Abstract
Corticosteroid receptors are critical for the maintenance of homeostasis after both psychological and physiological stress. To properly understand the different roles and interactions of the glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) during stress, it is necessary to dissect the role of corticosteroid signaling at both the system and sub-system level. A variety of GR transgenic mouse lines have recently been used to characterize the role of GR in the CNS as a whole and particularly in the forebrain. We will describe both the behavioral and cellular/molecular implications of disrupting GR function in these animal models and describe the implications of this data for our understanding of normal endocrine function and stress adaptation.
MRs in tight epithelia have a long established role in sodium homeostasis. Recently however, evidence has suggested that limbic MRs also play an important role in psychological stress. Just as with GR, targeted mutations in MR induce a variety of behavioral changes associated with stress adaptation. In this review, we will discuss the implications of this work on MR.
Finally, we will discuss the possible interaction between MR and GR and how future work using double mutants (through conventional means or virus based gene alteration) will be needed to fully understand how signaling through these two steroid receptors provides the adaptive mechanisms to deal with a variety of stressors.
INTRODUCTION
Any imbalance to an organism’s physical or psychological wellbeing creates a stress response that involves multiple systems and whose activation allows appropriate adaptation to the disturbance. Stress can be beneficial in that it can create a situation of increased arousal and emotional salience enabling the organism to appropriately respond to the stressor and ensure survival. However, under states of dysregulation or after chronic activation, stress can be maladaptive and can place the body in a state of increased susceptibility to illness or disease. For instance, early-life stress can induce changes in the endocrine stress response that lead to increased incidence of depression (Nemeroff, 2004). Furthermore, severe stress in adulthood is associated with precipitation of the onset of psychiatric illness (Corcoran et al., 2003). Of the many systems involved in the mammalian stress response, one of the most important and intensely studied is the endocrine system whose response is governed by the hypothalamic-pituitary-adrenal (HPA) axis.
The HPA axis
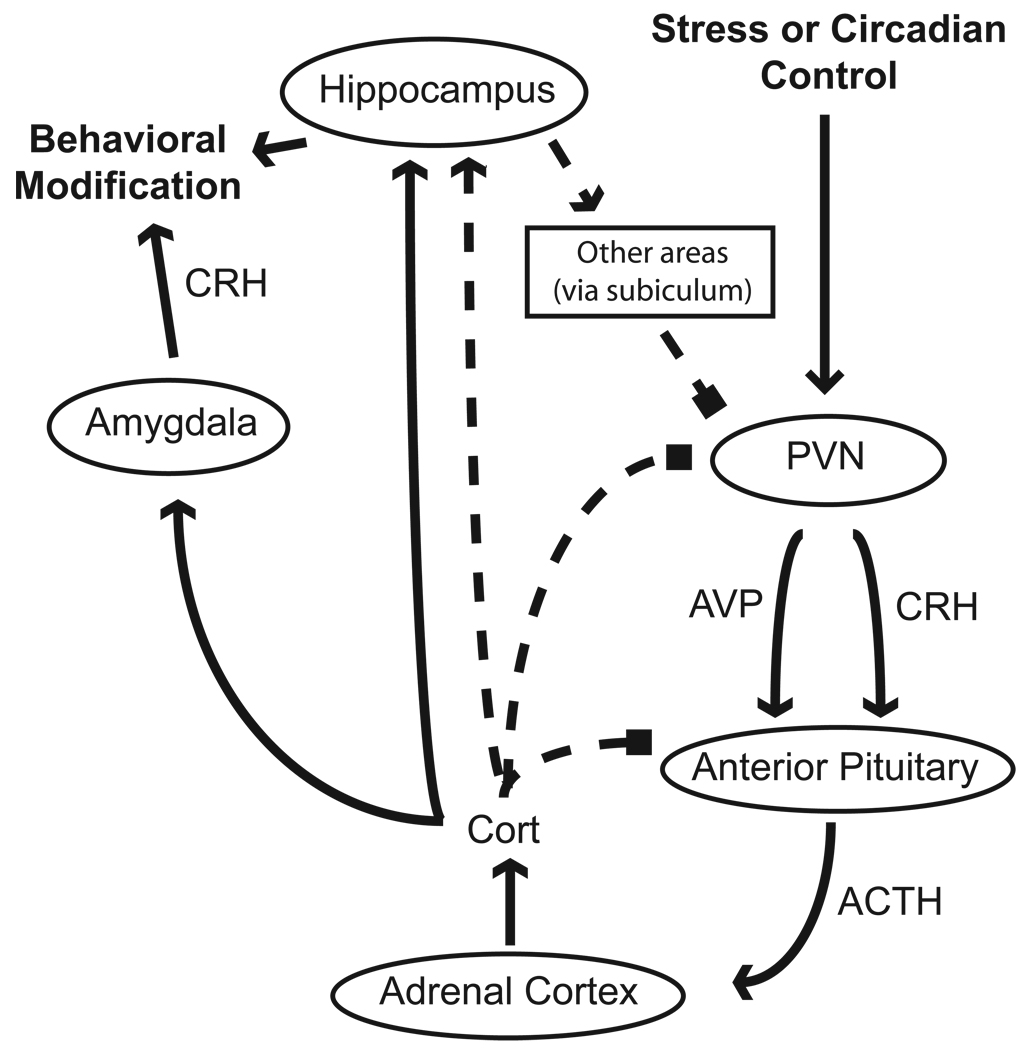
During stress, HPA axis activation through sympathetic, parasympathetic and limbic circuits induces parvocellular neurons in the paraventricular nucleus of the hypothalamus (PVN) to release two neuropeptides, corticotropin-releasing hormone (CRH) and vasopressin (AVP) which enter the hypothalamic-pituitary portal system (Figure 1). Binding of these neuropeptides to corticotrophs in the anterior pituitary gland causes the processing of pro-opiomelanocortin (POMC) into adrenalcorticotrophic hormone (ACTH) followed by the secretion of ACTH into the general circulation. The binding of ACTH in the adrenal cortex causes the systemic release of cortisol/corticosterone (cort). Cort levels are controlled through a negative feedback loop when cort binds to receptors at the level of the PVN, the anterior pituitary gland and the hippocampus causing a downregulation of HPA axis activity.
Figure 1. HPA axis activation and feedback.
Stressful stimuli and circadian gating stimulate neurons in the paraventricular nucleus (PVN) of the hypothalamus to secrete corticotropin-releasing hormone (CRH) and vasopressin (AVP) into the hypothalamic portal system. Binding of these peptides causes the release of adrenalcorticotrophic hormone (ACTH) from the pituitary gland, which then induces the release of corticosterone (cort) from the adrenal cortex. Cort then feeds back on the CNS at the level of the anterior pituitary gland, the PVN and the hippocampus to inhibit HPA axis activation. In addition, cort binds to additional GR populations in the amygdala and hippocampus to modulate a variety of behaviors. negative feedback loop = ¯ ¯ ¯ ¯; excitatory projection = ➔; inhibitory projection = ■
A variety of stressors can activate the HPA axis causing a non-circadian release of cort. Stressful circumstances force an organism to quickly adapt behavior and physiological processes to maintain a healthful existence. Chronic alterations in this adaptation are thought to promote a state of altered allostatic load that can result in the development of a maladaptive psychiatric state (Mcewen, 2001). The HPA axis responds to two main types of stressors, physiological and psychological stressors. Physiological or interoceptive stressors are usually homeostatic challenges sensed by the somatic, visceral or circumventricular pathways, while psychological or exteroceptive stressors are external challenges that contain species- and individual-specific characteristics.
In addition to the duration and type of stressor, timing of the onset of a stressor with respect to the circadian cycle can greatly influence the HPA response to stress. Cort and ACTH have been shown to display dynamic patterns of release throughout a 24-hour period that is characterized by increased hormone levels prior to daily activity onset (circadian peak in the morning for humans and in the evening for many rodents, including mice and rats). In addition, the circadian pattern exhibits smaller pulses during the circadian trough and higher pulses during the circadian peak (Carnes et al., 1989; Windle et al., 1998). This ultradian pulsatile release of cort has been shown to profoundly influence the cort response to stress. When acute stress coincides with the rising phase of a pulse, resulting cort concentrations exhibit a significantly greater increase than when the stress coincides with the falling phase (Windle et al., 1998). This suggests that the basal pulsatile activity of the HPA axis should be considered when interpreting the differential HPA responses seen after stress administration.
A number of lines of evidence have indicated that hyperactivity of the HPA axis is an important correlate of mood disorders. Compared with healthy individuals, depressed patients often have elevated levels of plasma cort (Carpenter, 1971; Brown, 2004), enlarged adrenal glands (Nemeroff, 1992), increased PVN CRH (Raadsheer et al., 1994; Blanchard et al., 2001) and impaired inhibition of the HPA axis as measured by the dexamethasone suppression test (DST) (Carroll et al., 1980; Holsboer et al., 1982). The DST interrogates the integrity of feedback inhibition on the HPA axis. In normal adults, a moderate (e.g. 1 mg) dose of dexamethasone, a corticosteroid receptor agonist, will induce a dramatic reduction in circadian peak plasma cort. However, depressed patients often maintain elevated levels of cort in the DST, thus implicating impaired negative feedback in depression. Furthermore, individuals afflicted with primary disorders of the HPA axis, such as Cushing’s disease (i.e. hypercortisolemia), are at a much greater risk for developing depression (Sonino and Fava, 2001), demonstrating that a dysregulation of the HPA axis may be involved in the pathogenesis of the disorder. Finally, successful antidepressant treatment is highly correlated with a reversal of HPA axis disruption (Pariante and Miller, 2001).
Glucocorticoids released in response to a stressor act at a variety of locations throughout the body. Systemically, cort has effects on metabolism, fat deposition, bone metabolism and immune responses. In the central nervous system, cort has a variety effects associated with different areas of the brain.
Corticosteroid receptors
Cort binding in the hippocampus can alter HPA axis drive and under a variety of situations modulate behavioral and cellular function. Injection of cort into the hippocampus can induce changes in cell number and morphology (Cameron and Gould, 1994). During stress, cort can alter learning and memory related processes such as long-term potentiation (LTP) and long-term depression (Avital et al., 2006). Cort action in amygdalar nuclei induces changes in anxiety and emotionally relevant learning and memory (Roozendaal and Mcgaugh, 1996; Shepard et al., 2000). It is likely that a combination of changes in corticosteroid receptor expression and activity (via changes in circulating cort) form the basis for the link between psychiatric disease and the HPA axis.
The release of glucocorticoids from the adrenal cortex can readily activate two types of corticosteroid receptors: the type I mineralocorticoid receptor (MR) and type II glucocorticoid receptor (GR) (Hollenberg et al., 1985; Arriza et al., 1987). MR and GR function as transcription factors that reside within the cytoplasm in the ligand-free state. Once bound by ligands, they dimerize and translocate to the nucleus, allowing for transcriptional control over a variety of target genes. Transcriptional control can happen directly through positive or negative regulation of targeted genes or indirectly through modulation of other transcription factors via protein-protein interactions (Yang-Yen et al., 1990; Stocklin et al., 1996). Additionally, rapid non-genomic effects can occur with bound receptors functioning as cytoplasmic monomers that can interact with a variety of cellular proteins or through interactions with membrane-bound G protein-coupled receptors (Tasker et al., 2006). These rapid non-genomic effects appear crucial for generating the immediate behavioral and physiological responses needed to maintain homeostasis as stress hormones can rise significantly within minutes after exposure to a stressor (Reichardt et al., 2001).
One specific way in which GR may exert control over transcription is through chromatin remodeling. Psychological stress through novel environment exposure or forced swim exposure leads to increases in phospho-acetylation of histone H3 within the dentate gyrus, which can be prevented by GR antagonist treatment, but not MR antagonist treatment (Bilang-Bleuel et al., 2005; Chandramohan et al., 2007). It is known that chromatin remodeling allows activation of previously silenced genes through post-translational modification of the N-terminus of core histone molecules. Therefore, this evidence suggests that GR, but not MR, can regulate gene expression via post-translational modification of histone H3.
Glucocorticoids activate both GR and MR, with MR having a 10-fold higher affinity for glucocorticoids than GR (Reul and De Kloet, 1985). Additionally, another adrenal steroid, aldosterone, can activate MR. Specifically, within peripheral tissues, such as the tight epithelia in kidney and colon, aldosterone activates MR as cort is enzymatically converted by 11 β-hydroxysteroid dehydrogenase type 2 to the inactive 11-dehydrocorticosterone (Funder et al., 1988; Seckl, 1997). The localization of MR within these tissues is important for MR’s role in sodium and water homeostasis.
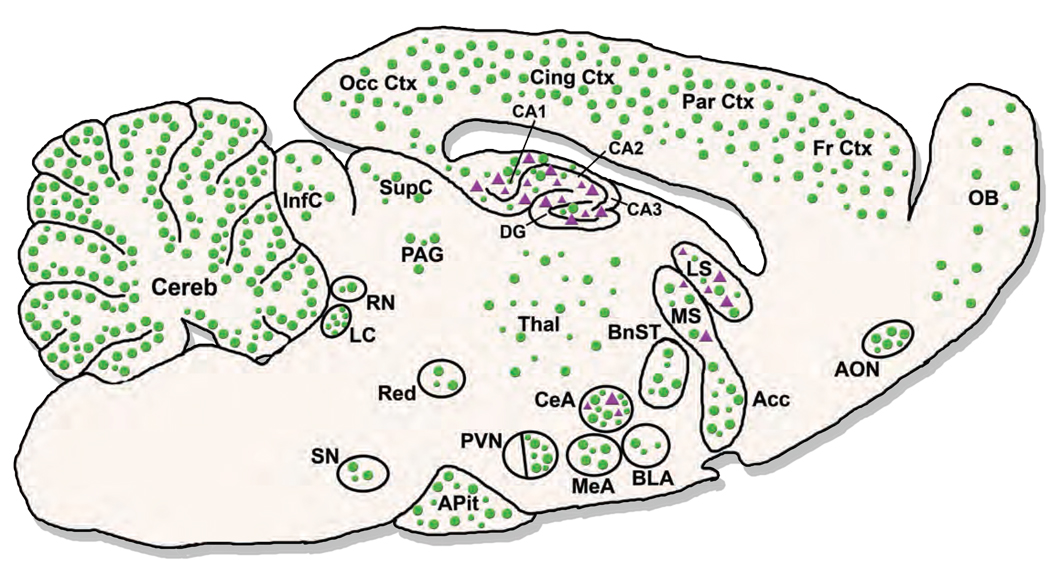
In addition to differential ligand binding affinities, there is differential expression of the receptors throughout the brain (Figure 2). GRs are found in both neurons and glial cells, and are ubiquitously distributed throughout the brain with the highest density occurring in hypothalamic CRH neurons and pituitary corticotrophs (Reul and De Kloet, 1985; Morimoto et al., 1996). Conversely, MRs have a more restricted distribution with the highest density in the limbic brain regions, such as the hippocampus and lateral septum, and a minimal density at hypothalamic sites (Reul and De Kloet, 1985; Kretz et al., 2001). Within the hippocampus, MRs are distributed throughout all pyramidal cell fields, while GRs are limited primarily to the CA1, CA2 and dentate gyrus (Van Eekelen et al., 1988).
Figure 2. Schematic illustration of GR and MR expression in the rodent brain.
GR is ubiquitously expressed throughout the brain, showing higher expression in a number of important limbic areas (e.g. CeA, PVN, hippocampus). MR expression is restricted to the hippocampus with minimal expression in amygdalar nuclei. Green circles ( ) represent glucocorticoid receptors (GR) and purple triangles (
) represent glucocorticoid receptors (GR) and purple triangles ( ) represent mineralocorticoid receptors (MR). Abundance of receptors is given by the relative density of circles or triangles in an area. Acc – nucleus accumbens; AON – anterior olfactory nucleus; APit – anterior pituitary gland; BLA – basolateral nucleus of the amygdala; BnST – bed nucleus of the stria terminalis; CA1, CA2, CA3 – hippocampal areas CA1 to CA3; CeA – central nucleus of the amygdala; Cereb – cerebellum; Cing Ctx – cingulate cortex; DG – dentate gyrus; Fr Ctx – frontal cortex; InfC – inferior colliculus; LC – locus coeruleus; LS – lateral septum; MeA – medial nucleus of the amygdala; MS – medial septum; OB – olfactory bulb; Occ Ctx – occipital cortex; PAG – periaqueductal gray; Par Ctx – parietal cortex; PVN – paraventricular hypothalamic nucleus; Red – red nucleus; RN – raphe nuclei; SupC – superior colliculus; SN – substantia nigra; Thal – thalamus. (Adapted from (Morimoto et al., 1996; Steckler and Holsboer, 1999a; Kretz et al., 2001)
) represent mineralocorticoid receptors (MR). Abundance of receptors is given by the relative density of circles or triangles in an area. Acc – nucleus accumbens; AON – anterior olfactory nucleus; APit – anterior pituitary gland; BLA – basolateral nucleus of the amygdala; BnST – bed nucleus of the stria terminalis; CA1, CA2, CA3 – hippocampal areas CA1 to CA3; CeA – central nucleus of the amygdala; Cereb – cerebellum; Cing Ctx – cingulate cortex; DG – dentate gyrus; Fr Ctx – frontal cortex; InfC – inferior colliculus; LC – locus coeruleus; LS – lateral septum; MeA – medial nucleus of the amygdala; MS – medial septum; OB – olfactory bulb; Occ Ctx – occipital cortex; PAG – periaqueductal gray; Par Ctx – parietal cortex; PVN – paraventricular hypothalamic nucleus; Red – red nucleus; RN – raphe nuclei; SupC – superior colliculus; SN – substantia nigra; Thal – thalamus. (Adapted from (Morimoto et al., 1996; Steckler and Holsboer, 1999a; Kretz et al., 2001)
It has been postulated that at basal cort levels, MR is primarily activated, while GR becomes activated when cort levels are elevated during the circadian peak or stress conditions. Therefore, the hypothesized interpretation is that MR modulates the tonic influences of cort to maintain basal activity, whereas GR modulates responses to increased cort levels (De Kloet and Reul, 1987; De Kloet et al., 1998). Evidence of MR’s role in modulating basal cort levels is evident by intracerebroventricular (icv) administration of an MR antagonist, which elevates basal trough levels of cort. Conversely, icv administration of a GR antagonist has no effect on basal trough levels of cort (Ratka et al., 1989), but icv administration during the circadian peak increases basal HPA activity (Van Haarst et al., 1997). It is postulated that an imbalance in MR/GR may enhance the vulnerability to psychiatric disorders in individuals who are genetically predisposed (De Kloet et al., 1998; De Kloet, 2000).
Over the last 15 years, a number of relevant genetic alterations in the corticosteroid receptor system have been developed. In this review, we will discuss how the data from these alterations has modified our understanding of corticosteroid signaling during and after stress.
GENETIC MODIFICATION OF GR IN MICE
A number of researchers have used transgenic and knockout approaches to investigate the role of GR activity during stress. In this section, we will describe the targeting strategies of these various models and then summarize the major cellular, HPA axis (see Table 1) and behavioral (see Table 2) changes associated with each model to provide a novel framework with which to easily compare individual parameters of the various models.
Table 1.
Phenotypic consequences including HPA axis dysregulation in mice with targeted mutations of GR and MR
| Circadian HPAAxis |
Non Circadian HPA axis |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Basal |
Peak |
Negative Feedback |
Acute Restraint Stress |
||||||||||||
| Transgenic Line | Onset of Modulation |
GR Brain Expression |
MR Brain Expression |
Hypothalamic CRH |
Pituitary POMC/ACTH |
Plasma Cort |
Plasma ACTH |
Plasma Cort |
Plasma ACTH |
CRH/ DST |
DST | Peack | Return to baseline |
Mild Stress |
References # |
| Mice with⇓ GR (AGR) | embryo | 50–70%⇓ | ⇓ | ⇓ | nc | nc | nc | nc | ⇑cort | ⇑ACTH; nc cort | Pepin, 1992;Montkowski, 1995; Barden, 1997; Dijkstra, 1998; Steckler, 2001; | ||||
| GR exon 2 Knockout (GRHyp°) | embryo | none * | nc | ⇑ | ⇑ | ⇑ | ⇑ | ⇑cort | Cole, 1995; Hesen, 1996; Reichardt, 1996; Oitzl, 1997; Kretz, 1999 | ||||||
| GR exon 3 Knockout (GRNuM) | embryo | none | Ridder, 2005 | ||||||||||||
| GR exon 3 heterozygote (GRNu” het) | embryo | 70%⇓ | nc | nc | ⇑ cort | ⇑cort | ⇑cort | delayed | Ridder, 2005 | ||||||
| GRDNA binding mutant .(GRDim) | embryo | nc** | nc | ⇑ | ⇑ | nc | ⇑ | ⇑cort | Derijk, 1997; Reichardt, 1998 | ||||||
| Panneural GR Knockout (GRNesCre) | embryo | none | ⇑ | ⇑ | ⇑ | ⇓ | ⇑ | nc | Tranche, 1999 | ||||||
| Forebrain GR Knockout (FBGRKO) | young adult | none forebrain | nc basal | nc basal | ⇑ | nc | ⇑ | ⇑ | ⇑cort | ⇑cort | delayed | ⇑cort ⇑ACTH | Boyle, 2005; Boyle, 2006 | ||
| Mice with⇑GR (YGR) | embryo | 50%⇑ | 30%⇓ | ⇓ | ⇓ | *** | *** | *** | *** | nc | ⇓cort | ⇓cort | nc | Reichardt, 2000; Ridder, 2005 | |
| Forebrain GR .Overexpressor .(GRov) | 1st post-natal week | 75%⇑ forebrain | ne | nc | nc | nc | nc | nc | nc | nc | Wei, 2004 | ||||
| MR Knockout .(MRNuN) | embryo | none | ⇑ | ⇑ | ⇑ | Berger, 1998; Bleich, 1999; Gass, 2000; Gass, 2001 | |||||||||
| Forebrain MR Knockout .(MRCamKCre) | 1st post-natal week | ⇑HPC | none forebrain | nc | nc | nc | nc | nc | Berger, 2006 | ||||||
| Forebrain MR .Overexpressor .(MRov) | ⇓HPC | 20–25%⇑ forebrain | nc | nc | 20– 27% ⇓ p>0.05) | ⇓female nc male | nc | nc | Rozeboom, 2007 | ||||||
| Forebrain MR .Overexpressor .(MR-Tg) | nc | >25%⇑ forebrain | nc | nc | nc | Lai, 2007 | |||||||||
Abbreviations: ⇑ - increase compared to control mice; ⇓ - decrease compared to control mice; nc = no change compared to control mice; ACTH - adrenalcorticotrophic hormone; cort - corticoste-rone; CRH - corticotropin releasing hormone; DST - dexamethasone suppression test; HPC - Hippocampus.
GRHypo mice express some aberrant GR;
GRDim mice express mutant GR to a similar level to wildtype GR in control mice;
conflicting results in literature.
References given by first author and year of publication.
Table 2.
Behavioral analysis of mice with targeted mutations of GR and MR
| Anxiety Tests |
Despair Tests |
Learning and Memory |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Transgenic Line | Locomotion | Elevated Plus Maze/Elevated O Maze | Light:Dark Preference | Open Field | Forced Swim Test | Tail Suspension Test | Learned Helplessness | MWM | RAM | Novel Object | References# |
| Mice with⇓GR (AGR) | ⇑(EPM; open field) | ⇓anxiety | ⇓despair | ⇓memory | ⇓memory | * | Beaulieu, 1994; Montkowski, 1995 Rochford, 1997; Rouse, 1997; Steckler, 1999b & Steckler, 2001 | ||||
| GR exon 2 Knockout (GRHypo) | ⇑ (open field) | nc anxiety | ⇓memory | Oitzl, 1997; | |||||||
| GR exon 3 heterozygote (GRNu” het) | nc (open field) | nc | nc | nc | nc | ⇑despair | Ridder, 2995 | ||||
| GRDNA binding mutant (GRDim) | ⇓(MWM); nc (open field) | nc | nc | ⇓memory | Oitzl, 2001 | ||||||
| Panneural GR Knockout (GRNesCre) | nc (open field) | ⇓anxiety | ⇓anxiety | nc | ⇓despair** | Tranche, 1999 | |||||
| Forebrain GR Knockout (FBGRKO) | ⇑(EPM); nc (open field) | ⇓anxiety | ⇑reactivity | nc | ⇑despair | ⇑despair | Boyle, 2005; Boyle, 2006 | ||||
| Mice with⇑GR (YGR) | nc (open field) | nc | nc | nc | nc | ⇓despair | Ridder, 2005 | ||||
| Forebrain GR Overexpressor (GRov) | nc (open field) | ⇑ anxiety | ⇑ anxiety | nc | ⇑despair | Wei, 2004 | |||||
| MR Knockout (MRNu”) | *** | *** | *** | Gass, 2001 | |||||||
| Forebrain MR Knockout (MRCamKCre) | ⇓(MWM; L:D pref; EOM); nc (open field) | nc | nc | nc | ⇓memory | ⇓memory | ⇑exploratory activity | Berger, 2006 | |||
| Forebrain MR Overexpressor (MRov) | nc (open field) | ⇓anxiety | ⇓anxiety | Rozeboom, 2007 | |||||||
| Forebrain MR Overexpressor (MR-Tg) | nc (open field) | ⇓anxiety | ⇓anxiety | ⇑memory | ⇓memory | Lai, 2007 | |||||
Abbreviations: ⇑ - increase compared to control mice; ⇓ - decrease compared to control mice; nc - no change compared to control mice; EPM - elevated plus maze; MWM - Morris water maze; RAM - radial arm maze.
Conflicting data on this test in literature;
show decreased immobility on 2nd day of testing;
preliminary data suggests increased anxiety (but details are unpublished ).
References are given by first author and year of publication.
Targeting strategies for genetic GR manipulation
The variety of GR models demonstrates the diversity of genetic approaches available for disruption of normal gene function. Researchers have generated knockouts, overexpressors and mutants with reduced or altered GR function.
Antisense GR mutant
The first published genetic model of glucocorticoid disruption involved the introduction of antisense GR cDNA into the mouse genome and is known as the antisense GR mouse (AGR) (Pepin et al., 1992). A 1.8kb fragment of the GR cDNA was inverted and placed under the control of the neurofilament promotor. This strategy was designed to force reduced expression of endogenous GR in the nervous system. However, inconsistent expression of the transgene induced differing amounts of reduced GR expression in neural (e.g. 50–70% decrease in the GR expression in the hypothalamus and cortex) and non-neural (e.g. 30–50% reduction in kidney and liver) tissue. Phenotypically, these mice exhibit increased weight and fat deposition with a concomitant reduction in food intake. Some of the important cellular changes seen in this model include a surprising decrease in CRH expression in the PVN and median eminence (Dijkstra et al., 1998) as well as a decrease in LTP (possibly a result of reduced MR expression) (Steckler et al., 2001). Finally, although this model is more representative of the human disease state as compared to a complete GR knockout, the cell-to-cell consequences of varying amounts of GR expression limit the interpretation of the data.
Conventional GR knockouts
To investigate the effects of loss-of-function in GR, two conventional knockout animals have been produced: Exon 2 targeted GRHypo (Cole et al., 1995) and Exon 3 targeted GRNull (Ridder et al., 2005). The GRHypo mice were developed by inserting a PGK-Neo cassette into Exon 2 of the GR gene, a region involved in transactivation, while the GRNull mice were developed using mutant mice containing loxP sites surrounding Exon 3, a region involved in DNA binding. It has been reported that most of the GRHypo mice and all of the homozygous GRNull mice die in the first hours of life from severe lung atelectasis. The fact that 5–10% of the GRHypo mice survive to adulthood prompted researchers to carefully look for aberrantly truncated GR proteins (Cole et al., 2001). Analysis showed that GRHypo mice on an outbred strain have a truncated GR with a ligand-binding domain that can bind the synthetic glucocorticoid dexamethasone. So, GRHypo mice may have some remaining GR function that could limit interpretation of findings, particularly when differences in action are not found. Heterozygotes of both models survive into adulthood and have been used to study a variety of physiological, endocrine and behavioral factors (Hesen et al., 1996; Oitzl et al., 1997; Ridder et al., 2005). GRHypo mice exhibit increased hypothalamic CRH and anterior pituitary gland POMC (Reichardt and Schutz, 1996; Kretz et al., 1999). Both of these models, as heterozygotes, offer the opportunity to investigate the effect of reduced but not complete loss of neural GR activity in stress-related questions.
GR DNA binding mutant
As mentioned previously, glucocorticoid binding to GR can induce cellular changes through dimerization-dependent and independent actions. To investigate these two types of GR activity on a variety of cellular processes, a GR mutant with a point mutation in Exon 4 was developed (GRDim) (Reichardt et al., 1998). The point mutation, A458T, had previously been shown to disrupt D loop formation causing a loss of GR dimerization and direct DNA binding (Heck et al., 1994). Interestingly, GRDim homozygous mice are born at the normal Mendelian ratios from heterozygote to heterozygote pairings. Despite the lack of dimerization-dependent DNA binding in GRDim mice, in vitro evidence suggests that GR with the GRDim mutation can still bind DNA and directly influence the transcription of genes (Adams et al., 2003). This in vitro work is consistent with the observation that the mRNA levels of a GR-dependent gene are normal in GRDim mice but are undetectable in GRHypo mice (Cole et al., 1995; Reichardt et al., 1998). On a cellular level, CRH in the median eminence is unaffected by the mutation, while POMC mRNA and ACTH are upregulated in the anterior pituitary gland demonstrating the importance of GR dimerization-dependent DNA binding effects on some but not all aspects of GR-mediated feedback on the HPA axis (Reichardt et al., 1998).
All of the above mutants have a number of peripheral changes in metabolism and immune function associated with the conventional, whole organism targeting strategies used. Although not fully characterized, these peripheral changes make it more difficult to derive specific conclusions about the role of GR in stress and nervous system function. To more precisely define the role of GR in the CNS, groups have produced panneural GR knockout (GRNesCre) (Tronche et al., 1999) and forebrain-specific GR knockout (FBGRKO) (Boyle et al., 2005) lines.
Panneural GR knockout
Tronche and colleagues (1999) generated mice with GR deleted throughout the brain beginning prenatally using the Cre-loxP system. In this model, Exon 3 of the GR gene was flanked with loxP sites. Mating of these mice with nestin-Cre recombinase mice results in the deletion of GR in all CNS neurons and glial cells. GRNesCre mice have normal survival but exhibit a Cushing’s syndrome-like phenotype. These mice have altered fat deposition with lowered weight gain and osteoporosis. GRNesCre mice show an increase in PVN CRH protein and POMC mRNA in the anterior pituitary gland. Investigation of GR downstream MAPK targets revealed a downregulation of p-ERK1/2, Ras, Raf-1 and Egr-1 with potential implications for stress responsiveness and fear-based learning and memory (Revest et al., 2005). The GRNesCre mutants, as the first neural-specific line, provide an opportunity to investigate the role of both neurons and glial cells in understanding the effects of GR activation on HPA axis function and behavior.
Forebrain GR knockout
Recently, our group produced forebrain-specific GR disruption by mating mice containing a floxed GR Exon 1C through 2 to CamKII-Cre recombinase mice (Boyle et al., 2005). In this targeting strategy, promoter elements at the normal translation start sites for GR are deleted. The potential exists for the production of truncated GR regions analogous to the GRHypo allele. However, such fragments have not been detected in tissues analyzed to date (Brewer et al., 2003). In these mice (FBGRKO), the floxed GR region is progressively deleted from age 3–6 months in the hippocampus, cortex, basolateral nucleus of the amygdala (BLA) and nucleus accumbens but GRs in the PVN, thalamus and central nucleus of the amygdala (CeA) are spared (Boyle et al., 2006). Associated with this specific disruption of GR, FBGRKO mice exhibit basal increases in PVN AVP and hippocampal (CA1 and DG) CRH receptor-1 expression. The additional spatial specificity (i.e. forebrain only) and temporal aspects of deletion (i.e. deletion after 3 months of age) make the FBGRKO mice a particularly interesting model to investigate the role of extrahypothalamic sites of GR on basal and stress-induced HPA axis activity as well as the role of GR in limbic modification of behavior in the absence of nonspecific developmental changes.
General GR overexpressor
To complement the loss-of-function studies, two models of GR overexpression have been generated. The first model (YGR) involved the addition of an extra copy of the full length GR gene (Reichardt et al., 2000). Although insertion-site effects of the extra GR cannot be fully appreciated, the inclusion of the known GR promoter and regulatory elements was predicted to induce overexpression in the normal areas of GR expression. Both GR mRNA and protein were only increased by about 50% despite the presence of two extra copies of the GR per mouse. In YGR mice, median eminence CRH protein, pituitary POMC mRNA and ACTH protein, hippocampal BDNF protein and MR expression are all decreased (Reichardt et al., 2000; Ridder et al., 2005). Despite the fact that GR is overexpressed throughout the nervous system and periphery of YGR mice, these mutants provide an interesting framework to look at the effects of increased GR activation on stress-mediated alterations.
Forebrain GR overexpressor
To improve the spatial specificity of GR overexpression, Wei and colleagues (2004) introduced a transgene containing the CamKII promoter driving expression of the GR cDNA. This model (GRov) exhibits about 78% overexpression of GR in the forebrain (including the cortex, hippocampus, CeA, BLA and nucleus accumbens) as well as the PVN and possibly includes ectopic expression of GR within groups of neurons not normally expressing GR in the CNS such as the suprachiasmatic nucleus (SCN) (Balsalobre et al., 2000). Importantly, expression excludes the cerebellum, thalamus and anterior pituitary gland as well as all peripheral organs. GRov mice show increases in CeA CRH mRNA and various neurotransmitter transporters (Wei et al., 2004). GRov mice offer the opportunity to investigate the role of increased GR in important limbic areas with the caveat that PVN overexpression of GR might make it difficult to disentangle hypothalamic versus extrahypothalamic GR modulation of HPA axis drive.
HPA axis analysis from genetic models of GR alteration
Change in HPA axis drive is one of the most important components of the link between psychiatric illness and the endocrine system. Understanding how GR activity changes drive during the normal circadian release of cort and during stress-induced cort release is crucial to understanding this link. The various GR models include a wide array of changes to the GR system, and when the various phenotypes from the strains are combined together the role of GR in modulating HPA axis activity can be more clearly understood.
HPA axis circadian activity and feedback
Cort and ACTH are normally modulated under non-stress conditions by the SCN, the center of circadian control. As previously mentioned, the circadian rhythmicity of cort release is such that cort levels are high at activity onset (lights off for mice) and low at the start of rest (lights on for mice). An important component of HPA axis activity during normal circadian cort release or after stress HPA axis activation is the negative feedback loop that returns the system to a state of homeostasis through the activation of GR in a variety of CNS areas (Figure 1). To assess feedback, researchers employ two tests, the DST and the dexamethasone/CRH challenge test. Both of these tests measure suppression of cort in response to a GR agonist and have both been shown to be altered in psychiatric illness (Carroll et al., 1980; Holsboer et al., 1982). The use of these tests in the GR mutant models has led to a better understanding of the effects of each specific genetic alteration on basal HPA axis drive and feedback inhibition.
When the AGR mice were originally subjected to analysis of circadian changes in cort production, initial results indicated an increase in circulating cort compared to control animals (Pepin et al., 1992), however subsequent and more in depth analysis failed to identify a difference between transgenic and control groups (Barden et al., 1997). While differences in circulating cort were not observed, the use of the DST showed reduced suppression of HPA axis activity consistent with the reduction but not elimination of GR activity in these mice.
Although not analyzed with respect to circadian rhythmicity, GRHypo postnatal day 1 mice exhibit increased cort (2.5-fold) and ACTH (15-fold) (Cole et al., 1995). While HPA axis activity for GRNull homozygotes has not been reported, GRNull heterozygotes exhibit normal cort levels during the light and dark phases and reduced dexamethasone suppression indicating impaired negative feedback (Ridder et al., 2005). Normal MR activity, even in the presence of reduced GR, may explain this lack of a circadian phenotype in the GRNull heterozygotes at least at basal levels. Alternatively, the presence of one GR allele may be sufficient to maintain circadian rhythmicity of HPA axis activity. However, when the system is challenged in the DST or during stress (as discussed below), the reduced GR may fail to fully control HPA axis activity causing elevated levels of cort through impaired feedback inhibition.
GRDim and GRNesCre mice demonstrate increased nadir (4- and 15-fold respectively) and peak (1.5- and 4-fold respectively) cort compared to control mice (Tronche et al., 1999; Oitzl et al., 2001). Paradoxically, both of these models actually exhibit unexpected levels of ACTH, a 1.5-fold reduction in GRNesCre mice and no change in GRDim mice. Interestingly, ACTH stimulation tests in the GRNesCre mice indicated that the sensitivity of the adrenal cortex is heightened in GRNesCre animals, a mechanism that could potentially account for the discrepancy in ACTH levels (Tronche et al., 1999). The increase in cort in the GRDim mice illustrates the relative importance of GR DNA binding-dependent versus -independent mechanisms in the modulation of cort-regulated negative feedback. It appears that while ACTH production in the pituitary gland is under transcriptionally-regulated control, the actual secretion of ACTH is regulated by DNA binding-independent means.
FBGRKO mice exhibit increased nadir, with a 2-fold increase in cort levels and small, but not statistically significant increase in ACTH, and peak, with a 1.5-fold increase in cort and ACTH levels, circadian HPA axis activity (Boyle et al., 2005). These results suggest that forebrain GR may play an important role in basal inhibition of HPA axis activity, and this inhibition is released when GR is deleted. In addition, FBGRKO mice show no dexamethasone suppression of cort. When compared to a reduction of suppression in AGR and GRNull heterozygotes, this result suggests that the hippocampus and other forebrain areas may comprise an important and necessary circuit in the negative feedback of the HPA axis. Alternately, the chronic loss of GR in the forebrain may cause downstream changes in other components of the negative feedback system. More region-restricted genetic approaches should be useful in determining the true necessity of forebrain GR in the DST.
At first glance, the two overexpression models appear to show different effects on HPA axis drive. GRov mice exhibit normal circadian rhythmicity of HPA axis drive (Wei et al., 2004). In contrast, initial evidence showed that YGR mice exhibit decreased nadir cort (4-fold) but increased ACTH (2-fold) (Reichardt et al., 2000). However, it should be noted that the control animal’s levels of basal cort in that experiment appear to be slightly elevated (40 ng/ml) compared to other published values. Since the YGR mice exhibit reduced cort in response to a strong stressor as compared to stressed control mice (discussed below), it may be that these basal levels are actually more likely to be representative of mild handling stress-induced cort release. In fact, subsequent analysis failed to find a difference in circadian cort or ACTH but did reveal elevated suppression in the DST (Ridder et al., 2005). The apparent lack of an HPA axis phenotype in GRov and YGR mice compared with elevations in circadian cort in FBGRKO mice may be explained by the possibility that the normal endogenous GR abundance is already at a level where further increases cause no changes.
Our findings with FBGRKO mice suggest that while resting levels of cort primarily occupy MR, some amount of GR are also activated and act to inhibit HPA axis activation. So, in both the FBGRKO and the GRNesCre mice, the lack of this small group of occupied GR releases the inhibition of the HPA axis causing increased cort. On the other hand, when GR is overexpressed in the forebrain, the increased amount of GR does not further potentiate this inhibition because of the limited amount of cort. This interpretation of forebrain GR in tonically inhibiting the HPA axis is further supported by the results from GRNull heterozygotes who exhibit reduced GR expression that may exceed the threshold for basal tonic inhibition in order to produce normal circadian cort release. Previous observations that GR antagonists do not alter basal cort in wildtype animals (Ratka et al., 1989) could be explained on the basis that the above observations in GR mutant mice occur after weeks or months of altered GR expression/activity and may therefore actually be representative of a chronically altered HPA axis control system or differences could be related to the fact that in the pharmacological experiments, GR agonists were delivered icv.
Stress induced activation of the HPA axis
As a dynamic system, the HPA axis is activated in rodents to different degrees when the stress is mild (e.g. mild handling, needle stick, time in elevated plus maze (EPM)), moderate (e.g. time in water maze) or severe (e.g. acute or chronic restraint). Using these different types of stressors in GR mutant mice, investigators have been able to dissect the role of GR in stress-associated HPA axis activity.
Evaluation of mild stress (10 min in EPM) in AGR mice revealed increased ACTH release but no difference in cort (Montkowski et al., 1995). In the GRHypo adult mice, there was evidence of a gene deletion dose effect on mild stress-induced (novel environment) HPA axis activation (Hesen et al., 1996). GRHypo homozygotes had the highest cort levels followed by GRHypo heterozygotes and wildtype animals.
In GRDim, a moderate stressor (1 min of swimming) caused an increase in cort release 30 min and 90 min after the stressor (Oitzl et al., 2001). However, the increase in cort at these time points was roughly the same (2-fold increase) as the basal changes in cort. Therefore, it cannot be concluded whether the HPA axis is more sensitive to stress or just hyperactive overall.
The stress-induced increase in GRDim mice is in contrast to the lack of a difference in GRNesCre mice after severe stress (40 min restraint) (Tronche et al., 1999). However, the absence of a difference here may simply be the result of a ceiling effect. The notion of which is well illustrated with data from FBGRKO mice (Boyle et al., 2006). These mice show increased cort and ACTH release after 5 min and 15 min of restraint but not after 30 min of restraint compared to control mice. Furthermore, FBGRKO mice show increased cort after a mild stressor (needle stick).
GRNull heterozygotes and YGR mice show increased and decreased cort, respectively after 30 min of restraint stress (Ridder et al., 2005). Finally, GRov mice exhibit no changes in cort (or ACTH) to a mild stressor (5 min in the EPM) but have changes in HPA axis function with more severe stressors (Wei et al., 2004).
The HPA axis under stressful conditions appears to be modulated by active receptor density with an inverse relationship between GR density and levels of cort secretion. Mutants with reduced GR activity (GRNull heterozygotes, GRDim and FBGRKO mice) show increases in stress-induced cort while the YGR mice show reduced cort after stress. The density of receptors probably modulates cort abundance through altered feedback inhibition (i.e. increased GR = increased negative feedback).
Behavioral analysis from genetic models of GR alteration
Thus far, we have discussed the physiological effects of genetic manipulations of GR as determined by alterations in HPA axis activity. In order to fully understand the role of GR in the whole organism’s response to normal or pathologic stress, one must assess the behavioral correlates of these physiological alterations as well. In this section, we will describe the behavioral phenotypes of mice with GR alterations. These phenotypes are divided into one of two categories: behavior associated with anxiety or depression and behavior associated with learning and memory (Table 2).
Changes in anxiety-related behavior in GR mice
Common tests for anxiety include the open field test, the EPM, the elevated zero maze (EOM) and light:dark (L:D) preference. Although open field is a classic measure of anxiety-like behavior in rodents, it also offers data on general locomotor output that can significantly confound the interpretation of results from other behavioral tests. None of the mice tested (e.g. GRHypo, GRNull heterozygotes, GRDim, GRNesCre, FBGRKO, YGR nor GRov) show any alterations in open field anxiety-related behavior (Oitzl et al., 1997; Tronche et al., 1999; Oitzl et al., 2001; Wei et al., 2004; Ridder et al., 2005; Boyle et al., 2006). However, GRHypo homozygotes and AGR mice exhibited increased locomotor activity compared to control mice which could result in increased-behavior in other tests being misinterpreted as an anxiety change when it is simply increased movement (Beaulieu, 1994; Oitzl et al., 1997). It should be noted that for the GRHypo mice, this test occurred following Morris water maze testing, which can be considered a moderate stressor.
Although none of the transgenic mice tested showed alterations in anxiety behavior in the open field, a number of changes were observed in EPM and EOM. GRNesCre mice show decreased anxiety-like behavior on the EOM; AGR and FBGRKO mice show an anxiolytic phenotype on the EPM (Montkowski et al., 1995; Rochford et al., 1997; Tronche et al., 1999; Boyle et al., 2006). However, for both the AGR and FBGRKO mice, other evidence complicates the interpretation of a simple anxiolytic phenotype. For instance, the AGR mice show increased anxiety on two other tests, the Thatcher Britton Novelty food test and the acoustic startle test (Rochford et al., 1997). In the EPM, diazepam (an anxiolytic) increased the anxiolytic phenotype (Rochford et al., 1997). These results suggest that the AGR mice exhibit an exaggerated exploratory response characterized by increased locomotor activity (in open field activity and in EPM total arm entries) that allows the animal to ignore potentially harmful stimuli (such as open arm time). In tests that do not involve a “safe” or “non-safe” choice, these mice have reduced anxiety-like behavior. Similarly, for the FBGRKO mice, the decrease in anxiety-like behavior (e.g. more time and entries into the open arms) was accompanied by an overall increase in activity in the closed and open arms and may represent exaggerated stress reactivity given that these mice show no alterations in open field locomotion. In contrast to the GRNesCre, AGR, and FBGRKO mice, GRov mice show significant increases in anxiety-related measures in the EPM (Wei et al., 2004). However, the fact that the YGR mice do not show any EOM differences complicates the interpretation of this finding that increased GR expression is correlated with increased behavior representative of an anxious state (Ridder et al., 2005).
The final major test for anxiety that has been thoroughly examined is the L:D preference test. This procedure pits a mouse’s innate interest in exploring novel areas against its fear of open-lighted environments. In this test, GRDim mice show no differences from control mice indicating that direct DNA binding activities of GR dimers may not be central to GR’s influence on anxiety-like behavior (Oitzl et al., 2001). GRNesCre mice show decreased latency to enter the light and longer overall time in light (both characteristic of decreased anxiety) indicating that decreased neural GR may reduce anxiety-like behavior (Tronche et al., 1999). FBGRKO mice show confusing behaviors in L:D preference. FBGRKO mice show decreased latency to enter the light and more entries into the light (both indices of anxiolytic responses) (Boyle et al., 2006). However, in contrast to the GRNesCre mice, FBGRKO mice spent an equivalent amount of time in the light compared to controls. A closer analysis of the L:D preference data using reversed lighting (animals start in light rather than dark), stress, and antidepressant treatment, shows that FBGRKO mice most likely exhibit increased stress reactivity and an enhanced flight behavior rather than more common changes in anxiety. Finally, as with EPM/EOM, GRov but not YGR mice show significant increases in anxiety-related measures in the L:D preference (Wei et al., 2004; Ridder et al., 2005). Since the GRov mice have no changes in circadian HPA axis activity or mild stress HPA axis activity, it appears that increased GR in the presence of a normal amount of cort is sufficient to increase anxiety-like behaviors.
Overall, increased anxiety-like behavior appears to be associated with increased GR (GRov mice) while decreased anxiety (AGR, GRNesCre) and altered anxiety responsiveness (FBGRKO) is related to reduced GR activity. Conflicts between models such as the lack of an anxiety-like phenotype in the YGR mice coupled with the anxiogenic phenotype in GRov mice may be explained by the differences in the amount of overexpression (50% YGR vs. 78% GRov) or the general areas of overexpression (endogenous sites of GR expression in YGR vs. forebrain only in GRov).
Changes in despair-related behavior in GR mice
The forced swim (FST) (Kitayama et al., 1988), tail suspension and learned helplessness tests, all measures for despair-like behavior, help illustrate the role of GR signaling in depression-like behavior. AGR mice exhibit reduced depression-like behavior in the FST, which is surprisingly reversed by antidepressant treatment (Montkowski et al., 1995). This may suggest that the AGR mice have difficulty interpreting a potentially fearful situation and that antidepressant treatment can help bring the mice back to a state of increased awareness. Alternatively, this finding may simply reflect the elevations in locomotor activity seen in other tests (e.g. open field and EPM). Increased depression-related behavior in both the FBGRKO mice (in the FST, tail-suspension test and sucrose preference) and GRov mice (in the FST) is reversed with antidepressant treatment (Wei et al., 2004; Boyle et al., 2005). Although GRNull heterozygotes show no differences compared to control mice in the FST, they exhibit more depression-like coping strategies including increased escape latencies and more escape failures when compared to control mice in learned helplessness (Ridder et al., 2005). Similarly, YGR mice show reduced depression-like behavior in learned helplessness but have no observed changes in the FST (Ridder et al., 2005). Finally, GRNesCre mice show increased mobility on the 2nd day of a repeated FST (Tronche et al., 1999). While increased mobility is often thought of as a measure for reduced despair, in the 2-day FST, it can also be a measure of cognitive impairment (Bilang-Bleuel et al., 2005). Interpreted in this fashion, the GRNesCre mice fail to learn that escaping from the FST is impossible while the control mice learn to conserve their energy and be immobile longer. It should be noted that some of the altered behavior in GRNesCre mice might be related to their Cushing’s syndrome-like phenotype. GRNesCre exhibit osteoporosis and altered fat metabolism, which may affect buoyancy and locomotor behavior in water.
If one compares all of the models of GR alteration, there appear to be contradictory conclusions for depression-like behavior. For instance, both FBGRKO and GRov mice show an increase in despair-like behavior that is reversed with antidepressant treatment. While the different regions affected in these models may account for some of these conflicting results, the data also illustrate the idea that the effects of glucocorticoid action can be modeled on an inverted “U” shaped curve where either too little or too much GR activity can be harmful. An inducible system in which levels of GR expression could be titrated (e.g. the tetracycline-inducible system) could be a useful method with which to more closely examine this model. Finally, differences in using knockout systems versus systems that are prone to ectopic alterations (e.g. non-GR promotor-driven overexpression systems) could cause abnormal non-specific changes in despair-like behavior.
Learning and memory behavior
Stress and glucocorticoids have a well-established role in modifying learning- and memory-related behavior. Specifically, emotionally relevant memories have been shown to be altered by GR signaling (Roozendaal and Mcgaugh, 1996). Interestingly, the effect of glucocorticoids on memory can be positive in some cases (e.g. acute stress) or detrimental in others (e.g. chronic stress, psychiatric illness, etc). Due to their varying degrees and specificity of GR manipulation, the genetic models of GR alteration provide an interesting and informative framework for understanding this dynamic process of GR modification of cognition.
In terms of learning and memory-related behavior, spatial memory tests such as the Morris water maze (MWM) and working memory tasks are some of the most common and well-established procedures to evaluate memory in rodents. All of the animals tested in the MWM (AGR, GRHypo and GRDim) show deficits in various aspects of the test. AGR mice show deficits in location acquisition of both a submerged and a visible platform (Rousse et al., 1997). Furthermore, when the platform is removed, AGR mice show no preference for the target quadrant. While still performing worse than control animals, AGR mice do begin to show improvement from one session to the next when the incentive to find the visible platform is increased (by lowering the water temperature). GRHypo homozygous and heterozygous mice both show deficits in probe trials (no platform) after training in the MWM (Oitzl et al., 1997). However, analysis of trials during training (submerged platform) or with a visible platform is difficult because the day-to-day variability is high. A similar deficit is seen when GRDim mice are tested in the MWM (Oitzl et al., 2001). In this case, GRDim mice exhibit clear deficits during training and during probe trials that are associated with decreased swimming speeds and an increase in cort release after swimming. To account for differences in cort, studies incorporated adrenalectomized GRDim and control mice (Oitzl et al., 2001). After adrenalectomy, mice were tested on the MWM with or without cort replacement. In the control group, cort replacement significantly improved performance. However, both adrenalectomized GRDim groups, regardless of cort treatment, exhibited significant deficits. These experiments suggest that these spatial memory deficits in GRDim mice are caused by the loss of GR dimerization-dependent DNA binding activity rather than increased cort acting through altered GR or MR. In the radial arm maze test (RAM), a measure of working memory, AGR mice show more errors than control animals (Rousse et al., 1997). Finally, in two slightly different tests of object recognition, AGR transgenic mice exhibit either no difference or a deficit in novel object interaction compared to control mice (Steckler and Holsboer, 1999b; Steckler et al., 2001).
In tests that involve more emotional processing (e.g. social recognition and conditioned fear) only a few of the GR mutants have been analyzed. In a social recognition task, AGR mice fail to recognize a previously encountered juvenile mouse (Montkowski et al., 1995). However, the AGR mice interact with the juvenile mouse less during the first exposure. So, it is unclear whether this is a memory deficit or a floor effect of interaction time. To evaluate the role of altering GR on fear-based learning and memory, YGR and GRNull heterozygotes were examined in conditioned fear (Ridder et al., 2005). Despite significant changes in a learned helplessness paradigm, neither YGR nor GRNull heterozygotes mice exhibited abnormal contextual or cued fear learning.
Unfortunately, the low number of learning and memory studies has thus far prevented the development of more robust conclusions concerning the role of GR in various types (e.g. working, spatial, emotional) of learning processes. At this point, reduced GR appears to cause significant deficits in MWM, a classic test of spatial memory. However, it is important to consider the fact that MWM is not a stress-free behavioral test. Immersion into water is likely to cause both physical and psychological stress that could confound the interpretation of any changes as being related strictly to spatial memory. All of the above mice with MWM deficits show increased cort secretion when subjected to a mild stressor. Therefore, the deficits in spatial memory may be a consequence of increased cort. In the future, it will be important for more in-depth characterization of all the models of GR modulation. In addition, more specific targeting strategies that manipulate GR expression only in specific brain areas (e.g. the hippocampus only) should be valuable in addressing the role of GR in different types of memory formation and retrieval.
Summary of HPA axis findings and behavioral phenotypes of GR mice
Although some variability between GR models exists, it can consistently be said that brain GRs are important for regulating basal and stress HPA axis function independent of pituitary GR activity. The spatial and temporal control of this regulation will have to be further elucidated as genetic techniques progress, some of which are described later in this review. GRs appear to be important for normal circadian HPA axis drive, with significant changes occurring only when DNA binding-dependent GR activity does not occur in the forebrain. Additional mechanisms of compensation that have not been fully appreciated include changes in MR expression or adrenal ACTH sensitivity. Finally, although mouse behavior is at best a moderate correlate for human affective disorder symptoms, it can be stated that GR contributes to behavior characteristic of anxiety, despair and learning phenotypes. As more models of GR manipulation continue to be analyzed, the precise mechanisms of GR control (e.g. GR expression levels, areas of GR expression, types of GR modulation on cellular functions) will become clearer.
GENETIC MODIFICATIONS OF MR IN MICE
Although less well understood than GR, MR’s role in stress-associated activity has begun to be investigated. A number of groups have now published data from transgenic and knockout animals for MR.
Targeting strategies to manipulate MR expression
Conventional MR knockout
Conventional MR knockout mice were developed by inserting a neo cassette into Exon 3 of the MR gene, a region involved in DNA binding (Berger et al., 1998). These mice (MRKO) develop pseudohypoaldosteronism, increases in plasma renin, angiotensin II, aldosterone hyperkalemia and hyponatremia, causing them to die around postnatal day 10. However, MRKO mice can be rescued by exogenous NaCl administration (Bleich et al., 1999). They display elevated CRH levels in the PVN and increased POMC and ACTH in the anterior pituitary gland (Gass et al., 2001). These mice display decreased granule cell density in the hippocampus suggesting a role for MR in neurogenesis. In addition, they exhibit increased glucocorticoid levels (Gass et al., 2000) making it difficult to conclusively decipher whether the decreased neurogenesis in MRKO mice is a direct effect of knocking out MR or an indirect effect of increased GR activation. Interestingly, some evidence points to a potential role for MR as an anti-apoptotic modulator because adrenalectomy-induced apoptosis in the dentate gyrus can be rescued by low levels of glucocorticoids that would be sufficient for complete MR but not GR binding (Hassan et al., 1996). Although the MRKO mice can be rescued, their continual problems associated with salt balance including chronic elevations in the renin-angiotensin system make analysis of these mice difficult to interpret.
Forebrain MR knockout
Researchers have used the Cre-loxP system to produce forebrain-specific MR knockout mice (MRCamKCre) (Berger et al., 2006). In this system, CamKII-Cre recombinase mice are mated to mice with an Exon 3 floxed MR allele. MR is completely deleted in these mice by P12. These mice exhibit normal CRH mRNA in the PVN, but upregulated GR in the hippocampus. They also exhibit a histological alteration in mossy fiber morphology. The forebrain specificity of these mice allows for a careful analysis of the role of MR in basal HPA axis control as well as behavioral modulation.
Forebrain MR overexpressor
Two different laboratories have recently generated transgenic mice with overexpression of forebrain MR (MR-Tg, Lai et al., 2007; MRov, Rozeboom et al., 2007) under the control of the CamKII promoter. MRov mice exhibit overexpression at levels 20–25% above endogenous levels in the cortex and hippocampus (Rozeboom et al., 2007). MR levels in the amygdala and PVN are normal. MRov mice show decreased CA1 GR expression and an increase in CA1 5-HT1a with no changes in PVN CRH. Lai and colleagues (2007) have generated MR-Tg mice using a similar system that exhibits overexpression at >25% above normal levels in the cortex, BLA and hippocampus. Unlike the MRov mice, MR-Tg mice show no change in GR levels in the hippocampus. Both of the MR overexpressors provide good models to investigate the hypothesized cellular role of MR as an anti-apoptotic factor and the systems level role as a modulator of behavior.
HPA axis analysis after MR alteration
MR has been thought to be primarily responsible for basal HPA axis tone. To investigate the role of MR in circadian HPA axis activity as well as stress-induced activity, researchers analyzed the various models of MR mutation (Table 1).
MRCamKCre mice show normal basal circadian HPA axis activity (Berger et al., 2006), implying that forebrain MR (e.g. MR in hippocampus, amygdala and septum) is not necessary for maintenance of the basal HPA axis activity, which would conflict with the increased cort seen in the conventional MRKO mice (Gass et al., 2000). A possible explanation is that increased cort in rescued MRKOs results from the chronic elevation in the renin-angiotensin system seen in these mice (Bleich et al., 1999). In addition, MRCamKCre mice show no differences in cort immediately after 40 min of restraint stress compared to control animals (Berger et al., 2006).
In both MRov and MR-Tg mice, basal HPA axis function appears unaffected; the CORT response to restraint stress is moderately suppressed in female, but not male MRov mice (Lai et al., 2007; Rozeboom et al., 2007). There is no change in cort release in MRov mice in response to mild stress (5 min in EPM) (Rozeboom et al., 2007).
The fact that three of four models of MR modulation exhibit no change in circadian cort argues that the classic model of MR control of basal HPA axis drive should be revisited. Considering the results reviewed above for GR mutants along with the lack of an HPA axis phenotype in the MR mutants, a new model that emphasizes a role for partial occupation of forebrain GR in inhibiting basal HPA axis drive should be considered. Again, the fact that the above models represent chronic alterations in MR may help explain the lack of phenotype as more acute alterations, such as MR antagonist treatment, can increase HPA axis drive (Ratka et al., 1989). Future work involving more direct targeting (e.g. viral mediated modulation of genes) or inducible targeting (e.g. tetracycline inducible system) of GR and MR populations within the forebrain should be useful in clarifying the roles of GR and MR in the basal control of HPA axis drive.
Behavioral phenotype of mice with MR alteration
Although MR has been largely thought of as a mediator of hippocampal cellular plasticity and basal HPA axis drive, recent evidence suggests a role for MR activity in modulating cognition (Table 2).
Preliminary studies in rescued MRKO mice suggest that these mice may display increased anxiety although no details have been published (Gass et al., 2001). In MRCamKCre mice, anxiety levels appear similar to controls on a variety of paradigms (e.g. open field, EOM, L:D preference), but decreased locomotor activity was seen in EOM and L:D preference (Berger et al., 2006). In contrast, MRov and MR-Tg mice display reduced anxiety-like behavior in open field (both mutants), L:D preference (MR-Tg only) and EPM (MRov only) (Lai et al., 2007; Rozeboom et al., 2007). The reduced anxiety phenotype may be partially explained by the increase in serotonin receptor 5HT-1a seen in MRov mice (Rozeboom et al., 2007).
MRCamKCre mice show memory impairments on the MWM with slower swim speeds and deficits in working memory on the RAM (Berger et al., 2006). In addition, hyperreactivity toward a novel object in a familiar environment was seen, suggesting an increased drive for exploration. MR-Tg mice show enhanced spatial memory in MWM and altered novel object recognition (Lai et al., 2007).
Increased MR activity in the forebrain is related to decreased anxiety and in some circumstances improved spatial memory capability. These anxiolytic effects of MR on anxiety appear to only occur with MR overexpression as no alterations were seen in MRCamKCre mice and the anxiety-like behavior reported in conventional MR knockouts may be related to increased cort acting on existing GR populations. This result suggests that GR may be sufficient for mediating the harmful effects of a dysregulated HPA axis on behavior as seen in some mood disorders. Additionally, targeted activation of MR in the forebrain could serve as a novel therapeutic route to alleviating anxiety-related symptoms. The contrasting effects on spatial memory in MRCamKCre (deficit) versus MR-Tg (enhancement) could be related to MR’s role as an anti-apoptotic agent in the hippocampus. It will be interesting to see whether more in-depth characterization of the MR-Tg mice reveals any changes in hippocampal circuitry.
Summary of MR phenotypes
In the past, pharmacological and lesion studies suggested that MR activity could play a role in modulating HPA axis drive as well as behavior associated with anxiety, depression, and learning and memory. However, the generation of transgenic MR mutants has identified the important role that signaling through MR has in modulating behavior while challenging the role of MR in basal HPA axis drive. As the role of MR in modulation of behavior is clarified, the interaction between MR and GR in each mutant background will have to be assessed. These studies should provide insight into the mechanism by which MR and GR function to regulate the HPA axis and behaviors associated with altered stress responsiveness. In fact, this additional level of complexity in understanding the role of GR and MR in stress adaptivity could help disentangle some of the results generated thus far.
CONCLUSIONS AND FUTURE DIRECTIONS
The use of transgenic and knockout genetic approaches has allowed a thorough and intriguing study of the role of both MR and GR in mediating HPA axis drive and stress-induced behavioral changes. These studies provide valuable insight into the link between the HPA axis, stress and psychiatric illness.
One important analysis that has only been partially characterized in a few of the GR and MR mutants is an evaluation of CRH and CRH receptor expression and function. While PVN CRH has a well-established role in activating the HPA axis, the role of CRH as a direct and crucial peptide modulator of behavior has emerged. An important component of this new role for CRH is in extrahypothalamic areas (including the amygdala, the bed nucleus of the stria terminalis and hippocampus). However, only GRov and FBGRKO mice have been analyzed for any changes in the extrahypothalamic CRH system. Additional analysis of the extrahypothalamic CRH system as well as other downstream targets of GR and MR activation should help to further clarify the role of corticosteroid receptor activity in the nervous system.
Taken together, the data from the GR and MR mutants indicates that GRs may play a role in the basal tonic inhibition that was once attributed solely to MR activity. Under stressful conditions, GR seems to be the predominant receptor in producing HPA axis feedback inhibition. With respect to behavior, increased GR is associated with increased anxiety- and despair-like behavior while decreased GR and increased MR are both associated with decreased behavior associated with anxiety and despair. The as yet incomplete characterization of behaviors associated with learning and memory in both sets of mutants hinders the formation of any strong conclusions but it is clear that the loss of either GR or MR can induce memory-like deficits while MR overexpression can facilitate MWM spatial memory.
While often mutually consistent, the various strains analyzed sometimes show conflicting or contradictory phenotypes. Given the subtle differences in targeting strategies, peripheral effects and possible developmental adaptations, these differences in phenotypes likely reflect the dynamic nature in which the endocrine system functions. Future work should include additional regional-specific and temporal targeting strategies. There is evidence for opposing roles of different region-specific GR populations in modifying behavior. If one deletes GR throughout the nervous system or even within the entire forebrain rather than within a more restricted region, the observed phenotype may be a combination of these opposing roles and thus be less clear and informative. Future targeting strategies will include newly developed promoters targeting dopaminergic neurons (Lemberger et al., 2007) or amygdalar/PVN areas (Balthasar et al., 2005). In addition, work with viral based vectors and stereotaxic delivery methods could allow sub-region specific targeting of populations of GRs and MRs. Temporally specific manipulations using inducible systems, such as the tetracycline inducible system, should allow researchers to avoid developmental complications as expression can be turned on after critical periods of development. Additionally, the system allows modulation of gene expression in an effort to demonstrate reversibility of the phenotype. Finally, the use of double GR and MR mutant mice (knockouts or overexpressors) will help reveal the poorly understood cellular and systems-level interactions between these two important steroid receptors.
Acknowledgements
We would like to thank Maria Elena Morales for manuscript review.
References
- Adams M, Meijer OC, Wang J, Bhargava A, Pearce D. Homodimerization of the glucocorticoid receptor is not essential for response element binding: activation of the phenylethanolamine N-methyltransferase gene by dimerization-defective mutants. Mol Endocrinol. 2003;17:2583–2592. doi: 10.1210/me.2002-0305. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237:268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- Avital A, Segal M, Richter-Levin G. Contrasting roles of corticosteroid receptors in hippocampal plasticity. J Neurosci. 2006;26:9130–9134. doi: 10.1523/JNEUROSCI.1628-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, Mcgovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Barden N, Stec IS, Montkowski A, Holsboer F, Reul JM. Endocrine profile and neuroendocrine challenge tests in transgenic mice expressing antisense RNA against the glucocorticoid receptor. Neuroendocrinology. 1997;66:212–220. doi: 10.1159/000127240. [DOI] [PubMed] [Google Scholar]
- Beaulieu S, Rousse I, Gratton A, Barden N, Rochford J. Behavioral and endocrine impact of impaired type II glucocorticoid receptor function in a transgenic mouse model. Ann N Y Acad Sci. 1994;746:388–391. doi: 10.1111/j.1749-6632.1994.tb39263.x. [DOI] [PubMed] [Google Scholar]
- Berger S, Bleich M, Schmid W, Cole TJ, Peters J, Watanabe H, Kriz W, Warth R, Greger R, Schutz G. Mineralocorticoid receptor knockout mice: pathophysiology of Na+ metabolism. Proc Natl Acad Sci U S A. 1998;95:9424–9429. doi: 10.1073/pnas.95.16.9424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S, Wolfer DP, Selbach O, Alter H, Erdmann G, Reichardt HM, Chepkova AN, Welzl H, Haas HL, Lipp HP, Schutz G. Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proc Natl Acad Sci U S A. 2006;103:195–200. doi: 10.1073/pnas.0503878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilang-Bleuel A, Ulbricht S, Chandramohan Y, De Carli S, Droste SK, Reul JM. Psychological stress increases histone H3 phosphorylation in adult dentate gyrus granule neurons: involvement in a glucocorticoid receptor-dependent behavioural response. Eur J Neurosci. 2005;22:1691–1700. doi: 10.1111/j.1460-9568.2005.04358.x. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Mckittrick CR, Blanchard DC. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol Behav. 2001;73:261–271. doi: 10.1016/s0031-9384(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Bleich M, Warth R, Schmidt-Hieber M, Schulz-Baldes A, Hasselblatt P, Fisch D, Berger S, Kunzelmann K, Kriz W, Schutz G, Greger R. Rescue of the mineralocorticoid receptor knock-out mouse. Pflugers Arch. 1999;438:245–254. doi: 10.1007/s004240050906. [DOI] [PubMed] [Google Scholar]
- Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y, Muglia LJ. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci U S A. 2005;102:473–478. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle MP, Kolber BJ, Vogt SK, Wozniak DF, Muglia LJ. Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J Neurosci. 2006;26:1971–1978. doi: 10.1523/JNEUROSCI.2173-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Khor B, Vogt SK, Muglia LM, Fujiwara H, Haegele KE, Sleckman BP, Muglia LJ. T-cell glucocorticoid receptor is required to suppress COX-2-mediated lethal immune activation. Nat Med. 2003;9:1318–1322. doi: 10.1038/nm895. [DOI] [PubMed] [Google Scholar]
- Brown ES, Varghese FP, Mcewen BS. Association of depression with mental illness: does cortisol play a role. Biol Psychiatry. 2004;55:1–9. doi: 10.1016/s0006-3223(03)00473-6. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Carnes M, Lent S, Feyzi J, Hazel D. Plasma adrenocorticotropic hormone in the rat demonstrates three different rhythms within 24 h. Neuroendocrinology. 1989;50:17–25. doi: 10.1159/000125197. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Bunney WE. Adrenal cortical activity in depressive illness. Am J Psychiatry. 1971;128:31–40. doi: 10.1176/ajp.128.1.31. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Greden JF, Feinberg M. Neuroendocrine disturbances and the diagnosis and aetiology of endogenous depression. Lancet. 1980;1:321–322. doi: 10.1016/s0140-6736(80)90826-0. [DOI] [PubMed] [Google Scholar]
- Chandramohan Y, Droste SK, Reul JM. Novelty stress induces phospho-acetylation of histone H3 in rat dentate gyrus granule neurons through coincident signalling via the N-methyl-d-aspartate receptor and the glucocorticoid receptor: relevance for c-fos induction. J Neurochem. 2007 doi: 10.1111/j.1471-4159.2006.04396.x. [DOI] [PubMed] [Google Scholar]
- Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schutz G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9:1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- Cole TJ, Myles K, Purton JF, Brereton PS, Solomon NM, Godfrey DI, Funder JW. GRKO mice express an aberrant dexamethasone-binding glucocorticoid receptor, but are profoundly glucocorticoid resistant. Mol Cell Endocrinol. 2001;173:193–202. doi: 10.1016/s0303-7207(00)00407-x. [DOI] [PubMed] [Google Scholar]
- Corcoran C, Walker E, Huot R, Mittal V, Tessner K, Kestler L, Malaspina D. The stress cascade and schizophrenia: etiology and onset. Schizophr Bull. 2003;29:671–692. doi: 10.1093/oxfordjournals.schbul.a007038. [DOI] [PubMed] [Google Scholar]
- De Kloet ER. Stress in the brain. Eur J Pharmacol. 2000;405:187–198. doi: 10.1016/s0014-2999(00)00552-5. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Reul JM. Feedback action and tonic influence of corticosteroids on brain function: a concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology. 1987;12:83–105. doi: 10.1016/0306-4530(87)90040-0. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Dijkstra I, Tilders FJ, Aguilera G, Kiss A, Rabadan-Diehl C, Barden N, Karanth S, Holsboer F, Reul JM. Reduced activity of hypothalamic corticotropin-releasing hormone neurons in transgenic mice with impaired glucocorticoid receptor function. J Neurosci. 1998;18:3909–3918. doi: 10.1523/JNEUROSCI.18-10-03909.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988;242:583–585. doi: 10.1126/science.2845584. [DOI] [PubMed] [Google Scholar]
- Gass P, Kretz O, Wolfer DP, Berger S, Tronche F, Reichardt HM, Kellendonk C, Lipp HP, Schmid W, Schutz G. Genetic disruption of mineralocorticoid receptor leads to impaired neurogenesis and granule cell degeneration in the hippocampus of adult mice. EMBO Rep. 2000;1:447–451. doi: 10.1093/embo-reports/kvd088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass P, Reichardt HM, Strekalova T, Henn F, Tronche F. Mice with targeted mutations of glucocorticoid and mineralocorticoid receptors: models for depression and anxiety? Physiol Behav. 2001;73:811–825. doi: 10.1016/s0031-9384(01)00518-2. [DOI] [PubMed] [Google Scholar]
- Hassan AH, Von Rosenstiel P, Patchev VK, Holsboer F, Almeida OF. Exacerbation of apoptosis in the dentate gyrus of the aged rat by dexamethasone and the protective role of corticosterone. Exp Neurol. 1996;140:43–52. doi: 10.1006/exnr.1996.0113. [DOI] [PubMed] [Google Scholar]
- Heck S, Kullmann M, Gast A, Ponta H, Rahmsdorf HJ, Herrlich P, Cato AC. A distinct modulating domain in glucocorticoid receptor monomers in the repression of activity of the transcription factor AP-1. Embo J. 1994;13:4087–4095. doi: 10.1002/j.1460-2075.1994.tb06726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesen W, Karst H, Meijer O, Cole TJ, Schmid W, De Kloet ER, Schutz G, Joels M. Hippocampal cell responses in mice with a targeted glucocorticoid receptor gene disruption. J Neurosci. 1996;16:6766–6774. doi: 10.1523/JNEUROSCI.16-21-06766.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, Thompson EB, Rosenfeld MG, Evans RM. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318:635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsboer F, Liebl R, Hofschuster E. Repeated dexamethasone suppression test during depressive illness. Normalisation of test result compared with clinical improvement. J Affect Disord. 1982;4:93–101. doi: 10.1016/0165-0327(82)90039-8. [DOI] [PubMed] [Google Scholar]
- Kitayama I, Janson AM, Cintra A, Fuxe K, Agnati LF, Ogren SO, Harfstrand A, Eneroth P, Gustafsson JA. Effects of chronic imipramine treatment on glucocorticoid receptor immunoreactivity in various regions of the rat brain. Evidence for selective increases of glucocorticoid receptor immunoreactivity in the locus coeruleus and in 5-hydroxytryptamine nerve cell groups of the rostral ventromedial medulla. J Neural Transm. 1988;73:191–203. doi: 10.1007/BF01250136. [DOI] [PubMed] [Google Scholar]
- Kretz O, Reichardt HM, Schutz G, Bock R. Corticotropin-releasing hormone expression is the major target for glucocorticoid feedback-control at the hypothalamic level. Brain Res. 1999;818:488–491. doi: 10.1016/s0006-8993(98)01277-3. [DOI] [PubMed] [Google Scholar]
- Kretz O, Schmid W, Berger S, Gass P. The mineralocorticoid receptor expression in the mouse CNS is conserved during development. Neuroreport. 2001;12:1133–1137. doi: 10.1097/00001756-200105080-00017. [DOI] [PubMed] [Google Scholar]
- Lai M, Horsburgh K, Bae SE, Carter RN, Stenvers DJ, Fowler JH, Yau JL, Gomez-Sanchez CE, Holmes MC, Kenyon CJ, Seckl JR, Macleod MR. Forebrain mineralocorticoid receptor overexpression enhances memory, reduces anxiety and attenuates neuronal loss in cerebral ischaemia. Eur J Neurosci. 2007;25:1832–1842. doi: 10.1111/j.1460-9568.2007.05427.x. [DOI] [PubMed] [Google Scholar]
- Lemberger T, Parlato R, Dassesse D, Westphal M, Casanova E, Turiault M, Tronche F, Schiffmann SN, Schutz G. Expression of Cre recombinase in dopaminoceptive neurons. BMC Neurosci. 2007;8:4. doi: 10.1186/1471-2202-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcewen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- Montkowski A, Barden N, Wotjak C, Stec I, Ganster J, Meaney M, Engelmann M, Reul JM, Landgraf R, Holsboer F. Long-term antidepressant treatment reduces behavioural deficits in transgenic mice with impaired glucocorticoid receptor function. J Neuroendocrinol. 1995;7:841–845. doi: 10.1111/j.1365-2826.1995.tb00724.x. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neurosci Res. 1996;26:235–269. doi: 10.1016/s0168-0102(96)01105-4. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry. 2004;65 Suppl 1:18–28. [PubMed] [Google Scholar]
- Nemeroff CB, Krishnan KR, Ree D, Leder R, Beam C, Dunnick NR. Adrenal gland enlargement in major depression: A computed tomographic study. Arch Gen Psychiatry. 1992;49:384–387. doi: 10.1001/archpsyc.1992.01820050048008. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, De Kloet ER, Joels M, Schmid W, Cole TJ. Spatial learning deficits in mice with a targeted glucocorticoid receptor gene disruption. Eur J Neurosci. 1997;9:2284–2296. doi: 10.1111/j.1460-9568.1997.tb01646.x. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, Reichardt HM, Joels M, De Kloet ER. Point mutation in the mouse glucocorticoid receptor preventing DNA binding impairs spatial memory. Proc Natl Acad Sci U S A. 2001;98:12790–12795. doi: 10.1073/pnas.231313998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Pepin MC, Pothier F, Barden N. Impaired type II glucocorticoid-receptor function in mice bearing antisense RNA transgene. Nature. 1992;355:725–728. doi: 10.1038/355725a0. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60:436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- Ratka A, Sutanto W, Bloemers M, De Kloet ER. On the role of brain mineralocorticoid (type I) and glucocorticoid (type II) receptors in neuroendocrine regulation. Neuroendocrinology. 1989;50:117–123. doi: 10.1159/000125210. [DOI] [PubMed] [Google Scholar]
- Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schutz G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- Reichardt HM, Schutz G. Feedback control of glucocorticoid production is established during fetal development. Mol Med. 1996;2:735–744. [PMC free article] [PubMed] [Google Scholar]
- Reichardt HM, Tuckermann JP, Gottlicher M, Vujic M, Weih F, Angel P, Herrlich P, Schutz G. Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. Embo J. 2001;20:7168–7173. doi: 10.1093/emboj/20.24.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt HM, Umland T, Bauer A, Kretz O, Schutz G. Mice with an increased glucocorticoid receptor gene dosage show enhanced resistance to stress and endotoxic shock. Mol Cell Biol. 2000;20:9009–9017. doi: 10.1128/mcb.20.23.9009-9017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JM, De Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Revest JM, Di Blasi F, Kitchener P, Rouge-Pont F, Desmedt A, Turiault M, Tronche F, Piazza PV. The MAPK pathway and Egr-1 mediate stress-related behavioral effects of glucocorticoids. Nat Neurosci. 2005;8:664–672. doi: 10.1038/nn1441. [DOI] [PubMed] [Google Scholar]
- Ridder S, Chourbaji S, Hellweg R, Urani A, Zacher C, Schmid W, Zink M, Hortnagl H, Flor H, Henn FA, Schutz G, Gass P. Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. J Neurosci. 2005;25:6243–6250. doi: 10.1523/JNEUROSCI.0736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochford J, Beaulieu S, Rousse I, Glowa JR, Barden N. Behavioral reactivity to aversive stimuli in a transgenic mouse model of impaired glucocorticoid (type II) receptor function: effects of diazepam and FG-7142. Psychopharmacology (Berl) 1997;132:145–152. doi: 10.1007/s002130050330. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Mcgaugh JL. Amygdaloid nuclei lesions differentially affect glucocorticoid-induced memory enhancement in an inhibitory avoidance task. Neurobiol Learn Mem. 1996;65:1–8. doi: 10.1006/nlme.1996.0001. [DOI] [PubMed] [Google Scholar]
- Rousse I, Beaulieu S, Rowe W, Meaney MJ, Barden N, Rochford J. Spatial memory in transgenic mice with impaired glucocorticoid receptor function. Neuroreport. 1997;8:841–845. doi: 10.1097/00001756-199703030-00007. [DOI] [PubMed] [Google Scholar]
- Rozeboom AM, Akil H, Seasholtz AF. Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. Proc Natl Acad Sci U S A. 2007;104:4688–4693. doi: 10.1073/pnas.0606067104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl JR. 11beta-Hydroxysteroid dehydrogenase in the brain: a novel regulator of glucocorticoid action? Front Neuroendocrinol. 1997;18:49–99. doi: 10.1006/frne.1996.0143. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 2000;861:288–295. doi: 10.1016/s0006-8993(00)02019-9. [DOI] [PubMed] [Google Scholar]
- Sonino N, Fava GA. Psychiatric disorders associated with Cushing's syndrome. Epidemiology, pathophysiology and treatment. CNS Drugs. 2001;15:361–373. doi: 10.2165/00023210-200115050-00003. [DOI] [PubMed] [Google Scholar]
- Steckler T, Holsboer F. Corticotropin-releasing hormone receptor subtypes and emotion. Biol Psychiatry. 1999a;46:1480–1508. doi: 10.1016/s0006-3223(99)00170-5. [DOI] [PubMed] [Google Scholar]
- Steckler T, Holsboer F. Enhanced conditioned approach responses in transgenic mice with impaired glucocorticoid receptor function. Behav Brain Res. 1999b;102:151–163. doi: 10.1016/s0166-4328(99)00003-0. [DOI] [PubMed] [Google Scholar]
- Steckler T, Rammes G, Sauvage M, Van Gaalen MM, Weis C, Zieglgansberger W, Holsboer F. Effects of the monoamine oxidase A inhibitor moclobemide on hippocampal plasticity in GR-impaired transgenic mice. J Psychiatr Res. 2001;35:29–42. doi: 10.1016/s0022-3956(00)00040-6. [DOI] [PubMed] [Google Scholar]
- Stocklin E, Wissler M, Gouilleux F, Groner B. Functional interactions between Stat5 and the glucocorticoid receptor. Nature. 1996;383:726–728. doi: 10.1038/383726a0. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Di S, Malcher-Lopes R. Minireview: rapid glucocorticoid signaling via membrane-associated receptors. Endocrinology. 2006;147:5549–5556. doi: 10.1210/en.2006-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Van Eekelen JA, Jiang W, De Kloet ER, Bohn MC. Distribution of the mineralocorticoid and the glucocorticoid receptor mRNAs in the rat hippocampus. J Neurosci Res. 1988;21:88–94. doi: 10.1002/jnr.490210113. [DOI] [PubMed] [Google Scholar]
- Van Haarst AD, Oitzl MS, De Kloet ER. Facilitation of feedback inhibition through blockade of glucocorticoid receptors in the hippocampus. Neurochem Res. 1997;22:1323–1328. doi: 10.1023/a:1022010904600. [DOI] [PubMed] [Google Scholar]
- Wei Q, Lu XY, Liu L, Schafer G, Shieh KR, Burke S, Robinson TE, Watson SJ, Seasholtz AF, Akil H. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc Natl Acad Sci U S A. 2004;101:11851–11856. doi: 10.1073/pnas.0402208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Wood SA, Lightman SL, Ingram CD. The pulsatile characteristics of hypothalamo-pituitary-adrenal activity in female Lewis and Fischer 344 rats and its relationship to differential stress responses. Endocrinology. 1998;139:4044–4052. doi: 10.1210/endo.139.10.6238. [DOI] [PubMed] [Google Scholar]
- Yang-Yen HF, Chambard JC, Sun YL, Smeal T, Schmidt TJ, Drouin J, Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990;62:1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]