Abstract
At neutral pH, dendronized deep-cavity cavitands were shown to form supramolecular nano-capsules via assembly around a range of guest molecules.
The majority of synthetic supramolecular assemblies rely on enthalpically powerful hydrogen-bonding1-5 and metal coordination to drive assembly;6-10 motifs that are most powerful in non-aqueous media. In contrast, Nature often relies on entropy - in the guise of the hydrophobic effect - to bring about assembly. Inspired by this point, one of our laboratories has reported on a supramolecular capsule formed by the dimerization of 1a around a guest(s) (Scheme 1 and 2).11-15 This capsule, soluble in aqueous base by virtue of a coat of sixteen carboxylates, has been shown to affect the separation of hydrocarbon gases,16 and act as a nano-scale reaction vessel for photochemical reactions.17-19 This type of encapsulation also offers an attractive route to modulating the physical properties of a drug without covalent modification, however for this water solubility close to neutral pH is required. Herein we report on the synthesis and assembly of dendronized cavitand 2 (Scheme 1 and 2). Coated with 128 hydroxy groups, the dimeric capsule formed by 2 encapsulates a range of guests at physiological pH.
Scheme 1.
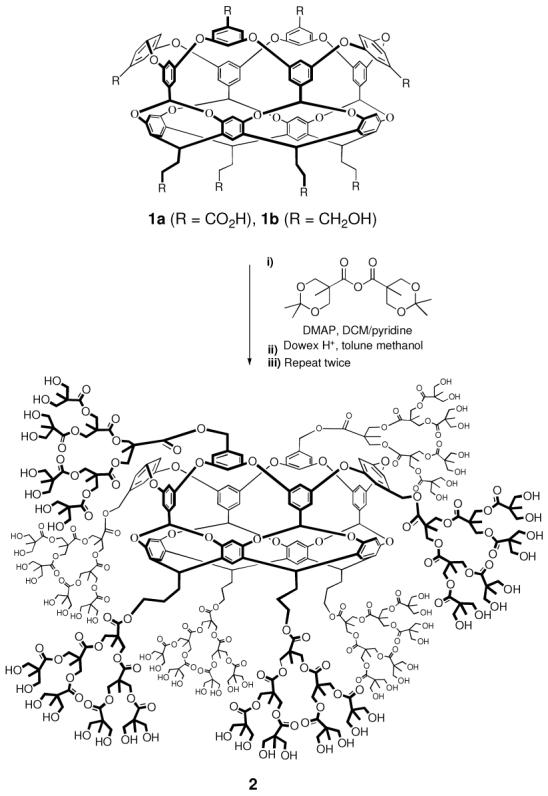
Synthesis of dendronized cavitand 2.
Scheme 2.
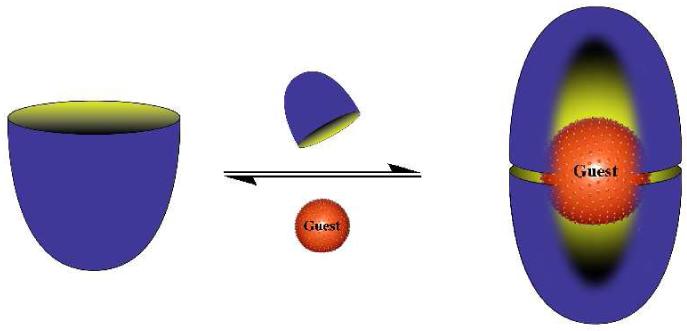
Representation of the dimerization of deep-cavity cavitands 1a and 2 around a single guest.
The attachment of hydroxyl-terminated aliphatic polyester dendrons onto the cavitand core was pursued to impart both pH-independent water solubility and high biocompatibility. The dendritic structure20 imparts an improved solubility over linear analogs,21 while the hydroxylated periphery affords a highly biocompatible surface.22 Similar biocompatible dendritic coats have been applied to small molecule cores,23 linear polymers,24 and solid surfaces.25
While a variety of protecting groups could be used for the diol monomer required for dendronization, the acid sensitive acetonide protecting group proved to be the most compatible with 1b. Thus, 1b treated with 1.5 equiv. per hydroxyl of acetonide-protected bis-(hydroxymethyl)-propanoic anhydride (DMAP catalyst) gave the resulting ester after precipitation from methanol in >90% yield, whilst subsequent deprotection (Dowex acid resin) cleanly affected the removal of the acetonide acetals in quantitative yield. Both the esterification and the deprotection reactions could be monitored by MALDI-TOF MS (supporting information, SI). The resulting firstgeneration (G-1) dendronized cavitand bearing 16 OH groups, Cav-([G-1]-OH2)8 was then subjected to a second repetition of coupling and deprotection to yield the G-2 cavitand Cav-([G-2]-OH4)8, and finally a third repetition of these steps to afford cavitand 2 Cav-([G-3]-OH8)8.
Solubility studies revealed that the G-1 cavitand was sparingly soluble in methanol, the G-2 soluble in alcohols and mixtures of water and methanol up to 80% water by volume, while the G-3 cavitand 2 was freely soluble in pure water.
Binding studies began with an NMR analysis of free host 2 (SI). Whereas the spectrum of 2 in MeOH showed well-resolved peaks, in pure D2O many signals were broad. This broadness was independent of concentration, but the peaks did sharpen upon capsule formation (vide infra) suggesting that free 2 undergoes some aggregation at mmol concentrations. In all probability, the large size of 2 (C376H528O192, avg. mw = 8120.10 amu) and a restricted mobility of the dendrons also contribute to peak broadening. The spectrum of 2 in D2O also exhibited broad peaks in its guest region (< 0 ppm). Titration with MeOH resulted in their disappearance but no free signals indicative of impurities being displaced. As models indicate it is possible for the third generation dendrons to bind into the pocket of 2, we attribute these upfield signals to self-inclusion.
At neutral pH, 2 proved to be a consummate host with a broad range of complexation properties. In the presence of 3 (Figure 1) it formed a well defined 1:1 complex.26 NMR signal shifts for the guest demonstrated that it adopted an orientation such that the carboxylate group resides at the portal of the host. Consequently, the propensity of the complex to be capped by another cavitand is inhibited.
Figure 1.
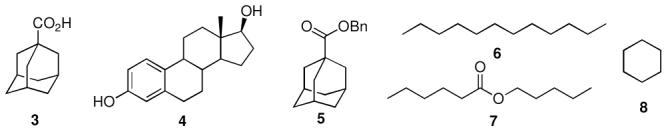
Guests investigated in this study.
In contrast, non-amphiphilic guests formed capsular complexes. Thus, rigid estradiol 4 readily formed a 2:1 host-guest complex, although some degree of broadening prevented a detailed analysis of the guest binding region. Furthermore, 1D and 2D NMR (NOESY and COSY) experiments revealed that more flexible 5 also led to capsule formation, as did highly flexible 6 and ester 7. In most of the complexes, NOE interactions between host and guest, and between one host hemisphere and the next, were apparent. The symmetry of 6 led to a particularly well-defined NMR spectrum (Figure 2). In the free state, signals for 6 are found over a narrow range, but in the capsule are spread over 3 ppm; “equatorially” located methylenes in the middle of the chain undergo small shifts, whereas the terminal methyls, deep within the “north/south polar” regions of the capsule, were shifted almost 4 ppm upfield. The NOESY NMR did not reveal any helical conformation of the guest. Finally, we wished to determine if small guests also formed kinetically stable complexes. Gratifyingly, the addition of excess 8 resulted in a slow exchanging, 2:2 capsular complex, with free and bound guest signals at 1.25 and -0.75 ppm.
Figure 2.
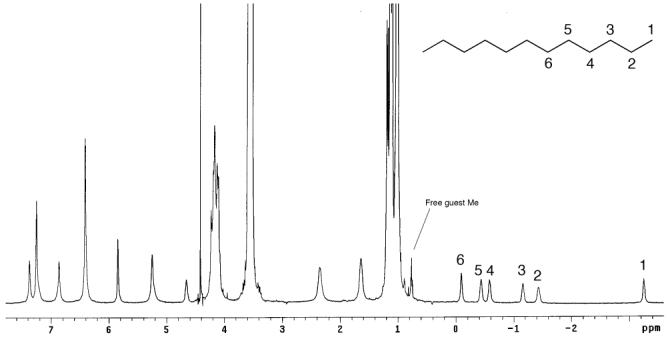
NMR spectrum of the complex 6@22
Even though more non-covalent contacts are possible between two molecules of 2, initial experiments (SI) suggest that 1a binds guests more strongly. Thus, in the presence of one equivalent of 7, a small amount of free guest is observed with 2 that was not observed with 1a. We hypothesize two reasons for this. First, self-inclusion by 2 would reduce its affinity for guests. Second, because of the relatively thick dendritic coat, the hydrophobic rim of cavitand 2 may be less solvated by water than its more “naked” counterpart 1a. Consequently, capsule formation would not result in the same degree of desolvation. We are currently studying the properties of host 2 further, and synthesizing other dendritic cavitands to shed light on this potentially important structural consideration of assemblies driven by the hydrophobic effect. We will report on these findings in due course.
Supplementary Material
Acknowledgment
BCG and SL authors gratefully acknowledge the financial support of the National Institutes of Health (GM074031). SMG and MDG acknowledge support from the Louisiana Board of Regents (LEQSF(2006-09)-RD-A-29), the National Aeronautics and Space Administration (NNC06AA18A), and Tulane University. RLE acknowledges support from LS-LAMP. In addition, the National Science Foundation (NSF-MRI 0619770) is acknowledged for enabling MALDI-TOF MS characterization. Special thanks also to Mary Cloninger for helpful discussions.
References
- 1.Sijbesma RP, Meijer EW. Chem. Commun. 2003:5–16. doi: 10.1039/b205873c. [DOI] [PubMed] [Google Scholar]
- 2.Rebek J., Jr. Angew. Chem. Int. Ed. 2005;44:2068–2078. doi: 10.1002/anie.200462839. [DOI] [PubMed] [Google Scholar]
- 3.Vriezema DM, Aragonès MC, Elemans JAAW, Cornelissen JJLM, Rowan AE, Nolte RJM. Chem. Rev. 2005;105:1445–1489. doi: 10.1021/cr0300688. [DOI] [PubMed] [Google Scholar]
- 4.Dalgarno SJ, Thallapally PK, Barbour LJ, Atwood JL. Chem. Soc. Rev. 2007;36:236–245. doi: 10.1039/b606047c. [DOI] [PubMed] [Google Scholar]
- 5.Davis JT, Spada GP. Chem. Soc. Rev. 2007;36:296–313. doi: 10.1039/b600282j. [DOI] [PubMed] [Google Scholar]
- 6.Gianneschi NC, Masar MS, III, Mirkin CA. Acc. Chem. Res. 2005;38:825–837. doi: 10.1021/ar980101q. [DOI] [PubMed] [Google Scholar]
- 7.Fujita M, Tominaga M, Hori A, Therrien B. Acc. Chem. Res. 2005;38:369–378. doi: 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]
- 8.Nitschke JR. Acc. Chem. Res. 2007;40:103–112. doi: 10.1021/ar068185n. [DOI] [PubMed] [Google Scholar]
- 9.Lehn J-M. Science. 2002;295:2400–2403. doi: 10.1126/science.1071063. [DOI] [PubMed] [Google Scholar]
- 10.Fiedler D, Leung DH, Bergman RG, Raymond KN. Acc. Chem. Res. 2005;38:351–360. doi: 10.1021/ar040152p. [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Gibb BC. Chem. Comm. 2008:3709–3716. doi: 10.1039/b805446k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibb BC. Nano-Capsules Assembled by the Hydrophobic Effect. In: Atwood JL, Steed JW, editors. Organic Nano-Structures. John Wiley and Sons; 2007. [Google Scholar]
- 13.Gibb CLD, Gibb BC. J. Am. Chem. Soc. 2004;126:11408–11409. doi: 10.1021/ja0475611. [DOI] [PubMed] [Google Scholar]
- 14.Gibb CLD, Gibb BC. Chem. Comm. 2007:1635–1637. doi: 10.1039/b618731e. [DOI] [PubMed] [Google Scholar]
- 15.Biros SM, Rebek J., Jr. Chem. Soc. Rev. 2007;36:93–104. doi: 10.1039/b508530f. [DOI] [PubMed] [Google Scholar]
- 16.Gibb CLD, Gibb BC. J. Am. Chem. Soc. 2006;128:16498–16499. doi: 10.1021/ja0670916. [DOI] [PubMed] [Google Scholar]
- 17.Gibb CLD, Sundaresan AK, Ramamurthy V, Gibb BC. J. Am. Chem. Soc. 2008;130:4069–4080. doi: 10.1021/ja7107917. [DOI] [PubMed] [Google Scholar]
- 18.Natarajan A, Kaanumalle LS, Jockusch S, Gibb CLD, Gibb BC, Turro NJ, Ramamurthy V. J. Am. Chem. Soc. 2007;129:4132–4133. doi: 10.1021/ja070086x. [DOI] [PubMed] [Google Scholar]
- 19.Kaanumalle LS, Gibb CLD, Gibb BC, Ramamurthy V. J. Am. Chem. Soc. 2004;126:14366–14367. doi: 10.1021/ja0450197. [DOI] [PubMed] [Google Scholar]
- 20.For a smaller cavitand made water-soluble by a second generation coat of ethylene glycol units, see:Middel O, Verboom W, Reinhoudt DN. Eur. J. Org. Chem. 2002:2587–2597. doi: 10.1021/jo010107k.
- 21.Wooley KL, Fréchet JMJ, Hawker CJ. Polymer. 1994;35:4489–4495. [Google Scholar]
- 22.Padilla de Jesus OL, Ihre H, Gagne HR, Szoka FC, Jr., Fréchet JMJ. Bioconjug. Chem. 2002;13:452–461. doi: 10.1021/bc010103m. [DOI] [PubMed] [Google Scholar]
- 23.Ihre H, Padilla de Jesus OL, Fréchet JMJ. J. Am. Chem. Soc. 2001;123:5908–5917. doi: 10.1021/ja010524e. [DOI] [PubMed] [Google Scholar]
- 24.Grayson SM, Fréchet JMJ. Macromolecules. 2001;34:6542–6544. [Google Scholar]
- 25.Benhabbour SR, Liu L, Sheardown H, Adronov A. Macromolecules. 2008;41:2567–2576. [Google Scholar]
- 26.For 1:1 complexation using host 1a, see:Sun H, Gibb CLD, Gibb BC. Supramolecular Chem. 2008;20:141–147.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


