Abstract
Purpose
To improve treatment efficacy and tumor cell selectivity of δ-aminolevulinic acid (ALA)-based photodynamic therapy (PDT), via pretreatment of cells and tumors with methotrexate (MTX) to enhance intracellular photosensitizer levels.
Experimental Design
Skin carcinoma cells, in-vitro and in-vivo, served as the model system. Cultured human SCC13 and HEK1 cells, normal keratinocytes, and in-vivo skin tumor models (see below) were preconditioned with MTX for 72 h, then incubated with ALA for 4 h. Changes in PpIX levels and in cell survival after light exposure were assessed.
Results
MTX-preconditioning of monolayer cultures preferentially raised intracellular PpIX levels 2- to 4-fold in carcinoma cells versus normal keratinocytes. Photodynamic killing was synergistically enhanced by the combined therapy, compared to PDT alone. MTX enhancement of PpIX levels was achieved over a broad MTX concentration range (0.0003 – 1.0 mg/L; 0.6 nM – 2 mM). PpIX enhancement correlated with changes in protein expression of key porphyrin pathway enzymes, i.e. ~4-fold increase in coproporphyrinogen oxidase, and stable or slightly decreased expression of ferrochelatase. Differentiation markers (E-cadherin, involucrin, filaggrin) were also selectively induced by MTX in carcinoma cells. In-vivo relevance was established by showing that MTX preconditioning enhances PpIX accumulation in three models: (1) organotypic cultures of immortalized keratinocytes; (2) chemically-induced skin tumors in mice; and (3) human A431 squamous cell tumors implanted subcutaneously in mice.
Conclusion
Combination therapy using short-term exposure to low-dose MTX followed by ALA-PDT should be further investigated as a new combination modality to enhance efficacy and selectivity of PDT for epithelial carcinomas.
Keywords: ALA, PDT, methotrexate, differentiation, porphyrin
INTRODUCTION
Photodynamic therapy (PDT) is a cancer treatment modality increasingly utilized for precancerous lesions of the skin, and also for thin nonmelanoma skin cancers and for palliative therpy of advanced internal malignancies. A popular type of PDT utilizes 5-aminolevulinic acid (ALA), or its methly ester (methyl-ALA), small molecule precursors that are taken up as a prodrug by cancer cells and then converted into an intracellular photosensitizer (protoporphyrin IX; PpIX) that is activated by visible light; reviewed in (1–3). ALA- PDT offers an opportunity to selectively target tumors through several mechanisms, including preferential uptake and accumulation of ALA in tumor versus normal tissues, preferential synthesis of PpIX in cancer cells versus normal cells, and targeted illumination to selectively encompass the lesion and spare normal tissue (4–6). Unfortunately, current ALA-PDT protocols have proven insufficient for the treatment of large or biologically aggressive tumors. In cutaneous oncology, ALA-PDT is successfully used for the treatment of sun-induced precancers (actinic keratoses) (7, 8) and squamous carcinomas in-situ (7), but the success rate for nodular basal cell carcinoma (BCC) and invasive squamous cell carcinoma remains inadequate (9). The reasons for incomplete success are not entirely clear. Topical delivery of ALA and its more lipophilic esters are now largely optimized (10, 11) so that depth of penetration of the prodrug is thought to be adequate for effective therapy (12, 13). Similarly, modern light sources including lasers (14) and intense pulsed light (IPL) (15) generate high energies at long wavelengths, and should therefore be expected to penetrate well into the dermis. However, one factor that is not yet optimized is the intracellular production of the photosensitizer. Inadequate production of PpIX, and a nonhomogeneous distribution of PpIX within tumors, could scuttle any attempt to provide reliable photodynamic killing of all cancer cells within a population.
Given the fact that ALA-PDT is unlikely to succeed as a monotherapy, our group since the late 1990’s has conducted a series of studies on the use of common pharmacological agents that we have found to enhance PpIX production in cancer cells. Beginning with an observation that calcium-induced differentiation can lead to enhanced accumulation of PpIX in keratinocytes (16), we found several other agents (mostly hormones) that can induce cellular differentiation and at the same time increase PpIX in epithelial cancers. For example, androgens stimulate a large increase in PpIX levels in LNCaP prostate carcinoma cells (17, 18). Likewise, Vitamin D or 9-cis-retinoic acid raise PpIX levels significantly in those cells (18). Why most agents capable of elevating PpIX levels also promote cellular differentiation remains an interesting but yet unexplained association. The term ‘differentiation therapy’ has been used before in oncology (retinoic acid, for example, as used for promyelocytic leukemia to drive immature cancer cells to a more mature and differentiated state (19–21) ), but the notion of using a differentiating agent in combination with ALA-PDT to enhance therapy has not been explored.
Methotrexate (MTX) is a familiar chemotherapeutic agent that inhibits cell proliferation due to its potent inhibition of dihydrofolate reductase and thymidylate synthesis (22). MTX also triggers cellular differentiation (23). MTX induces differentiation in normal human keratinocytes (24) and in cancer cell lines including human HL-60 promyelocytic cells (25), neuroblastoma LA-N1 cells (26), and choriocarcinoma cells (27, 28); it is probably through this pro-differentiating mechanism that MTX can successfully control aggressive human choriocarcinoma tumors (29). Because of the possible association between pro-differentiating properties and an ability to promote PpIX accumulation (see above), we tested MTX as a preconditioning agent during ALA-PDT of prostate tumor (LNCaP) cells and found that such preconditioning led to enhanced PpIX levels and enhanced photodynamic killing in monolayer cultures (30).
Despite our earlier in-vitro work, the question of whether or not PpIX-enhancing effects of MTX may occur selectively in tumor cells relative to normal cells had not been addressed. Also, it was unknown whether MTX preconditioning combined with ALA-PDT might provide benefit for actual tumors in-vivo. In this paper, we address these two questions in cell culture models and animal models of skin cancer. First, we demonstrate that MTX exerts a highly selective upregulatory effect on PpIX production in skin carcinoma cells as compared to normal keratinocytes. The mechanism underlying selective PpIX upregulation involves MTX-inducible enhancement of a key PpIX-synthetic enzyme, coproporphyrinogen oxidase (CPO). Extending these studies into an organotypic skin model, we show that MTX can enhance PpIX accumulation in a 3-dimensional tissue. Taking this to the in-vivo level, studies in two different models, namely, carcinogen-induced skin tumors in mice, and subcutaneous human tumors (A431 cells) in nude mice, confirm that MTX pretreatment can significantly and selectively enhance PpIX accumulation in squamous skin tumors in-vivo.
MATERIALS AND METHODS
Culture of primary keratinocytes and carcinoma lines
Normal human epidermal keratinocytes (NHEKs) from Cascade Biologics (Portland, OR) were cultured at 37 °C in a humidified CO2 incubator in EpiLife medium with human keratinocyte growth supplement (Cascade Biologics), calcium chloride (0.06 mM), and Pen-Strep (penicillin, 100 units/mL; and streptomycin; 100 μg/mL). For passaging, subconfluent cells were released with trypsin-EDTA solution (0.05 % trypsin, 53 mM EDTA), and when cells had just begun to detach, an equal volume of trypsin neutralizer (Cascade Biologics) was added. Detached cells were centrifuged at 180 × g in a conical tube, the supernatant was aspirated and the cell pellet was replated in serum free NHEK medium.
SCC13 cells (31) were kindly provided by Dr. Jonathan Garlic (Tufts University, Boston, MA) and cultured at 37 °C in SCC13 growth medium, consisting of: keratinocyte serum-free medium (Invitrogen Corp., Carlsbad, CA) supplemented with EGF (0.33 ng/ml), bovine pituitary extract (2.5 μl/ml), calcium chloride (0.3 mM) and Pen-Strep.
HEK1 cells (32), also called HEK001, were obtained from ATCC (Manassas, VA) and cultured 37 °C in HEK1 growth medium consisting of keratinocyte serum-free medium (Invitrogen Corp.) supplemented with EGF (8.4 ng/ml) and Pen-Strep.
Immortalized rat epidermal keratinocytes (REK cells) were maintained as previously described (33), for use in organotypic 3-D cultures (see below).
A431, a human squamous cell carcinoma cell line, was obtained from ATCC (Manassas, VA) and were cultured at 37 °C in high glucose DMEM (ATCC) supplemented with 10% FBS (Biowhittaker, Walkersville, MD) and Pen-Strep. They were used in the subcutaneous nude mouse model (see below).
Pretreatment with MTX, and measurement of PpIX in cell lysates from monolayer cultures
For each experimental condition, four 25 cm2 flasks were plated at 200,000 cells per flask. On the second day, the medium was replaced with medium containing different doses of MTX (Sigma-Aldrich, St Louis, MO) for an additional 72 h. The medium was aspirated and replaced with media containing ALA (Sigma-Aldrich; 1 mM for NHEK and SCC13, and 0.5 mM for HEK1) for an additional 4 h. Medium without ALA was added to control flasks. One flask from each set of treatments was observed and photographed on a phase contrast microscope and cells were counted from five random fields. Cells were lysed and vortexed in 1 ml of Solvable (PerkinElmer Life and Analytical Sciences, Boston, MA) and centrifuged at 10,000 × G at 4 °C. PpIX content of a 100 μl aliquot of the supernatant was measured in triplicate in clear plastic 96 well plates (Corning Inc., Corning, NY) using a SpectraMAX GeminiXS spectrophotofluorimeter (Molecular Devices, Sunnyvale, CA) at the excitation and emission wavelengths of 395 and 633 nm, respectively. In every experiment, four 25 cm2 flasks were plated with 200,000 cells and processed as described in methods section.
Fluorescence analysis of PpIX in living cells and in frozen tissue sections
PpIX-specific fluorescence was analyzed by fluorescence microscopy on a confocal laser scanning microscope (Leica Microscopy Systems GmbH, Wetzlar, Germany). Cells, plated on sterile microscope coverslips in 35 mm dishes at 50,000 cells/dish, were conditioned with MTX or control media as above. PpIX-specific fluorescence in the living cells was collected on the confocal microscope (excitation 488 nm, emission 550–700 nm) and the digital images analyzed using IPLab image processing software (Signal Analytics, Vienna, VA). The background signal threshold was set to reveal only specific (mitochondrial) PpIX fluorescence in controls. This setting was then applied to all images within the same series. Total integrated area was calculated and divided by number of cells in the field (counted in corresponding phase-contrast images). PpIX levels were expressed as arbitrary fluorescence units (total pixels) per cell. In other experiments described below, frozen tissue sections from organotypic tissues, from chemically-induced mouse skin tumors, or from subcutaneous A431 tumors in nude mice, were analyzed by confocal imaging as follows. Cryosections (10 μ) were placed on glass slides, briefly air dried, coverslipped with Vectashield (Vector laboratories, Burlingame, CA), and viewed on the confocal microscope with a 40x lens under oil using excitation at 633 nm and image collection in the red channel (emission 650–780 nm).
Light exposure, and FDA-EB survival assay
Cells were plated 50,000 cells per 35 mm dish, and after 24 h, were incubated with/without MTX for an additional 72 h. Fresh ALA-containing media were added for 4 h. Cells were irradiated using a custom-built monochromatic light source consisting of four light-emitting diodes with a peak output at 395 nm and bandwidth (FWHM) of 14 nm. Dishes received 4 mJ/cm2 (power density 0.1 mW/cm2 at 5 cm distance, as measured with an IL1700 radiometer (International light, Newburyport, MA). At 24 h after light exposure, the cells were PBS-rinsed, 50 μg/ml fluorescein diacetate (FDA) and 4 μg/ml ethidium bromide(EB) added, and the cells observed under epifluorescence using a FITC/TRITC filter (34).
Western blot analyses
Cells were lysed in lysis buffer (7 M urea, 2% IGEPAL and 5% β-mercaptoethanol) supplemented with protease inhibitor cocktail (Sigma-Aldrich). Samples with equal quantities of protein, as determined by Bradford’s method (Bio-Rad, Hercules, CA), were denatured in sample buffer and sample reducing agent (Invitrogen) for 10 min at 70 °C, then resolved on a 4–12% Bis-Tris acrylamide gel (Invitrogen) along with molecular size markers (MagicMark, Invitrogen). Electrophoresis was performed at constant voltage (200 volts) at room temperature. Proteins were electrophoretically transferred to Immobilon PVDF membrane (Millipore, Bedford, MA), at constant voltage (100 volts) for 1 h at 4 °C. Following transfer, the blot was stained with Ponceau Red-S to check transfer efficiency. Blots were then blocked with 10% non-fat dry milk, and incubated with the following primary antibodies. A rabbit polyclonal antiserum against a C-terminal peptide of murine CPO, consisting of the amino acid sequence CLEVLRHKPDWVH, which is 83% homologous to human, was produced as described previously (30). Incubation with anti-CPO (1:5000), or with antibodies against ferrochelatase (gift of H. Dailey, 1:5000), GAPDH (Santa Cruz, SC-25778; 1:5000), p27 (Santa Cruz Biotech, Santa Cruz, CA, SC-528; 1:2000), PCNA (Santa Cruz SC-7907; 1:2000), E-cadherin (Santa Cruz, SC-7870; 1:2000), filaggrin (Covance, PRB-417P; 1:2000), Involucrin (Santa Cruz, SC-15234; 1:2000) or Keratin-10 (Covance, PRB-159; 1:2000) was followed by peroxidase-conjugated goat anti-rabbit IgG or donkey anti-goat IgG (Jackson ImmunoResearch, West Grove, PA; 1:20,000), and the blot developed using enhanced chemiluminescence reagents (ECL kit, Amersham Biosciences, Piscataway, NJ).
Organotypic 3-D epidermal cultures
Rat epidermal keratinocytes (REK) were cultured at the air-media interface to induce stratification (lift cultures) as previously described (33). At 48 h after lifting, REK medium was replaced with medium containing different doses of MTX and the cultures preconditioned for 72 h. The medium was aspirated, medium containing 1 mM ALA was added for another 4 h, and the cultures frozen-embedded for cryosectioning and confocal analysis of PpIX.
Chemically-induced tumors in mouse skin
Tumors in SKH-1 mice (Charles River Laboratories, Wilmington, MA) were generated by topical application of DMBA (50 μg/100 μl in acetone, one time application) and TPA (5 μg/200 μl acetone, twice a week for up to 20 weeks) on the flanks. These papillomas were preconditioned with MTX treatment (2 mg/kg, intramuscular, daily for 1 or 3 days), or with saline for 3 days (no-MTX control). ALA (Levulan Kerastick™, DUSA Pharmaceuticals) was topically applied for 4 h. The amount of PpIX in the tumors was estimated by noninvasive dosimetry (see below). Mice were then euthanized and tumors harvested. Skin biopsies were embedded in OCT compound, and 10 μm frozen sections analyzed for PpIX levels by confocal microscopy as described earlier.
In vivo measurement of PpIX in papillomas by Aurora fluorescence dosimetry
Non invasive, real-time measurements of PpIX fluorescence in normal skin and tumors on the skin surface were performed at 0, 2 and 4 h post ALA application using an Aurora dosimeter (Aurora Optics, Hanover, NH). This device employs a optical fiber-based probe in which a small 2 mm area receives excitation from a diode laser at ~ 400 nm, and PpIX emission in the red range is collected (35). Five readings were averaged for each tumor (Fig. 5B).
Figure 5. PpIX in chemically-induced skin tumors in mice.
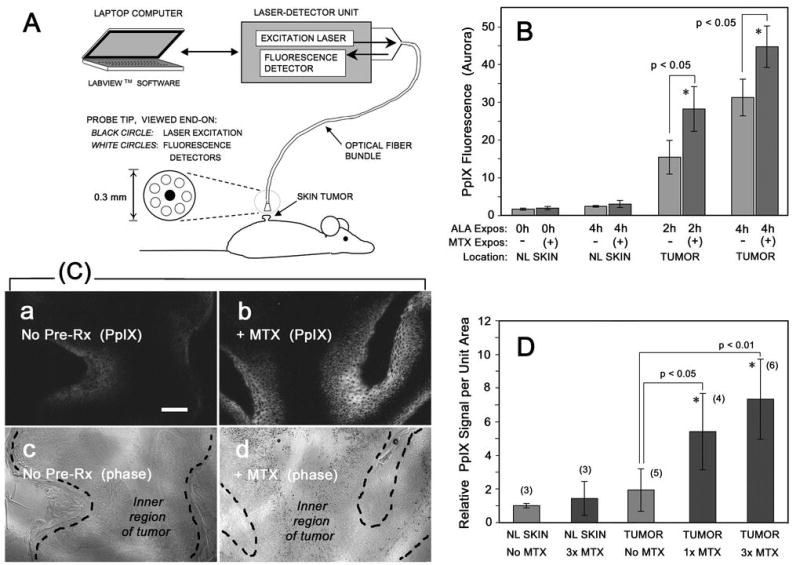
Benign, low-grade papillomas were generated by chemical carcinogenesis on the dorsum of SKH-1 hairless mice, then pretreated with systemic MTX (or saline vehicle) for 1 or 3 days, followed by injection of ALA for 2h or 4 h prior to measurement of PpIX by noninvasive dosimetry (A, B), or by tissue biopsy (C, D). (A) Schematic diagram of the Aurora noninvasive fluorescence dosimeter. (B) Fluorescence signal is significantly increased in tumors from mice pretreated with MTX for 3 d, followed by ALA for the times indicated, as compared to normal skin (NL Skin) on the same mouse. Each bar, mean of 3 tumors (5 readings/tumor) ± SEM. (C) Mice were treated with/without MTX and with ALA, then euthanized and the tumors harvested and PpIX analyzed by confocal microscopy of frozen sections; typical PpIX signals (a, b) and corresponding tumor morphology (c, d) are shown. (D) Quantitation of the PpIX signal from digital confocal images, from tumors preconditioned with no MTX, 1 day of MTX, or 3 days of MTX prior to harvest. Shown are the mean ± SD of images from at least three independent tumors (numbers in parentheses).
Generation of subcutaneous tumors by dermal injection of human SCC cells in nude mice
Immunocompromised nude mice (Charles River Labs, Wilmington, MA) received intradermal injections of 2 × 106 A431 cells (an SCC line originally obtained from a human cutaneous squamous carcinoma) in each flank. After 6–10 days, visible nodules were observed. For the preconditioning therapy, mice received systemic MTX (2 mg/kg, intramuscular, daily for 1 or 3 days) for half of the animals; all others received sham injections of PBS. Subsequently, ALA was administered in PBS (75 mg/kg, intramuscular) for 24 h, and the mice euthanized for tumor harvest. Frozen tumor sections were analyzed by confocal microscopy, as described above. Some sections were post-fixed in 4% paraformaldehyde for 1h at room temperature and stained with hematoxylin-eosin to visualize the tumor morphology.
Statistics
Two-sample t-tests were used to compare differences in PpIX accumulation, or survival after PDT, between treated and untreated controls. A p value of 0.05 or less was considered statistically significant. In order to determine whether the cytotoxic effect of combining MTX and PDT was additive or synergistic (data in Fig. 3B), the fractional product technique (Bliss synergy) was employed as described by Duska et al. (36). Calculations are shown in Supplemental Table 2.
Fig 3. Preconditioning with MTX significantly enhances photocytotoxicity with ALA-PDT.
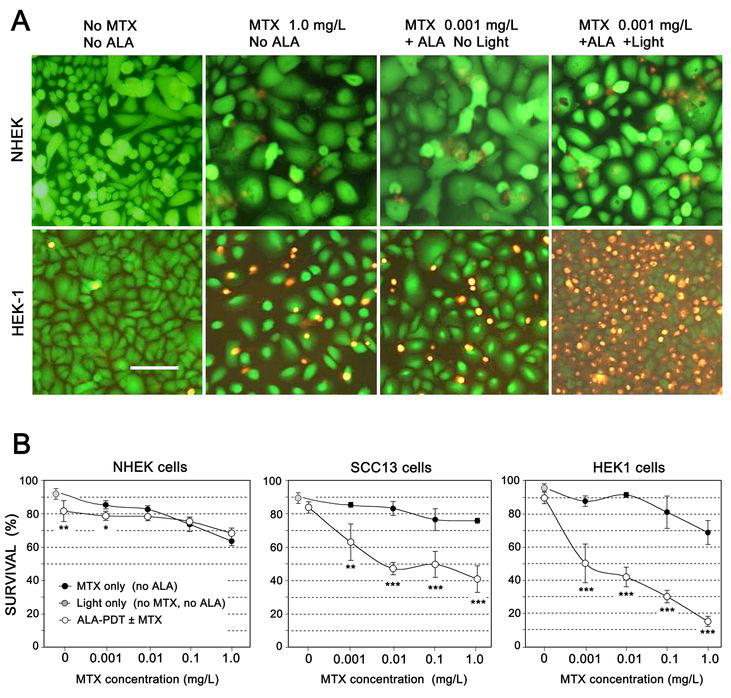
(A) Cell survival assays (using the fluorescent dyes, FDA and EB) following ALA-mediated photodynamic therapy with or without preconditioning with MTX. Monolayer cultures of cells (NHEK, top row; HEK1 cells, bottom row) were incubated for 72 h in the absence or presence of MTX, then were left unexposed or irradiated with intense blue light (395 nm, 4 mJ/cm2). At 24 h after irradiation, cells were incubated for 2 min with FDA and EB as described in Methods, then photographed using an epifluorescent microscope. Scale bar, 100 μm.
(B) MTX leads to PpIX accumulation and enhanced photodynamic killing of skin carcinoma cells, but has little effect upon normal keratinocytes. Cell cultures grown at the same time under similar conditions of confluency, were pretreated with MTX for 72 hr at the indicated concentrations, followed by 1 mM ALA for 4 hr. PpIX was visualized with confocal microscopy in some dishes to confirm PpIX induction (not shown), while other dishes were irradiated using a laser diode source. At 24 h post-irradiation, survival was analyzed using the FDA/EB assay; ~200 cells per field were counted to determine survival. Each data point represents mean ± SD of 3 micrographs. Open (white) circles: Cells pretreated with MTX, followed by ALA-PDT. Solid black circles: Cells given MTX pretreatment, but not exposed to ALA-PDT. Gray circle: Controls exposed to light only (no MTX, no ALA). (*) p<0.05; (*) p<0.01; (***) p <0.005.
RESULTS
Preincubation with metrotrexate selectively enhances the production of PpIX in skin carcinoma cell lines relative to normal keratinocytes
For therapeutic success, ALA-PDT requires selective accumulation of high amounts of PpIX within tumor cells. Normal human keratinocytes (NHEK), when incubated with ALA and visualized by confocal microscopy, accumulated negligible amounts of PpIX (near the level of background autoflorescence; Fig. 1A, panel a). Carcinoma cells, on the other hand, developed relatively high PpIX signals when incubated in ALA (Fig. 1A, panels b and c). Although higher baseline levels of PpIX might be expected to confer some selective therapeutic advantage in carcinoma cells, we wished to ask a different question: Can pretreatment with methotrexate cause an even more pronounced, selective increase in PpIX levels in the carcinoma cells? Accordingly, SCC13 cells, HEK-1 cells, and normal keratinocytes were pretreated with MTX for 72 h prior to receiving ALA. As shown in Fig. 1A (panels d–f, second row), MTX-preconditioned carcinoma cells produced higher cytoplasmic amounts of PpIX relative to the ALA-only controls (Fig. 1A, first row). Almost no induction of PpIX was seen in primary keratinocytes (panel d).
Figure 1. Intracellular PpIX selectively accumulates in skin carcinoma cell lines (SCC13 and HEK1) following preconditioning with MTX, relative to normal human keratinocytes (NHEK).

(A) Confocal microscopic imaging of three cell types: normal keratinocytes NHEK, SCC13 cells, or HEK1 cells that received either no preconditioning (panels a–c), or preconditioning with 0.01 mg/L MTX for 72 hr (panels d–f), followed by incubation with ALA (4 hr) and visualization with confocal microscopy. Phase-contrast images (panels g–i) correspond to PpIX images directly above. White-boxed insets are from dishes that did not receive ALA (negative controls, -ALA). Note that PpIX levels are preferentially elevated in the carcinoma cells pretreated with MTX (e, f) relative to NHEK (d). Scale bar, 50 μm. (B) Confocal images at higher magnification to illustrate localization of PpIX in normal NHEK (j) versus carcinoma SCC13 (k) cells; both had received MTX preconditioning. PpIX signal is observable in plasma membranes (arrows) and cytoplasm (Cy), but not in the nucleus (Nu). Scale bar, 50 μm.
Following MTX pretreatment, a qualitative difference in the intracellular location of PpIX was observed between normal cells and carcinoma cells. In primary keratinocytes, bright PpIX signals were found only in cell membranes (Fig. 1B, panel j). In the carcinoma lines, however, strong PpIX signal was found principally in cytoplasmic perinuclear regions that contain mitochondria (Fig. 1B, panel k). Therefore, MTX appears to induce tumor cell-selective accumulation of PpIX in intracellular organelles that represent traditional targets for PDT.
To quantify cellular increases in PpIX, two different methods were employed (Fig. 2A,B). In the first, flasks of cells were pretreated with MTX or vehicle alone, followed by measurement of PpIX in cell lysates using spectrofluorimetry (Fig. 2A). MTX pretreatment increased PpIX levels by 3 to 4-fold. In the second method, confocal fluorescent images of cells grown on coverslips were digitally analyzed over the entire microscopic field, with correction for autofluorescent background and for cell number (Fig. 2B). While estimates of PpIX with this method are inherently less linear than are measurements done after cell solubilization (to break up porphyrin aggregates that may cause quenching), the confocal method still permits the detection of large and significant relative changes in PpIX levels, even if PpIX amounts are potentially underestimated. Importantly, direct microscopic observation of PpIX in individual, living cells is essential to normalize PpIX measurements for changes in cell number. As documented in Supplemental Table 1, cell density declined significantly with MTX concentration, by up to 50% at the highest MTX dose. Therefore, all subsequent experiments used the confocal method in order to normalize PpIX levels to the number of cells per dish.
Figure 2. Cell type-specificity and dose-dependence of PpIX accumulation following MTX pre-conditioning, and correlation with changes in PpIX-metabolic enzymes.

(A) Quantification of PpIX signal in the different cell lines using spectrofluorimetry. Cells were seeded in replicate 25 cm2 flasks at ~60% confluence and induced with MTX (0.01 mg/L) for 72 h. Following incubation with ALA, cell numbers were determined by photography and manual counting; then cells were lysed and the lysates measured in a spectrofluorimeter (see Methods). PpIX levels per cell are shown relative to untreated controls. Each bar represents mean ± SD of 3 separate flasks; differences (MTX versus no MTX) are significant at the p < 0.005 level (**). (B) Quantification of PpIX signal by analysis of digital confocal images. Living cells on coverslips were analyzed with confocal microscopy and image processing, as described in Methods. Integrated fluorescence intensities (arbitrary fluorescence units) were calculated from 4 images/condition, corrected for cell number, and expressed relative to zero-MTX controls. Differences are significant at the p < 0.05 (*) or p < 0.005 (**) level.
(C) Methotrexate dose-ranging experiments to examine induction of PpIX in normal human keratinocytes, SCC13 cells, and HEK1 cells. Experiments in which the confocal image-processing method was used to determine relative PpIX concentration per cell were performed after 72 h incubation in MTX (concentrations shown on the x-axis as a logarithmic scale). Each data point is the mean ± SEM of 3 or more independent experiments (actual number in parentheses), with at least 3 confocal fields per experiment. All PpIX values were normalized to the zero-MTX NHEK control, which was arbitrarily set at 1.0.
(D) Methotrexate preconditioning selectively increases the expression of coproporphyrinogen oxidase (CPO) in carcinoma cells, while not affecting ferrochelatase (FC). Cells were incubated for 72 h in the presence of the following concentrations of MTX: Lane 1, 0 mg/L; Lane 2, 0.001 mg/L; Lane 3, 0.01 mg/L; Lane 4, 0.1 mg/L; Lane 5, 1.0 mg/L. Cells were then harvested and the lysates analyzed on Western blots using antisera specific to CPO, FC, or GAPDH (with bands detected at the expected sizes of ~38 kD, ~40 kD, and ~37 k, respectively). Graphs show the relative changes in band intensity at each MTX concentration, relative to the no-MTX control for each given cell type, as measured by gel densitometry.
PpIX production is enhanced by very low concentrations of methotrexate in squamous carcinoma cells
To examine the MTX dose dependence of MTX-induced enhancement of PpIX levels, a large number experiments were performed as summarized in Fig 2C. MTX had only a slight impact upon normal human keratinocytes (NHEK), leading to no more than a 2.5-fold PpIX increase at MTX concentrations of 0.003 mg/L and higher (Fig. 2C, dotted line). For the two carcinoma cell lines, however, PpIX levels were 3-fold higher than NHEK even at baseline, and accumulated to very high relative levels (10–12-fold higher than NHEK at a MTX concentration of 0.01 mg/L). Above 0.01 mg/L MTX, levels of PpIX trended downward, probably due to increasing cytoxicity of the MTX. We should note that the range of MTX concentrations that appear optimally effective here, between 0.001 and 0.1 mg/L (2 to 200 nanomolar), is lower than maximal serum levels, and well within the range of steady-state plasma concentrations, that are typically achieved in clinical settings (see Discussion).
Methotrexate-induced accumulation of PpIX, in a combined photodynamic regimen, leads to selective killing of squamous carcinoma cells in-vitro
To ask whether MTX-induced elevation of PpIX translates into enhanced cell killing, we employed an in-situ survival assay (34); (Fig. 3). Living cells were tagged with fluorescein (green), dead cells with ethidium (orange), and survival was expressed as the proportion of total cells with green fluorescence. When MTX and ALA-PDT were administered to normal keratinocytes, either singly or in combination, very little toxicity was observed (Fig. 3A, first row). However, in HEK1 cancer cells, greater cytotoxity was observed with the combination treatment than with either agent alone (Fig. 3A, second row). To test the hypothesis that the combination of MTX and PDT selectively enhances treatment efficacy in the cancer cells, experiments were performed in which all cell lines were preconditioned with MTX at various doses, followed by administration of ALA-PDT. Cell survival was assessed 24 h later (Fig. 3B). With MTX treatment alone, a MTX dose-dependent loss of cell viability was observed that appeared to be similar in all cell lines (Fig. 3B, solid circles). Photodynamic therapy alone (without MTX) had relatively little effect upon any of the cell lines at the sublethal (LD20) light dose employed in the experiment (Fig. 3B, open circles, far left). However, after combination therapy (MTX followed by ALA-PDT), the SCC13 and HEK1 cells were markedly more sensitive than normal keratinocytes (Fig. 3B, white circles). Negative controls exposed only to light in the absence of ALA (gray circles) showed minimal cell death.
To determine whether the combination of MTX and ALA-PDT provides synergistic (as opposed to an additive) enhancement of cytotoxicity, a formal analysis of synergy was performed (Supplemental Table 2). The results showed that the enhancement of cytotoxicity with the combination therapy was synergistic for SCC13 and for HEK1 carcinoma cells, but not for normal keratinocytes.
The mechanism of MTX-enhanced PpIX accumulation involves changes in expression of heme enzymes in the setting of increased cellular differentiation
Based upon evidence that suggested a possible link between cellular differentiation, increased synthesis of PpIX, and elevated levels of the porphyrin-synthetic enzyme CPO (16, 18, 30), we examined the expression of two enzymes (CPO and FC) located immediately upstream and downstream from PpIX in the heme-synthesis pathway, and that therefore might be rate-limiting. In MTX-treated cells, both carcinoma lines showed a robust increase in CPO protein levels, whereas the CPO level in normal cells remained essentially unchanged (Fig. 2D). Ferrochelatase (FC), immediately downstream of PpIX, was either slightly decreased or unchanged in cells incubated with MTX (Fig. 2D).
To evaluate the growth and differentiation status of the cells in response to MTX, some general markers of cell cycle progression (PCNA), growth arrest (p27/kip1) and cell differentiation (E-cadherin) were also examined. PCNA, expressed in proliferating cells in S-phase (37), was slightly induced at low MTX concentrations (0.001 mg/L) in all cell types, reflected by a slight increase in cell number (Supplemental Table 1). However, at higher MTX doses PCNA was selectively decreased in carcinoma cell lines relative to normal keratinocytes, suggesting growth arrest in the carcinoma cells (Supplemental Fig. 1A). Two general markers of growth arrest and differentiation, p27 and E-cadherin, respectively, were selectively induced by MTX in the carcinoma lines (SCC13 ≫ HEK1 > NHEK), particularly at low MTX doses (Supplemental Fig. 1B, C). Specific epidermal differentiation markers, such as involucrin, K10, and filaggrin (38–40) were reduced in squamous carcinoma cells relative to normal keratinocytes, reflecting neoplasia-associated loss of differentiation. However, those markers were re-expressed after incubation with MTX (Supplemental Fig. 1D–F). Collectively, these MTX-related changes in differentiation markers confirmed an interesting association between MTX-inducibility of CPO and induction of cellular differentiation, occurring selectively in squamous carcinoma cells and not in normal epidermal keratinocytes.
MTX pretreatment enhances photosensitizer levels in an organotypic, three-dimensional epithelial model tissue
Monolayer cell cultures do not always mimic the physiological behavior of living cells in an intact organ such as the skin. To ask whether effects of MTX observed in monolayer cultures are relevant in a three-dimensional (3-D) tissue, experiments were performed in an organotypic model. In these 3-D cultures of immortalized keratinocytes that have lost their dependence upon exogenous paracrine support, we had shown previously that pretreatment with another differentiation-promoting agent (Vitamin D) supports PpIX accumulation and improves ALA-PDT efficacy (33). As seen in Fig. 4A, preincubation of the lift cultures with MTX followed by incubation with ALA significantly enhanced the intracellular porphyrin levels in the tissue. Relative increases in PpIX were significantly greater than controls, even at the lowest MTX concentration examined (Fig. 4B). Because keratinocytes did not show any significant PpIX-induction with MTX when grown in proliferating monolayers (Figs. 1–3), yet showed MTX-induced PpIX accumulation in stratifying lift cultures, the response in Fig. 4 appears to be related to the state of keratinocyte differentiation.
Figure 4. MTX pretreatment increases PpIX levels in organotypic 3-D cultures of immortalized keratinocytes (REK cells).

Fully-stratified organotypic cultures of the REK cell line were pretreated with MTX or with media alone for 72 hr at the concentration indicated, followed by incubation with ALA (1 mM, 4 hr) prior to frozen-sectioning. (A) Frozen sections visualized for PpIX by confocal microscopy. Note the increased intensity of the bright PpIX-specific signal in cultures pretreated with methotrexate. (B) Quantification of PpIX signal. Integrated fluorescence was analyzed from 6 confocal images per condition, using IPLab image-processing software. (*), significant difference from control, p < 0.005 level.
Chemically-induced skin tumors, when preconditioned with MTX, produce relatively high amounts of PpIX
Tumors were generated by chemical carcinogenesis on the dorsum of SKH-1 hairless mice. Mice were then pretreated systemically with either MTX or saline vehicle for 1 or 3 days, followed by systemic ALA for up to 4 h to allow production of PpIX. PpIX levels were analyzed in two ways. First, a noninvasive surface dosimetry technique was used in the living animals (Fig. 5A), and enhancement of PpIX within tumors was observed in the MTX-preconditioned mice (Fig. 5B). In the second technique, tumors were harvested and the amount of PpIX in frozen tissue sections was analyzed by confocal microscopy (Fig. 5C). Again, a higher level of PpIX was seen in tumors preconditioned with MTX than in saline-only controls (Fig. 5D). These increases in PpIX were statistically significant for both the 1-day and the 3-day pretreatments.
Human squamous tumors (subcutaneous A431 cells in mice) accumulate high amounts of PpIX as a result of MTX preconditioning
Subcutaneous tumors were generated by intradermal injection of human A431 cells. Mice with palpable tumors were preconditioned with intramuscular MTX, or saline alone. PpIX production was initiated by systemic ALA injection, and tumors harvested for analysis of PpIX by confocal microscopy as described in Methods. In tissue cryosections, a bright PpIX signal was observed preferentially in MTX-treated tumors as compared to saline-treated controls (Fig. 6A). Quantitatively, an increase in PpIX was observed following 1 day (single mouse examined) or 3 days (statistically significant increase) of daily MTX injections (Fig. 6B).
Figure 6. PpIX in human A431 subcutaneous tumors in nude mice.

Subcutaneous tumors, produced by intradermal injection of human A431 cells, were preconditioned with intramuscular MTX or with saline for 1 d or 3 d, and then PpIX production was initiated by intraperitoneal ALA injection as described in Methods. (A) Harvested tumors were cryosectioned and analyzed by PpIX-specific confocal microscopy (a, c) and by post-fixation and H&E staining to visualize the corresponding region (b, d). Bright PpIX signal (asterisk) was observed preferentially in MTX-treated tumors, which despite some areas of necrosis, had high PpIX levels in living tumor areas. (B) Quantitation of PpIX signal in digital confocal images, as a function of MTX pre-conditioning for 1 day (1x MTX) or for 3 days (3x MTX). Values are mean ± SD; the number of fields and number of mice (in parentheses) examined are shown above each bar.
DISCUSSION
Data presented in this paper show that the efficacy and cancer-selectivity of ALA-based photodynamic therapy can be enhanced by using methotrexate (MTX) as a preconditioning agent. MTX, when used at concentrations too low to provide any significant tumoricidal activity on its own, significantly and selectively enhances the production of PpIX within carcinoma cells. PpIX then serves as the target for light. Although the skin carcinoma cell lines intrinsically produced ~3-fold higher levels of PpIX than did normal epithelial keratinocytes, this difference did not appear to offer any selective advantage for photodynamic killing in the absence of MTX (see Fig. 3). However, when cells were preconditioned with MTX, a significant induction of PpIX levels occurred only in the cancer lines (Fig. 2), and this induction translated into selective and efficient photodynamic killing of the carcinoma cells (Fig. 3). Cytotoxic enhancement due to the combination of MTX and ALA-PDT was more than additive (i.e., synergistic) in the monolayer cultures (Table 2). Importantly, very low MTX concentrations (~ 2 nM) proved to be quite effective at enhancing ALA-PDT.
In a whole-tissue setting, the effects of MTX preconditioning were explored in three models that, together, indicate the existence of a significant PpIX-enhancing effect in-vivo. First, MTX was shown to enhance PpIX levels ~2-fold in a differentiating (organotypic) epidermal model. Second, PpIX enhancement of ~6-fold was shown in skin tumors produced by chemical carcinogenesis in mice. Third, PpIX enhancement of ~3-fold was demonstrated in subcutaneous human A431 tumors in nude mice. While tumor survival/regression experiments in animals were beyond the scope of this study, previous work on Vitamin D as an adjuvant for PDT, in the same organotypic model as that used here, demonstrated an enhancement in photodynamic killing that ran parallel to the increase in PpIX, with a similar magnitude of effect (2.5-fold increase in PpIX, 2.0-fold increase in lethality) (33). Experiments to test whether MTX enhances therapeutic PDT-efficacy in the two animal models are being planned.
One of the most notable features of our study is the finding that PpIX levels are enhanced at very low, nontoxic concentrations of MTX. This has important clinical implications. Based upon current clinical protocols, concentrations of MTX measured in the serum and tissues of patients undergoing treatment for psoriasis or rheumatoid arthritis using oral MTX are similar to concentrations found to be effective in our study. For example, plasma drug concentrations achieved when treating rheumatoid arthritis (41) and psoriasis (42) with low-dose oral MTX fall within the range of 10 and 100 nM (41, 42). In a psoriasis study using 15 mg weekly, the MTX peak was 105 – 150 nM, and the steady state concentration was ~10 – 20 nM at 12 h (42). Thus, tissue levels of MTX that fall within the broad range of MTX concentrations (2 – 2000 nM) effective for inducing PpIX, seem readily obtainable. While more preclinical and clinical work will be required, a combination of oral MTX and ALA-PDT for skin carcinoma could lead to a safe and effective new treatment regimen.
In considering possible mechanisms for MTX-mediated accumulation of PpIX, we felt that an increase in prodrug (ALA) uptake into cells was unlikely to be important because (i), no differential effect of MTX upon ALA uptake was found in our previous work with prostate carcinoma cells (30), and (ii), no significant differences in ALA uptake between several colon carcinoma lines was observed despite significant differences in PpIX production (43). Instead, we focused upon evidence that changes in PpIX synthesis play a crucial role. CPO, a major enzyme for protoporphyrin synthesis, was previously shown to be induced in MTX-treated prostate cancer cells; however, no comparison to normal cells was available at that time (30). In the current study, two lines of cutaneous carcinoma were compared to normal keratinocytes. MTX-mediated increases in CPO expression, PpIX levels, and photodynamic killing were all shown to occur selectively in the carcinoma cells.
Since CPO is increased by MTX, it is important to consider another heme-metabolic enzyme, ferrochelatase (FC), which lies downstream from PpIX and might be relatively rate-limiting. FC is unequivocally rate-liming after chelation of iron with desferoximine (44). In other studies, reduced activity of ferrochelatase was invoked to explain higher relative accumulation of PpIX observed in tumor cells versus normal host cells (45, 46). Our data show that FC either remains unchanged or decreases slightly in response to MTX in our system (Fig. 2D). A third enzyme reported to be rate-limiting in some situations is porphobilinogen deaminase (PBGD). Zvi Malik’s group showed that in cultured B16 melanoma cells, treatment with differentiation-altering agents (HMBA, or butyrate) caused decreases or increases that correlated with enzyme levels and enzyme activity, suggesting that PBGD is rate-limiting in that system (47). Although PBGD message levels by RT-PCR were unchanged after MTX pretreatment in our system (data not shown), we did not have access to a suitable anti-PBGD antibody, so the question of whether MTX affects PBGD remains open. What we can say is that altered expression of two enzymes (CPO, leading to increased PpIX synthesis; and FC, leading to decreased PpIX degradation) together contribute to enhanced accumulation of PpIX after methotrexate preconditioning.
Cancer-selective enhancement of PDT due to MTX might also result from differential localization of PpIX. In normal keratinocytes, PpIX is detected mainly in plasma membranes (Fig. 1j, arrows), but localizes primarily within cytoplasmic/perinuclear granules in carcinoma cells (Fig. 1k). Cervical and urothelial carcinoma cells that featured higher PpIX levels then normal epithelial cells, also showed preferential PpIX elevation in mitochondrial versus membranous compartments (48, 49). Our observation that MTX induces PpIX in mitochondrial regions is consistent with those reports. Likewise, the literature indicates that PpIX (44, 50) or other porphyrin derivatives (51) will cause more damage when localized and photoactivated within mitochondria than within lysosomal or plasma. Thus, the selective killing of carcinoma cells vs. normal keratinocytes in our study may be occurring through preferential targeting of mitochondria.
Schwartz et al. (24) reported that NHEK undergo growth-arrest and differentiation in response to MTX at 50 nM (within the range used here), whereas we found NHEK to be largely unresponsive to MTX. This apparent discrepancy may be due to the fact that thymidine-free culture media were used in their experiments to amplify the MTX blockade upon thymidylate synthesis and to enhance subsequent DNA synthesis inhibition and growth arrest (24). In our work, higher MTX sensitivity of the skin-derived squamous cell carcinoma cells relative to normal keratinocytes may be due to a loss of thymidine salvage pathways in the carcinoma cells. Other researchers have shown that in the presence of methotrexate, NHEK are able to obtain thymidylate from alternative sources and thus maintain their DNA synthesis, whereas skin-derived carcinoma cells cannot do this (52, 53).
In summary, this study reports the following important observations: (1) PpIX levels can be induced in carcinoma cells using low, nontoxic doses of MTX. (2) Inducible changes in mitochondrial CPO expression and subsequent accumulation of PpIX appear to underlie this response. (3) The cytotoxic (therapeutic) effect achieved by combining MTX and ALA-PDT is synergistic in cell culture, and is also significant in organotypic and in-vivo animal models. (4) The beneficial effect of MTX is tumor-selective, occurring preferentially in carcinoma cells and not in normal epithelial cells. Futures studies will be required to translate MTX-PDT combination therapy into a useful modality for human skin carcinomas.
Supplementary Material
Figure 1 SUPPLEMENTAL. Methotrexate preferentially affects markers of epidermal proliferation and differentiation in carcinoma cells as compared to primary keratinocytes. Cells were incubated for 72 h in the presence of the following concentrations of MTX: Lane 1, 0 mg/L; Lane 2, 0.001 mg/L; Lane 3, 0.01 mg/L; Lane 4, 0.1 mg/L; Lane 5, 1.0 mg/L, then harvested and analyzed on Western blots using antisera specific to: (A) PCNA; (B) p27/kip-1; (C) E-cadherin; (D) Involucrin; (E) keratin 10; (F) Profilaggrin (top) and its proteolytic fragments (bottom). The approximate MW of each proteins is specified in parentheses above the figure, and the relative changes are shown graphically at the right. In (F), the graph is only for the pro-form of filaggrin.
Acknowledgments
NIH grant CA84203
We thank Harry A. Dailey and Tammy Dailey (U. of Georgia, Athens) for a kind gift of anti-ferrochelatase antibody. We thank Ellen Lee for assistance with the initial methotrexate titration studies, and Anatoly Prokvohit for help with the spectrophotometric PpIX assays. We also thank Christine Baran for statistical support, and Judy Drazba for help with confocal microscopy.
Abbreviations
- ALA
5-aminolevulinic acid
- DMBA
dimethylbenzanthracene
- CPO
coproporphyrinogen oxidase
- FBS
fetal bovine serum
- FC
ferrochelatase
- HEK1
a human squamous cell carcinoma cell line
- MTX
methotrexate
- NHEK
normal human epidermal keratinocytes
- PBS
phosphate buffered saline
- PDT
photodynamic therapy
- PpIX
protoporphyrin IX
- REK
rat epidermal keratinocyte
- SCC13
a human squamous cell carcinoma cell line
- TPA
tetraphorbol acetate
Footnotes
STATEMENT OF TRANSLATIONAL RELEVANCE
Photodynamic therapy (PDT) using aminolevulinic acid (ALA), a precursor for the accumulation of intracellular photosensitizers (PpIX) within carcinoma cells, is an emerging cancer treatment. Currently, however, the efficacy of ALA-PDT for large tumors is limited. Here, in cell culture and animal experiments using squamous skin carcinoma as the model, we describe a new paradigm in which intracellular PpIX levels are enhanced several fold by three days of pretreatment with low-dose methotrexate (MTX). This preconditioning improves tumor cell selectivity and overall treatment efficacy. These enhancements are achieved at low, nontoxic concentrations of MTX, similar to the plasma concentrations achieved clinically during treatment of psoriasis. Our data suggest a future clinical protocol in which MTX would be taken orally for three days, followed by topical ALA and exposure of the tumor to light. Such a combination protocol could make PDT more competitive with surgery for the treatment of nonmelanoma skin cancers.
References
- 1.Hasan T, Ortel B, Solban N, Pogue BW. Photodynamic therapy of cancer. In: Kufe D, Bast R, Hait W, et al., editors. Cancer Medicine. 7. Hamilton, Ontario: BC Decker, Inc; 2006. pp. 537–48. [Google Scholar]
- 2.Brown SB, Brown EA, Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004;5(8):497–508. doi: 10.1016/S1470-2045(04)01529-3. [DOI] [PubMed] [Google Scholar]
- 3.MacCormack MA. Photodynamic therapy. Adv Dermatol. 2006;22:219–58. doi: 10.1016/j.yadr.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy JC, Pottier RH, Pross DC. Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience. J Photochem Photobiol B. 1990;6(1–2):143–8. doi: 10.1016/1011-1344(90)85083-9. [DOI] [PubMed] [Google Scholar]
- 5.Morton CA, Brown SB, Collins S, et al. Guidelines for topical photodynamic therapy: report of a workshop of the British Photodermatology Group. Br J Dermatol. 2002;146(4):552–67. doi: 10.1046/j.1365-2133.2002.04719.x. [DOI] [PubMed] [Google Scholar]
- 6.Bickers DR, Pathak MA, Lim HW. The Porphyrias. In: Freedberg IM, Eisen AZ, Wolff K, et al., editors. Dermatology in General Medicine. 5. New York: McGraw-Hill; 1999. pp. 1766–803. [Google Scholar]
- 7.Morton CA, Whitehurst C, McColl JH, Moore JV, MacKie RM. Photodynamic therapy for large or multiple patches of Bowen disease and basal cell carcinoma. Arch Dermatol. 2001;137(3):319–24. [PubMed] [Google Scholar]
- 8.Stefanidou M, Tosca A, Themelis G, Vazgiouraki E, Balas C. In vivo fluorescence kinetics and photodynamic therapy efficacy of delta-aminolevulinic acid-induced porphyrins in basal cell carcinomas and actinic keratoses; implications for optimization of photodynamic therapy. Eur J Dermatol. 2000;10(5):351–6. [PubMed] [Google Scholar]
- 9.Marmur ES, Schmults CD, Goldberg DJ. A review of laser and photodynamic therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2004;30(2 Pt 2):264–71. doi: 10.1111/j.1524-4725.2004.30083.x. [DOI] [PubMed] [Google Scholar]
- 10.Juzeniene A, Juzenas P, Iani V, Moan J. Topical application of 5-aminolevulinic acid and its methylester, hexylester and octylester derivatives: considerations for dosimetry in mouse skin model. Photochem Photobiol. 2002;76(3):329–34. doi: 10.1562/0031-8655(2002)076<0329:taoaaa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Palsson S, Gustafsson L, Bendsoe N, Soto Thompson M, Andersson-Engels S, Svanberg K. Kinetics of the superficial perfusion and temperature in connection with photodynamic therapy of basal cell carcinomas using esterified and non-esterified 5-aminolaevulinic acid. Br J Dermatol. 2003;148(6):1179–88. doi: 10.1046/j.1365-2133.2003.05268.x. [DOI] [PubMed] [Google Scholar]
- 12.Ahmadi S, McCarron PA, Donnelly RF, Woolfson AD, McKenna K. Evaluation of the penetration of 5-aminolevulinic acid through basal cell carcinoma: a pilot study. Exp Dermatol. 2004;13(7):445–51. doi: 10.1111/j.0906-6705.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 13.Peng Q, Soler AM, Warloe T, Nesland JM, Giercksky KE. Selective distribution of porphyrins in skin thick basal cell carcinoma after topical application of methyl 5-aminolevulinate. J Photochem Photobiol B. 2001;62(3):140–5. doi: 10.1016/s1011-1344(01)00173-7. [DOI] [PubMed] [Google Scholar]
- 14.Alexiades-Armenakas M. Laser-mediated photodynamic therapy. Clin Dermatol. 2006;24(1):16–25. doi: 10.1016/j.clindermatol.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Clark C, Bryden A, Dawe R, Moseley H, Ferguson J, Ibbotson SH. Topical 5-aminolaevulinic acid photodynamic therapy for cutaneous lesions: outcome and comparison of light sources. Photodermatol Photoimmunol Photomed. 2003;19(3):134–41. doi: 10.1034/j.1600-0781.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- 16.Ortel B, Chen N, Brissette J, Dotto GP, Maytin E, Hasan T. Differentiation-specific increase in ALA-induced protoporphyrin IX accumulation in primary mouse keratinocytes. Br J Cancer. 1998;77(11):1744–51. doi: 10.1038/bjc.1998.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Momma T, Hamblin MR, Hasan T. Hormonal modulation of the accumulation of 5-aminolevulinic acid-induced protoporphyrin and phototoxicity in prostate cancer cells. Int J Cancer. 1997;72(6):1062–9. doi: 10.1002/(sici)1097-0215(19970917)72:6<1062::aid-ijc22>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Ortel B, Sharlin D, O’Donnell D, Sinha AK, Maytin EV, Hasan T. Differentiation enhances aminolevulinic acid-dependent photodynamic treatment of LNCaP prostate cancer cells. Br J Cancer. 2002;87(11):1321–7. doi: 10.1038/sj.bjc.6600575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glasser L, Fiederlein RL, Shamdas GJ, Brothman AR. Functional characteristics of in vivo induced neutrophils after differentiation therapy of acute promyelocytic leukemia with all-trans-retinoic acid. Cancer. 1994;73(4):1206–12. doi: 10.1002/1097-0142(19940215)73:4<1206::aid-cncr2820730414>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.Douer D. Acute promyelocytic leukemia. Curr Treat Options Oncol. 2000;1(1):31–40. doi: 10.1007/s11864-000-0013-1. [DOI] [PubMed] [Google Scholar]
- 21.Fenaux P, Chomienne C, Degos L. All-trans retinoic acid and chemotherapy in the treatment of acute promyelocytic leukemia. Semin Hematol. 2001;38(1):13–25. doi: 10.1016/s0037-1963(01)90002-2. [DOI] [PubMed] [Google Scholar]
- 22.Nelson DL, Cox MM. Lehninger Principles of Biochemistry. 4. New York: W.H. Freeman and Company; 2005. [Google Scholar]
- 23.Hatse S, De Clercq E, Balzarini J. Role of antimetabolites of purine and pyrimidine nucleotide metabolism in tumor cell differentiation. Biochem Pharmacol. 1999;58(4):539–55. doi: 10.1016/s0006-2952(99)00035-0. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz PM, Barnett SK, Atillasoy ES, Milstone LM. Methotrexate induces differentiation of human keratinocytes. Proc Natl Acad Sci U S A. 1992;89(2):594–8. doi: 10.1073/pnas.89.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bodner AJ, Ting RC, Gallo RC. Induction of differentiation of human promyelocytic leukemia cells (HL-60) by nucleosides and methotrexate. J Natl Cancer Inst. 1981;67(5):1025–30. [PubMed] [Google Scholar]
- 26.Ross SA, Jones CS, De Luca LM. Retinoic acid and methotrexate specifically increase PHA-E-lectin binding to a 67-kDa glycoprotein in LA-N-1 human neuroblastoma cells. Int J Cancer. 1995;62(3):303–8. doi: 10.1002/ijc.2910620312. [DOI] [PubMed] [Google Scholar]
- 27.Friedman SJ, Skehan P. Morphological differentiation of human choriocarcinoma cells induced by methotrexate. Cancer Res. 1979;39(6 Pt 1):1960–7. [PubMed] [Google Scholar]
- 28.Hatse S, Naesens L, De Clercq E, Balzarini J. Potent differentiation-inducing properties of the antiretroviral agent 9-(2-phosphonylmethoxyethyl) adenine (PMEA) in the rat choriocarcinoma (RCHO) tumor cell model. Biochem Pharmacol. 1998;56(7):851–9. doi: 10.1016/s0006-2952(98)00058-6. [DOI] [PubMed] [Google Scholar]
- 29.Berkowitz RS, Goldstein DP, Bernstein MR. Ten year’s experience with methotrexate and folinic acid as primary therapy for gestational trophoblastic disease. Gynecol Oncol. 1986;23(1):111–8. doi: 10.1016/0090-8258(86)90123-x. [DOI] [PubMed] [Google Scholar]
- 30.Sinha AK, Anand S, Ortel BJ, et al. Methotrexate used in combination with aminolevulinic acid for photodynamic killing of prostate cancer cells. Brit J Cancer. 2006;95:485–95. doi: 10.1038/sj.bjc.6603273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W, Vaccariello MA, Wang Y, Alt-Holland A, Fusenig NE, Garlick JA. Escape from microenvironmental control and progression of intraepithelial neoplasia. Int J Cancer. 2005;116(6):885–93. doi: 10.1002/ijc.21103. [DOI] [PubMed] [Google Scholar]
- 32.Sugerman PB, Bigby M. Preliminary functional analysis of human epidermal T cells. Arch Dermatol Res. 2000;292(1):9–15. doi: 10.1007/pl00007461. [DOI] [PubMed] [Google Scholar]
- 33.Sato N, Moore B, Keevey S, Drazba J, Hasan T, Maytin EV. Vitamin D enhances ALA-mediated protoporphyrin IX production and photodynamic cell death in 3-D organotypic cultures of keratinocytes. J Invest Dermatol. 2007;127:925–34. doi: 10.1038/sj.jid.5700595. [DOI] [PubMed] [Google Scholar]
- 34.Maytin EV, Murphy LA, Merrill MA. Hyperthermia induces resistance to ultraviolet light B in primary and immortalized epidermal keratinocytes. Cancer Res. 1993;53(20):4952–9. [PubMed] [Google Scholar]
- 35.Zhou X, Pogue BW, Chen B, et al. Pretreatment photosensitizer dosimetry reduces variation in tumor response. Int J Radiat Oncol Biol Phys. 2006;64(4):1211–20. doi: 10.1016/j.ijrobp.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Duska LR, Hamblin MR, Miller JL, Hasan T. Combination photoimmunotherapy and cisplatin: effects on human ovarian cancer ex vivo. J Natl Cancer Inst. 1999;91(18):1557–63. doi: 10.1093/jnci/91.18.1557. [DOI] [PubMed] [Google Scholar]
- 37.Gehen SC, Vitiello PF, Bambara RA, Keng PC, O’Reilly MA. Downregulation of PCNA potentiates p21-mediated growth inhibition in response to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2007;292(3):L716–24. doi: 10.1152/ajplung.00135.2006. [DOI] [PubMed] [Google Scholar]
- 38.Mack JA, Anand S, Maytin EV. Proliferation and cornification during development of the mammalian epidermis. Birth Defects Res C Embryo Today. 2005;75(4):314–29. doi: 10.1002/bdrc.20055. [DOI] [PubMed] [Google Scholar]
- 39.Presland RB, Dale BA. Epithelial structural proteins of the skin and oral cavity: function in health and disease. Crit Rev Oral Biol Med. 2000;11(4):383–408. doi: 10.1177/10454411000110040101. [DOI] [PubMed] [Google Scholar]
- 40.Pearton DJ, Dale BA, Presland RB. Functional analysis of the profilaggrin N-terminal peptide: identification of domains that regulate nuclear and cytoplasmic distribution. J Invest Dermatol. 2002;119(3):661–9. doi: 10.1046/j.1523-1747.2002.01831.x. [DOI] [PubMed] [Google Scholar]
- 41.Hartmann SN, Rordorf CM, Milosavljev S, et al. Lumiracoxib does not affect methotrexate pharmacokinetics in rheumatoid arthritis patients. Ann Pharmacother. 2004;38(10):1582–7. doi: 10.1345/aph.1E044. [DOI] [PubMed] [Google Scholar]
- 42.Chladek J, Grim J, Martinkova J, et al. Pharmacokinetics and pharmacodynamics of low-dose methotrexate in the treatment of psoriasis. Br J Clin Pharmacol. 2002;54(2):147–56. doi: 10.1046/j.1365-2125.2002.01621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krieg RC, Messmann H, Rauch J, Seeger S, Knuechel R. Metabolic characterization of tumor cell-specific protoporphyrin IX accumulation after exposure to 5-aminolevulinic acid in human colonic cells. Photochem Photobiol. 2002;76(5):518–25. doi: 10.1562/0031-8655(2002)076<0518:mcotcs>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 44.Iinuma S, Farshi SS, Ortel B, Hasan T. A mechanistic study of cellular photodestruction with 5-aminolaevulinic acid-induced porphyrin. Br J Cancer. 1994;70(1):21–8. doi: 10.1038/bjc.1994.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kondo M, Hirota N, Takaoka T, Kajiwara M. Heme-biosynthetic enzyme activities and porphyrin accumulation in normal liver and hepatoma cell lines of rat. Cell Biol Toxicol. 1993;9(1):95–105. doi: 10.1007/BF00755143. [DOI] [PubMed] [Google Scholar]
- 46.Ohgari Y, Nakayasu Y, Kitajima S, et al. Mechanisms involved in delta-aminolevulinic acid (ALA)-induced photosensitivity of tumor cells: relation of ferrochelatase and uptake of ALA to the accumulation of protoporphyrin. Biochem Pharmacol. 2005;71(1–2):42–9. doi: 10.1016/j.bcp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 47.Ickowicz Schwartz D, Gozlan Y, Greenbaum L, Babushkina T, Katcoff DJ, Malik Z. Differentiation-dependent photodynamic therapy regulated by porphobilinogen deaminase in B16 melanoma. Br J Cancer. 2004;90(9):1833–41. doi: 10.1038/sj.bjc.6601760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gallegos ER, DeLeon Rodriguez I, Martinez Guzman LA, Perez Zapata AJ. In vitro study of biosynthesis of protoporphyrin IX induced by delta-aminolevulinic acid in normal and cancerous cells of the human cervix. Arch Med Res. 1999;30(3):163–70. doi: 10.1016/s0188-0128(99)00013-5. [DOI] [PubMed] [Google Scholar]
- 49.Seidl J, Rauch J, Krieg RC, Appel S, Baumgartner R, Knuechel R. Optimization of differential photodynamic effectiveness between normal and tumor urothelial cells using 5-aminolevulinic acid-induced protoporphyrin IX as sensitizer. Int J Cancer. 2001;92(5):671–7. doi: 10.1002/1097-0215(20010601)92:5<671::aid-ijc1240>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 50.Morgan J, Oseroff AR. Mitochondria-based photodynamic anti-cancer therapy. Adv Drug Deliv Rev. 2001;49(1–2):71–86. doi: 10.1016/s0169-409x(01)00126-0. [DOI] [PubMed] [Google Scholar]
- 51.Kessel D, Luguya R, Vicente MG. Localization and photodynamic efficacy of two cationic porphyrins varying in charge distributions. Photochem Photobiol. 2003;78(5):431–5. doi: 10.1562/0031-8655(2003)078<0431:lapeot>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 52.Firestone WM, FitzGerald GB, Wick MM. A comparison of the effects of antitumor agents upon normal human epidermal keratinocytes and human squamous cell carcinoma. J Invest Dermatol. 1990;94(5):657–61. doi: 10.1111/1523-1747.ep12876228. [DOI] [PubMed] [Google Scholar]
- 53.Lee MM, Ratliff J, FitzGerald GB, Wick MM. The mechanism of differential sensitivity to methotrexate of normal and malignant human epidermal cells. Cancer Chemother Pharmacol. 1991;28(3):181–4. doi: 10.1007/BF00685506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1 SUPPLEMENTAL. Methotrexate preferentially affects markers of epidermal proliferation and differentiation in carcinoma cells as compared to primary keratinocytes. Cells were incubated for 72 h in the presence of the following concentrations of MTX: Lane 1, 0 mg/L; Lane 2, 0.001 mg/L; Lane 3, 0.01 mg/L; Lane 4, 0.1 mg/L; Lane 5, 1.0 mg/L, then harvested and analyzed on Western blots using antisera specific to: (A) PCNA; (B) p27/kip-1; (C) E-cadherin; (D) Involucrin; (E) keratin 10; (F) Profilaggrin (top) and its proteolytic fragments (bottom). The approximate MW of each proteins is specified in parentheses above the figure, and the relative changes are shown graphically at the right. In (F), the graph is only for the pro-form of filaggrin.


