Abstract
A study on the synthesis of a 4-hydroxyquinoline derivative using the Conrad-Limpach reaction led to the identification of inexpensive and user-friendly solvents for this thermal condensation.
Keywords: Conrad-Limpach, 4-hydroxyquinoline, thermal condensation
INTRODUCTION
Molecules containing the quinoline nucleus often display biological activity and several quinolines are marketed drugs. Indeed, quinoline derivatives have shown significant biological activity in the treatment of many diseases including malaria,[1] arthritis,[2] diabetes,[3] HIV[4] and heart disease.[5] The alkaloid quinine, once claimed as “the drug to have relieved more human suffering than any other in history”, reflects the importance of quinoline-containing compounds.[6]
The chemical synthesis of quinolines has received a substantial amount of attention for more than a century. Many classical syntheses have been developed, including the Skraup synthesis, the Friedlander synthesis, and the Combes synthesis. The Conrad-Limpach synthesis, similarly, is useful for the synthesis of quinolones. These classical syntheses have been reviewed.[7,8] The importance attributed to the synthesis of quinolines relates to the significant biological activities of the quinolines and also to the challenges that these syntheses represent.
RESULTS AND DISCUSSION
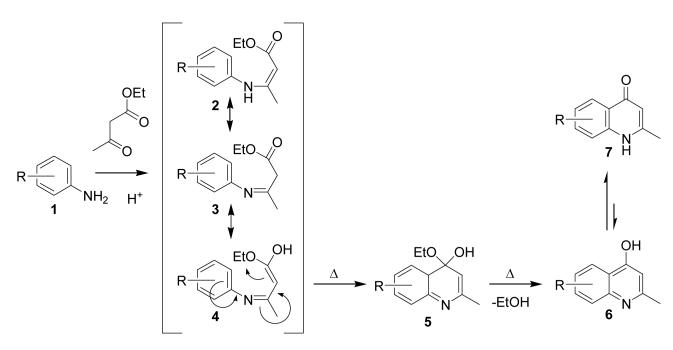
In many of the classical quinolines syntheses, the formation of a high-energy intermediate is often achieved using high boiling solvents and/or strong acids. The Conrad-Limpach reaction, used to prepare 4-quinolones, is shown in Scheme 1. Because the ultimate substrate for the cyclization must be in the high-energy imine-enol tautomer (4), and because the cyclization into the hemiketal 5 breaks the aromaticity of the phenyl ring, solvents with very high boiling points are traditionally used for this reaction. (Alternatively, a ketene-imine intermediate formed via direct elimination of EtOH from the imine ester 3 is an alternative reaction pathway; the cyclization of this intermediate would also require the breaking of aromaticity and necessitate the same high temperature solvents) Indeed, mineral oil (BP > 275 °C), diphenyl ether (BP = 259 °C) and more recently, Dowtherm A (BP = 257 °C) are the most widely referenced solvents.[7-11] Although mineral oil is inexpensive, it is an extremely inconvenient solvent to employ because of its physical characteristics. Diphenyl ether is inexpensive, but it is a solid at room temperature, and has an unpleasant odor. Dowtherm A (aeutectic mixture of diphenyl ether and biphenyl) is more convenient because it is a liquid a room temperature, but it is more expensive, and also has an unpleasant odor.
Scheme 1.
Mechanism of classical Conrad-Limpach reaction.
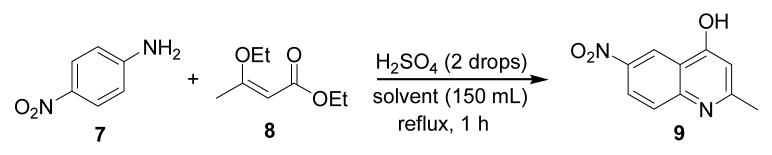
For one of our synthetic programs, we required the synthesis of large quantities of the 4-quinolone 9. Despite literature reports of high yields using the classical Conrad-Limpach conditions, we were unable to replicate these results. A substantial improvement in the yield was seen after substituting the traditional method for a one-pot modification in which the usual ethyl acetoacetate was replaced by the vinyl ether 8[12] (Scheme 2). However, we also wanted to avoid the expense and other limitations of the usual solvent choices. A serendipitous discovery lead to the finding that ethyl benzoate was an adequate replacement for the usual high-boiling solvents. Ethyl benzoate is inexpensive, can easily be removed from the product of the reaction, and does not have the unpleasant odor associated with the other solvents traditionally used.
Scheme 2.
Preparation of 4-hydroxy-2-methyl-6-nitroquinoline (9).
Using this information, we decided to investigate variations on the Conrad-Limpach synthesis to improve this process by screening several solvents of different boiling points and polarity. We began with an exploration of different alkyl benzoates with increasing boiling points. We then also explored a number of other high-boiling solvents that are not traditionally used for thermal cyclization reactions.
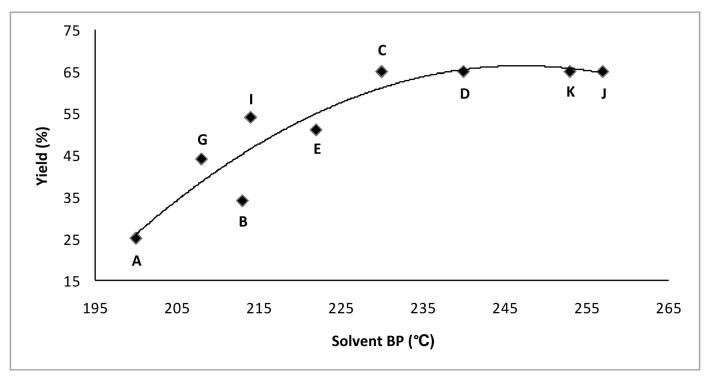
It was confirmed that the yield of 4-hydroxyquinoline increased with solvents of increasing boiling point and that the best yields were obtained with solvents of boiling points above 250 °C, as shown in Table 1. Initially, a series of alkyl benzoates was chosen as solvents. Although alkyl benzoates are typically considered as reagents, they behave well as solvents in this thermal cyclization. Increasing the size of the alkyl substituent from methyl to iso-butyl improved the yield from 25% to 66%. Unfortunately, the yield has been improved in a costly manner since iso-butyl benzoate is 13 times more expensive than methyl benzoate. Other high boiling point solvents were also screened. 2-Nitrotoluene and 1,2,4-trichlorobenzene were comparable to iso-butyl benzoate in terms of yield but are also less expensive. Surprisingly, 2,6-di-tert-butylphenol was found to be one of the best solvents for this reaction since it gave a clean product in reasonable yield (65%) and is significantly less expensive than Dowtherm A, and has no unpleasant odor. In Figure 2 is shown a plot of solvent boiling point versus yield for several solvents used in the Conrad-Limpach thermal cyclization shown in Scheme 3. Yields generally increase with the reaction temperature, until a maximum value of about 65% is achieved.
Table 1.
Summary of Conrad-Limpach reaction results with selected solvents.
| Solvent a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | K | |
| Solvent BP (°C) b |
200 | 213 | 230 | 240 | 222 | 230 | 208 | 190 | 213 | 257 | 253 |
| Cost ($/L)c |
26 | 35 | 340 | 340 | 20 | 22 | 21 | 45 | 32 | 122 | 30 |
| Yield | 25% | 34% | 65% | 66% | 51% | n/ad | 44% | n/ae | 54% | 65% | 65% |
| Product color |
light brown |
dark brown |
black | black | light brown |
n/ad | dark brown |
n/ae | dark brown |
light brown |
light brown |
A , m ethyl benzoate; B , ethyl benzoate; C , propyl benzoate; D , isobutyl benzoate; E , 2-nitrotoluene; F , 1,4-butanediol; G , tetrahydronaphthalene; H , decahydronaphthalene; I, 1,2,4-trichlorobenzene; J, D ow therm A ; K , 2,6-di-tert-butylphenol.
Obtained from Lange’s Handbook, 14 thedition.
Obtained from Aldrich catalog, 2007 -2008 edition.
Reaction with 1,4-butanediol did not produce any product.
Reaction with decahydronaphthalene yielded only non -cyclized enam ine product.
Figure 2.
Yield as a function of solvent boiling point for the Conrad-Limpach reaction.
CONCLUSION
A group of atypical solvents was used for the preparation of a 4-hydroxyquinoline derivative using the Conrad-Limpach thermal cyclization reaction. The yield of the reaction generally improved with higher-boiling solvents. Several alternative solvents, including 1,2,4-trichlorobenzene, 2-nitrotoluene, and 2,6-di-tert-butylphenol could be useful, inexpensive alternative solvents for the preparation of quinoline derivatives on a large scale.
EXPERIMENTAL
A 1 L round bottom flask was charged with 4-nitroaniline (7, 10.0 g, 72 mmol), ethyl 3-ethoxybut-2-enoate[12] (8, 29.0 g, 183 mmol, 2.5 eq.) and the solvent (150 mL). Concentrated sulfuric acid (2 drops) was added to the stirred mixture. The reaction vessel was equipped with a short distillation apparatus to remove the ethanol produced during the reaction. The reaction mixture was heated at reflux for 1 hour (with the exception of iso-butyl benzoate, 2,6-di-tert-butylphenol and Dowtherm A, which required only 35 min. of heating), and the ethanol was removed by distillation as the reaction progressed. During the reflux period, 2-methyl-6-nitro-4-quinolone (9) precipitated out of solution. Once the reaction mixture was cooled to room temperature, the product was collected and washed with toluene and hexanes, then dried in a vacuum oven (60 °C, 5 mmHg) to constant weight.
Figure 1.
Quinine, a quinoline-containing natural product
REFERENCES
- 1.Schmidt LH. Chemotherapy of the drug-resistant malarias. Ann. Rev. Microbiol. 1969;23:427–454. doi: 10.1146/annurev.mi.23.100169.002235. [DOI] [PubMed] [Google Scholar]
- 2.Baba A, Kawamura N, Makino H, Ohta Y, Taketomi S, Sohda T. Studies on disease-modifying antirheumatic drugs: Synthesis of novel quinoline and quinazoline derivatives and their anti-inflammatory activity. J. Med. Chem. 1996;39:5176–5182. doi: 10.1021/jm9509408. [DOI] [PubMed] [Google Scholar]
- 3.Cantin L-D, Magnuson S, Gunn D, Barucci N, Breuhaus M, Bullock WH, Burke J, Claus TH, Daly M, DeCarr L, Gore-Willse A, Hoover-Litty H, Kumarasinghe ES, Li Y, Liang S, Livingston JN, Lowinger T, MacDougall M, Ogutu HO, Olague A, Ott-Morgan R, Schoenleber RW, Tersteegen A, Wickens P, Zhang Z, Zhu J, Zhu L, Sweet LJ. PDE-10A inhibitors as insulin secretagogues. Bioorg. Med. Chem. Lett. 2007;17:2869–2873. doi: 10.1016/j.bmcl.2007.02.061. [DOI] [PubMed] [Google Scholar]
- 4.Oh Y-S, Lee C-W, Chung Y-H, Yoon S-J, Cho S-H. Syntheses of ney pyridonecarboxylic acid derivatives containing 3-, 5-, or 6-quinolyl substituents at N-1 and their anti-HIV-RT activities. J. Heterocycl. Chem. 1998;35:541–550. [Google Scholar]
- 5.Musser JH, Chakraborty U, Bailey K, Sciortino S, Whyzmuzis C, Amin D, Sunderland CA. Synthesis and antilipolytic activities of quinolyl carbanilates and related analogues. J. Med. Chem. 1987;30:62–67. doi: 10.1021/jm00384a011. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman TS, Ruveda EA. The quest for quinine: Those who won the battles and those who won the war. Angew. Chem. Int. Ed. 2005;44:854–885. doi: 10.1002/anie.200400663. [DOI] [PubMed] [Google Scholar]
- 7.Bergstrom FW. Heterocyclic nitrogen compounds. Part IIA. hexacyclic compounds: pyridine, quinoline, and isoquinoline. Chem. Rev. 1944;35:77–277. [Google Scholar]
- 8.Reitsema RH. The chemistry of 4-hydroxyquinolines. Chem. Rev. 1948;43:43–68. doi: 10.1021/cr60134a002. [DOI] [PubMed] [Google Scholar]
- 9.Kaslow CE, Stayner RD. Substituted Quinolines. J. Am. Chem. Soc. 1948;70:3350–3351. doi: 10.1021/ja01190a039. [DOI] [PubMed] [Google Scholar]
- 10.Horchler CL, McCauley JP, Jr., Hall JE, Snyder DH, Moore WC, Hudzik TJ, Chapdelaine MJ. Synthesis of novel quinolone and quinoline-2-carboxylic acid (4-morpholin-4-yl-phenyl)amides: A late-stage diversification approach to potent 5HT1B antagonists. Bioorg. Med. Chem. 2007;15:939–950. doi: 10.1016/j.bmc.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 11.Huang CQ, Wilcoxen K, McCarthy JR, Haddach M, Webb TR, Gu J, Xie Y-F, Grigoriadis DE, Chen C. Synthesis and SAR of 8-arylquinolines as potent corticotrophin-releasing factor1 (CRF1) receptor antagonists. Bioorg. Med. Chem. Lett. 2003;13:3375–3379. doi: 10.1016/s0960-894x(03)00684-x. [DOI] [PubMed] [Google Scholar]
- 12.Carbonnel S, Fayet C, Gelas J. Introduction of a carboxyl group through an acetal as a new route to carboxylic acid derivatives of sugars. Carbohydr. Res. 1999;319:63–73. doi: 10.1016/s0008-6215(99)00118-4. [DOI] [PubMed] [Google Scholar]