Abstract
Objective
Cardiac dysfunction after aneurysmal subarachnoid hemorrhage (SAH) is associated with elevation of serum cardiac troponin I (cTnI) levels. Elevation of cTnI predicts cardiopulmonary and neurological complications, and poor outcome.
Methods
We retrospectively reviewed the medical and radiologic records of 114 (male : 30, female : 84) patients who developed aneurysmal SAH between January 2006 and June 2007 and had no history of previous cardiac problems. We evaluated their electrocardiography and cTnI level, which had been measured at admission. A cTnI level above 0.5 µg/L was defined as an indicator of cardiac injury following SAH. We examined various clinical factors for their association with cTnI elevation and analyzed data using chi-square test, t-test and logistic regression test with SPSS version 12.0. The results were considered significant at p < 0.05.
Results
The following parameters shows a correlation with cTnI elevation : higher Hunt-Hess (H-H) grade (p = 0.000), poor Glasgow Outcome Scale (GOS) score (p = 0.000), profound pulmonary complication (p = 0.043), higher heart rate during initial three days following SAH (p = 0.029), ruptured aneurysm on communicating segment of internal carotid artery (p = 0.025), incidence of vasospasm (p = 0.421), and duration of hyperdynamic therapy for vasospasm (p = 0.292). A significant determinants for outcome were cTnI elevation (p = 0.046) and H-H grade (p = 0.000) in a multivariate study.
Conclusion
A cTnI is a good indicator for cardiopulmonary and neurologic complications and outcome following SAH. Consideration of variable clinical factors that related with cTnI elevation may be useful tactics for treatment of SAH and concomitant complications.
Keywords: Subarachnoid hemorrhage, Cardiac troponin I, Complications, Vasospasm
INTRODUCTION
The annual incidence of aneurysmal subarachnoid hemorrhage (SAH) was 8.25 per 100,000 in South Korea8). Medical complications of SAH are common and contribute substantially to morbidity and mortality. One of the most common and important medical complications of SAH are cardiac problems, including myocardial injury and cardiac dysfunction. Left ventricular systolic dysfunction and other electrocardiography (ECG) changes occur frequently, while the elevation of several cardiac enzymes occurs in 20-40% of patients1,5,9).
Pathologically, the form of reversible myocardial injury that follows SAH is a type of subendocardial contraction band necrosis, which is thought to result from the excessive release of catecholamines within the myocardium5). Recently, numerous studies have shown that serum cardiac troponin I (cTnI) is a sensitive and specific marker for this form of cardiac dysfunction. In addition, cTnI elevation has been considered as a predictor of various cardiac complications, pulmonary problems, cerebral infarction and overall poor outcome5-7,9). In this report, we discuss about the risk factors and clinical importances including vasospasm associated with cTnI elevation.
MATERIALS AND METHODS
Our 114 (male : 30, female : 84) patients were selected from hospitalized referrals based on the following criteria : 1) patients with an aneurysmal SAH; 2) the absence of a history of cardiac problems (coronary artery disease, heart failure, arrythmia, cardiomyopathy, conduction abnormality, or other causes); and 3) the absence of surgical complications after clipping or coiling that result in neurological deterioration.
After patient selection, we collected clinical data including age, sex, history of hypertension, Hunt-Hess (H-H) grade (I-V), Fisher grade (1-4), peak blood pressure (systolic and diastolic) and peak heart rate during the three days following SAH, aneurysm location [Group 1 : anterior cerebral artery and anterior communicating artery; Group 2 : middle cerebral artery; Group 3 : communicating segment of the internal carotid artery, Group 4 : ophthalmic segment of the internal carotid artery (ICA); Group 5 : posterior cerebral circulation; Group 6 : unknown origin], outcome at discharge as defined by the Glasgow Outcome Scale (GOS, 1-5), admission days on intensive care unit, presence of profound pulmonary complications (caused by pulmonary edema and pneumonia) defined by imaging studies, presence of symptomatic vasospasm, duration of hyperdynamic (hypertensive-hypervolemic) therapy for symptomatic vasospasm, ECG, and a peak cTnI level for three days following SAH.
The patients were divided into two groups based on the cTnI level (Group A ≥ 0.5 µg/L, Group B < 0.5 µg/L) following SAH. We assessed the relationship of the above clinical factors between the two groups and analyzed the determinants that influenced the GOS score. A cTnI level above 0.5 µg/L was defined as an indicator of cardiac injury following SAH.
Data analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 12.0. Descriptive statistics were performed to determine the influence of clinical factors on cTnI level elevation. Chi-square test, t-test and logistic regression test were conducted to assess the overall relationship between clinical factors and cTnI elevation. The results were considered significant at p < 0.05.
RESULTS
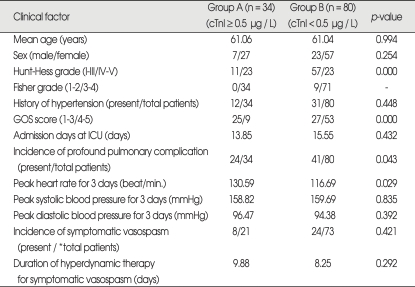
Clinical characteristics of the patients related with cTnI and clinical factors are summarized in Table 1. The incidence of cTnI ≥ 0.5 µg/L was 29.8% (34/114) in our participants, and statistically significant differences between Group A (cTnI ≥ 0.5 µg/L) and Group B (cTnI < 0.5 µg/L) in age (p = 0.994) and sex (p = 0.254) were not detected.
Table 1.
The relationship between clinical facors and cTnI level
p < 0.05. *20/114 patients with a severe consciousness deterioration (semicoma or coma) or early death that could not present symptomatic vaso-spasm were excluded. cTnI : serum cardiac troponin I, GOS : Glasgow outcome scale, ICU : Intensive care unit
There was a statistically significant difference in H-H grade between Groups A and B in that a higher H-H grade (IV-V) group was related with cTnI elevation (p = 0.000). In a Fisher grade, all of the participants in Group A had higher grades (3-4). The relationship between a history of hypertension and cTnI elevation did not have a statistically significant difference (p = 0.448). There was a statistically significant difference in GOS score between Group A and B, the overall outcome defined as GOS score related with cTnI was better in Group B than in Group A (p = 0.000). There was no statistically significant difference in the admission days to the intensive care unit between Groups A (13.85 days) and B (15.55 days) (p = 0.432). In Group A, profound pulmonary complications, caused by pulmonary edema and pneumonia, defined by imaging studies (chest simple X-ray or computed tomography) developed in 70.6% (24/34) of the participants [p = 0.043, 51.3% (41/80) in Group B].
For the peak heart rate for three days following SAH, there was a statistically significant difference between Groups A (130.59/min.) and B (116.69/min.) in that a higher peak heart rate was related with cTnI elevation (p = 0.029). No significant differences were found in peak blood pressure (systolic and diastolic) for three days following SAH between Group A and Group B [peak systolic (p = 0.835) and peak diastolic (p = 0.392) pressure].
There were no statistically significant differences for the incidence of symptomatic vasospasm (p = 0.421) and the duration of hyperdynamic therapy in patients with symptomatic vasospasm (p = 0.292) between Groups A and B.
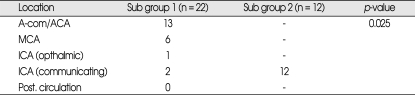
The most common aneurysmal location in Group A was a communicating segment of the internal carotid artery (p = 0.025) (Table 2).
Table 2.
Location of aneurysm in patients with cTnI elevation (Group A, ≥ 0.5 µg/L)
p < 0.05. A-com : anterior communicating artery, cTnI : serum cardiac troponin I, ICA : internal carotid artery, MCA : middle cerebral artery
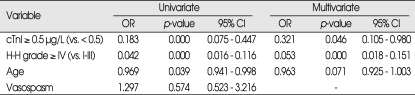
The determinants associated with a good GOS score (4-5) are presented in Table 3. Among independent predictors, cTnI elevation (≥ 0.5 µg/L) [p = 0.000, Odds ratio (OR) = 0.183], higher H-H grades (IV-V) (p = 0.000, OR = 0.420) and older age (p = 0.039, OR = 0.969) were determinants with a negative effect on a good GOS score (4-5). However, the presence of vasospasm was not a statistically significant independent predictor (p = 0.574). Additionally, cTnI elevation (≥ 0.5 µg/L) (p = 0.046, OR = 0.321) and higher H-H grades (IV-V) (p = 0.000, OR = 0.053) were determinants with a negative effect on a good GOS score (4-5) in a multivariate logistic regression analysis. However, age was not a statistically significant determinant in a multivariate study (p = 0.071).
Table 3.
Determinants for a good GOS score (4-5)
p < 0.05. CI : confidence interval, cTnI : serum cardiac troponin I, GOS : Glasgow outcome scale, H-H : Hunt-Hess, OR : odds ratio
The relationship between cTnI and the outcome in patients with symptomatic vasospasm following SAH is presented in Table 4. There was a 32 patients with a symptomatic vasospasm, and a statistically significant difference according to cTnI level that a cTnI elevation (≥ 0.5 µg/L) group was related with a poor outcome (GOS 1-3) (p = 0.037).
Table 4.
The relationship between cTnI level and outcome in patients with symptomatic vasospasm
p < 0.05. cTnI : serum cardiac troponin I, GOS : Glasgow outcome scale
DISCUSSION
Among cardiac laboratory parameters, the strongest and only independent predictor of poor outcome identified by the literature is the elevation of serum cardiac troponin I level9). The serum cardiac troponin I is used to diagnose myocardial damage, including acute myocardial infarction and cardiomyopathy, that increase rapidly in the first 3-12 hours following myocardial damage then peak at 24 hours and may stay elevated for about 5-7 days. In a previous article, Tung et al.9) reported that 20% of patients with aneurysmal SAH went on to develop elevated cTnI level. Tung and colleagues9) also showed that the severity of the neurological injury was related to the degree of myocardial necrosis, which supports the hypothesis that SAH-associated sympathetic tone and the resulting level of circulating catecholamines may play a critical role following head trauma and aneurysmal SAH. According to Tung et al.9), all patients with SAH and abnormal cardiac manifestations had normal coronary arteries both at percutaneous cardiac catheterization and at autopsy. This finding demonstrates that myocytolysis and contraction band necrosis resulting in global ventricular hypokinesis do not correlate geographically with regional wall motion abnormalities seen on ECG10).
It is possible that cardiovascular dysfunction may result from the myocardial stress caused by hyperdynamic therapy, which is routinely used to improve cerebral perfusion in patients with symptomatic vasospasm. Interestingly, an elevated cTnI level may help to identify patients at increased risk for cardiac decompensation when hyperdynamic therapy is indicated5).
In this study, patients with symptomatic vasospasm and elevated cTnI levels generally had worse outcomes. Patients with elevated cTnI levels have stressed hearts and myocardial decompressed states caused by the sympathetic effect (mentioned below) following SAH. Additional hyperdynamic effects from vasopressors (dopamine, epinephrine and others) may aggravate cardiac problems, pulmonary complications (pulmonary edema, pneumonia and others) and eventually poor outcomes. We think that patients with elevated cTnI levels should be considered poor candidates for hyperdynamic therapy for symptomatic vasospasm, and early chemical or mechanical angioplasty may be an appropriate alternative.
Changes in blood pressure and heart rate accompanied by ECG abnormalities are often observed in patients after SAH2). Sudden death following SAH is frequently reported, suggesting that the functional anatomical connections between the central nervous system and the heart may be involved2). Some authors have reported that the rostral midbrain area is essential to produce hypertension and premature ventricular contractions under conditions of simulated SAH, while the other parts of brain stem can produce bradycardia and other arrhythmias2). This mechanism results from an activation of the sympathetic nervous system secondary to an elevation of intracranial pressure4). For these reasons, we believe that the location of the ruptured aneurysm plays an important role because it applies direct pressure on specific brain regions, which may cause tachycardia, arrhythmia and an ischemic heart state after rupture. In this study, ruptured aneurysms on a communicating segment of the internal carotid artery were most strongly correlated with an elevated cTnI of ≥ 0.5 µg/L and may stimulate specific brain regions, including the midbrain. Nevertheless, the pathophysiologic relationship between aneurysm location and cTnI elevation is not fully understood and requires further investigation.
We also analyzed the relationship between heart rate, blood pressure and cTnI level. We found that an increased heart rate is related to cTnI elevation. In particular, our results showed that peak heart rate during the initial three days following SAH was significantly correlated with cTnI elevation. Interestingly, some reports have found that a β-receptor blockade reverses ECG changes, prevents myocardial necrosis and reduces mortality caused by increased sympathetic activity and the resulting release of circulating catecholamines following SAH3). One study reported that the myocardium of patients with SAH who had been given α-blockers and β-blockers had significantly less damage at autopsy, including less focal necrosis and inflammatory cell infiltration4).
In previous literature1,5), reduction of cardiac output in severely affected patients with cTnI elevation may increase the risk of cerebral ischemia related to vasospasm. Experimental studies have shown that cerebral blood flow (CBF) in ischemic areas can vary passively with changes in blood pressure and cardiac output. We analyzed the incidence of symptomatic vasospasm and duration of hyperdynamic therapy according to cTnI elevation. There were no statistically significant differences for the incidence and duration of hyperdynamic therapy. We believe that the incidence of symptomatic vasospasm is related with the previously mentioned general risk factors for vasospasm, however vasospasm with cTnI elevation was associated with a poor outcome. Additionally, no statistically significant differences for the duration of hyperdynamic therapy between Groups A and B may have been observed because Group A had a higher early death rate caused by cardiopulmonary complications and other causes during hyperdynamic therapy.
CONCLUSION
We can conclude that it is useful to monitor cTnI level following SAH, especially during the initial three days to estimate cardiopulmonary complications and outcomes. The cTnI and H-H grade were determinants for outcome, and development of vasospasm concomitant with cardiac complications as defined by cTnI elevation is related with a poor outcome. Additionally, a ruptured aneurysm on communicating segment of ICA is related with cTnI elevation. However, further research is essential to determine the mechanisms underlying the results of this study and to choose optimal treatment.
Acknowledgments
This research was supported by the Yeungnam University research grants in 2008.
References
- 1.Armin S. Cardiovascular therapy of neurosurgical patients. Best Practice Res Clin Anaesthesiol. 2007;21:483–496. doi: 10.1016/j.bpa.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Lacy PS, Earle AM. Central neural control of blood pressure and cardiac arrhythmias during subarachnoid hemorrhage in rats. Stroke. 1985;16:998–1002. doi: 10.1161/01.str.16.6.998. [DOI] [PubMed] [Google Scholar]
- 3.Lambert E, Du XJ, Percy E, Lambert G. Cardiac response to norepinephrine and sympathetic nerve stimulation following experimental subarachnoid hemorrhage. J Neurol Sci. 2002;198:43–50. doi: 10.1016/s0022-510x(02)00073-4. [DOI] [PubMed] [Google Scholar]
- 4.Masuda T, Sato K, Yamamoto S, Matsuyama N, Shimohama T, Matsunaga A, et al. Sympathetic nervous activity and myocardial damage immediately after subarachnoid hemorrhage in a unique animal model. Stroke. 2002;33:1671–1676. doi: 10.1161/01.str.0000016327.74392.02. [DOI] [PubMed] [Google Scholar]
- 5.Naidech AM, Kreiter KT, Janjua N, Ostapkovich ND, Parra A, Commichau C, et al. Cardiac troponin elevation, cardiovascular morbidity, and outcome after subarachnoid hemorrhage. Circulation. 2005;112:2851–2856. doi: 10.1161/CIRCULATIONAHA.105.533620. [DOI] [PubMed] [Google Scholar]
- 6.Parekh N, Venkatesh B, Cross D, Leditschke A, Atherton J, Miles W, et al. Cardiac troponin I predicts myocardial dysfunction in aneurysmal subarachnoid hemorrhage. J Am Coll Cardiol. 2000;36:1328–1335. doi: 10.1016/s0735-1097(00)00857-3. [DOI] [PubMed] [Google Scholar]
- 7.Schuiling WJ, Dennesen PJ, Tans JT, Kingma LM, Algra A, Rinkel GJ. Troponin I in predicting cardiac or pulmonary complications and outcome in subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2005;76:1565–1569. doi: 10.1136/jnnp.2004.060913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sim JH. Intracranial aneurysms in Korea. Neurol Med Chir (Tokyo) 1998;38(Suppl):118–121. doi: 10.2176/nmc.38.suppl_118. [DOI] [PubMed] [Google Scholar]
- 9.Tung P, Kopelnik A, Banki N, Ong K, Ko N, Lawton MT, et al. Predictors of neurocardiogenic injury after subarachnoid hemorrhage. Stroke. 2004;35:548–551. doi: 10.1161/01.STR.0000114874.96688.54. [DOI] [PubMed] [Google Scholar]
- 10.Urbaniak K, Merchant AI, Amin-Hanjani S, Roitberg B. Cardiac complications after aneurysmal subarachnoid hemorrhage. Surg Neurol. 2007;67:21–28. doi: 10.1016/j.surneu.2006.08.065. discussion 28-29. [DOI] [PubMed] [Google Scholar]