. Skull Base. 2002;12:77–86. doi: 10.1055/s-2002-31570-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiltshaw E. The natural history of extramedullary plasmacytoma and its relation to solitary myeloma of bone and myelomatosis. Medicine (Baltimore) 1976;55:217–238. doi: 10.1097/00005792-197605000-00002. [DOI] [PubMed] [Google Scholar]