Abstract
A critical role for arachidonic acid in the regulation of calcium entry during agonist activation of calcium signals has become increasingly apparent in numerous studies over the past 10 years or so. In particular, low concentrations of this fatty acid, generated as a result of physiologically relevant activation of appropriate receptors, induces the activation of a unique, highly calcium-selective conductance now known as the ARC channel. Activation of this channel is specifically dependent on arachidonic acid acting at the intracellular surface of the membrane, and is entirely independent of any depletion of internal calcium stores. Importantly, a specific role of this channel in modulating the frequency of oscillatory calcium signals in various cell types has been described. Recent studies, subsequent to the discovery of STIM1 and the Orai proteins and their role in the store-operated CRAC channels, have revealed that these same proteins are also integral components of the ARC channels and their activation. However, unlike the CRAC channels, activation of the ARC channels depends on the pool of STIM1 that is constitutively resident in the plasma membrane, and the pore of these channels is comprised of both Orai1 and Orai3 subunits. The clear implication is that CRAC channels and ARC channels are closely related, but have evolved to play unique roles in the modulation of calcium signals – largely as a result of their entirely distinct modes of activation. Given this, although the precise details of how arachidonic acid acts to activate the channels remain unclear, it seems likely that the specific molecular features of these channels that distinguish them from the CRAC channels – namely Orai3 and/or plasma membrane STIM1 – will be involved.
1. Introduction – ARC channels and agonist-activated Ca2+ entry
An enhanced entry of Ca2+ is a critical component of agonist-induced cytosolic Ca2+ signals in most cell types. In electrically nonexcitable cells, the study of such entry has been largely dominated by the so-called store-operated channels, activation of which is, by definition, dependent on the depletion of internal Ca2+ stores, notably the endoplasmic reticulum (ER) [1]. Given that the maintenance of adequate Ca2+ levels in the ER is critical for a variety of essential cellular functions (e.g. correct folding and processing of synthesized proteins), it is not surprising that store-operated Ca2+ entry has been observed in almost every cell examined. However, and despite frequent claims to the contrary, it would be wrong to conclude that this ubiquitous presence indicates that such entry underlies all, or even most, of the receptor-activated calcium signals seen in such cells. In reality, examination of the literature indicates that direct demonstration of the essential role of store-operated conductances in normal physiologically relevant responses is largely limited to hematopoetic cells – most notably lymphocytes and mast cells [2–5]. Here, it is clear that the activation of the highly Ca2+-selective store-operated CRAC channels is an essential component of the normal physiological responses of these cells to relevant agonists .
In contrast to these specific cases, almost all studies of such entry in other cell types have involved the depletion of the internal stores using either pharmacological agents (e.g. thapsigargin, cyclopiazonic acid, ionomycin), or high concentrations of internally applied InsP3 (or its potent analog adenophostin A), or simply by inducing passive store depletion by the introduction of highly buffered Ca2+-free intracellular solutions. Whilst such studies certainly demonstrate the presence of store-operated Ca2+ entry in these cells, their relevance to actual physiological responses is far from clear. Moreover, in the few examples where actual agonists have been used, either maximal or supramaximal concentrations were employed. Given that the ability of simple increases in cytosolic Ca2+ to signal effectively, in a discrete and tightly regulated fashion, to a diverse range of effectors would seem to demand some form of finely-tuned, graded response to agonist concentration, the physiological relevance of such protocols is certainly questionable. Indeed, the simple fact that gating of these channels is entirely dependent on, and subsequent to, the depletion of intracellular Ca2+ stores imposes certain constraints on their activation and function that raise questions as to whether such channels can provide the exclusive source of agonist-activated Ca2+ entry at all levels of stimulation. For example, the various activation protocols mentioned above only serve to emphasize that depletion of the intracellular Ca2+ stores can be achieved by a variety of different means, many of which may be unrelated to any receptor activation (e.g. changes in SERCA pump activity, in ATP levels, or in the poorly understood constitutive ER leak pathways). This indicates that simple store depletion itself might lack the required specificity to act as a reliable means for the accurate relaying of information from receptor activation to the induction of the appropriate response. Instead, it suggests that this pathway may be best suited to function as a “safety net” ensuring repletion of any Ca2+ stores that have become depleted as a result of, for example, various pathological or stress conditions.
Given this, it should not be surprising that evidence from a variety of different cell types has accumulated over the past several years demonstrating that alternative, store-independent pathways exist, and that it is these that often provide the predominant route of Ca2+ entry – particularly at lower, more physiologically relevant, levels of stimulation. Under these conditions, activation of the cells often results in the generation of oscillatory Ca2+ signals, and here the principal role of Ca2+ entry is to act as a modulator of the oscillation frequency [6–9]. Due largely to the greater fidelity, “band-width”, and signal-to-noise characteristics inherent in such oscillatory signals, it is widely held that they are critical in enabling the cell to regulate a diverse range of effects and responses in an appropriate manner, and therefore likely represent the physiologically relevant response of many cells to a range of different agonists. A search for conductances that might be responsible for such store-independent agonist-activated Ca2+ entry eventually led to the discovery of the ARC channels (arachidonate-regulated Ca2+ channels) – a novel highly Ca2+-selective conductance whose activation is independent of any store depletion and is, instead, specifically dependent on the receptor-mediated generation of low levels of intracellular arachidonic acid (20:4, cis-5,8,11,14) [10–13].
2. ARC channels – biophysical properties and molecular identity
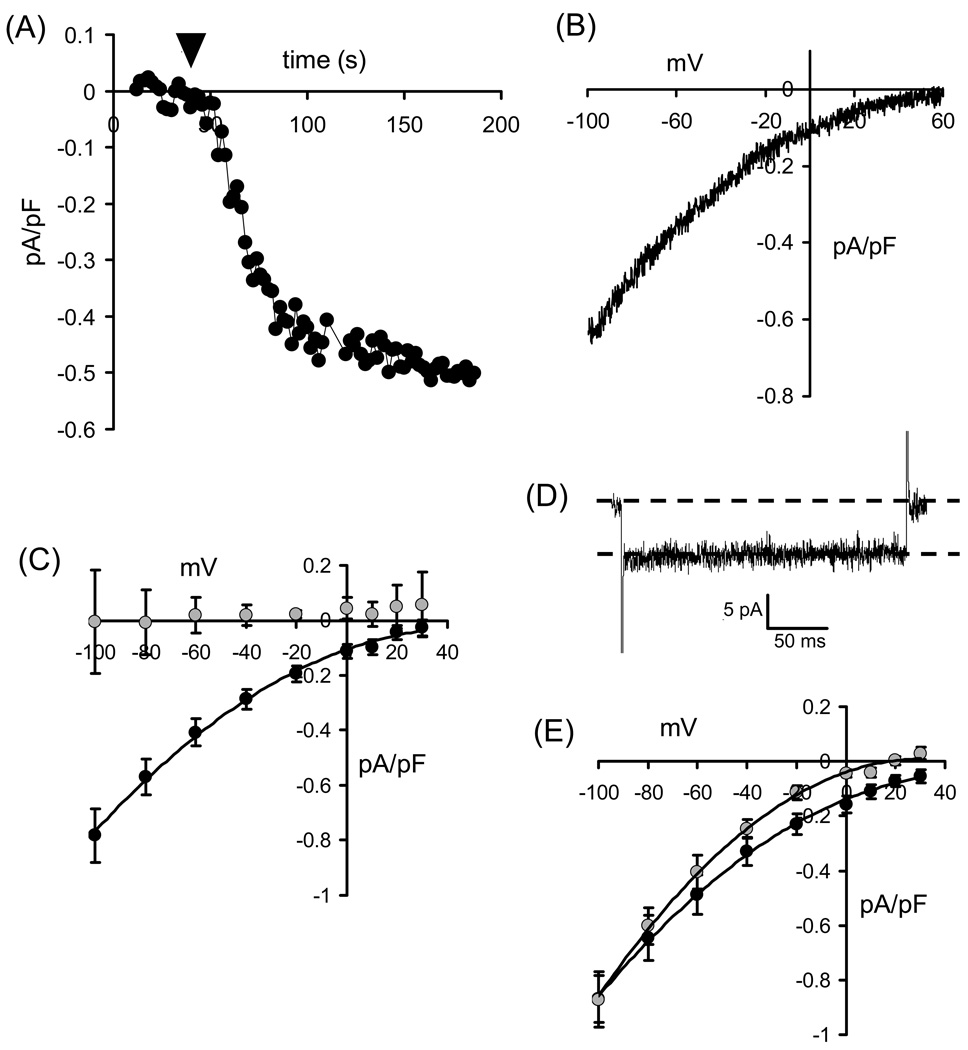
Biophysically, the ARC channels display many features in common with the store-operated CRAC channels (Fig. 1). Like the CRAC channels, ARC channels display small conductance values, with whole-cell current densities typically in the range of 0.5–1 pA/pF at −80 mV. Similarly, as would be expected of highly Ca2+-selective conductances, their current-voltage relationships show marked inward rectification and very positive reversal potentials (> +60 mV) (Fig. 1A, B). Moreover, both are inhibited by external La3+ (50–100 µM), as well as by low concentrations of Gd3+ (1–5 µM) (Fig. 1C). However, ARC channels show none of the Ca2+-dependent fast inactivation seen in CRAC channels during brief depolarization to negative potentials (Fig. 1D). Also, unlike the CRAC channels, they are largely unaffected by reduction of external pH, and are insensitive to the drug 2APB (Fig. 1E). Moreover, separate sequential activation of these two conductances in the same individual cell shows that their macroscopic currents are strictly additive – confirming that they represent distinct biophysical entities. Currents displaying features consistent with ARC channel activity have now been independently reported by several groups in various cell lines (HEK293, HeLa, RBL, COS, SY5Y, and K562 cells) as well as in primary cells (parotid and pancreatic acinar cells) [14–17]. Critically, specific activation of these channels occurs at the same low concentrations of agonists that generate oscillatory Ca2+ signals, and is graded over the precise range of concentrations that induce oscillatory signals of increasing frequency [18]. As such, this stands in marked contrast to the situation in CRAC channels, where existing evidence indicates that activation occurs in a strictly “all-or-none” manner to increasing agonist concentrations [19,20].
Figure 1.
Biophysical and pharmacological characteristics of the ARC channels. (A) Activation of ARC channel currents in a HEK293 cell by exogenous addition of arachidonic acid. Inward currents were measured at −80mV. Arachidonic acid (8 µM) was added to the external medium where indicated (arrowhead). (B) Current-voltage relationship of the arachidonic acid-induced currents in HEK293 cells. (C) Effect of gadolinium on ARC channel currents. Mean (± SE) ARC channel current-voltage relationships are shown for control cells (black symbols), and in cells exposed to Gd3+ (5 µM) (grey symbols). (D) Absence of any fast-inactivation in ARC channel currents. Shown is a representative recording of ARC channel current during a brief (250 ms) pulse to −80 mV. (E) Lack of any significant effect of 2-APB on ARC channel currents. Mean (± SE) ARC channel current-voltage relationships are shown for control cells (black symbols), and in cells exposed to 2-APB (100 µM) (grey symbols). Data in (C) and (E) redrawn from [14].
Although the essential biophysical features of the ARC channels have been known since 2000, further insight into the nature of these channels was hampered by the lack of any clear indication of their molecular identity. Recently, however, this situation has been dramatically changed by studies revealing the key role of the stromal interacting molecule (STIM) proteins and the Orai proteins, first in CRAC channels [21–23], and then subsequently in ARC channels [24,25]. With regard to the store-operated CRAC channels, studies from various groups have established that STIM1 located in the ER membrane acts as the critical sensor of the status of the intracellular Ca2+ stores via a N-terminal Ca2+-binding EF-hand domain that would be located in the ER lumen [26–29]. Dissociation of Ca2+ from this site leads to the oligomerization of STIM1 and its translocation to regions of the ER that lie close to the plasma membrane. Here, they signal to the CRAC channels, the pore of which is formed from Orai1 subunits [30–32] assembled in a homotetrameric structure [33,34]. Based on these original studies, the idea rapidly developed that the action of STIM1 on Ca2+ entry and the involvement of Orai proteins in such entry, were specifically, and exclusively, associated with the store-operated mode of entry. However, at the time that this idea was developed, any possible role of STIM and/or Orai proteins in other modes of Ca 2+ entry had not been examined.
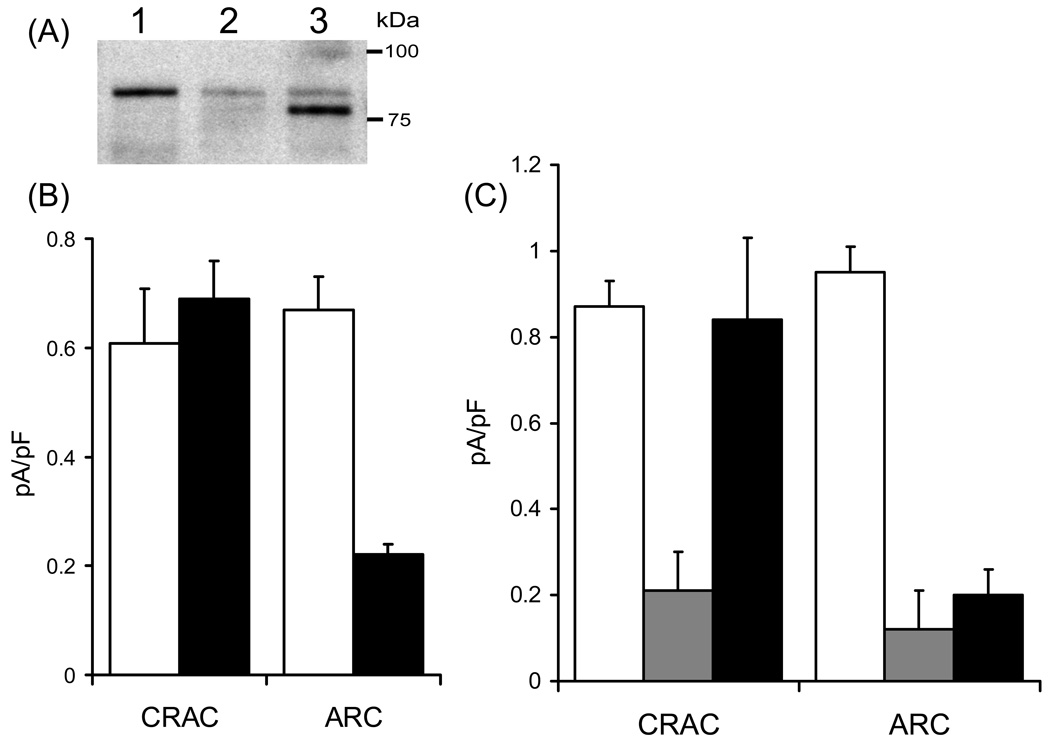
Given that activation of the ARC channels is independent of store-depletion, it was expected that STIM1 would play no role in the regulation of their activity. Consequently, we were surprised to find that changing STIM1 protein levels (either by siRNA or by overexpression) had effects on the activity of the ARC channels that were essentially indistinguishable from those seen with CRAC channels [24]. However, further studies revealed that the mechanisms involved were completely different for these two channel types. Thus, in marked contrast to the role of ER STIM1 in regulating CRAC channels, regulation of the ARC channels is entirely independent of the Ca 2+-binding ability of the EF-hand domain and, instead, exclusively involves the pool of STIM1 that constitutively resides in the plasma membrane [24]. The demonstration of a pool of STIM1 that is constitutively resident in the plasma membrane of cells actually dates back to the original publications identifying this protein [35–38], before its role in store-operated calcium entry was known. The size of this plasma membrane pool of STIM1 seems to vary somewhat in different cell types, but is typically in the range of 15–25% of the total cellular STIM1 [24,36]. The key role of plasma membrane STIM1 in the regulation of the activity of the ARC channels was perhaps most clearly illustrated by experiments involving mutants of STIM1 that were designed to impair its constitutive expression in the plasma membrane. Previous studies had shown that such expression is critically dependent on a process of N-linked glycosylation at two sites (N131 and N171) [38]. We therefore generated a STIM1 construct in which these two sites were mutated to glutamines (N→Q mutant). In order to minimize the contribution of the endogenous wild-type STIM1, cells were transfected with an siRNA targeting STIM1, along with the N→Q mutant STIM1 in a siRNA-resistant construct (generated by insertion of two silent mutations in the siRNA recognition site), In this way, the endogenous wild-type STIM1 is essentially replaced by the glycosylation site mutant STIM1 (Fig. 2A). The result was that currents through the CRAC channels were indistinguishable from those recorded in cells expressing the siRNA-resistant wild-type STIM1, whereas the corresponding ARC channel currents were reduced by ~70% compared to cells expressing the siRNA-resistant wild-type STIM1 (Fig. 2B) [24]. Clearly, such data demonstrate that the activity of the ARC channels is critically and exclusively dependent on the expression of STIM1 in the plasma membrane. Moreover, they indicate that STIM1 must be a far more universal regulator of Ca2+ entry pathways than had previously been assumed, and that the initial assumption that STIM1 is only involved in store-operated Ca2+ entry was incorrect.
Figure 2.
The involvement of plasma membrane STIM1 and Orai proteins in ARC channel function. (A) Western blot showing STIM1 protein in control cells (lane 1), in cells transfected with the STIM1 siRNA (lane 2), and in siRNA-transfected cells expressing the siRNA-resistant N-glycosylation mutant STIM1 (lane 3). In the latter, the residual endogenous STIM1 after siRNA treatment can be seen as a faint band running at the same point as in lanes 1 and 2, whilst the expressed siRNA-resistant glycosylation mutant runs at a lightly lower molecular weight. (B) Expression of the N-glycosylation mutant STIM1 specifically inhibits ARC channel currents. Values represent mean (± SE) inward CRAC channel and ARC channel current densities measured at −80 mV in control cells (white bars), and in siRNA-treated cells expressing the N-glycosylation mutant STIM1 (black bars), n = 6–11. (C) Expression of the dominant-negative Orai1 inhibits both CRAC and ARC channel currents, whilst expression of the dominant-negative Orai3 inhibits only the ARC channel currents. Values represent mean (± SE) inward current densities measured at −80 mV in cells stably expressing STIM1 (white bars), and in the same cells transfected with the E106Q mutant Orai1 (grey bars), or the E81Q mutant Orai3 (black bars), n = 4–6. Data redrawn from [24] and [25].
We have already noted that the basic biophysical properties of the conductances represented by the ARC channels and the CRAC channels are very similar, with the most obvious and profound difference being only in their mode of activation. This, together with the finding that they share the ability to be regulated by STIM1, albeit in distinct ways, suggests that these two conductances might be molecularly related. On this basis, we examined whether Orai1, or any of the other two Orai family members present in mammals, might have a similar role in the ARC channels. The result was that, like the store-operated CRAC channels, the molecular composition of the ARC channels also involves members of the Orai proteins. However, whilst the CRAC channel pore appears to be comprised of only Orai1, both Orai1 and Orai3 (but not Orai2) appear to contribute to the ARC channel pore [25]. For example, overexpression of Orai1 results in significant increases in both the CRAC channel currents, and the ARC channel currents. Similarly, expression of a dominant-negative Orai1 mutant (E106Q) profoundly inhibits currents through both the CRAC and ARC channels. However, expression of the corresponding dominant-negative Orai3 mutant (E81Q) completely obliterates the currents through the ARC channels, without affecting those through the CRAC channels (Fig. 2C). As yet, the detailed organization and stoichiometry of the heteromeric Oria1/Orai3 assembly that presumably forms the ARC channel pore remains to be determined. However, it seems clear that the CRAC and ARC channels should now be considered as founding members of an entirely new family of channel proteins, the “Orai-based channels”. Despite this, our functional studies have demonstrated that these two channels evolved to operate under distinct conditions of stimulation and to serve unique roles in the regulation of agonist-activated Ca2+ signals – features that derive largely from their entirely distinct modes of activation.
3. Activation by arachidonic acid (I) – sensitivity, and fatty acid specificity
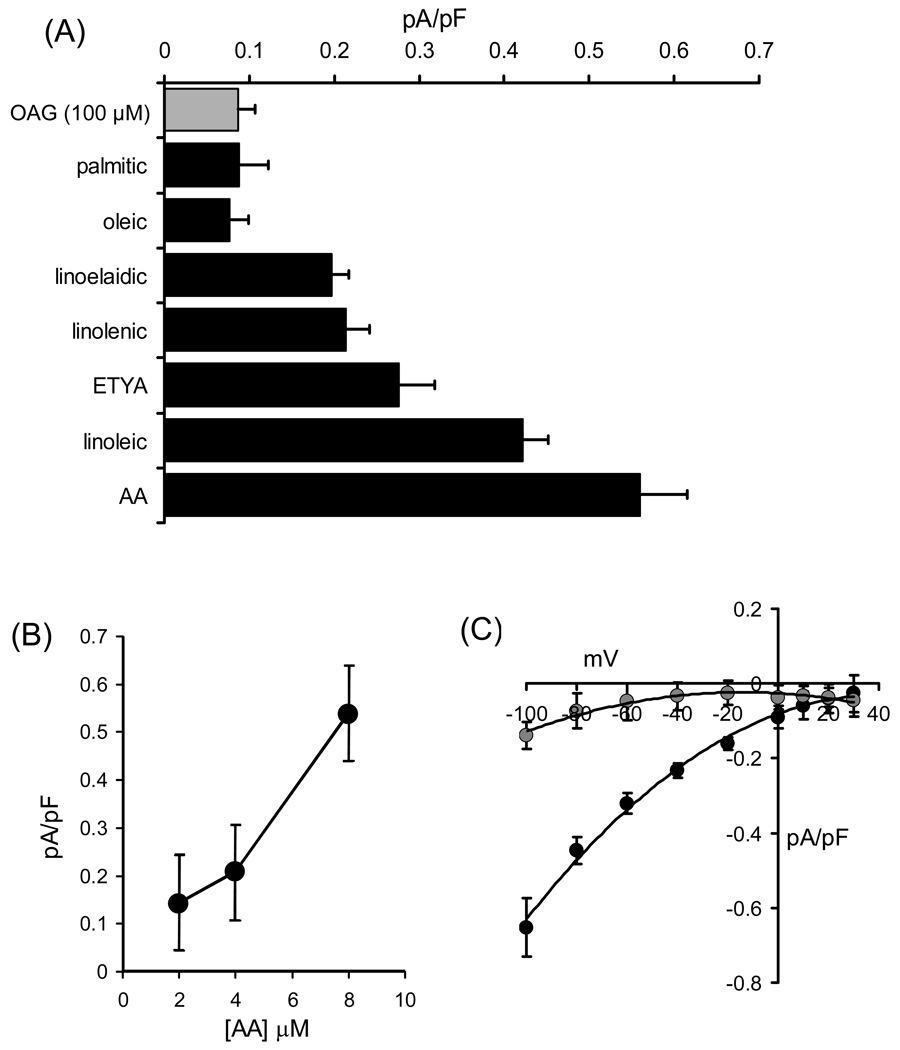
As already emphasized, the key feature of ARC channels that distinguishes them from the highly Ca2+-selective CRAC channels, as well as other store-operated conductances, is that their activation is entirely dependent on either the agonist-activated generation, or exogenous application, of arachidonic acid. However, before the physiological relevance or validity of such an effect can be evaluated it is necessary to consider several criteria beyond the simple demonstration that arachidonic acid induces an entry of Ca2+ in the absence of detectible store depletion. In this context, we were able to demonstrate that the generation of arachidonic acid was enhanced at the same low concentrations of agonists that induced the entry of Ca2+, and that specific pharmacological inhibition of this agonist-induced arachidonic acid generation blocked both the simultaneous entry of Ca2+ [11], and the activation of the ARC channels [18]. Moreover, experiments involving pharmacological inhibition of the major enzymes that metabolize the fatty acid revealed that the effects observed were dependent on arachidonic acid itself, rather than one of its many metabolites [11]. Detectible currents displaying all the characteristic features of the ARC channels could be recorded with concentrations of exogenous arachidonic acid as low as 2 µM [14] (Fig. 3B). Importantly, such concentrations of arachidonic acid are in same range as the Kd’s for the enzymes that metabolize the fatty acid in cells (3–8 µM), indicating that the channels are activated by concentrations of the fatty acid within the cell that are likely to exist during agonist stimulation. The particular importance of this is discussed further below.
Figure 3.
(A) Mean (±SE) inward current densities measured at −80 mV induced by exogenous application of the diacylglycerol OAG (100 µM, grey bar), and various fatty acids (black bars, all at 8 µM), n = 4–11. (B) ARC channel current magnitudes induced by different concentrations of exogenous arachidonic acid. Values are mean (± SE) inward current density at −80 mV (n = 4–6). (C) Comparison of internally and externally applied arachidonyl coenzyme A on currents through ARC channels. Shown are the mean (± SE) current-voltage relationships of the currents activated by application of arachidonyl coenzyme A (8 µM) added in the pipette solution (black symbols, n = 6), or in the external bath (grey symbols, n = 5). Data in (B) and (C) redrawn from [14].
In reality, the finding of an agonist-activated Ca2+ entry pathway that was dependent on the generation of arachidonic acid was unexpected, and immediately raised several critical questions regarding its specificity, and its possible mode of action. With regard to specificity, exogenous addition of either the saturated fatty acid palmitic acid (16:0) or the mono-unsaturated fatty acid oleic acid (18:1, cis-9) revealed that neither had any significant effect on the activity of the channels [14] (Fig. 3A). Moreover, examination of a series of polyunsaturated fatty acids (both cis and trans) with different chain lengths and numbers of double bonds, showed that only linoleic acid (18:2, cis-9,12) was capable of inducing significant currents (Fig. 3A). These currents were equivalent to approximately 75% of the normal arachidonic acid-stimulated current and, given that linoleic acid is a metabolic precursor of arachidonic acid, may simply reflect the conversion of this fatty acid in the cell. The triple-bond non-metabolizable analog of arachidonic acid, ETYA (20:4, yne-5,8,11,14), acts as an effective blocker of lipoxygenase, cyclo-oxygenase, and P450 pathways for the metabolism of the fatty acid [39] – an action that reflects its close structural analogy to arachidonic acid. Despite this, its ability to activate the ARC channels is significantly impaired (~50%) compared to arachidonic acid itself [14]. Finally, it is also worth noting that ARC channels are not activated by the diacylgycerol analogue 1-oleoyl-2-acetyl-sn-glycerol (OAG), even at concentrations as high as 100 µM (Fig. 3A). The significance of this is that certain members of the mammalian TRPC family (especially TRPC3 TRPC6, and TRPC7) have been reported to be activated by diacylglycerols (including OAG) in a manner that is independent of store-depletion [40]. Clearly this is not the case for ARC channels.
As to its mode of action, it is necessary to consider the fact that arachidonic acid can affect the properties of membrane proteins, such as ion channels, by directly acting on the proteins themselves, or indirectly by inducing modifications in the properties or integrity of the lipid bilayer in which the proteins are incorporated. Possible indirect effects on the properties of the lipid membrane can be loosely categorized into two types – changes in membrane fluidity, and detergent effects [41]. Importantly, changes in membrane fluidity are a particular property of the cis-unsaturated fatty acids like arachidonic acid and, of course, depend on the concentration of the fatty acid used. Consequently, the low concentrations at which arachidonic acid is effective in the activation of the ARC channels is particularly important. Moreover, the likely involvement of these types of effect are, to some extent, counter-indicated by the demonstrated high degree of specificity, among a range of PUFAs, for the arachidonic acid moiety itself. Moreover, the effects of ETYA are particularly revealing in this context. Although structurally similar to arachidonic acid, the presence of an additional double bond in ETYA would predict an even more potent effect on membrane fluidity than arachidonic acid itself [42]. However, as noted above, it is a far less effective activator of the ARC channels, suggesting that general membrane fluidity is unlikely to be a critical part of the activation of the channels. As for potential detergent effects, these generally center on the ability of amphiphilic molecules, including arachidonic acid, to form micelles at higher concentrations (~10 µM) [43]. These micelles can have a variety of effects including the formation of lipid pores capable of allowing the indiscriminate passage of large ions across membranes [44]. Again, such effects are very concentration-dependent emphasizing the importance of the low effective concentrations at which arachidonic acid acts on the ARC channels. As a simple illustration of this, concentrations of exogenously added arachidonic acid greater than 25 µM routinely induce large, highly nonselective leak conductances capable of passing even the very large cation NMDG+. Clearly, such effects indicate a significant perturbation of the integrity of the cell membrane. No such non-discriminating “leak” currents are observed at the concentrations of arachidonic acid (2–8 µM) that are effective in the activation of the ARC channels. Taken together, the above data suggest that the ability of arachidonic acid to activate the ARC channels most likely reflects some kind of, as yet undefined, specific “molecular” action – either on the channel itself, or on some regulator of the channels. Of course, the fact that the CRAC channel is entirely unaffected by arachidonic acid, despite its obvious close molecular relationship, is further evidence for either a specific action on the channel itself, on an action on a specific modulator of the channel.
As already discussed, the normal physiological activation of the ARC channels is dependent on the intracellular generation of arachidonic acid as a result of agonist action. Given this, we would predict that the fatty acid is most likely acting exclusively at the inner face of the membrane. Of course, the extracellular application of exogenous arachidonic acid in the experiments described above, does not allow such discrimination of “sidedness” because arachidonic acid, along with other fatty acids, is readily able to “flip” between outer and inner leaflets of the cell membrane [45]. We therefore examined the ability of the arachidonic acid analog arachidonyl coenzyme A (ACoA) to activate the ARC channels when applied either outside of the cell or intracellularly via the patch pipette. This molecule differs from arachidonic acid by the presence of a large charged head-group which effectively prevents its ability to cross the plasma membrane. The results obtained showed that ACoA was able to activate macroscopic currents that were qualitatively and quantitatively indistinguishable from those seen with application of exogenous arachidonic acid, but only when applied to the inside of the cell [14]. Addition of ACoA to the bath produced negligible currents that were indistinguishable from background noise (Fig. 3C). This confirms that activation of the ARC channels by arachidonic acid specifically involves an action of the fatty acid either in, or at the cytosolic face of, the inner leaflet of the membrane.
Finally, it is also worth noting that certain ion channels that are sensitive to arachidonic acid, are also sensitive to changes in levels of the phosphoinositide PIP2 (phosphatidylinositol 4,5-bisphosphate). The most obvious example of this is the human inwardly-rectifying potassium channel hKir2.3. Here arachidonic acid increases the channel activity, but does so indirectly by increasing the affinity of the channel for PIP2 [46]. However, evidence indicates that such PIP2-arachidonic acid interactions can likely be excluded in the case of the ARC channels. For example, during the normal agonist-induced activation of the channels, we would predict that receptor-mediated increases in arachidonic acid generation, would be co-incidental with the activation of PLC and the consequent metabolism of PIP2. This is most clearly shown in the example of a HEK293 cell line stably expressing the muscarinic m3 receptor, where activation of this single receptor type with carbachol results in the generation of both arachidonic acid and InsP3 [11]. If activation of the channels required an arachidonic acid-dependent increase in PIP2 affinity, then any agonist-induced activation would result in the simultaneous contradictory actions of increasing arachidonic acid levels and decreasing PIP2 levels. An alternative possibility is that, in the case of the ARC channels, PIP2 is inhibitory. However, the fact that agonist-induced activation of the ARC channels is blocked by the selective pharmacological inhibition of the generation of arachidonic acid, independent of any effect on PLC activity [18], argues against such a possibility. Of course, it is possible that arachidonic acid exerts its actions via a pool of PIP2 that is independent of PLC activity. However, as yet, we have no evidence for any such pool.
4. Activation by arachidonic acid (II) – the role of phosphorylation
Although activation of the ARC channels is essentially dependent on arachidonic acid, the activity of the channels is also profoundly influenced by the opposing actions of PKA-dependent phosphorylation and calcineurin-dependent dephosphorylation [47,48]. However, neither the calcineurin-dependent inhibition, nor the PKA-dependent activation are themselves arachidonic acid dependent. These phosphorylation/dephosphorylation effects are of critical physiological relevance, as they are key to the phenomenon we have described as the “reciprocal regulation of Ca2+ entry”. In this, the sustained elevation in cytosolic Ca2+ levels that result from the activation of store-operated Ca2+ entry (e.g. via the CRAC channels), leads to the activation of calcineurin and the consequent inhibition of the ARC channels [18]. In this way, the predominant mode of Ca2+ entry is switched from the store-independent ARC channels, to the store-operated channels as agonist concentrations increase. Further studies revealed that, not only was the calcineurin-dependent dephosphorylation able to inhibit previously activated ARC channels, but it also renders the channels insensitive to subsequently applied arachidonic acid – in other words, it blocks the activation of the channels by the fatty acid. This suggested that phosphorylation is necessary for the effective stimulation of the ARC channels by arachidonic acid. Examination of the underlying nature of this phosphorylation revealed that neither PKC, protein kinase G, or CaM-kinase were effective, and that it was specifically dependent on the activity of protein kinase A. In addition, the PKA-dependent phosphorylation effects on ARC channel activity were dependent on a member of the family of A-kinase anchoring proteins (AKAPs) [48]. Thus, the Ht31 peptide which specifically disrupts the interactions between AKAPs and PKA, inhibited both the ability of ARC channels to be activated by arachidonic acid, and the PKA-dependent restoration of ARC channel activity following a calcineurin-induced dephosphorylation. In this context, it is interesting that of the various AKAP family members, AKAP79 is unique in that it acts as a scaffold for both PKA and calcineurin. Moreover, AKAP79 is present in the HEK293 cells used in the above studies [48,49].
Although it is clear that PKA-dependent phosphorylation plays a key role in activation of the ARC channels, it is important to emphasize that phosphorylation does not, itself, activate the channels – rather, it is a prerequisite for arachidonic acid to do so. The simplest explanation for this is that the channel, or some regulator of the channel, must be in a phosphorylated state in order for arachidonic acid to induce the activation. Alternatively, it is the possible that, once activated by arachidonic acid, phosphorylation results in a markedly higher overall activity of the channels, presumably via increases in open probability and/or single channel conductance. In either case, it is clear the PKA-dependent phosphorylation is necessary, but not sufficient for the full effective activation of the ARC channels.
5. Future directions – the molecular basis of activation by arachidonic acid
Together, data accumulated over the past 8–10 years have revealed much about the arachidonic acid-dependent activation of a novel store-independent mode of agonist-activated Ca2+ entry, the role and molecular identity of the ARC channels that mediate such entry, and its actions in modulating the frequency of oscillatory Ca2+ signals. However, it is equally apparent that much remains to be determined regarding the precise nature of this arachidonic acid-dependent effect on Ca2+ signaling.
Of course, a critical goal is the identification of the actual site, or sites, of action of arachidonic acid in activating the ARC channels. We have already discussed the evidence that the actions of arachidonic acid on the channels are unlikely to reflect any essential nonspecific effects on the physical properties, or integrity, of the cell membrane, or any possible interacting actions of the fatty acid with PIP2 – suggesting some form of direct molecular interaction either with the channel protein itself or with a modulator of the channel. A problem here is that, although there are numerous precedents for the direct regulation of various channel types by arachidonic acid [50], the structural determinants of such modulation (in the cases where they have been mapped), reveal no obvious sequence analogy – i.e. there is no conserved consensus motif. However, this lack of specificity at the level of amino acid sequence does not mean that specificity is not achieved by other means. Indeed, that such specificity exists is clearly demonstrated by the fact that, although molecularly closely related, the CRAC channel is entirely unaffected by arachidonic acid. However, it does mean that any search for “arachidonic acid consensus sequences” in any potential target protein is likely to be fruitless.
Nevertheless, in the case of the ARC channels, it may still be possible to narrow down the key participants in this activation, particularly in light of the new information available on the actual molecular identity of, at least some, of the key players. Thus, we know that the overall activity of the ARC channels is influenced by a variety of different molecular entities including STIM1 [24], both Orai1 and Orai3 [25], and an AKAP, possibly AKAP79 [48]. However, in a practical sense, this in itself raises some important problems. For example, at a minimum, any one of these may have the potential to act as a limiting factor for overall channel activity. Because of this, the effects (or lack thereof) of the simple overexpression of one or more of these components must obviously be interpreted with caution. In addition, any overexpression of these potentially interacting components must be carefully regulated. Thus, we can assume that, for example, STIM1, Orai1, and/or Orai3 all have at least some level of affinity for each other. Whilst some of these affinities may only be very low, and therefore essentially physiologically “irrelevant” in the endogenous situation, they could become effective at increasing levels of overexpression, resulting in the formation of artificial (i.e. non-native) complexes. It may be expected that these would likely display, at least, some features of the “real” channels and may, in this way, be very misleading – hence the need to avoid protocols involving excessive overexpression.
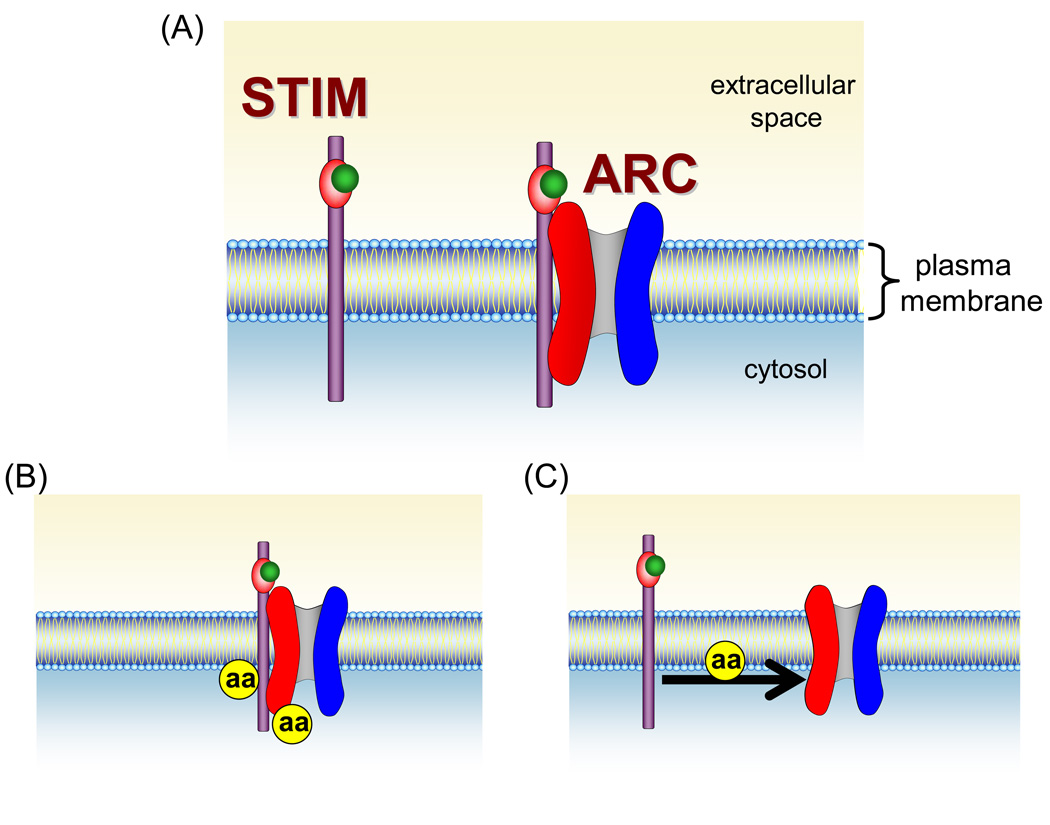
Despite these potential problems it seems clear that the specific activation of the ARC channels by arachidonic acid likely reflects some unique molecular feature of the channels and/or their regulators. Two such features are immediately apparent. The first is the requirement of Orai3 in the molecular structure of the ARC channel pore, and the second is the specific involvement of the pool of STIM1 that is constitutively present in the plasma membrane (Fig. 4A). These, then, currently represent the most likely candidates for the key target of arachidonic acid (Fig. 4B). With respect to the role of STIM1 in the activation of the ARC channels, it is important to note that the STIM1 in the plasma membrane is likely to behave rather differently than STIM1 in the ER [51]. Most obviously, because of the extracellular location of the N-terminal EF-hand, it would never, under normal circumstances, lose its bound Ca2+. This implies that the reported Ca2+-dependent changes in the conformation of the adjacent sterile α-motif (SAM) domain of STIM1, and the subsequent cytosolic domain-dependent oligomerization of STIM1 [52,53] are unlikely to play any role in the activation of the ARC channels. The potential relevance of this is that it is thought that the specific conformation of the SAM domain, when Ca2+ is bound to the EF-hand, is such that it acts to prevent the oligomerization and aggregation of STIM1 [53–55] – a process that is dependent on the cytosolic domains of STIM1. During the activation of the CRAC channels, dissociation of Ca2+ from the EF-hand, results in conformational changes in the SAM domain, which lead to the loss of this inhibitory ability, and the ultimate activation of the CRAC channels [54]. It would seem likely that such potential conformational changes will play no part in the STIM1-dependent activation of the ARC channels.
Figure 4.
Potential sites of arachidonic acid action in the activation of ARC channels. (A) The unique features of ARC channel activity include the presence of Orai3 (red), along with Orai1 (blue), as the pore forming components of the channel itself, and the specific dependence on the pool of STIM1 that is resident in the plasma membrane. Significantly, the extracellular location of the N-terminal EF hand of this plasma membrane STIM1 means that it would, under normal circumstances, always have calcium (green) bound to it. (B) Based on these unique components, activation of the channels is likely to involve arachidonic acid (aa) acting on the plasma membrane STIM1, and/or on the Orai3 protein of the channel. (C) Alternatively, arachidonic acid may act to induce an interaction between these proteins.
As for the specific involvement of Orai3 in the molecular structure of the ARC channels [25], simple sequence comparisons demonstrate that Orai3 possesses features that are not seen in either Orai1, or Orai2. The most obvious of these is a greatly extended (by ~30 aa) extracellular region that lies between the third and fourth transmembrane domains. However, how this extended extracellular loop might be affected by intracellular arachidonic acid is far from clear. Instead, such action leads us to focus on the cytosolic portions of the protein. Here, it appears that Orai3 lacks both the arginine-rich domain, and the two flanking proline-rich sequences, seen in the N-terminal portion of Orai1. In addition, analyses indicate that the putative C-terminal coiled-coil domain (aa266–295) in Orai3 represent a much “stronger” coiled-coil compared to that in the corresponding sequence in Orai1 [56]. In the CRAC channels, both of the N-terminal and C-terminal regions in Orai1 appear to play a key role in activation of the currents, but only the C-terminus is critical for interactions with STIM1.
Of course, rather than acting on the individual proteins themselves, arachidonic acid may act on some process involved in the interactions between these two proteins (Fig. 4C). In other words, does arachidonic acid induce the interaction between plasma membrane STIM1 and the Orai proteins that make up the channel pore, or do STIM1 and the Orai proteins exist in a preassembled “channel complex” that is activated by arachidonic acid? In this context, the role of phosphorylation may also need to be considered. As already discussed, the evidence indicates that the PKA-dependent phosphorylation step is not dependent on arachidonic acid and lies upstream of the actual activation of the channel by the fatty acid. This suggests that it is unlikely that either PKA, or the scaffolding AKAP protein are, themselves, the target for arachidonic acid. Nevertheless, because the PKA-dependent phosphorylation is a necessary prerequisite for activation of the channels by arachidonic acid, it is therefore an integral part of the whole activation process. This raises the possibility that the opposing actions of phosphorylation and dephosphorylation might affect the ability of STIM1 in the plasma membrane to interact, or assemble, with Orai3, and that only after such interaction has occurred is arachidonic acid able to act on the resulting complex.
Acknowledgements
The author thanks the efforts of Dr. Olivier Mignen, and Jill Thompson for all their contributions to the studies described here. Thanks are also due to Pauline Leakey for excellent technical assistance. Work in the author’s laboratory is supported by grants GM 040457 and DE 016999 from the National Institutes of Health, and funds from the Alfred and Eleanor Wedd Endowment to the Department of Pharmacology and Physiology at the University of Rochester Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 2.Lewis RS, Cahalan MD. Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regul. 1989;1:99–112. doi: 10.1091/mbc.1.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 4.Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J. Physiol. (Lond. ) 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc. Natl. Acad. Sci. USA. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rooney TA, Thomas AP. Intracellular calcium waves generated by Ins(1,4,5)P3-dependent mechanisms. Cell Calcium. 1993;14:674–690. doi: 10.1016/0143-4160(93)90094-m. [DOI] [PubMed] [Google Scholar]
- 7.Girard S, Clapham D. Acceleration of intracellular calcium waves in Xenopus oocytes by calcium influx. Science. 1993;260:229–232. doi: 10.1126/science.8385801. [DOI] [PubMed] [Google Scholar]
- 8.Shuttleworth TJ, Thompson JL. Ca2+ entry modulates oscillation frequency by triggering Ca2+ release. Biochem. J. 1996;313(Pt 3):815–819. doi: 10.1042/bj3130815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bootman MD, Young KW, Young JM, Moreton RB, Berridge MJ. Extracellular calcium concentration controls the frequency of intracellular calcium spiking independently of inositol 1,4,5-trisphosphate production in HeLa cells. Biochem. J. 1996;314:347–354. doi: 10.1042/bj3140347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shuttleworth TJ. Arachidonic acid activates the noncapacitative entry of Ca2+ during [Ca2+]i oscillations. J. Biol. Chem. 1996;271:21720–21725. doi: 10.1074/jbc.271.36.21720. [DOI] [PubMed] [Google Scholar]
- 11.Shuttleworth TJ, Thompson JL. Muscarinic receptor activation of arachidonate-mediated Ca2+ entry in HEK293 cells is independent of phospholipase C. J. Biol. Chem. 1998;273:32636–32643. doi: 10.1074/jbc.273.49.32636. [DOI] [PubMed] [Google Scholar]
- 12.Mignen O, Shuttleworth TJ. (ARC), a novel arachidonate-regulated, noncapacitative Ca(2+) entry channel. J. Biol. Chem. 2000;275:9114–9119. doi: 10.1074/jbc.275.13.9114. [DOI] [PubMed] [Google Scholar]
- 13.Shuttleworth TJ, Thompson JL, Mignen O. ARC channels: a novel pathway for receptor-activated calcium entry. Physiology (Bethesda. ) 2004;19:355–361. doi: 10.1152/physiol.00018.2004. [DOI] [PubMed] [Google Scholar]
- 14.Mignen O, Thompson JL, Shuttleworth TJ. Ca2+ selectivity and fatty acid specificity of the noncapacitative, arachidonate-regulated Ca2+ (ARC) channels. J. Biol. Chem. 2003;278:10174–10181. doi: 10.1074/jbc.M212536200. [DOI] [PubMed] [Google Scholar]
- 15.Mignen O, Thompson JL, Yule DI, Shuttleworth TJ. Agonist activation of arachidonate-regulated Ca2+-selective (ARC) channels in murine parotid and pancreatic acinar cells. J. Physiol. 2005;564:791–801. doi: 10.1113/jphysiol.2005.085704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo AS, Cheng I, Chung S, et al. Presenilin-mediated modulation of capacitative calcium entry. Neuron. 2000;27:561–572. doi: 10.1016/s0896-6273(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 17.Li YS, Wu P, Zhou XY, et al. Formyl-peptide receptor like 1: a potent mediator of the Ca2+ release-activated Ca2+ current ICRAC. Arch. Biochem. Biophys. 2008;478:110–118. doi: 10.1016/j.abb.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Mignen O, Thompson JL, Shuttleworth TJ. Reciprocal regulation of capacitative and arachidonate-regulated noncapacitative Ca2+ entry pathways. J. Biol. Chem. 2001;276:35676–35683. doi: 10.1074/jbc.M105626200. [DOI] [PubMed] [Google Scholar]
- 19.Parekh AB, Fleig A, Penner R. The store-operated calcium current ICRAC: Nonlinear activation by InsP3 and dissociation from calcium release. Cell. 1997;89:973–980. doi: 10.1016/s0092-8674(00)80282-2. [DOI] [PubMed] [Google Scholar]
- 20.Chang WC, Di Capite J, Nelson C, Parekh AB. All-or-none activation of CRAC channels by agonist elicits graded responses in populations of mast cells. J. Immunol. 2007;179:5255–5263. doi: 10.4049/jimmunol.179.8.5255. [DOI] [PubMed] [Google Scholar]
- 21.Roos J, DiGregorio PJ, Yeromin AV, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang SYL, Yu Y, Roos J, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feske S, Gwack Y, Prakriya M, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 24.Mignen O, Thompson JL, Shuttleworth TJ. STIM1 regulates Ca2+ entry via rachidonate-regulated Ca2+-selective (ARC) channels without store depletion or translocation to the plasma membrane. J. Physiol. 2007;579:703–715. doi: 10.1113/jphysiol.2006.122432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mignen O, Thompson JL, Shuttleworth TJ. Both Orai1 and Orai3 are essential components of the arachidonate-regulated Ca2+-selective (ARC) channels. J. Physiol. 2008;586:185–195. doi: 10.1113/jphysiol.2007.146258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liou J, Kim ML, Heo WD, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J. Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang SL, Yu Y, Roos J, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spassova MA, Soboloff J, He LP, et al. STIM1 has a plasma membrane role in the activation of store-operated Ca(2+) channels. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prakriya M, Feske S, Gwack Y, et al. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 31.Yeromin AV, Zhang SL, Jiang W, et al. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peinelt C, Vig M, Koomoa DL, et al. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat. Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mignen O, Thompson JL, Shuttleworth TJ. Orai1 subunit stoichiometry of the mammalian CRAC channel pore. J. Physiol. 2008;586:419–425. doi: 10.1113/jphysiol.2007.147249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji W, Xu P, Li Z, et al. Functional stoichiometry of the unitary calcium-release-activated calcium channel. Proc. Natl. Acad. Sci. U. S. A. 2008;105:13668–13673. doi: 10.1073/pnas.0806499105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oritani K, Kincade PW. Identification of stromal cell products that interact with pre-B cells. J. Cell Biol. 1996;134:771–782. doi: 10.1083/jcb.134.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manji SS, Parker NJ, Williams RT, et al. STIM1: a novel phosphoprotein located at the cell surface. Biochim. Biophys. Acta. 2000;1481:147–155. doi: 10.1016/s0167-4838(00)00105-9. [DOI] [PubMed] [Google Scholar]
- 37.Williams RT, Manji SS, Parker NJ, et al. Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins. Biochem. J. 2001;357:673–685. doi: 10.1042/0264-6021:3570673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams RT, Senior PV, Van Stekelenburg L, et al. Stromal interaction molecule 1 (STIM1), a transmembrane protein with growth suppressor activity, contains an extracellular SAM domain modified by N-linked glycosylation. Biochim. Biophys. Acta. 2002;1596:131–137. doi: 10.1016/s0167-4838(02)00211-x. [DOI] [PubMed] [Google Scholar]
- 39.Tobias LD, Hamilton JG. The effect of 5,8,11,14-eicosatetraynoic acid on lipid metabolism. Lipids. 1979;14:181–193. doi: 10.1007/BF02533870. [DOI] [PubMed] [Google Scholar]
- 40.Hardie RC. TRP channels and lipids: from Drosophila to mammalian physiology. J. Physiol. 2007;578:9–24. doi: 10.1113/jphysiol.2006.118372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meves H. Modulation of Ion Channels By Arachidonic Acid. prog neur. 1994;43:175–186. doi: 10.1016/0301-0082(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 42.Brown M, Anderson KM, Patel H, Hopfinger AJ, Harris JE. Eicosatetraynoic and arachidonic acid-induced changes in cell membrane fluidity consonant with differences in computer-aided design-structures. Biochim. Biophys. Acta. 1992;1105:285–290. doi: 10.1016/0005-2736(92)90206-2. [DOI] [PubMed] [Google Scholar]
- 43.Corey SJ, Rosoff PM. Unsaturated fatty acids and lipoxygenase products regulate phagocytic NADPH oxidase activity by a nondetergent mechanism. J. Lab Clin. Med. 1991;118:343–351. [PubMed] [Google Scholar]
- 44.Sawyer DB, Andersen OS. Platelet-activating factor is a general membrane perturbant. Biochim. Biophys. Acta. 1989;987:129–132. doi: 10.1016/0005-2736(89)90464-1. [DOI] [PubMed] [Google Scholar]
- 45.Kamp F, Zakim D, Zhang F, Noy N, Hamilton JA. Fatty acid flip-flop in phospholipid bilayers is extremely fast. Biochemistry. 1995;34:11928–11937. doi: 10.1021/bi00037a034. [DOI] [PubMed] [Google Scholar]
- 46.Wang C, Mirshahi UL, Liu B, et al. Arachidonic acid activates Kir2.3 channels by enhancing channel-phosphatidyl-inositol 4,5-bisphosphate interactions. Mol. Pharmacol. 2008;73:1185–1194. doi: 10.1124/mol.107.043067. [DOI] [PubMed] [Google Scholar]
- 47.Mignen O, Thompson JL, Shuttleworth TJ. Calcineurin directs the reciprocal regulation of calcium entry pathways in nonexcitable cells. J. Biol. Chem. 2003;278:40088–40096. doi: 10.1074/jbc.M306365200. [DOI] [PubMed] [Google Scholar]
- 48.Mignen O, Thompson JL, Shuttleworth TJ. Arachidonate-regulated Ca2+-selective (ARC) channel activity is modulated by phosphorylation and involves an A-kinase anchoring protein. J. Physiol. 2005;567:787–798. doi: 10.1113/jphysiol.2005.090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gardner LA, Tavalin SJ, Goehring AS, Scott JD, Bahouth SW. AKAP79-mediated targeting of the cyclic AMP-dependent protein kinase to the beta1-adrenergic receptor promotes recycling and functional resensitization of the receptor. J. Biol. Chem. 2006;281:33537–33553. doi: 10.1074/jbc.M601809200. [DOI] [PubMed] [Google Scholar]
- 50.Meves H. Arachidonic acid and ion channels: an update. Br. J. Pharmacol. 2008;155:4–16. doi: 10.1038/bjp.2008.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shuttleworth TJ, Thompson JL, Mignen O. STIM1 and the noncapacitative ARC channels. Cell Calcium. 2007;42:183–191. doi: 10.1016/j.ceca.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. J. Biol. Chem. 2006;281:35855–35862. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 53.Baba Y, Hayashi K, Fujii Y, et al. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc. Natl. Acad. Sci. U. S. A. 2006;103:16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dziadek MA, Johnstone LS. Biochemical properties and cellular localisation of STIM proteins. Cell Calcium. 2007;42:123–132. doi: 10.1016/j.ceca.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 55.Li Z, Lu J, Xu P, et al. Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J. Biol. Chem. 2007;282:29448–29456. doi: 10.1074/jbc.M703573200. [DOI] [PubMed] [Google Scholar]
- 56.Muik M, Frischauf I, Derler I, et al. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J. Biol. Chem. 2008;283:8014–8022. doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]