Abstract
AIM: To evaluate the chemoradiotherapy for locally advanced pancreatic cancer utilizing low dose gemcitabine as a radiation sensitizer administered twice weekly.
METHODS: We performed a retrospective analysis of chemoradiotherapy utilizing gemcitabine administered twice weekly at a dose of 40 mg/m2. After that, maintenance systemic chemotherapy with gemcitabine, at a dose of 1000 mg/m2, was administered weekly for 3 wk with 1-wk rest until disease progression or unacceptable toxicity developed.
RESULTS: Eighteen patients with locally advanced unresectable pancreatic cancer were enrolled. Three of those patients could not continue with the therapy; one patient had interstitial pneumonia during radiation therapy and two other patients showed liver metastasis or peritoneal metastasis during an early stage of the therapy. The median survival was 15.0 mo and the overall 1-year survival rate was 60%, while the median progression-free survival was 8.0 mo. The subgroup which showed the reduction of tumor development, more than 50% showed a tendency for a better prognosis; however, other parameters including age, gender and performance status did not correlate with survival. The median survival of the groups that died of liver metastasis and peritoneal metastasis were 13.0 mo and 27.7 mo, respectively.
CONCLUSION: Chemoradiotherapy with low-dose gemcitabine administered twice weekly could be effective to patients with locally advanced pancreatic cancer; however, patients developing liver metastases had a worse prognosis. Another chemoradiotherapy strategy might be needed for those patients, such as administrating one or two cycles of chemotherapy initially, followed by chemoradiotherapy for the cases with no distant metastases.
Keywords: Advanced pancreatic cancer, Chemoradiotherapy, Gemcitabine, Radiosensitizer, Tumor marker
INTRODUCTION
Pancreatic cancer is one of the leading causes of cancer death in the world and in most patients the tumor is surgically unresectable at the time of diagnosis[1]. Even in the patient with complete surgical resection, both distant and local patterns of recurrence are common[2]. In approximately 50% of resected pancreatic tumors, the surgical margins are involved with tumor cells, so it can be assumed that most patients are harboring occult metastases at the time of diagnosis[3]. Recently, studies for adjuvant chemotherapy or chemoradiotherapy, and those for neoadjuvant chemotherapy or chemoradiotherapy have been investigated[3-6].
For patients with locally advanced pancreatic cancer, chemoradiotherapy has been accepted as a standard treatment[7]. The results of previous randomized trials have indicated that external-beam radiation therapy and 5-fluorouracil (5-FU) therapy results in a significantly longer survival time than radiotherapy[8] or chemotherapy alone[9]. Gemcitabine, a deoxycytidine analog that functions as an antimetabolite, has been approved for use in patients with advanced pancreatic cancer[10,11]. In a randomized study, gemcitabine improved survival in inoperable pancreatic cancer in comparison with 5-FU[12]. Gemcitabine has also been shown to exert an effect in 5-FU-refractory pancreatic cancer[13]. Gemcitabine has also been shown to be a potent radiosensitizer, both in vivo and in vitro[14]. The vast majority of the reported phase I-III clinical trials have used gemcitabine as a single agent given weekly in a single dose[7,15,16] (i.e. 250 mg/m2).
Several preclinical data, including animal studies[17], would suggest that maximum radiation sensitization with gemcitabine is observed at a lower dose administered twice weekly[15,17]. Blackstock et al[14,15] and Magnino et al[18] reported on a phase II study of chemoradiotherapy in which the patient’s were treated with gemcitabine twice weekly at 40 mg/m2 and 50 mg/m2, respectively, associated with radiotherapy. Therefore, in the present study, we analyzed the results of retrospective analysis of chemoradiotherapy for locally advanced pancreatic cancer, utilizing gemcitabine as a radiation sensitizer administered twice weekly at a dose of 40 mg/m2, followed by maintenance systemic chemotherapy with gemcitabine.
MATERIALS AND METHODS
Eligibility criteria included (1) locally advanced unresectable pancreatic cancer confirmed histologically or by imaging techniques including systemic computed tomography; (2) 20-74 years of age; (3) ECOG performance status of 0-2; (4) adequate hematological function, and adequate renal function, and (5) no prior anti-cancer treatment. A total dose of 40-50.4 Gy was delivered using 1.8-2.0 Gy daily fractions. Treatment planning was determined by a three-dimensional treatment planner. The targeted irradiation volume included the tumor, possible surrounding edema, and 1-cm margin. Gemcitabine, at a dose of 40 mg/m2, was administered as a 30-min intravenous infusion twice weekly (80 mg/m2 per week) for 4-5 wk. Gemcitabine was given within 2 h before radiation treatment. At 2 wk after the completion of chemoradiotherapy, maintenance systemic chemotherapy of gemcitabine at a dose of 1000 mg/m2 was administered as a 30-min intravenous infusion weekly for 3 wk with 1-wk rest until disease progression or unacceptable toxicity. Both radiation therapy and chemotherapy were suspended for grade 3 hematological toxicities or grade 2 non-hematological toxicities (according to the National Cancer Institute Common Toxicity Criteria) during the treatment course, and treatment was resumed when toxicity was resolved. The objective tumor response, as defined by the WHO criteria, was assessed every 2 mo or 3 mo by computed tomography scan or earlier if clinically indicated.
The Kaplan-Meier method was used to estimate the distribution of overall survival and progression free survival. Progression free survival was calculated from the first day of treatment until there was evidence of clinical progression, tumor progression assessed by computed tomography scan measurement or death. Overall survival was calculated from the first day of treatment until the date of death. In this study, there is no control arm to treat the locally advanced pancreatic cancer.
RESULTS
Clinical data
Eighteen patients were enrolled in this study. Three of those patients could not continue with the therapy under this protocol; one patient had interstitial pneumonia during radiation therapy and two other patients showed liver metastasis or peritoneal metastasis in an early stage of this protocol. Fifteen patients, including nine males and six females, completed therapy as planned and patient characteristics are shown in Table 1. The mean age was 62.2 years old (range, 50-73). The mean diameter of the tumor was 4.8-cm and the tumor was located in the pancreatic head in seven patients. Twelve patients received radiotherapy at a total of 40-Gy, two patients at a total dose of 50-Gy and one patient with 50.4-Gy. In general, therapy was well tolerated, one patient suffered AGML and another patient had an eruption. All the patients showed elevation of tumor markers, including CA19-9, Span-1 and DUPAN-2, at the enrollment for this study.
Table 1.
Patient characteristics
| Number of patients completing the protocol | 15 |
| Gender | |
| Male | 9 (60%) |
| Female | 6 (40%) |
| Age (yr) | |
| Mean (range) | 62.2 (50-73) |
| Tumor location | |
| Head | 7 (46.7%) |
| Head-Body | 1 (6.6%) |
| Body-Tail | 7 (46.7%) |
| Total radiation dose | |
| 40.0 Gy | 12 (80%) |
| 50.0 Gy | 1 (6.6%) |
| 50.4 Gy | 2 (13.4% |
| Response | |
| Complete response | 1 (6.6%) |
| Partial response | 4 (26.7%) |
| Stable disease | 9 (60 %) |
| Progressive disease | 1 (6.6%) |
| Cause of death | |
| Liver metastasis | 10 (66.7%) |
| Peritoneal metastasis | 3 (20%) |
Survival
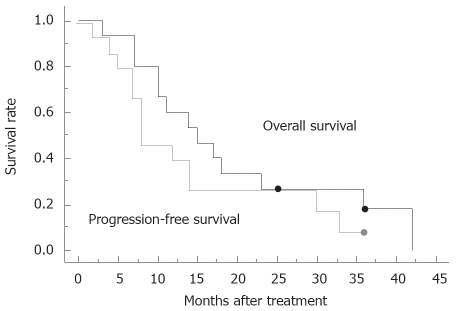
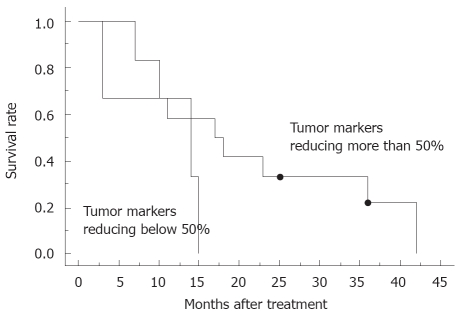
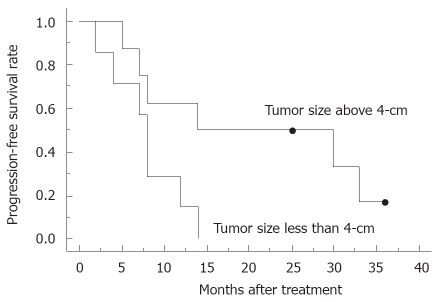
Regarding overall response, there was one complete response, 4 partial responses, 9 stable diseases and one progressive disease; the response rate was 33%. No patients could undergo tumor resection even after the completion of chemoradiotherapy, because of infiltration of the adjacent large vessels. The median survival was 15.0 mo and the overall 1-year survival rate was 60%, while the median progression-free survival was 8.0 mo, estimated by the Kaplan-Meier method (Figure 1). In 80% of the patients, the level of tumor marker, including CA19-9, Span-1 and DUPAN-2, was reduced more than 50% compared to that of pretreatment. The subgroup where the tumor marker was reduced more than 50% had a tendency for a better prognosis (Figure 2), compared to the group with reduced tumor marker below 50% of pretreatment. Blackstock et al[15] postulated previously that the extended median survival observed in the CA19-9 responding patients might reflect the impact of the improved local control. However, a recent study demonstrated that pretreatment serum CA19-9 concentration was an independent prognostic factor for survival for advanced pancreatic cancer, but a decrease in concentration during chemotherapy was not significantly associated with lengthened survival compared with those who did not have a corresponding decrease[19]; therefore, the importance of decreasing in serum tumor marker concentration during therapy requires further discussion. In the subgroup with a tumor size less than 4-cm in diameter, median progression-free survival was 14.0 mo, which was better than those above 4-cm in diameter (8.0 mo, Figure 3). Other parameters, including age, gender and performance status, did not correlate with survival. The major causes of death were liver metastasis and peritoneal metastasis. The median survival of the groups that died of liver metastasis and peritoneal metastasis were 13.0 mo and 27.7 mo, respectively.
Figure 1.
Overall survival curve and progression-free survival curve for 15 patients who received chemoradiotherapy under this study protocol. Dot indicates censored cases.
Figure 2.
Overall survival curve for patients with tumor markers reducing more than 50% compared to that of pretreatment (n = 12) and those reducing below 50% (n = 3). Dot indicates censored cases.
Figure 3.
Progression free-survival curve for patients with tumor size less than 4-cm (n = 6) and those with a size above 4-cm (n = 9). Dot indicates censored cases.
DISCUSSION
A recent retrospective comparison of the toxicity and efficacy of concurrent gemcitabine-based chemoradio-therapy with that of 5-FU based chemoradiotherapy for the patients with unresectable pancreatic cancer[20], showed a significantly higher toxicity rate in patients treated with gemcitabine and similar median survival times between the two arms. Investigators in Taiwan[21] reported favorable results for chemoradiotherapy with concurrent gemcitabine administration (600 mg/m2 once a week); however, this needs further confirmation by larger multi-institutional clinical trials.
Although this study, using a twice weekly gemcitabine infusion schedule for locally advanced pancreatic cancer was not a controlled study, the results of the median survival time, median disease free survival time and overall 1-year survival rate was found to be preferable compared to previous studies[7-9,15]. Okusaka et al[7] presented data of a phase II study for locally advanced pancreatic cancer treated with external-beam radiation (50.4 Gy) and weekly gemcitabine (250 mg/m2 once a week) followed by maintenance chemotherapy using gemcitabine. The median survival time, median progression-free survival time and 1-year survival rate was 9.5 mo, 4.4 mo and 28%, respectively[7]. An expanded retrospective review of patients receiving gemcitabine-based chemoradiotherapy at the M. D. Anderson Cancer Center reflected the difficulties combining the systemic toxicities of 200-500 mg/m2 doses of gemcitabine with the local-regional toxicities associated with chemoradiotherapy to the upper abdomen[22]. Furthermore, in the original GITSG trial of radiation and 5-FU based chemotherapy, 18% and 21% of the patients randomized into the 40-Gy and the 60-Gy treatment arms, respectively, were unable to complete all planned radiation[8]. For those patients completing the chemoradiotherapy, almost one-third were unable to initiate the planned maintenance 5-FU chemotherapy[8]. In this study, 15 of 18 patients could complete the planned protocol which might have come from the treatment with gemcitabine administered via a twice weekly infusion with radiation therapy and that most patients received radiation therapy at a total dose of 40-Gy. This might have resulted in the successful initiation in the maintenance of gemcitabine chemotherapy and to obtain a feasible survival rate in this trial. Some investigators did not propose maintenance chemotherapy after chemoradiotherapy[1]. Several studies of chemoradiotherapy used a therapeutic sequence with prior chemoradiotherapy and then chemotherapy until disease progression, but increased toxicity of chemotherapy after chemoradiotherapy limits this strategy[23,24], which might partially contribute to the total dose of radiation.
Two of the three patients enrolled initially who did not continue with the therapy under this protocol showed liver metastasis or peritoneal metastasis in the early stage of this protocol. Blackstock et al[15] pointed out in their study that the radiation sensitizing properties of twice weekly gemcitabine were important for improving the local control, and did not impact the survival for patients harboring micrometastatic disease at the initiation treatment. Hugutet et al[1] discussed that, an important concern about administrating chemoradiotherapy as first-line treatment in patients with locally advanced pancreatic cancer was that approximately 30% of them had occult metastatic disease at diagnosis and thus, they would clearly not benefit from this locoregional treatment. Furthermore, another investigator demonstrated that a fraction of patients with locally advanced pancreatic cancer developed metastases within a few weeks and died very quickly despite the type of treatment[25]. In this study, the patients who developed liver metastasis had a worse prognosis, which might owe to the miss-diagnosis of the staging of the disease at the initiation of the therapy, because of failure to detect micrometastasis by conventional imaging modalities. In this situation, we might need another strategy for the chemoradiotherapy for locally advanced pancreatic cancer, such as one in which the patients receive one or two cycles of systemic chemotherapy using gemcitabine at a dose of 1000 mg/m2 weekly for 3 wk with 1-wk rest, and then re-evaluated the staging of the disease, initiating the chemoradiotherapy under the protocol in this study. A recent study suggested that after control of disease by initial chemotherapy for at least 3 mo using combination of leucovorin, fluorouracil and gemcitabine, or gemcitabine and oxaliplatin, chemoradiotherapy with 5-FU, could significantly improve survival in patients with locally advanced pancreatic cancer compared with chemotherapy alone[1].
In conclusion, chemoradiotherapy with low-dose gemcitabine given twice weekly could be effective to patients with locally advanced pancreatic cancer; however, patients developing liver metastases had a worse prognosis. We might need another strategy for the chemoradiotherapy for those patients. Further investigations are required in the near future.
COMMENTS
Background
Pancreatic cancer is the fifth most common cause of cancer death in Japan. The prognosis is extremely poor because it is difficult to detect this disease in the early stage and also the postoperative incidence of recurrence is still high. We do not have any effective treatment for inoperable patients. Recently, chemoradiotherapy has been regarded as one of the standard therapies for locally advanced pancreatic cancer and it has improved the survival and presented a clinical benefit.
Research frontiers
In the early 1980s, fluorouracil-based concomitant chemoradiotherapy was shown to be better than radiotherapy alone for patients with locally advanced pancreatic cancer. Gemcitabine has improved the outcome of patients with advanced disease by improving survival with a clinical benefit. Gemcitabine also has been shown to be a potent radiosensitizer, both in vivo and in vitro. The vast majority of the reported phaseI-III clinical trials have used gemcitabine as a single agent given weekly in a single dose (i.e. 250 mg/m2), and there is no consensus of the protocol of the administration of gemcitabine.
Innovations and breakthroughs
Several preclinical data, including animal studies, would suggest that maximum radiation sensitization with gemcitabine is observed at a lower dose administered twice weekly. In this study, we show that we could obtain the feasible results of survival compared to previous studies using our protocol. There existed some patients who could not continue the therapy, because of developing metastases. One reason could be the failure to detect micrometastasis by conventional imaging modalities at the beginning of chemoradiotherapy.
Applications
Chemoradiotherapy, with low-dose gemcitabine given twice weekly, could be effective to patients with locally advanced pancreatic cancer. To improve this survival data, we may need stricter selection of the cases suitable for this chemoradiotherapy; however, using conventional imaging modalities, it seems to be hard to diagnose the micrometastasis, especially in the liver before this chemoradiotherapy. Another strategy that may be useful is where patients receive one or two cycles of systemic chemotherapy using gemcitabine at a dose of 1000 mg/m2 weekly for 3 wk with 1-wk rest, and then be re-evaluated for the staging of the disease, and then initiating the chemoradiotherapy under the protocol in this study.
Peer review
This is a nicely written paper that looks at the use of gemcitabine as a radiation sensitizer for pancreatic cancer. They report on twice-weekly doses. This dose contributes new information to the literature.
Footnotes
Peer reviewer: Michael E Zenilman, MD, Clarence and Mary Dennis Professor and Chairman, Department of Surgery, SUNY Downstate Medical Center, Box40, 450, Clarkson Avenue, Brooklyn, NY 11202, United States
S- Editor Zhong XY L- Editor Rippe RA E- Editor Ma WH
References
- 1.Huguet F, Andre T, Hammel P, Artru P, Balosso J, Selle F, Deniaud-Alexandre E, Ruszniewski P, Touboul E, Labianca R, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 2.White RR, Shah AS, Tyler DS. Pancreatic cancer since Halsted: how far have we come and where are we going? Ann Surg. 2003;238:S132–S144; discussion S145-S147. doi: 10.1097/01.sla.0000097793.68830.5e. [DOI] [PubMed] [Google Scholar]
- 3.Takai S, Satoi S, Yanagimoto H, Toyokawa H, Takahashi K, Terakawa N, Araki H, Matsui Y, Sohgawa M, Kamiyama Y. Neoadjuvant chemoradiation in patients with potentially resectable pancreatic cancer. Pancreas. 2008;36:e26–e32. doi: 10.1097/mpa.0b013e31814b229a. [DOI] [PubMed] [Google Scholar]
- 4.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 5.Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Kudrimoti MR, Fromm ML, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 6.Vento P, Mustonen H, Joensuu T, Karkkainen P, Kivilaakso E, Kiviluoto T. Impact of preoperative chemoradiotherapy on survival in patients with resectable pancreatic cancer. World J Gastroenterol. 2007;13:2945–2951. doi: 10.3748/wjg.v13.i21.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okusaka T, Ito Y, Ueno H, Ikeda M, Takezako Y, Morizane C, Kagami Y, Ikeda H. Phase II study of radiotherapy combined with gemcitabine for locally advanced pancreatic cancer. Br J Cancer. 2004;91:673–677. doi: 10.1038/sj.bjc.6602001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moertel CG, Frytak S, Hahn RG, O'Connell MJ, Reitemeier RJ, Rubin J, Schutt AJ, Weiland LH, Childs DS, Holbrook MA, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer. 1981;48:1705–1710. doi: 10.1002/1097-0142(19811015)48:8<1705::aid-cncr2820480803>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. J Natl Cancer Inst. 1988;80:751–755. [PubMed] [Google Scholar]
- 10.Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB 3rd. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20:3270–3275. doi: 10.1200/JCO.2002.11.149. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto H, Kitano M, Suetomi Y, Takeyama Y, Ohyanagi H, Nakai T, Yasuda C, Kudo M. Comparison of standard-dose and low-dose gemcitabine regimens in pancreatic adenocarcinoma patients: a prospective randomized trial. J Gastroenterol. 2006;41:70–76. doi: 10.1007/s00535-005-1724-7. [DOI] [PubMed] [Google Scholar]
- 12.Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 13.Rothenberg ML. New developments in chemotherapy for patients with advanced pancreatic cancer. Oncology (Williston Park) 1996;10:18–22. [PubMed] [Google Scholar]
- 14.Blackstock AW, Bernard SA, Richards F, Eagle KS, Case LD, Poole ME, Savage PD, Tepper JE. Phase I trial of twice-weekly gemcitabine and concurrent radiation in patients with advanced pancreatic cancer. J Clin Oncol. 1999;17:2208–2212. doi: 10.1200/JCO.1999.17.7.2208. [DOI] [PubMed] [Google Scholar]
- 15.Blackstock AW, Tepper JE, Niedwiecki D, Hollis DR, Mayer RJ, Tempero MA. Cancer and leukemia group B (CALGB) 89805: phase II chemoradiation trial using gemcitabine in patients with locoregional adenocarcinoma of the pancreas. Int J Gastrointest Cancer. 2003;34:107–116. doi: 10.1385/ijgc:34:2-3:107. [DOI] [PubMed] [Google Scholar]
- 16.Crane CH, Varadhachary G, Pisters PW, Evans DB, Wolff RA. Future chemoradiation strategies in pancreatic cancer. Semin Oncol. 2007;34:335–346. doi: 10.1053/j.seminoncol.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Fields MT, Eisbruch A, Normolle D, Orfali A, Davis MA, Pu AT, Lawrence TS. Radiosensitization produced in vivo by once- vs. twice-weekly 2'2'-difluoro-2'-deoxycytidine (gemcitabine) Int J Radiat Oncol Biol Phys. 2000;47:785–791. doi: 10.1016/s0360-3016(00)00447-8. [DOI] [PubMed] [Google Scholar]
- 18.Magnino A, Gatti M, Massucco P, Sperti E, Faggiuolo R, Regge D, Capussotti L, Gabriele P, Aglietta M. Phase II trial of primary radiation therapy and concurrent chemotherapy for patients with locally advanced pancreatic cancer. Oncology. 2005;68:493–499. doi: 10.1159/000086993. [DOI] [PubMed] [Google Scholar]
- 19.Hess V, Glimelius B, Grawe P, Dietrich D, Bodoky G, Ruhstaller T, Bajetta E, Saletti P, Figer A, Scheithauer W, et al. CA 19-9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol. 2008;9:132–138. doi: 10.1016/S1470-2045(08)70001-9. [DOI] [PubMed] [Google Scholar]
- 20.Crane CH, Abbruzzese JL, Evans DB, Wolff RA, Ballo MT, Delclos M, Milas L, Mason K, Charnsangavej C, Pisters PW, et al. Is the therapeutic index better with gemcitabine-based chemoradiation than with 5-fluorouracil-based chemoradiation in locally advanced pancreatic cancer? Int J Radiat Oncol Biol Phys. 2002;52:1293–1302. doi: 10.1016/s0360-3016(01)02740-7. [DOI] [PubMed] [Google Scholar]
- 21.Li CP, Chao Y, Chi KH, Chan WK, Teng HC, Lee RC, Chang FY, Lee SD, Yen SH. Concurrent chemoradiotherapy treatment of locally advanced pancreatic cancer: gemcitabine versus 5-fluorouracil, a randomized controlled study. Int J Radiat Oncol Biol Phys. 2003;57:98–104. doi: 10.1016/s0360-3016(03)00435-8. [DOI] [PubMed] [Google Scholar]
- 22.Crane CH, Janjan NA, Evans DB, Wolff RA, Ballo MT, Milas L, Mason K, Charnsangavej C, Pisters PW, Lee JE, et al. Toxicity and efficacy of concurrent gemcitabine and radiotherapy for locally advanced pancreatic cancer. Int J Pancreatol. 2001;29:9–18. doi: 10.1385/IJGC:29:1:09. [DOI] [PubMed] [Google Scholar]
- 23.Epelbaum R, Rosenblatt E, Nasrallah S, Faraggi D, Gaitini D, Mizrahi S, Kuten A. Phase II study of gemcitabine combined with radiation therapy in patients with localized, unresectable pancreatic cancer. J Surg Oncol. 2002;81:138–143. doi: 10.1002/jso.10159. [DOI] [PubMed] [Google Scholar]
- 24.Schneider BJ, Ben-Josef E, McGinn CJ, Chang AE, Colletti LM, Normolle DP, Hejna GF, Lawrence TS, Zalupski MM. Capecitabine and radiation therapy preceded and followed by combination chemotherapy in advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63:1325–1330. doi: 10.1016/j.ijrobp.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 25.Andre T, Balosso J, Louvet C, Hannoun L, Houry S, Huguier M, Colonna M, Lotz JP, De Gramont A, Bellaiche A, et al. Combined radiotherapy and chemotherapy (cisplatin and 5-fluorouracil) as palliative treatment for localized unresectable or adjuvant treatment for resected pancreatic adenocarcinoma: results of a feasibility study. Int J Radiat Oncol Biol Phys. 2000;46:903–911. doi: 10.1016/s0360-3016(99)00478-2. [DOI] [PubMed] [Google Scholar]