Abstract
In mammals, imprinted genes have parent-of-origin–specific patterns of DNA methylation that cause allele-specific expression. At Rasgrf1 (encoding RAS protein-specific guanine nucleotide-releasing factor 1), a repeated DNA element is needed to establish methylation and expression of the active paternal allele1. At Igf2r (encoding insulin-like growth factor 2 receptor), a sequence called region 2 is needed for methylation of the active maternal allele2,3. Here we show that replacing the Rasgrf1 repeats on the paternal allele with region 2 allows both methylation and expression of the paternal copy of Rasgrf1, indicating that sequences that control methylation can function ectopically. Paternal transmission of the mutated allele also induced methylation and expression in trans of the normally unmethylated and silent wild-type maternal allele. Once activated, the wild-type maternal Rasgrf1 allele maintained its activated state in the next generation independently of the paternal allele. These results recapitulate in mice several features in common with paramutation described in plants4.
Rasgrf1 is methylated on the paternal allele in a differentially methylated domain (DMD) 30 kb 5′ of the promoter. Expression is from the paternal allele in neonatal brain5. This imprinting requires a 1.6-kb repeated element located immediately downstream of the DMD consisting of a 41-mer repeated 40 times that regulates establishment of methylation at the DMD1,6. The DMD is a methylation-sensitive enhancer-blocking element, which, together with the repeats, functions as a binary switch that regulates imprinting. Sequences regulating DNA methylation have been identified for one other locus, Igf2r. In intron 2 of Igf2r, region 2 controls methylation and allele-specific expression2,3,7.
We generated mice containing Igf2r region 2 in place of the Rasgrf1 repeats to determine if their activities overlap. Reciprocal crosses were done between mice heterozygous with respect to this allele (Rasgrf1tm3.1Pds, Fig. 1) and PWK mates to monitor expression from the two alleles in neonatal brain5. Similar crosses were done with C57BL/6 mates to evaluate changes in methylation of the Rasgrf1 DMD.
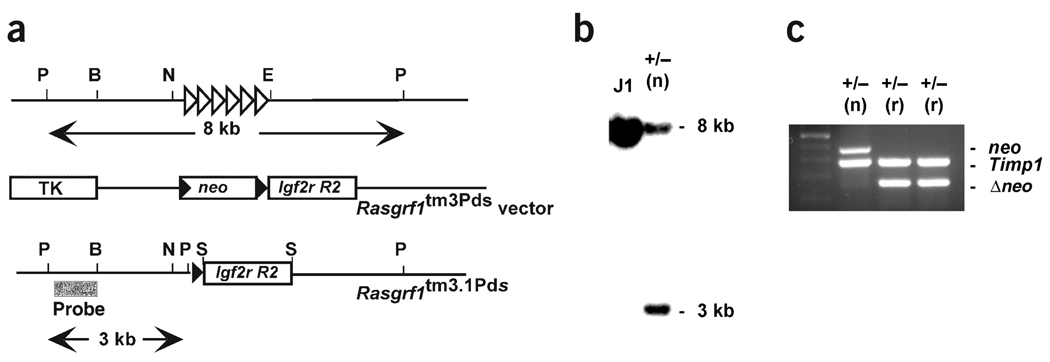
Figure 1.
Generation of mice with the Rasgrf1tm3.1Pds allele. (a) The wild-type Rasgrf1 locus, targeting vector and structure of the Rasgrf1tm3.1Pds allele are shown. Open triangles represent the Rasgrf1 repeats 30 kb 5′ of the transcription start site. Immediately upstream of the repeats is the 350-bp DMD. The neo cassette flanked by loxP sites (filled triangles) and Igf2r region 2 (R2) replaced the repeats in the targeting vector. Cre-mediated excision of the neo cassette produced the Rasgrf1tm3.1Pds allele used in this study. PstI (P), BamHI (B), NotI (N), EcoRV (E) and SalI (S) sites are shown. (b) Hybridization of the probe in a to a Southern blot containing PstI-digested DNA from parental J1 ES cells and a targeted clone containing the neo cassette (+/−(n)) produced bands of 8.0 kb and 3.0 kb from the wild-type and mutated alleles, respectively. (c) DNA from progeny of a cross between males with the +/− (n) allele and Zp3-cre transgenic females was analyzed by a PCR assay that showed loss of the neo cassette (Δneo) and acquisition of a new band diagnostic of the Rasgrf1tm3.1Pds neo-deleted allele (+/− (r)). Timp1 was used as a positive control for amplification.
Maternal transmission of the Rasgrf1tm3.1Pds allele (Rasgrf1−/+) had no effect on methylation or expression of the locus, which remained paternal allele–specific and was expressed at levels comparable to those in wild-type mice (Fig. 2a and data not shown). Mice with a paternally transmitted repeat deletion lacked both methylation and expression of the paternal allele1, but paternal transmission of the Rasgrf1tm3.1Pds mutation (Rasgrf1+/−) permitted expression of the locus, albeit at lower levels than in wild-type mice (Fig. 2b). The paternal Rasgrf1tm3.1Pds allele also caused derepression of the normally silent wild-type maternal allele. Expression of the mutated paternal allele in Rasgrf1+/− mice showed that region 2 was able, in part, to replace the function of the Rasgrf1 repeats.
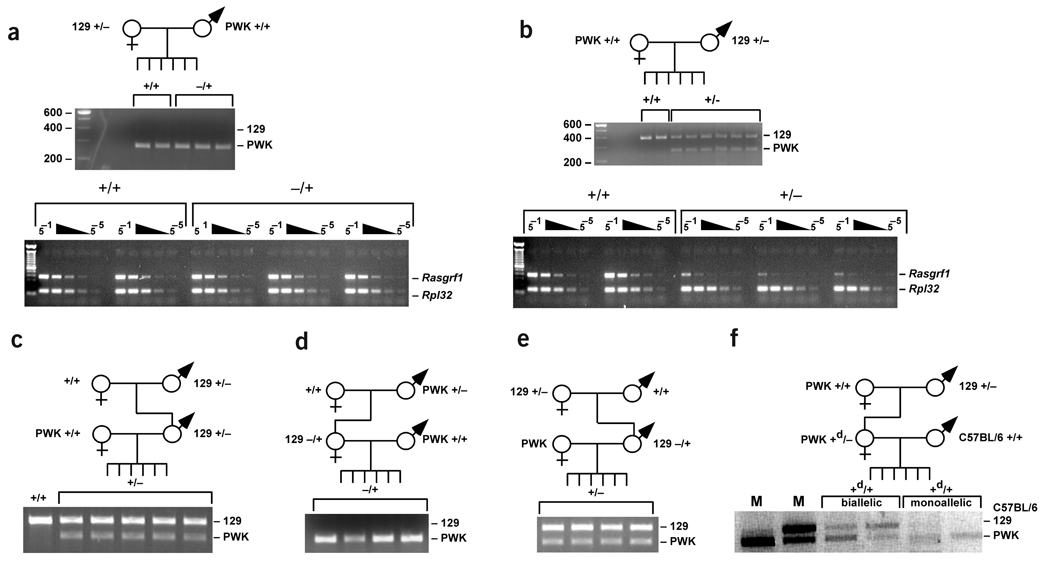
Figure 2.
Paternal transmission of the Rasgrf1tm3.1Pds allele caused derepression of the normally silent wild-type maternal allele and paramutation. Allele-specific and quantitative expression analysis of Rasgrf1 was done using neonatal brain from (a) wild-type (+/+) and mutant (−/+) progeny of heterozygous mothers and (b) wild-type (+/+) and mutant (+/−) progeny of heterozygous fathers. Assays identifying the expressed allele (middle) were done as described1,6. RNA levels (bottom) were measured by RT–PCR using a dilution series (5−1 to 5−5) of cDNA and primers for Rasgrf1 and Rpl32 (an internal control) as described1. Allelespecific expression was determined after (c) transmission of the mutated allele for two generations through the male germ line; (d) when transmission for the second generation was through the female germ line; and (e) after a two-generation cross where the mode of inheritance was through the female germ line for the first generation and through the male germ line for the second generation. A two generation cross served as a test for paramutation (f). Wild-type PWK females were crossed with +/− 129 males. Female progeny with a wild-type maternal PWK allele that had been derepressed (Rasgrf1+d) were crossed to wild-type C57BL/6 males. The maternal and paternal alleles in resulting wild-type (Rasgrf1+d/+) mice were from PWK and C57BL/6, respectively. Allele-specific expression analysis was done on Rasgrf1+d/+ mice. Mice expressing both alleles (biallelic) or predominantly the maternal allele (monoallelic) are indicated. Markers (M) were from mice with monoallelic PWK (left lane) or biallelic PWK and 129 expression (adjacent lane). In a through f, the top panels depict the cross.
Trans activation of the wild-type allele was heritable and reversible. Pups with a paternally transmitted Rasgrf1tm3.1Pds allele had biallelic expression of Rasgrf1 regardless of which grandparent transmitted the mutation. Pups that had inherited the mutated allele from the mother had normal monoallelic expression (Fig. 2c–e).
Trans activation of the normally silent maternal allele in Rasgrf1+/− mice is reminiscent of transvection in Drosophila melanogaster8,9 and paramutation in maize (Zea mays; refs. 4,10). In paramutation, expression of a paramutable allele may be altered by a paramutagenic allele on the homologous chromosome. Once affected, altered expression of the paramutable allele persists through meiosis independently of its homolog. In some cases, an affected paramutable allele can behave as a paramutagenic allele in the next generation, an effect termed secondary paramutation11
To determine if trans allele activation of the wild-type maternal allele in Rasgrf1+/− mice is stable through meiosis, as is the case with paramutation, we carried out crosses using Rasgrf1+/− females that had inherited the Rasgrf1tm3.1Pds allele from strain 129 fathers and the wild-type allele from PWK mothers. We crossed these females, which were shown to have biallelic expression of Rasgrf1 (Fig. 2b,c,e), with wild-type C57BL/6 males. The wild-type progeny carried a C57BL/6 allele from their fathers (Rasgrf1+) and a wild-type PWK allele from their mothers that had been biallelically expressed in the mother (Rasgrf1+d). These Rasgrf1d/+ progeny fell into two clear phenotypic classes (Fig. 2f). In one, biallelic expression was preserved, indicating that aberrant expression of the maternal Rasgrf1+d allele was retained through meiosis. In the other class, the maternal allele retained its aberrant expression and the Rasgrf1+ wild-type paternal allele was inappropriately silenced.
This showed that transmission of the Rasgrf1tm3.1Pds allele from father to daughter modified the daughter’s Rasgrf1+d wild-type allele in a manner that allowed it to affect expression of both parental alleles in the grandchildren. This recapitulates the two key properties of paramutation: regulation of expression of one allele by the other and stability of this phenotype through meiosis. The class of offspring with a silenced paternal allele showed secondary paramutation, a third property seen in some cases of paramutation11. The paramutation-like effects were observed only when the Rasgrf1+d allele was maternally transmitted, recapitulating a fourth feature of paramutation noted at the r1 locus in maize, where paramutation was observed only on transmission of the paramutated allele from males12. Paternal transmission of the wild-type allele by Rasgrf1+/− males led to normal monoallelic expression from the paternal allele (Fig. 2c).
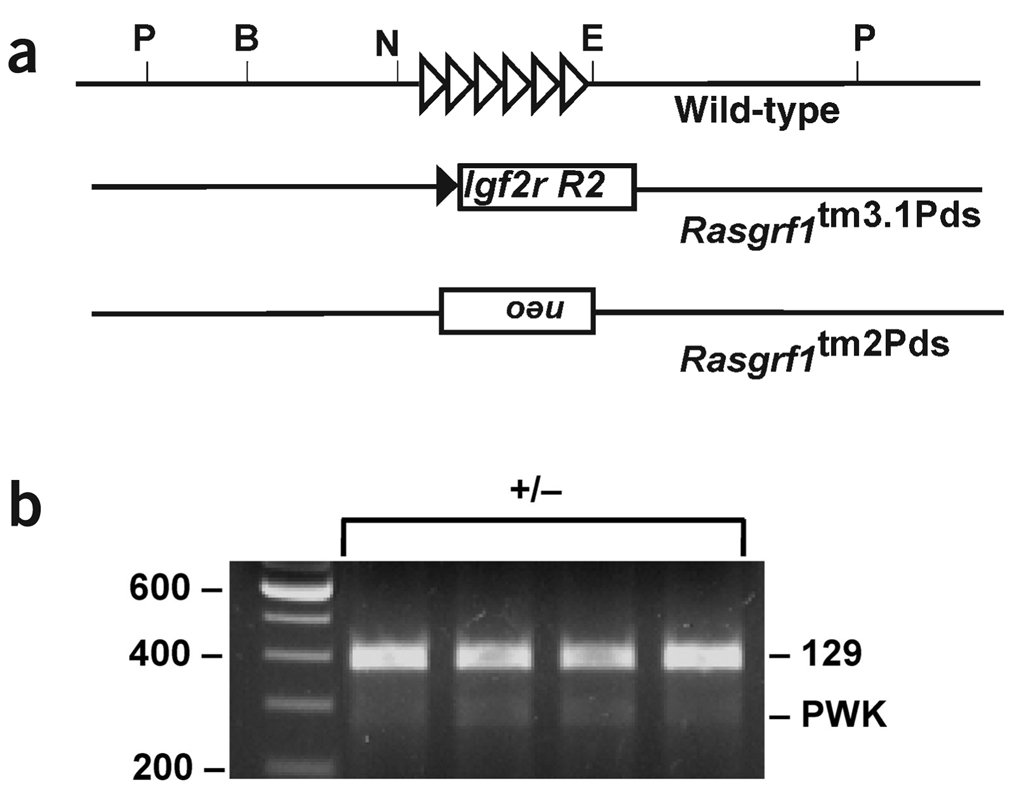
To determine if paramutation required region 2 specifically, we tested the ability of another Rasgrf1 allele to cause paramutation. The Rasgrf1tm2Pds allele contained the same deletion as Rasgrf1tm3.1Pds but a 2.3-kb Pgk-neo cassette insertion instead of the 2.8-kb insertion in Rasgrf1tm3.1Pds (B.J.Y., H.H. and P.D.S., unpublished data). None of the heterozygous progeny of Rasgrf1+/tm2Pds males expressed Rasgrf1 from the maternal allele (Fig. 3), showing that the phenotype associated with Rasgrf1tm3.1Pds resulted from an activity specific to the Igf2r sequences.
Figure 3.
Trans allele effects caused by insertion of Igf2r region 2 are due to the specific sequence changes. (a) Males with the Rasgrf1tm2Pds allele (bottom line) were crossed with PWK females. (b) Allele-specific expression analysis was done on the Rasgrf1+/tm2Pds progeny.
We have previously shown that Rasgrf1 expression depends on methylation of the DMD at the expressed locus, which ablates its CTCF-binding and enhancer-blocking activity (ref. 1 and B.J.Y., H.H., Y.C. and P.D.S., unpublished data). Therefore, we hypothesized that the expressed wild-type and mutated alleles of Rasgrf1+/− mice should be methylated on the DMD. To test this, we carried out methylation-specific PCR (MS–PCR; ref. 13) using allele-specific primer pairs and analyzed the PCR products by gel electrophoresis (Fig. 4a,b) and direct sequencing (Fig. 4c). The positive control for detecting wild-type allele methylation was DNA from wild-type mice, which is methylated on the paternal allele. The negative control was DNA from mice carrying a simple deletion of the Rasgrf1 repeats on the paternal chromosome (Rasgrf1+/tm1Pds), which prevents establishment of methylation on either DMD1. MS–PCR was done using a dilution series of bisulfite-treated DNA to permit comparisons of methylation levels. Paternal transmission of the mutation resulted in methylation of the mutated paternal allele (Fig. 4b), indicating that Igf2r region 2 can replace the Rasgrf1 repeats, causing methylation and expression of the paternal allele, albeit less efficiently than the native repeats. Furthermore, paternal transmission of the Rasgrf1tm3.1Pds allele imparted methylation to the wild-type maternal allele in trans, resulting in its aberrant expression.
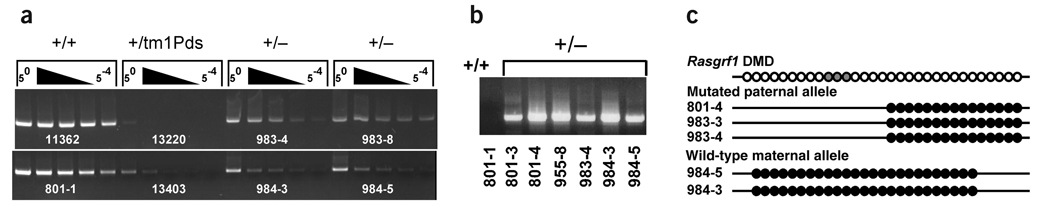
Figure 4.
Paternal transmission of the Rasgrf1tm3.1Pds allele leads to methylation of both alleles. (a) Wild-type allele–specific MS–PCR was done using a dilution series (50 to 5−4) of bisulfite-treated DNA isolated from wild-type mice (+/+), mice with a paternally transmitted Rasgrf1tm3.1Pds allele (+/−) or mice with a paternally transmitted allele previously shown to cause a complete loss of Rasgrf1 methylation1 (+/tm1Pds). (b) MS–PCR was done using primers specific for the mutated paternal allele. (c) Selected products from a and b were sequenced directly or cloned and sequenced to verify that bands in a and b arose from methylated DNA. Numbers identify specific mice. The 31 CpG dinucleotides in the DMD are shown as open circles, and the three lightly shaded circles represent CpGs in the NotI site (top line). The CpGs assayed on the mutated and wild-type alleles are shown as circles on the lower five lines. Methylated CpGs are depicted as filled circles.
Trans allele methylation has been reported in Ascobolus immersus14, maize15 and mice. Mice with a U2af1-rs transgene acquired aberrant methylation on the endogenous paternal allele16. A deletion at the maternal H19 allele reduced methylation at the linked wild-type paternal Igf2 allele17. At the Rosa26 locus modified with a loxP-stop-loxP cassette, Cre-mediated recombination led to methylation of the modified locus that was transferred to the unrecombined loxP-stop-loxP allele on the homologous chromosome in the next generation. Transferred methylation was stable for multiple generations. This was originally characterized as transvection but may represent paramutation18.
In our system, trans methylation and activation of the wild-type maternal Rasgrf1 allele was initiated after fertilization (Fig. 2). Initiation required a paternal Rasgrf1tm3.1Pds allele, but communication between wild-type Rasgrf1+d and Rasgrf1+ alleles persisted in the next generation in the absence of region 2 (Fig. 2f). This suggested that despite the artificial means of initiating the trans allelic interactions, such interactions are normal genomic events. It is not known if initiation involved physical interactions between homologous alleles19, the sequences in region 2 implicated in de novo methylation and allele discrimination2, the paternal-specific Air transcript from the endogenous Igf2r locus3 or the extensive inverted repeat structure in region 2.
Trans allele phenomena are relevant to human disease. Susceptibility to type 1 diabetes has been shown to be sensitive to a trans allele effect during male meiosis20. Details of the mechanisms underlying methylation control and trans allele regulation at Rasgrf1 may assist in understanding related processes involving alleles associated with disease.
METHODS
Preparation of mutant mice
The Rasgrf1tm3Pds vector (pML4-2) was a modified form of the Rasgrf1tm1Pds vector (pBJR3; ref. 1). We filled in a SalI site at the 3′ end of the 3′ arm of pBJR3 and replaced it with a unique AscI site to produce pML3. We modified a 2.8-kb plasmid containing Igf2r region 2 (P4, a gift from D. Barlow7, University of Vienna, Vienna, Austria) to include SalI sites on either side of the Igf2r sequences and inserted the SalI fragment at the single remaining SalI site of pML3. In pML4-2, the Igf2r sequences assumed the same 5′ to 3′ orientation with respect to Rasgrf1 that is assumed at Igf2r. We electroporated embryonic stem (ES) cells with pML4-2 linearized by AscI, and we used homologous recombinants identified by Southern-blot hybridization1 to prepare germline chimeras. We excised the neo cassette by crossing male mutants with Zp3-cre transgenic mice21 to produce the Rasgrf1tm3.1Pds allele. We verified excision using three primer pairs (sequences available on request). We did all experiments with the approval of the Institutional Animal Care and Use Committees at Roswell Park Cancer Institute and Cornell University.
RNA analysis
We prepared RNA and carried out allele-specific assays for Rasgrf1 as described previously5,22.
Methylation analysis
We carried out nested MS–PCR on bisulfite-treated DNA, prepared from tail or limb tissue as described1. We amplified the wild-type allele using primers WF686 and WR1083, which amplified both methylated and unmethylated templates, followed by a second round of amplification with primers WF714M and WR1005M, which amplified only methylated DNA and resulted in a product of 292 bp. Primer sequences are available on request. We analyzed the products by electrophoresis, direct sequencing using WF714M and WR1005m as the sequencing primers and sequencing after cloning in the TopoTA vector (InVitrogen) using T3 and T7 sequencing primers. We amplified the mutated allele using either one or two rounds of PCR using primers WF803M and LuR1087M (sequences available on request).
ACKNOWLEDGMENTS
The authors thank D. Barlow for the Igf2r region 2 clone, G. Martin for Zp3-cre mice and M. Stam and V.L. Chandler for helpful comments and sharing unpublished data. This work was supported by grants to P.D.S. from the US National Eye Institute, US National Cancer Institute, US Department of Defense and the Roswell Park Alliance and by a US National Cancer Institute Core Grant to Roswell Park Cancer Institute.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
References
- 1.Yoon BJ, et al. Regulation of DNA methylation of Rasgrf1. Nat. Genet. 2002;30:92–96. doi: 10.1038/ng795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birger Y, Shemer R, Perk J, Razin A. The imprinting box of the mouse Igf2r gene. Nature. 1999;397:84–88. doi: 10.1038/16291. [DOI] [PubMed] [Google Scholar]
- 3.Sleutels F, Zwart R, Barlow DP. The non-coding Air RNA is required for silencing autosomal imprinted genes. Nature. 2002;415:810–813. doi: 10.1038/415810a. [DOI] [PubMed] [Google Scholar]
- 4.Brink RA. A genetic change associated with the R locus in maize which is directed and potentially reversible. Genetics. 1956;41:872–889. doi: 10.1093/genetics/41.6.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plass C, et al. Identification of Grf1 on mouse chromosome 9 as an imprinted gene by RLGS-M. Nat. Genet. 1996;14:106–109. doi: 10.1038/ng0996-106. [DOI] [PubMed] [Google Scholar]
- 6.Pearsall RS, et al. A direct repeat sequence at the Rasgrf1 locus and imprinted expression. Genomics. 1999;55:194–201. doi: 10.1006/geno.1998.5660. [DOI] [PubMed] [Google Scholar]
- 7.Stoger R, et al. Maternal-specific methylation of the imprinted mouse Igf2r locus identifies the expressed locus as carrying the imprinting signal. Cell. 1993;73:61–71. doi: 10.1016/0092-8674(93)90160-r. [DOI] [PubMed] [Google Scholar]
- 8.Lewis EB. The theory and application of a new method of detecting chromosomal rearrangements in Drosophila melanogaster. Am. Nat. 1954;88:225–239. [Google Scholar]
- 9.Kennison JA, Southworth JW. Transvection in Drosophila. Adv. Genet. 2002;46:399–420. doi: 10.1016/s0065-2660(02)46014-2. [DOI] [PubMed] [Google Scholar]
- 10.Chandler VL, Eggleston WB, Dorweiler JE. Paramutation in maize. Plant Mol. Biol. 2000;43:121–145. doi: 10.1023/a:1006499808317. [DOI] [PubMed] [Google Scholar]
- 11.Patterson GI, Thorpe CJ, Chandler VL. Paramutation, an allelic interaction, is associated with a stable and heritable reduction of transcription of the maize b regulatory gene. Genetics. 1993;135:881–894. doi: 10.1093/genetics/135.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker EL. Paramutation of the r1 locus of maize is associated with increased cytosine methylation. Genetics. 1998;148:1973–1981. doi: 10.1093/genetics/148.4.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylationspecific PCR: a novel PCR assay for methylation status of CpG islands. Proc. Natl. Acad. Sci. USA. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colot V, Maloisel L, Rossignol JL. Interchromosomal transfer of epigenetic states in Ascobolus: transfer of DNA methylation is mechanistically related to homologous recombination. Cell. 1996;86:855–864. doi: 10.1016/s0092-8674(00)80161-0. [DOI] [PubMed] [Google Scholar]
- 15.Stam M, Belele C, Dorweiler JE, Chandler VL. Differential chromatin structure within a tandem array 100 kb upstream of the maize b1 locus is associated with paramutation. Genes Dev. 2002;16:1906–1918. doi: 10.1101/gad.1006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatada I, et al. Aberrant methylation of an imprinted gene U2af1-rs1(SP2) caused by its own transgene. J. Biol. Chem. 1997;272:9120–9122. doi: 10.1074/jbc.272.14.9120. [DOI] [PubMed] [Google Scholar]
- 17.Forne T. Loss of the maternal H19 gene induces changes in Igf2 methylation in both cis and trans. Proc. Natl. Acad. Sci. USA. 1997;94:10243–10248. doi: 10.1073/pnas.94.19.10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rassoulzadegan M, Magliano M, Cuzin F. Transvection effects involving DNA methylation during meiosis in the mouse. EMBO J. 2002;21:440–450. doi: 10.1093/emboj/21.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaSalle JM, Lalande M. Homologous association of oppositely imprinted chromosomal domains. Science. 1996;272:725–728. doi: 10.1126/science.272.5262.725. [DOI] [PubMed] [Google Scholar]
- 20.Bennett ST, et al. The IMDIAB Group. Insulin VNTR allele-specific effect in type 1 diabetes depends on identity of untransmitted paternal allele. Nat. Genet. 1997;17:350–352. doi: 10.1038/ng1197-350. [DOI] [PubMed] [Google Scholar]
- 21.Lewandoski M, Wassarman KM, Martin GR. Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr. Biol. 1997;7:148–151. doi: 10.1016/s0960-9822(06)00059-5. [DOI] [PubMed] [Google Scholar]
- 22.de la Puente A, et al. Structural characterization of Rasgrf1 and a novel linked imprinted locus. Gene. 2002;291:287–297. doi: 10.1016/s0378-1119(02)00601-7. [DOI] [PubMed] [Google Scholar]