Abstract
The thyroid hormone receptors (TRs) are transcription factors that mediate the pleiotropic activities of the thyroid hormone, T3. Four T3-binding isoforms, TRα1, TRβ1, TRβ2, and TRβ3, are encoded by two genes, THRA and THRB. Mutations and altered expression of TRs have been reported in human cancers. A targeted germline mutation of the Thrβ gene in the mouse leads to spontaneous development of follicular thyroid carcinoma (TRβPV/PV mouse). The TRβPV mutant has lost T3 binding activity and displays potent dominant negative activity. The striking phenotype of thyroid cancer exhibited by TRβPV/PV mice has recently led to the discovery of novel non-genomic actions of TRβPV that contribute to thyroid carcinogenesis. These actions involve direct physical interaction of TRβPV with cellular proteins, namely the regulatory subunit of the phosphatidylinositol 3-kinase (p85α), the pituitary tumor-transforming gene (PTTG) and β-catenin, that are critically involved in cell proliferation, motility, migration, and metastasis. Thus, a TRβ mutant (TRβPV), via a novel mode of non-genomic action, acts as an oncogene in thyroid carcinogenesis.
Keywords: thyroid hormone receptor mutants, thyroid cancer, non-genomic action, phosphatidylinositol 3 kinase, pituitary tumor transforming gene, β-catenin, mouse model
1. Introduction
Thyroid hormone receptors (TRs) are members of the nuclear receptor superfamily that mediate the pleiotropic activities of the thyroid hormone, T3, in differentiation, growth, and metabolism (Yen, 2001). There are four major T3-binding receptor isoforms: TRα1, TRβ1, TRβ2, and TRβ3. They are encoded by two genes, THRA and THRB, that are located on two different chromosomes. Numerous efforts have been made over the past decade to uncover the underlying mechanism of action of these nuclear receptors. Classically, TRs bind to specific DNA sequences on the promoter of T3-target genes (thyroid hormone response elements, or TREs) to activate or repress basal gene transcription (Yen, 2001). The regulation of their transcriptional activity is complex: it depends not only on T3 binding but also on the type of TREs on the promoter of T3-target genes.
There are many T3-target tissues, including bone, heart, adipose tissue, liver, pituitary, and brain. The broad spectrum of T3 action is well illustrated by patients with resistance to thyroid hormone (RTH). RTH is a syndrome characterized by reduced sensitivity of tissues to the action of thyroid hormones, and it is inherited in an autosomal dominant manner. The hallmark of RTH is elevated thyroid hormone associated with nonsuppressible thyroid stimulating hormone (TSH). Other clinical signs are goiter, delayed bone growth and maturation, decreased weight, tachycardia, hearing loss, attention deficit disorder, and hyperactivity disorder (Weiss and Refetoff, 2000; Yen, 2003). Numerous mutations in the ligand-binding domain of TRβ have been identified in RTH families (Adams et al., 1994; Refetoff et al., 1993). Most patients are heterozygous for the mutation, and the clinical symptoms are mild (Weiss and Refetoff, 2000; Yen, 2003). Only one patient homozygous for a mutant TRβ has been reported (Ono et al., 1991). That patient, who died young, displayed extreme RTH with high levels of thyroid hormones and TSH (Ono et al., 1991).
Several findings support the notion that mutations of TRs can be associated with human cancer. Early evidence to suggest that mutated TRs could be involved in carcinogenesis came from the discovery that v-erbA, a highly mutated chicken TRα1 that has lost the ability to activate gene transcription, leads to neoplastic transformation in erythroleukemia and sarcomas (Sap et al., 1989; Thormeyer and Baniahmad, 1999; Wallin et al., 1992). That male transgenic mice overexpressing v-erbA develop hepatocellular carcinomas is evidence that v-erbA oncoprotein can promote neoplasia in mammals through its dominant negative activity (Barlow et al., 1994). Abnormal expression and somatic mutations of TRs have been observed in human cancers. To date, TR alteration has been reported in thyroid cancer (Wallin et al., 1992; Bronnegard et al., 1994; Puzianowska-Kuznicka et al., 2002), liver cancer (Lin et al., 1999), kidney cancer (Kamiya et al., 2002; Puzianowska-Kuznicka et al., 2000), pituitary tumor (Safer et al., 2001; Ando et al., 2001a; Ando et al., 2001b), and breast cancer (Li et al., 2002; Silva et al., 2002). Knock-in mice harboring a germ-line mutation in TRβ (TRβPV), leading to the abolition of T3 binding and dominant negative activity, develop thyroid cancer and pituitary tumor (Furumoto et al., 2005; Suzuki et al., 2002). These studies suggest that partial loss of normal TR function due to reduced expression, or complete loss or alteration of TR activity, provides an opportunity for cells to proliferate, invade, and metastasize. In this context, TR could act as a tumor suppressor.
Taken together, these studies raise two important questions. First, what genes or signaling pathways critical for carcinogenesis are affected by loss of function of TR? Second, by what mechanisms do the mutated TRs alter the activity of the affected genes or signaling pathways to mediate carcinogenesis?
The availability of a mouse model of thyroid cancer harboring the TRβPV mutation provides us with the possibility to explore the role of TRβ mutants in tumor progression and metastasis of thyroid cancer. Our earlier studies sought to understand how TRβPV alters signaling pathways by affecting gene expression profiles in the thyroid of the TRβPV/PV mice (Ying et al., 2003). However, our most recent studies of the TRβPV oncogenic actions in TRβPV/PV mice showed that this TR mutant not only exerts dominant activity on gene transcription, but also acts via non-genomic action to mediate thyroid carcinogenesis (Furuya et al., 2006; Ying et al., 2006; Furuya et al., 2007a; Guigon et al., 2008). The aim of this review is to highlight recent advances in the understanding of the critical role of the novel non-genomic actions of mutations of TR in thyroid carcinogenesis.
2. Novel modes of non-genomic actions of a TRβ mutant
2.1. The TRβPV/PV mouse as a model of thyroid cancer
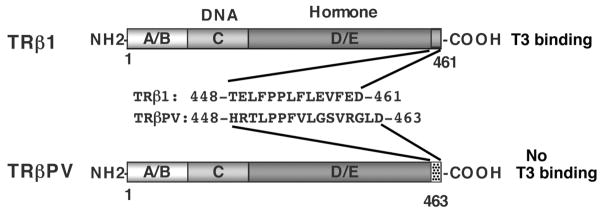
The TRβ mutant proteins identified in RTH have either low or no T3-binding affinity and transcriptional activity (Yen, 2003). The creation of a mouse model harboring a C-terminal 14 amino acid frameshift mutation (Kaneshige et al., 2000) led to a better understanding of the molecular mechanisms underlying RTH. This mutation, which was called PV after an RTH patient, leads to a complete loss of T3 binding (TRβ mutant) (Meier et al., 1992; Parrilla et al., 1991) and exhibits potent dominant negative activity (Meier et al., 1992) (Figure 1). The TRβPV mouse model faithfully reproduces RTH in humans with the loss of the feedback regulation in the pituitary-thyroid axis (Kaneshige et al., 2000). Consistent with the disorders associated with RTH in humans, TRβPV mice also display growth retardation, thyrotoxic skeletal phenotype, and neurological dysfunction (Kaneshige et al., 2000; O’Shea et al., 2003; Siesser et al., 2005).
Figure 1. Schematic comparison of TRβ1 and TRβPV structure.
The TRβPV mutation was identified in a patient with resistance to thyroid hormone. The TRβPV mutation is a frameshift mutation due to a C-insertion at codon 448 of TRβ1. The carboxyl-terminal sequences of the wild-type TRβ and the TRβPV mutant are indicated.
Notably, as homozygous TRβPV (TRβPV/PV), but not heterozygous TRβPV (TRβPV/+) mice age, they spontaneously develop thyroid cancer (Suzuki et al., 2002). TRβPV/PV mice display thyroid hyperplasia as early as 2 months of age, which is followed by follicular thyroid carcinoma (FTC) with progression from capsular and vascular invasion to anaplasia and distant metastases (Suzuki et al., 2002). The similarities in FTC progression between this mouse model and humans provide us with a unique opportunity to identify and characterize the molecular pathways underlying thyroid carcinogenesis and metastasis.
2.2. Overactivation of phosphatidylinositol 3-kinase (PI3K) signaling by TRβPV
Dysregulation of PI3K signaling contributes to abnormal cell growth, cellular transformation in a variety of neoplasms, including thyroid cancer. PI3Ks consist of a catalytic subunit of about 110 kD (p110) and a tightly associated regulatory subunit (p85α, p85β or p55γ). The subunit regulates the association of PI3K with membrane-associated signaling complexes. Upon activation by membrane receptors, PI3K phosphorylates phosphatidylinositol-4,5 biphosphate [PIP2] to form phosphatidylinositol-3,4,5- triphosphate [PIP3]. Through phosphatidylinositol-dependent kinases, the downstream effectors of PI3K, the serine/threonine kinase AKT is phosphorylated and activated to further phosphorylate downstream protein substrates (Neri et al., 2002; Shepherd et al., 1997; Wymann and Marone, 2005). The activity of PI3K is negatively regulated by PTEN (phosphatase and tensin homolog deleted on chromosome 10), a protein phosphatase that dephosphorylates PIP3 to form PIP2 (Eng, 2002).
Recent studies have shown aberrant PI3K/AKT signaling in thyroid carcinogenesis. Inactivating mutations of the tumor suppressor gene PTEN were observed in patients with Cowden syndrome, a genetically inherited disorder associated with higher risk to develop several types of tumors, including follicular thyroid carcinoma (Nelen et al., 1997; Steck et al., 1997). Studies of human thyroid cancer by several groups have shown AKT overactivation and overexpression in primary thyroid cancers (Ringel et al., 2001; Miyakawa et al., 2003). Similar to findings in human thyroid cancer, we determined that the PI3K/AKT signaling pathway was overactivated in the thyroid tumors of TRβPV/PV mice (Furuya et al., 2006). Consistent with a major role for PI3K signaling in thyroid cancer, the treatment of TRβPV/PV mice with the potent PI3K inhibitor LY294002 significantly delayed thyroid tumor progression and blocked metastatic spread (Furuya et al., 2007b).
Most studies aimed at understanding the molecular actions of TRβ mutants, including TRβPV, have focused on their interference with the genomic actions of wild-type TRβ. By contrast, our study of the molecular mechanisms involved in the overactivation of AKT in thyroid cancer of TRβPV/PV mice led to the identification of a novel non-genomic mode of action of a mutant thyroid hormone receptor that drives thyroid carcinogenesis (Furuya et al., 2006). Indeed, we found that the p85α regulatory subunit of PI3K physically interacts with TRβ1 or the TRβPV mutant. Notably, p85α interacted two to three times more strongly with TRβPV than with TRβ1, resulting in a great increase of PI3K activity associated with the overactivation of AKT and phosphorylation of its downstream effectors.
Recent studies have indicated the presence of PI3Ks, their lipid substrates and products, and AKT in the nuclear compartment (Neri et al., 2002; Irvine, 2003). Consistent with these observations, we found that sequestration of TRβPV by p85α occurred in both the nuclear and the cytoplasmic compartments, suggesting that the regulation of PI3K signaling by TRβPV could occur in both. Indeed, activation of AKT and downstream signaling pathways occurred in the nuclear and cytosolic fraction of thyroid extracts, but with a higher activity in the nuclear fraction (Furuya et al., 2006). Given the critical role of PI3K in regulating multiple signaling pathways, it is likely that its activation by TRβPV in both compartments affects diverse cellular signaling pathways. This hypothesis would be consistent with our earlier studies using cDNA microarrays that showed complex alteration of multiple signaling pathways to be associated with thyroid carcinogenesis in TRβPV/PV mice (Ying et al., 2003).
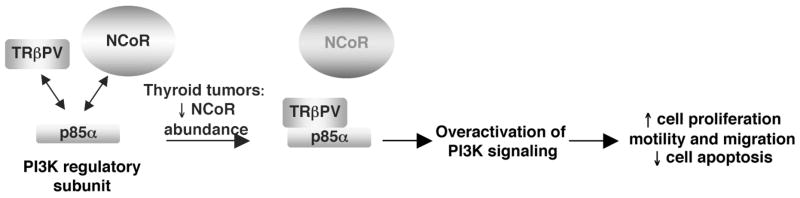
Additionally, we found that the nuclear receptor corepressor (NCoR) was involved in the modulation of TRβPV-induced PI3K activation (Furuya et al., 2007a). NCoR is well known to regulate the genomic actions of nuclear receptors, as it was reported for TRβ and TRβ mutants in vitro and in vivo; however, recent studies have also shown that NCoR can be involved in transcription-independent mechanisms. NCoR is found not only in the nucleus, but also in the cytoplasm (Hermanson et al., 2002; Sardi et al., 2006). The redistribution of NCoR to the cytoplasm provides a mechanism for controlling differentiation of neural stem cells into astrocytes (Hermanson et al., 2002). Moreover, cytoplasmic NCoR included in protein complexes can be translocated to the nucleus to regulate astrogenesis (Sardi et al., 2006). These studies led us to evaluate the potential involvement of NCoR in regulating PI3K signaling. Notably, we found that NCoR interacts with p85α in the nucleus and the cytoplasm, and thereby competes with TRβPV (Figure 2). Experimental alteration of cellular NCoR protein levels by overexpression led to reduced PI3K signaling. Conversely, knocking down cellular NCoR by small interfering RNA (siRNA) approaches increased PI3K activity. In thyroid tumors, cellular NCoR protein abundance was decreased as compared with wild-type thyroids, thereby favoring the interaction between p85α and TRβPV to activate PI3K signaling (Furuya et al., 2007a). Therefore, NCoR, via protein-protein interaction, is a novel regulator of PI3K signaling that could modulate thyroid tumor progression.
Figure 2. Proposed model for the overactivation of PI3K signaling by TRβPV in the thyroid cancer of TRβPV/PV mice.
TRβPV physically interacts with the regulatory subunit of PI3K, p85α, to activate PI3K signaling, thereby affecting cell proliferation, motility, migration, and apoptosis (Furuya et al., 2006). TRβPV competes with NCoR to interact with p85α, but in thyroid cancer of TRβPV/PV mice, NCoR protein abundance is lower than in normal thyroid, thus favoring the physical interaction of TRβPV and p85α and the overactivation of PI3K signaling (Furuya et al., 2007b).
2.3. Stabilization of the pituitary tumor-transforming gene (PTTG) by the TRβPV mutant
PTTG is a mammalian securin that is a critical mitotic checkpoint protein involved in maintaining sister chromatids cohesiveness before entering anaphase (Yu and Melmed, 2001). Its association with separin (a protease) prevents the proteolytic activity of separin from acting on the proper separation of sister chromatids. At each cell cycle, degradation of PTTG by proteasome pathways frees separin, thus leading to chromatid separation at the anaphase. Alteration of normal levels of PTTG may disrupt this orderly process of chromatid segregation with subsequent aneuploidy in the daughter cells (Zou et al., 1999). Indeed, increased expression of PTTG has been demonstrated in human thyroid carcinoma (Heaney et al., 2001; Kim et al., 2005), colon carcinoma (Heaney et al., 2000), and pituitary adenoma (Zhang et al., 1999; McCabe et al., 2003). Aneuploidy is a common feature of thyroid follicular adenoma and carcinoma (Joensuu et al., 1986; Joensuu and Klemi, 1988), and chromosomal instability may underlie the progression to anaplastic thyroid cancer, the more aggressive stage of thyroid cancer.
We discovered chromosomal alterations in seven cell lines derived from thyroid tumors of TRβPV/PV mice (Zimonjic et al., 2005). These cell lines exhibit abnormal karyotypes and a variety of structural chromosomal aberrations, including translocations and deletions, raising the possibility that induction of chromosomal aberrations could be one of the key genetic events involved in thyroid carcinogenesis in TRβPV/PV mice (Zimonjic et al., 2005). The search for genes underlying the chromosomal aberrations in TRβPV/PV mice by cDNA microarray led to the discovery that Pttg mRNA levels were significantly increased in thyroid cancer (Ying et al., 2003). In addition, cellular PTTG protein levels were markedly increased in the primary lesions of thyroid as well as lung metastasis of TRβPV/PV mice (Ying et al., 2006). Consistently, our cell-based studies further showed that aberrant accumulation of PTTG induced by TRβPV inhibits mitotic progression (Ying et al., 2006). In TRβPV/PVPttg−/− as compared with TRβPV/PV mice, there was a consistent decrease in thyroid cell proliferation. The thyroids of TRβPV/PVPttg−/− mice were significantly smaller, and the occurrence of metastasis spread to the lung was less frequent (Kim et al., 2007).
Altogether, these data indicate that abnormal accumulation of PTTG in the thyroids of TRβPV/PV mice contributes to thyroid carcinogenesis by affecting cell cycle progression and inducing genetic instability. Still, the molecular mechanisms underlying elevated abundance of PTTG proteins have not been fully elucidated. It is likely that the increase in Pttg mRNA levels in TRβPV/PV mice (Ying et al., 2003) leads, at least partially, to the aberrant accumulation of PTTG proteins. Because TRs and PTTG are both involved in proteasome-mediated degradation pathways (Dace et al., 2000; Yu et al., 2000), we investigated whether TRβ and TRβPV could regulate PTTG protein levels through such mechanisms (Ying et al., 2006). A series of cell-based and molecular studies showed that the DNA binding domain of TRβ1 or TRβPV interacts with the amino-terminal region of PTTG (Ying et al., 2006) and that T3 induces the degradation of TRβ1 concomitantly with that of PTTG via the proteasomal machinery (Ying et al., 2006). In contrast, T3 does not induce TRβPV degradation, consistent with the fact that TRβPV loses T3 binding capacity. Along with the stability of TRβPV, PTTG protein levels are not altered by T3 treatment and remain high (Ying et al., 2006). Therefore, the liganded TRβ1 regulates PTTG stability; this regulatory function is lost in TRβPV that fails to bind T3.
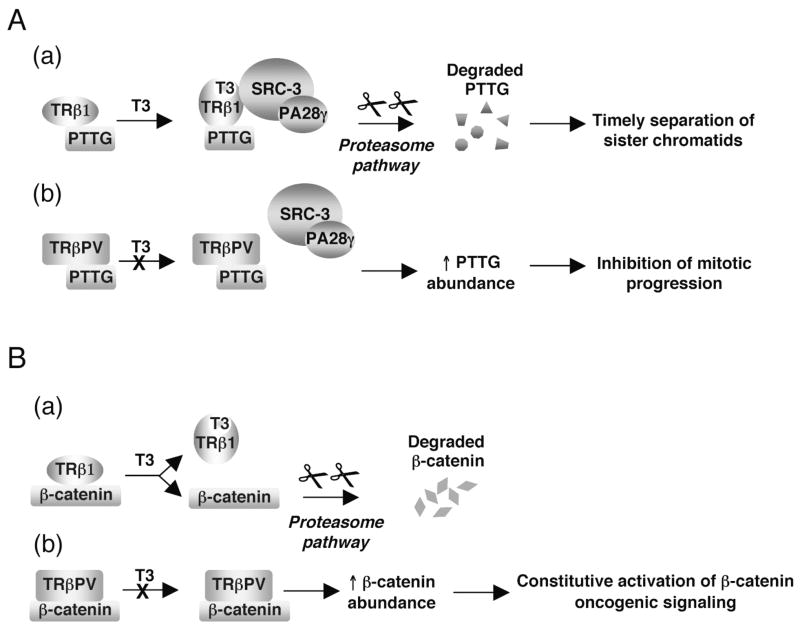
To understand how TRβPV fails to regulate the stability of PTTG as the liganded TRβ1 does, we considered the possibility that the protein complexes TRβ1/PTTG and TRβPV/PTTG recruit proteasome activators differently. It was reported that steroid receptor co-activator-3 (SRC-3) is degraded via 19S proteasome through its physical interaction with proteasome activator 28γ (PA28γ), an activator of the trypsin-like activity of the proteasome (Li et al., 2006). Similar to other steroid receptors (Lonard and O’Malley B, 2007), the liganded TRβ1 recruits SRC-3, but the unliganded TRβ1 does not (Ying et al., 2006). TRβPV does not bind SRC-3, whether T3 is present or not (Ying et al., 2006). We therefore tested the possibility of the existence of a differential recruitment of TRβ/PTTG and TRβPV/PTTG complexes by SRC-3/PA28γ. Indeed, we made the remarkable finding that the liganded TRβ1/PTTG recruits SRC-3/PA28γ through the direct interaction of TRβ1 with SRC-3 (Figure 3A-a), whereas the unliganded TRβ1 and TRβPV fail to recruit SRC-3/PA28γ (Figure 3A-b) (Ying et al., 2006). Altogether, this study indicates that TRβPV, via protein-protein interaction, affects PTTG degradation by the proteasome pathway and thereby results in mitotic abnormalities, contributing to thyroid carcinogenesis.
Figure 3. TRβPV, via protein-protein interaction, interferes with proteasomal pathway regulatory function.
A) A proposed molecular model for the accumulation of PTTG protein in the thyroid cancer of TRβPV/PV mice(a) The direct interaction of TRβ with SRC-3 induced by the binding of T3 induces the formation of TRβ/PTTG/SRC3/PA28γ complexes that activate the degradation of PTTG and lead to timely separation of sister chromatids at anaphase. (b) TRβPV physically interacts with PTTG but as it does not bind T3, it does not interact with SRC-3/PA28γ to activate PTTG degradation. The absence of degradation leads to PTTG accumulation and inhibition of mitotic progression (Ying et al., 2006).
B) A proposed molecular model for the accumulation of β-catenin in the thyroid cancer of TRβPV/PV mice. (a) The physical interaction of TRβ with β-catenin is weakened by T3 binding and favors the degradation of β-catenin by proteasomal pathways. (b) TRβPV strongly interacts with β-catenin and the loss of its T3 binding activity inhibits the release of β-catenin from TRβPV/β-catenin complexes in the presence of T3, thereby preventing β-catenin degradation. The absence of β-catenin degradation leads to β-catenin accumulation and constitutive activation of its oncogenic signaling (Guigon et al., 2008).
2.4. Stabilization and constitutive activation of β-catenin by the TRβPV mutant
β-catenin is a central mediator of the Wnt signaling pathway, which is critical for a variety of cellular processes including cell proliferation, cell apoptosis, and cell migration (Polakis, 2007). Upon increased β-catenin cellular levels and nuclear accumulation, β-catenin together with the T-cell factor/lymphoid enhancer factor (TCF/LEF) binds to the promoters of downstream target genes (Moon et al., 2002). Induction of these genes has significant effects on tissue development and oncogenesis; abnormal subcellular localization and aberrant accumulation of β-catenin have been reported in a number of human cancers, including thyroid cancers (Garcia-Rostan et al., 1999).
The cellular levels of β-catenin are tightly regulated by two distinct adenomatous polyposis coli (APC)-dependent proteasomal pathways. One includes the glycogen synthase kinase 3β (GSK3β)-regulated pathway involving the APC/axin complex (Polakis, 2002) and the other a p53-inducible pathway involving APC/Siah-1 (Liu et al., 2001). An additional mode of β-catenin cellular level regulation is mediated by nuclear receptors, namely retinoid X receptor α (RXRα) and peroxisome proliferator-activated receptor γ (PPARγ). These nuclear receptors regulate β-catenin protein abundance via APC/GSK3β/p53-independent mechanisms (Sharma et al., 2004; Xiao et al., 2003).
The finding that the cellular abundance of β-catenin was aberrantly elevated in thyroid tumors of TRβPV/PV mice gave us the opportunity to understand how TRβ and its TRβPV mutant regulate the cellular levels of β-catenin and, furthermore, what effect β-catenin accumulation has on the activation of β-catenin oncogenic signaling in the thyroids of TRβPV/PV mice.
We found that, similar to RXRα and PPARγ2, TRβ and its mutant TRβPV physically interact with β-catenin in vitro and in cells (Sharma et al., 2004; Guigon et al., 2008; Xiao et al., 2003). As reported for RXRα and PPARγ, β-catenin protein level regulation by TRβ and TRβPV occurs via APC/GSK3β/p53-independent mechanisms. However, in contrast to RXRα and PPARγ2, binding of T3 to TRβ weakens the interaction of the receptor with β-catenin, thereby leading more uncomplexed β-catenin to be degraded by proteasomal pathways (Guigon et al., 2008) (Figure 3B-a). Importantly, the interaction of β-catenin with TRβPV is not affected by T3, because TRβPV does not bind T3. Therefore, the constitutive association of TRβPV with β-catenin leads to the stabilization of β-catenin (Figure 3B-b).
Although there is a commonality in the early steps of the APC-independent regulation of β-catenin mediated by TRβ and by PPARγ2 and RXRα in that they all physically interact with β-catenin, ligand binding differently affects the subsequent mode of actions between TR β and PPARγ2 or RXRα. The reason for these different modes of nuclear receptor actions in β-catenin cellular level regulation is currently unknown. The A/B domain of RXR (Xiao et al., 2003) and the ligand binding domain of PPARγ2 (Liu et al., 2006) were reported to be necessary for their interaction with β-catenin. In contrast, the DNA binding domain of TRβ is important in its interaction with β-catenin3 (Guigon et al., 2008). It is thus tempting to speculate that these different interacting regions in TRβ, PPARγ2, and RXRα may play a role in the different modes of β-catenin regulation.
Our mouse model also gave us the opportunity to study in vivo the effect of β-catenin aberrant accumulation on the activity of the β-catenin signaling pathway in the thyroid cancer of TRβPV/PV mice. We found that the increased β-catenin cellular levels in the thyroids of TRβPV/PV mice was associated with a significant increase in its nuclear translocation and transcriptional activity (Guigon et al., 2008), as shown by the increased cellular abundance of β-catenin phosphorylated on serine 552 (P552-β-catenin) as well as of β-catenin transcriptional downstream targets such as c-myc and cyclin D1, both involved in cell cycle progression, and MT1-MMP (matrix metalloprotease-1) that is involved in cell migration. Given the critical role of β-catenin in mediating epithelial-mesenchymal transition (EMT) (Lee et al, 2006;Hubert et al, 2005), it is very likely that β-catenin constitutive activation in TRβPV/PV thyroids plays a role in the tumorigenic progression to metastasis. It would be of interest to determine whether defining features of EMT that were reported in human invasive thyroid carcinomas such as loss of E-cadherin and gain of vimentin and fibronectin expression (Vasko et al, 2007), are also found in TRβPV/PV thyroid cancers.
3. Conclusions and future challenges
Increasing evidence supports the notion that TRs could play an important role in carcinogenesis (Cheng, 2003). The availability of the TRβPV/PV mouse as a model of thyroid carcinogenesis provides a valuable tool to understand the role of TRβ mutants in tumor progression and metastasis of thyroid cancer. Studies so far have shown that the deleterious effects of TRβ mutants in causing RTH are mediated via dominant negative actions at the transcriptional level (Yen, 2003; Cheng, 2005). However, the striking phenotype of thyroid cancer displayed by TRβPV/PV mice led to the identification of novel modes of TRβ mutant actions that are beyond nucleus-initiated transcription. The direct protein-protein interaction of TRβPV with the PI3K regulatory subunit p85α increases the activity of PI3K, leading to an activated cascade of downstream signaling to affect cell proliferation, apoptosis, migration, and metastasis. The direct protein-protein interaction of TRβPV with PTTG or β-catenin affects their degradation by the proteasomal pathways, thus leading to PTTG and β-catenin aberrant accumulation. That contributes to genetic instability and constitutively active β-catenin oncogenic signaling to affect cell proliferation and migration. Therefore, the development and progression of thyroid tumors in TRβPV/PV mice involve not only impaired transcriptional activity but also derailed post-transcriptional mechanisms. Altogether, our studies uncovered novel molecular mechanisms by which a TRβ mutant affects critical signaling pathways for cell proliferation, cell apoptosis, EMT transition and cell migration, to drive thyroid carcinogenesis. It would be of interest to determine whether additional non-genomic mechanisms that are reported to mediate T3/TR actions in other cellular models (Lei et al, 2004; Lei et al, 2008) could also contribute to thyroid tumors in TRβPV/PV mice.
A future challenge will be to elucidate whether, in addition to thyroid cancer, these novel modes of action of TR mutants could underlie the development and progression of tumors in other tissues where TR mutations have been reported, such as the pituitary gland and the mammary gland. Of interest, other studies have reported the interaction of wild-type TRβ1 or TRα with p85α to activate PI3K signaling pathway in pancreatic beta cells, human fibroblasts, vascular endothelial cells, indicating that this mode of PI3K regulation by TRs may well occur in a number of T3-target tissues to regulate key cellular events (Cao et al., 2005; Hiroi et al., 2006; Verga Falzacappa et al., 2007). Given that context, it may be hypothesized that TR mutations could significantly affect PI3K activity and contribute to the development of pathologies. Within that framework, the TRβPV/PV mouse model should help advance understanding of the possible effect of TR mutation on the development of human cancer.
Acknowledgments
We regret any reference omissions due to length limitation. We wish to thank all colleagues and collaborators who have contributed to the work described in this review. The present research was supported by the Intramural Research Program of Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Abbreviations
- NCoR
nuclear receptor co-repressor
- PI3K
phosphatidylinositol 3 kinase
- PPARγ
peroxisome proliferator-activatedγ
- PTEN
phosphatase and tensin homolog deleted from chromosome 10
- PTTG
pituitary tumor transforming gene
- RTH
resistance to thyroid hormone
- RXRα
retinoid X receptorα
- SRC-3
steroid receptor co-activator-3
- T3
thyroid hormone
- TR
thyroid hormone receptor
- TSH
thyroid stimulating hormone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams M, Matthews C, Collingwood TN, Tone Y, Beck-Peccoz P, Chatterjee KK. Genetic analysis of 29 kindreds with generalized and pituitary resistance to thyroid hormone. Identification of thirteen novel mutations in the thyroid hormone receptor beta gene. J Clin Invest. 1994;94:506–15. doi: 10.1172/JCI117362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando S, Sarlis NJ, Krishnan J, Feng X, Refetoff S, Zhang MQ, Oldfield EH, Yen PM. Aberrant alternative splicing of thyroid hormone receptor in a TSH-secreting pituitary tumor is a mechanism for hormone resistance. Mol Endocrinol. 2001a;15:1529–38. doi: 10.1210/mend.15.9.0687. [DOI] [PubMed] [Google Scholar]
- Ando S, Sarlis NJ, Oldfield EH, Yen PM. Somatic mutation of TRbeta can cause a defect in negative regulation of TSH in a TSH-secreting pituitary tumor. J Clin Endocrinol Metab. 2001b;86:5572–6. doi: 10.1210/jcem.86.11.7984. [DOI] [PubMed] [Google Scholar]
- Bronnegard M, Torring O, Boos J, Sylven C, Marcus C, Wallin G. Expression of thyrotropin receptor and thyroid hormone receptor messenger ribonucleic acid in normal, hyperplastic, and neoplastic human thyroid tissue. J Clin Endocrinol Metab. 1994;79:384–9. doi: 10.1210/jcem.79.2.8045952. [DOI] [PubMed] [Google Scholar]
- Cao X, Kambe F, Moeller LC, Refetoff S, Seo H. Thyroid hormone induces rapid activation of Akt/protein kinase B-mammalian target of rapamycin-p70S6K cascade through phosphatidylinositol 3-kinase in human fibroblasts. Mol Endocrinol. 2005;19:102–12. doi: 10.1210/me.2004-0093. [DOI] [PubMed] [Google Scholar]
- Cheng S-y. Thyroid hormone receptor mutations in cancer. Mol Cell Endocrinol. 2003;213:23–30. doi: 10.1016/j.mce.2003.10.051. [DOI] [PubMed] [Google Scholar]
- Cheng S-y. Thyroid hormone receptor mutations and disease: beyond thyroid hormone resistance. Trends Endocrinol Metab. 2005;16:176–82. doi: 10.1016/j.tem.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Dace A, Zhao L, Park KS, Furuno T, Takamura N, Nakanishi M, West BL, Hanover JA, Cheng S. Hormone binding induces rapid proteasome-mediated degradation of thyroid hormone receptors. Proc Natl Acad Sci U S A. 2000;97:8985–90. doi: 10.1073/pnas.160257997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng C. Role of PTEN, a lipid phosphatase upstream effector of protein kinase B, in epithelial thyroid carcinogenesis. Ann NY Acad Sci. 2002;968:213–21. doi: 10.1111/j.1749-6632.2002.tb04337.x. [DOI] [PubMed] [Google Scholar]
- Furumoto H, Ying H, Chandramouli GV, Zhao L, Walker RL, Meltzer PS, Willingham MC, Cheng S-y. An unliganded thyroid hormone beta receptor activates the cyclin D1/cyclin-dependent kinase/retinoblastoma/E2F pathway and induces pituitary tumorigenesis. Mol Cell Biol. 2005;25:124–35. doi: 10.1128/MCB.25.1.124-135.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya F, Guigon CJ, Zhao L, Lu C, Hanover JA, Cheng S-y. Nuclear receptor corepressor is a novel regulator of phosphatidylinositol 3-kinase signaling. Mol Cell Biol. 2007a;27:6116–26. doi: 10.1128/MCB.00900-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya F, Hanover JA, Cheng S-y. Activation of phosphatidylinositol 3-kinase signaling by a mutant thyroid hormone beta receptor. Proc Natl Acad Sci U S A. 2006;103:1780–5. doi: 10.1073/pnas.0510849103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya F, Lu C, Willingham MC, Cheng S-y. Inhibition of phosphatidylinositol 3-kinase delays tumor progression and blocks metastatic spread in a mouse model of thyroid cancer. Carcinogenesis. 2007b;28:2451–8. doi: 10.1093/carcin/bgm174. [DOI] [PubMed] [Google Scholar]
- Garcia-Rostan G, Tallini G, Herrero A, D’Aquila TG, Carcangiu ML, Rimm DL. Frequent mutation and nuclear localization of beta-catenin in anaplastic thyroid carcinoma. Cancer Res. 1999;59:1811–5. [PubMed] [Google Scholar]
- Guigon CJ, Zhao L, Lu C, Willingham MC, Cheng S-y. Regulation of beta-catenin by a novel nongenomic action of thyroid hormone beta receptor. Mol Cell Biol. 2008;28:4598–608. doi: 10.1128/MCB.02192-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney AP, Nelson V, Fernando M, Horwitz G. Transforming events in thyroid tumorigenesis and their association with follicular lesions. J Clin Endocrinol Metab. 2001;86:5025–32. doi: 10.1210/jcem.86.10.7886. [DOI] [PubMed] [Google Scholar]
- Heaney AP, Singson R, McCabe CJ, Nelson V, Nakashima M, Melmed S. Expression of pituitary-tumour transforming gene in colorectal tumours. Lancet. 2000;355:716–9. doi: 10.1016/S0140-6736(99)10238-1. [DOI] [PubMed] [Google Scholar]
- Hermanson O, Glass CK, Rosenfeld MG. Nuclear receptor coregulators: multiple modes of modification. Trends Endocrinol Metab. 2002;13:55–60. doi: 10.1016/s1043-2760(01)00527-6. [DOI] [PubMed] [Google Scholar]
- Hiroi Y, Kim HH, Ying H, Furuya F, Huang Z, Simoncini T, Noma K, Ueki K, Nguyen NH, Scanlan TS, Moskowitz MA, Cheng SY, Liao JK. Rapid nongenomic actions of thyroid hormone. Proc Natl Acad Sci U S A. 2006;103:14104–9. doi: 10.1073/pnas.0601600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–58. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Irvine RF. Nuclear lipid signalling. Nat Rev Mol Cell Biol. 2003;4:349–60. doi: 10.1038/nrm1100. [DOI] [PubMed] [Google Scholar]
- Joensuu H, Klemi P, Eerola E. DNA aneuploidy in follicular adenomas of the thyroid gland. Am J Pathol. 1986;124:373–6. [PMC free article] [PubMed] [Google Scholar]
- Joensuu H, Klemi PJ. DNA aneuploidy in adenomas of endocrine organs. Am J Pathol. 1988;132:145–51. [PMC free article] [PubMed] [Google Scholar]
- Kamiya Y, Puzianowska-Kuznicka M, McPhie P, Nauman J, Cheng S-y, Nauman A. Expression of mutant thyroid hormone nuclear receptors is associated with human renal clear cell carcinoma. Carcinogenesis. 2002;23:25–33. doi: 10.1093/carcin/23.1.25. [DOI] [PubMed] [Google Scholar]
- Kaneshige M, Kaneshige K, Zhu X, Dace A, Garrett L, Carter TA, Kazlauskaite R, Pankratz DG, Wynshaw-Boris A, Refetoff S, Weintraub B, Willingham MC, Barlow C, Cheng S. Mice with a targeted mutation in the thyroid hormone beta receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc Natl Acad Sci U S A. 2000;97:13209–14. doi: 10.1073/pnas.230285997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Pemberton H, Stratford AL, Buelaert K, Watkinson JC, Lopes V, Franklyn JA, McCabe CJ. Pituitary tumour transforming gene (PTTG) induces genetic instability in thyroid cells. Oncogene. 2005;24:4861–6. doi: 10.1038/sj.onc.1208659. [DOI] [PubMed] [Google Scholar]
- Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–81. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei J, Mariash CN, Ingbar DH. 3,3′,5-Triiodo-L-thyronine up-regulation of Na, K-ATPase activity and cell surface expression in alveolar epithelial cells is Src kinase- and phosphoinositide 3-kinase-dependent. J Biol Chem. 2004;279:47589–600. doi: 10.1074/jbc.M405497200. [DOI] [PubMed] [Google Scholar]
- Lei J, Mariash CN, Bhargava M, Wattenberg EV, Ingbar DH. T3 increases Na-K-ATPase activity via a MAPK/ERK1/2-dependent pathway in rat adult alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L749–54. doi: 10.1152/ajplung.00335.2007. [DOI] [PubMed] [Google Scholar]
- Li X, Lonard DM, Jung SY, Malovannaya A, Feng Q, Qin J, Tsai SY, Tsai MJ, O’Malley BW. The SRC-3/AIB1 coactivator is degraded in a ubiquitin- and ATP-independent manner by the REGgamma proteasome. Cell. 2006;124:381–92. doi: 10.1016/j.cell.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Li Z, Meng ZH, Chandrasekaran R, Kuo WL, Collins CC, Gray JW, Dairkee SH. Biallelic inactivation of the thyroid hormone receptor beta1 gene in early stage breast cancer. Cancer Res. 2002;62:1939–43. [PubMed] [Google Scholar]
- Lin KH, Shieh HY, Chen SL, Hsu HC. Expression of mutant thyroid hormone nuclear receptors in human hepatocellular carcinoma cells. Mol Carcinog. 1999;26:53–61. doi: 10.1002/(sici)1098-2744(199909)26:1<53::aid-mc7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Liu J, Stevens J, Rote CA, Yost HJ, Hu Y, Neufeld KL, White RL, Matsunami N. Siah-1 mediates a novel beta-catenin degradation pathway linking p53 to the adenomatous polyposis coli protein. Mol Cell. 2001;7:927–36. doi: 10.1016/s1097-2765(01)00241-6. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang H, Zuo Y, Farmer SR. Functional interaction between peroxisome proliferator-activated receptor gamma and beta-catenin. Mol Cell Biol. 2006;26:5827–37. doi: 10.1128/MCB.00441-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonard DM, O’Malley BW. Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell. 2007;27:691–700. doi: 10.1016/j.molcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- McCabe CJ, Khaira JS, Boelaert K, Heaney AP, Tannahill LA, Hussain S, Mitchell R, Olliff J, Sheppard MC, Franklyn JA, Gittoes NJ. Expression of pituitary tumour transforming gene (PTTG) and fibroblast growth factor-2 (FGF-2) in human pituitary adenomas: relationships to clinical tumour behaviour. Clin Endocrinol (Oxf) 2003;58:141–50. doi: 10.1046/j.1365-2265.2003.01598.x. [DOI] [PubMed] [Google Scholar]
- Meier CA, Dickstein BM, Ashizawa K, McClaskey JH, Muchmore P, Ransom SC, Menke JB, Hao EH, Usala SJ, Bercu BB, Cheng S-yWeintraub BD. Variable transcriptional activity and ligand binding of mutant beta 1 3,5,3′-triiodothyronine receptors from four families with generalized resistance to thyroid hormone. Mol Endocrinol. 1992;6:248–58. doi: 10.1210/mend.6.2.1569968. [DOI] [PubMed] [Google Scholar]
- Miyakawa M, Tsushima T, Murakami H, Wakai K, Isozaki O, Takano K. Increased expression of phosphorylated p70S6 kinase and Akt in papillary thyroid cancer tissues. Endocr J. 2003;50:77–83. doi: 10.1507/endocrj.50.77. [DOI] [PubMed] [Google Scholar]
- Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–6. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- Nelen MR, van Staveren WC, Peeters EA, Hassel MB, Gorlin RJ, Hamm H, Lindboe CF, Fryns JP, Sijmons RH, Woods DG, Mariman EC, Padberg GW, Kremer H. Germline mutations in the PTEN/MMAC1 gene in patients with Cowden disease. Hum Mol Genet. 1997;6:1383–7. doi: 10.1093/hmg/6.8.1383. [DOI] [PubMed] [Google Scholar]
- Neri LM, Borgatti P, Capitani S, Martelli AM. The nuclear phosphoinositide 3-kinase/AKT pathway: a new second messenger system. Biochim Biophys Acta. 2002;1584:73–80. doi: 10.1016/s1388-1981(02)00300-1. [DOI] [PubMed] [Google Scholar]
- O’Shea PJ, Harvey CB, Suzuki H, Kaneshige M, Kaneshige K, Cheng S-y Williams GR. A thyrotoxic skeletal phenotype of advanced bone formation in mice with resistance to thyroid hormone. Mol Endocrinol. 2003;17:1410–24. doi: 10.1210/me.2002-0296. [DOI] [PubMed] [Google Scholar]
- Ono S, Schwartz ID, Mueller OT, Root AW, Usala SJ, Bercu BB. Homozygosity for a dominant negative thyroid hormone receptor gene responsible for generalized resistance to thyroid hormone. J Clin Endocrinol Metab. 1991;73:990–4. doi: 10.1210/jcem-73-5-990. [DOI] [PubMed] [Google Scholar]
- Parrilla R, Mixson AJ, McPherson JA, McClaskey JH, Weintraub BD. Characterization of seven novel mutations of the c-erbA beta gene in unrelated kindreds with generalized thyroid hormone resistance. Evidence for two “hot spot” regions of the ligand binding domain. J Clin Invest. 1991;88:2123–30. doi: 10.1172/JCI115542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P. Casein kinase 1: a Wnt’er of disconnect. Curr Biol. 2002;12:R499–R501. doi: 10.1016/s0960-9822(02)00969-7. [DOI] [PubMed] [Google Scholar]
- Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Puzianowska-Kuznicka M, Krystyniak A, Madej A, Cheng S-y, Nauman J. Functionally impaired TR mutants are present in thyroid papillary cancer. J Clin Endocrinol Metab. 2002;87:1120–8. doi: 10.1210/jcem.87.3.8296. [DOI] [PubMed] [Google Scholar]
- Puzianowska-Kuznicka M, Nauman A, Madej A, Tanski Z, Cheng S, Nauman J. Expression of thyroid hormone receptors is disturbed in human renal clear cell carcinoma. Cancer Lett. 2000;155:145–52. doi: 10.1016/s0304-3835(00)00416-x. [DOI] [PubMed] [Google Scholar]
- Refetoff S, Weiss RE, Usala SJ. The syndromes of resistance to thyroid hormone. Endocr Rev. 1993;14:348–99. doi: 10.1210/edrv-14-3-348. [DOI] [PubMed] [Google Scholar]
- Ringel MD, Hayre N, Saito J, Saunier B, Schuppert F, Burch H, Bernet V, Burman KD, Kohn LD, Saji M. Overexpression and overactivation of Akt in thyroid carcinoma. Cancer Res. 2001;61:6105–11. [PubMed] [Google Scholar]
- Safer JD, Colan SD, Fraser LM, Wondisford FE. A pituitary tumor in a patient with thyroid hormone resistance: a diagnostic dilemma. Thyroid. 2001;11:281–91. doi: 10.1089/105072501750159750. [DOI] [PubMed] [Google Scholar]
- Sap J, Munoz A, Schmitt J, Stunnenberg H, Vennstrom B. Repression of transcription mediated at a thyroid hormone response element by the v-erb-A oncogene product. Nature. 1989;340:242–4. doi: 10.1038/340242a0. [DOI] [PubMed] [Google Scholar]
- Sardi SP, Murtie J, Koirala S, Patten BA, Corfas G. Presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain. Cell. 2006;127:185–97. doi: 10.1016/j.cell.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Sharma C, Pradeep A, Wong L, Rana A, Rana B. Peroxisome proliferator-activated receptor gamma activation can regulate beta-catenin levels via a proteasome-mediated and adenomatous polyposis coli-independent pathway. J Biol Chem. 2004;279:35583–94. doi: 10.1074/jbc.M403143200. [DOI] [PubMed] [Google Scholar]
- Shepherd PR, Nave BT, Rincon J, Haigh RJ, Foulstone E, Proud C, Zierath JR, Siddle K, Wallberg-Henriksson H. Involvement of phosphoinositide 3-kinase in insulin stimulation of MAP-kinase and phosphorylation of protein kinase-B in human skeletal muscle: implications for glucose metabolism. Diabetologia. 1997;40:1172–7. doi: 10.1007/s001250050803. [DOI] [PubMed] [Google Scholar]
- Siesser WB, Cheng S-y, McDonald MP. Hyperactivity, impaired learning on a vigilance task, and a differential response to methylphenidate in the TRbetaPV knock-in mouse. Psychopharmacology (Berl) 2005;181:653–63. doi: 10.1007/s00213-005-0024-5. [DOI] [PubMed] [Google Scholar]
- Silva JM, Dominguez G, Gonzalez-Sancho JM, Garcia JM, Silva J, Garcia-Andrade C, Navarro A, Munoz A, Bonilla F. Expression of thyroid hormone receptor/erbA genes is altered in human breast cancer. Oncogene. 2002;21:4307–16. doi: 10.1038/sj.onc.1205534. [DOI] [PubMed] [Google Scholar]
- Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–62. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Willingham MC, Cheng S-y. Mice with a mutation in the thyroid hormone receptor beta gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid. 2002;12:963–9. doi: 10.1089/105072502320908295. [DOI] [PubMed] [Google Scholar]
- Thormeyer D, Baniahmad A. The v-erbA oncogene. Int J Mol Med. 1999;4:351–8. (review) [PubMed] [Google Scholar]
- Vasko V, Espinosa AV, Scouten W, He H, Auer H, Liyanarachchi S, Larin A, Savchenko V, Francis GL, de la Chapelle A, Saji M, Ringel MD. Gene expression and functional evidence of epithelial-to-mesenchymal transition in papillary thyroid carcinoma invasion. Proc Natl Acad Sci U S A. 2007;104:2803–8. doi: 10.1073/pnas.0610733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verga Falzacappa C, Petrucci E, Patriarca V, Michienzi S, Stigliano A, Brunetti E, Toscano V, Misiti S. Thyroid hormone receptor TRbeta1 mediates Akt activation by T3 in pancreatic beta cells. J Mol Endocrinol. 2007;38:221–33. doi: 10.1677/jme.1.02166. [DOI] [PubMed] [Google Scholar]
- Wallin G, Bronnegard M, Grimelius L, McGuire J, Torring O. Expression of the thyroid hormone receptor, the oncogenes c-myc and H-ras, and the 90 kD heat shock protein in normal, hyperplastic, and neoplastic human thyroid tissue. Thyroid. 1992;2:307–13. doi: 10.1089/thy.1992.2.307. [DOI] [PubMed] [Google Scholar]
- Weiss RE, Refetoff S. Resistance to thyroid hormone. Rev Endocr Metab Disord. 2000;1:97–108. doi: 10.1023/a:1010072605757. [DOI] [PubMed] [Google Scholar]
- Wymann MP, Marone R. Phosphoinositide 3-kinase in disease: timing, location, and scaffolding. Curr Opin Cell Biol. 2005;17:141–9. doi: 10.1016/j.ceb.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Xiao JH, Ghosn C, Hinchman C, Forbes C, Wang J, Snider N, Cordrey A, Zhao Y, Chandraratna RA. Adenomatous polyposis coli (APC)-independent regulation of beta-catenin degradation via a retinoid X receptor-mediated pathway. J Biol Chem. 2003;278:29954–62. doi: 10.1074/jbc.M304761200. [DOI] [PubMed] [Google Scholar]
- Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- Yen PM. Molecular basis of resistance to thyroid hormone. Trends Endocrinol Metab. 2003;14:327–33. doi: 10.1016/s1043-2760(03)00114-0. [DOI] [PubMed] [Google Scholar]
- Ying H, Furuya F, Zhao L, Araki O, West BL, Hanover JA, Willingham MC, Cheng S-y. Aberrant accumulation of PTTG1 induced by a mutated thyroid hormone beta receptor inhibits mitotic progression. J Clin Invest. 2006;116:2972–84. doi: 10.1172/JCI28598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying H, Suzuki H, Furumoto H, Walker R, Meltzer P, Willingham MC, Cheng Sy. Alterations in genomic profiles during tumor progression in a mouse model of follicular thyroid carcinoma. Carcinogenesis. 2003;24:1467–79. doi: 10.1093/carcin/bgg111. [DOI] [PubMed] [Google Scholar]
- Yu R, Heaney AP, Lu W, Chen J, Melmed S. Pituitary tumor transforming gene causes aneuploidy and p53-dependent and p53-independent apoptosis. J Biol Chem. 2000;275:36502–5. doi: 10.1074/jbc.C000546200. [DOI] [PubMed] [Google Scholar]
- Yu R, Melmed S. Oncogene activation in pituitary tumors. Brain Pathol. 2001;11:328–41. doi: 10.1111/j.1750-3639.2001.tb00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Horwitz GA, Heaney AP, Nakashima M, Prezant TR, Bronstein MD, Melmed S. Pituitary tumor transforming gene (PTTG) expression in pituitary adenomas. J Clin Endocrinol Metab. 1999;84:761–7. doi: 10.1210/jcem.84.2.5432. [DOI] [PubMed] [Google Scholar]
- Zimonjic DB, Kato Y, Ying H, Popescu NC, Cheng S-y. Chromosomal aberrations in cell lines derived from thyroid tumors spontaneously developed in TRbetaPV/PV mice. Cancer Genet Cytogenet. 2005;161:104–9. doi: 10.1016/j.cancergencyto.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Zou H, McGarry TJ, Bernal T, Kirschner MW. Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science. 1999;285:418–22. doi: 10.1126/science.285.5426.418. [DOI] [PubMed] [Google Scholar]