Abstract
An elevated level of Homocysteine (Hcy) is a risk factor for vascular dementia (VA) and stroke. Cysthathionine βSynthase (CBS) gene is involved in the clearance of Hcy. Homozygous individuals for (CBS-/-) dies early but heterozygous (CBS-/+) survive having high Hcy. γ-Amino Butyric Acid (GABA) is present in the central nervous system and functions as an inhibitory neurotransmitter. Hcy competes with GABA at the GABAA receptor and effects the central nervous system regulation. We hypothesize that Hcy causes a decrease in blood flow to the brain due to increased vascular resistance (VR) and remodeling in carotid artery (CA). Blood pressure and blood flow in CA of WT, CBS -/+, CBS-/+ GABAA -/-double knockout, and GABAA -/- was measured. CA was stained with trichrome stain and brain permeability/edema was measured. Matrix Metalloproteinases (MMP-2 and MMP-9), Tissue Inhibitor of metalloproteinase (TIMP-3, TIMP-4), Elastin, and Collagen-III expression were measured by real-time PCR. Results showed an increase in VR in CBS-/+/GABAA -/-double knockout > CBS -/+/ > GABAA -/- compared to WT mice. Reduced cerebral blood flow (ischemic episode) is caused by an increase in VR, leading to edema. Increased MMP-2, MMP-9, Collagen-III and TIMP-3 mRNA levels were found in GABAA -/- , CBS -/+, CBS -/+/GABAA double knockouts compared to WT. The levels of TIMP-4 and elastin were decreased which indicative of arterial is remodeling. These results suggested that Hcy caused arterial remodeling, in part by increase in collagen/elastin ratio thereby increasing vascular resistance leading to decrease in CA blood flow.
Keywords: carotid artery, Edema, MMP, Vascular resistance
1. Introduction
Homocysteine (Hcy) is a sulfhydryl amino acid, structurally similar to cysteine with an additional methylene group (McCully, 1969). The normal Hcy level in blood plasma is 5-8 μmol /l. It exists in the blood as a predominantly protein bound form (70-80%), a reduced form (5%), and an oxidized form (Seshadri, 2002; Haorah, et al., 2007). Hyperhomocysteinemia (HHcy) leads to a wide variety of diseases ranging from mental retardation to renal diseases (Herzlich, et al., 1996; Hashimoto, et al., 2003; MacLellan, et al., 2007). The vitamin B6-dependent CBS (Cysthathionine βSynthase) gene is involved in the clearance of Hcy in the mammalian system (Malinow, 1996).
Mc Cully first reported the association of HHcy and vascular wall changes. Hcy has been shown to be responsible for both arterial changes and damage; moreover, extensive research has proved that HHcy is an independent risk factor for cardiovascular, neurovascular and renal diseases (Kieseier, et al., 1999; Vine, et al., 1991; Liu, et al., 1997). Hcy-induced vascular remodeling is increasingly being attributed to dysfunctions in BBB permeability (Bigg, 1997). Similarly, HHcy has also been causally associated with stroke, a disease of the blood vessels in the brain that typically results in a reduction or disruption of blood flow to the brain. The physiological parameters used to describe cerebrovacular physiology include the permeability of blood brain barrier, cerebral blood flow, and blood pressure (Durand, 2001). HHcy has been reported to cause vascular resistance (VR), vascular injury and remodeling in animals; this is the basis for HHcy acting as a risk factor for coronary, cerebral, and peripheral arterial occlusive disease (MacLellan, et al., 2007; Erdo, 1985; Tyagi, et al., 2007; Kumar, et al., 2008).
The matrix metalloproteinases (MMPs) constitute a large family of proteolytic enzymes responsible for extracellular matrix (ECM) degradation and remodeling under normal and pathological conditions (Shastry, et al., 2006). Activation of MMPs and alterations in the basement membrane in BBB injury is well-documented (Durand, et al., 2001). Several studies have addressed the expression of the MMPs after vascular injury is counter-balanced by their endogenous inhibitors, the tissue inhibitors of matrix metalloproteinases (TIMPs) (Shapiro, et al., 1965). The imbalance in TIMP/MMP ratio results in vascular remodeling which affects the collagen and elastin content. TIMP-4 is a 23-kDa protein that inhibits MMP-1, MMP-3, MMP-7, and MMP-9 (Lominadze, et al., 2006) and shows a particular interaction with MMP-2 (Griffiths, et al.,1983). TIMP-3 is a 26kDa protein that inhibits and interacts with MMP-2 and MMP-9 (Erdo, 1985). An endogenous amino acid, γ-Amino Butyric Acid (GABA) is present in the central nervous system and functions as an inhibitory neurotransmitter (Lazzarini, et al., 2001). Hcy competes with GABA at the GABAA receptor and acts as an excitatory neurotransmitter (Korshunov, et al., 2006). GABA receptors play a significant role in brain microvascular permeability; its inhibition leads to ECM degradation and an increase in brain permeability (Korshunov, et al., 2006; Malemud, 2006; Wald, 2002).
Hcy causes oxidative stress that leads to activation of MMP-2 and MMP-9 in the ECM. These conditions resulted in an imbalance in the MMPs/TIMPs ratio causing a change in the collagen/elastin ratio (Herzlich, et al., 1996). We hypothesized that HHcy results in an increased VR in carotid artery (CA), leading to reduce cerebral blood flow due to arterial remodeling.
2. Experimental procedures
2.1. Generation of DKO animals
Mice (Wild type C57BL/6J, GABAA-/- and CBS-/+) were obtained from Jackson Laboratories (Bar Harbor, ME) and housed in the animal care facility at University of Louisville. The CBS-/+ and GABAA-/- mice were crossbred for 6 generations to create CBS-/+/GABAA-/- double knock out. Mice were genotyped for each group with a specific set of primers. Briefly tail genomic DNA was isolated by DNA isolation kit from Invitrogen. DNA was amplified using PCR with CBS primers: 5-GCCTCTGTCTGCTAACCTA-3′ and 5′-GAGGTCGACGGTATCGATA-3′; and GABA-A receptor primers: I3 5′- AAG AGC CCA GCA GAA TGA ACA -3′; P10 5′-GGT CTG GAA TTC ACT ATG TAGC-3′; Neo 5′-GAG GTC GAC GGT ATC GAT A-3′; P10 is the common primer. I3 primer was used to identify wild type allele and Neo primer was used to identify knockout allele. PCR product was identified on 1.2% agarose gels. Molecular weight markers (Mr) were loaded in the left lane. All animal procedures were in accordance with the National Institute of Health Guidelines for animal research. The Institutional Animal Care and Use Committee of the University Of Louisville School Of Medicine approved this study.
2.2. Measurement of blood flow and blood pressure in carotid artery
Mice were anesthetized with Pentabarbitol (100mg/kg). After ventilation, tissue surrounding CA was cleaned with fine forceps and was calibrated using transonic probe (0.5 PSB 303) was placed in between the carotid artery and flow was measured using TS 420 transit time perivascular flow meter (Transonic System Inc. NY, USA). Only those mice were selected in which the blood pressure were normal. The VR was measure by dividing the arterial blood pressure by the blood flow.
2.3. Measurement of blood flow in brain microcirculation
Blood flow in brain microvasculature was measured in anesthetized mice. Briefly, mice were injected with FITC conjugated to bovine albumin (FITC-albumin, 70 KDa) through tail vein and after 30 minutes 14 μM hole was made in skull using high speed micro drill (FST). The exposed area was observed using in vivo imaging fluorescent microscopy (Olympus, Japan).
2.4. RNA isolation and expression study
Total RNA from mouse CA was isolated using Trizol Reagent, Gibco BRL (Cat No. 15596-026) following the instructions provided by the manufacturer. cDNA was prepared using promega kit. Expression levels of MMP-2, MMP-9, TIMP-3, TIMP-4, Elastin and Collagen-III was measured using SYBR green assay kit on Mx3000p QPCR. Primers were synthesized from Invitrogen. Primers used for analysis are listed below:
| MMP-2 | 5'-TGTGGGTGGAAATTCAGAAGGT-3' |
| 5'-TTGTTGCCCAGGAAAGTGAAG-3 | |
| MMP-9 | 5'-TCGCGTGGATAAGGAGTTCTC-3' |
| 5'-ATGGCAGAAATAGGCTTTGTC-3' | |
| TIMP-3 | 5'-GGCCTCAATTACCGCTACCA-3' |
| 5'-CTGATAGCCAGGGTACCCAAA-3' | |
| TIMP-4 | 5'-TGCAGAGGGAGAGCCTGAA-3' |
| 5'-GGTACATGGCACTGATAGCA-3' | |
| Collagen-III | 5'-GACGCCATCAAGGTCTACTG-3' |
| 5'-GAATCCATCGGTCATGCTCT-3' | |
| Elastin | 5'-CCTGGACATCATCTGGGTC-3' |
| 5'-TGTGGCCTTATTGGGAAG-3' |
Optimal concentration of primer used for the assay was between (750 nmol/L) to (1000nmol/L). PCR amplification was performed using a cyclic pattern: 94 °C for 10 minutes followed by 35 cycles of 94°C for 1minute, 58°C for 1 minute, 72°C for 1minute and final extension at 72°C for 5 minutes. For the determination of SYBR Green based melting peak (Tm) of the products, an additional cycle of 95°C for 1 minute, 55°C for 30sec, 95°C for 30sec was performed. TIMP-4 gene cloned in pIRES2-AcGFP1 vector system (Clontech) was used to normalize the mRNA. Standard curve was generated by amplification of known amount of TIMP-4 gene in pIRES2-AcGFP1 vector system depend on Ct (threshold cycle). The parameter Ct is defined as the fractional cycle number at which the fluorescence passes the fixed threshold. Data were expressed as copy number based on plasmid standard curve and normalized by dividing the copy number for each sample. Samples and control were run in duplicate for each gene.
2.5. Histological studies
Mice were sacrificed and CA was taken out. A part of CA freezed in freezing media and was sectioned on cryostat (15μm). CA was stained with trichrome stain for collagen content using standard protocol provided by vendor (Masson's Trichrome kit, Biocompare)
2.6. Measurement of edema
Edema was determined in all four groups by calculating the percent water content after drying the brain at 100 °C for 24 hour. Water content was expressed as the percentage change between wet weight (WW) and the dry weight (DW): [(WW-DW)/WW] X100.
2.7. Statistical analysis
The means and standard errors of mean (SEM) in four experimental groups of mice (WT, CBS-/+, CBS-/+/GABA-/- and GABA-/-) were determined. There were eight responses observed: VR, brain permeability, expression study for MMP-2, MMP-9, TIMP-3, TIMP-4, Elastin, and Collagen-III. For each of these eight responses, we checked normality assumptions using Shapiro-Wilk test (Lo, et al., 2003) and found data to be valid. ANOVA procedure analyzes the data to be compared between the groups, and declared results significant at alpha level of 0.05, (p<0.05).
3. Results
3.1. Genotyping CBS and GABA gene
Representative PCR analysis of WT, CBS-/+, GABAA-/- and CBS-/+/GABAA-/- alleles is shown (Figure 1A and B). To determine whether HCy effect blood flow to brain, the VR was measured in all four groups using ultrasonic meter and blood pressure analyzer. Results suggested that the VR in CA was significantly increased in CBS-/+GABAA -/- double KO, CBS-/+, and GABAA -/- mice compared with WT (Figure 2A and B). CA was stained from each group with trichrome stain to see the pattern of collagen. Blue color signifies collagen. The result suggested that there was an increase in collagen in CBS -/+ mice compared to the WT (Figure 3A). CBS-/+/GABA-/- KO mice had significant increases in collagen content. No significant difference was observed in GABAA-/- mice compared to WT. Collagen deposition leads to narrowing of arterial lumen and an increase in VR. These changes result in less supply of blood, leading to an increase in brain permeability/ water content in brain. There were significant increases in water content in CBS -/+, CBS-/+/GABAA -/- double KO and GABAA -/- mice compared to the WT type (Figure 3B). Increased water content and edema was observed in: CBS -/+, CBS-/+/GABAA -/- double KO and GABAA -/- mice. The In Vivo fluorescent microscopy revealed that in HHcy mice there was decreased blood flow in brain microcirculation and the increased brain permeability in brain interstitial (Figure 4A and B)
Figure 1.

Genotype GABAA-/+ and CBS-/- mice with specific sets of primers. Mr is 100 bp ladder. GABA has 280bp product. CBS -/+ has 1500bp and 800bp product.
Figure 2.
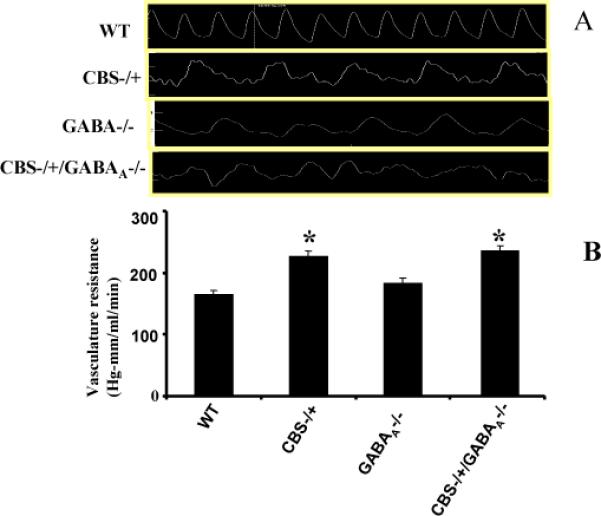
Vascular flow and vascular resistance in carotid artery. (A) Flow was measured in WT, CBS-/+, CBS-/+ / GABAA -/-, GABAA -/- (A) by transonic probe snuggled around carotid artery. There was significant increase in VR in CBS-/+ and CBS-/+ / GABAA -/- mice compared to WT. (B)The bar graph represents vascular resistance mean ± SEM in three experiments. *p<0.05 compared to WT.
Figure 3.

(A) Histological analysis of carotid artery in wild type and CBS -/+ mice. Tissue section was labeled with trichrome for collagen. Note: the arrow indicates increased blue stain for collagen in CBS -/+ mice. Magnification x40. (B) Bar graph represents water content (%) in the brain cortex in WT, CBS-/+, CBS-/+ / GABAA -/-, GABAA -/- and CBS-/+ mice. Mean ± SEM (n=3). *p<0.05 represent compared to WT, # p<0.05 compared to CBS-/+ /GABAA -/- double knockout mice.
Figure 4.

Photograph taken on in vivo fluorescent microscope. Fluorescence signal shows that in HHcy (B) mice the blood flow deceases and increase in leakage for FITC in brain interstitial.
These events were associated with changes in the expression of MMPs in CA. Standard curve was plotted using TIMP-4 gene cloned gene and the amplification of each gene was measured against the standard plot. Each of the genes analyzed have different melting temperature. MMP-2 and MMP-9 mRNA level was significantly increased in all the groups compared to WT (p<0.05) (Figure 5A and B). When comparing between MMP-2 and MMP-9 expression levels, the MMP-9 expression was higher than MMP-2. Lower Ct value signifies greater expression. One Ct difference equals two-fold difference in mRNA level. The data show that there was significant increase in the expression of MMPs in CA. The activities of MMPs are controlled by TIMPs; therefore, we measured the expression of TIMPs using SYBR green chemistry on Real Time PCR. TIMP-3 expression was significantly increased in all the groups compared with WT group p<0.05 (Figure 5C). In TIMP-4, Ct values were comparatively lower compared with TIMP-3, showing a significant increase in expression of TIMP-4. When compared with WT the TIMP-4 expression was significantly decreased in CBS-/+ and CBS-/+/GABAA-/- mice (Figure 5D). However, in GABAA -/- no change in expression level was observed compared with WT group. The data suggested TIMP-3 expression level is increased, whereas the TIMP-4 is decreased in all the groups compared to WT. The imbalance in MMPs and TIMPs or remodeling leads to change in collagen and elastin expression level. The collagen and elastin expression levels were measured in CA. There was a significant increase in collagen level in all groups compared to WT (Figure 6A). Elastin expression level was decreased in CBS-/+, GABA-/- and CBS-/+/ GABA-/- compared with WT (Figure 6B).
Figure 5.

Represents Ct values for MMP-2 (A), MMP-9 (B), TIMP-3 (C) and TIMP-4(D) in WT, CBS-/+, CBS-/+ / GABAA -/- and GABAA -/-. Lesser the value of Ct represents more the quantity of gene. One Ct difference signifies two fold increases in gene quantity. There is significant increase in MMP-2, MMP-9 activity or decrease in Ct values. There is significant increase in TIMP-4 and decrease in TIMP-3 expression or decrease in Ct values for TIMP-4 and increase for TIMP-3. The bar graph represents mean ± SEM in three experiments. *p<0.05 compared to WT.
Figure 6.
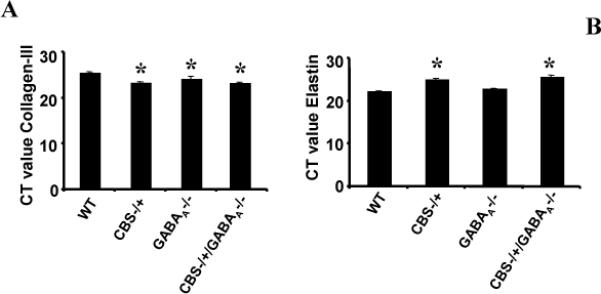
Represents Ct values for Collagen-III (A) and Elastin (B) in WT, CBS-/+, CBS-/+ / GABAA -/- and GABAA -/-. Lesser the value of Ct represents more the quantity of gene. One Ct difference signifies two fold increases in gene quantity. There is significant increase in collagen-III and decrease in elastin expression or decrease in Ct values for collagen-III and increase for elastin. The bar graph represents mean ± SEM in three experiments. *p<0.05 compared to WT.
4. Discussion
Microcirculation in the brain may be more susceptible to Hcy thus effecting permeability (Bendeck, et al., 1994). Still it is not clear what factors are affecting the increase in the permeability/edema in brain. In extension to our previous study, GABAA receptor agonist mitigated Hcy -induced cerbrovascular remodeling in knockout mice (Malemud, 2006) we planned to explore the role of Hcy in CA remodeling and blood flow. GABA is the primary inhibitory neurotransmitter in the brain (Korshunov, et al., 2006). Hcy specifically binds to the GABAA receptor (Folstein, et al., 2007) and behaves as an excitatory neurotransmitter. GABAA receptor play significant role in brain microvascular permeability, which if altered in function, results in matrix degradation and edema (Lenz, et al., 2006). Reports are available showing VR increases in vascular injuries (Tyagi, et al., 2005).
MMPs and TIMPs are involved in ECM remodeling that is essential in development and morphogenesis (Dollery, et al., 1999). MMPs degrade proteins and polysaccharides that compose the arterial matrix (collagen, laminin, elastin and fibronectin) (Wald, 2002; Raymond, 2004). In the present study, Hcy mice showed a significant increase in the MMP-2, MMP-9 protein expression in CA. Matrix-in activities are regulated by activation of the precursor zymogens and inhibition by the endogenous inhibitors, TIMPs. Thus, the balance between MMPs and TIMPs are critical for the eventual arterial remodeling (Wald, 2002). The expression level of TIMP-4 was significantly decreased in Hcy mice. On the other hand, TIMP-3 increased in the Hcy mice. MMPs are thought to be critical to the migration of smooth muscle cells into the arterial neointima after vascular balloon injury (Holven, 2003). Recently, it has been reported that hyperhomocysteinemia may lead to an elevated risk of depression, mainly via acting as a cerebrovascular risk factor and by causing neurotransmitter deficiency (Lentz, 1997). Acute Hcy treatment has been shown to cause misregulation of different gene-specific promoters and changes of corresponding messenger ribonucleic acid (mRNA) levels (Sen, et al., 2007). The present study shows that VR increased in CBS- /+ and CBS-/+/GABA-/- due to narrowing of lumen due to increases in collagen content. Further, elastin content was decreased, leading to a loss in elasticity of CA. Thus VR increased, which led to a lesser supply of nutrients and oxygen to brain.
It is well documented that Hcy causes cell detachment and brain cell death by creating oxidative stress [21, Bar-Or,et al., 2004). Our data supports earlier reports that TIMP-4 is the cardiospecific molecule capable of inhibiting the MMPs, and that TIMP-4 is unregulated by vascular injury (Moshal, et al., 2008). MMPs have a central role in degrading extracellular and basement membranes, a necessary step in angiogenesis, which could also be important in remodeling.
Present study along with earlier reports suggested substantial increases in MMP-2 and MMP-9 with stable or decreased expression of their inhibitors (TIMP-1, TIMP-3)(Refsum, et al., 1998). TIMP-4 expressed after vascular injury and associated with collagen accumulation, was significantly elevated only in the artery (Moshal, et al., 2008). Thus, the present study is important in understanding the mechanisms of vascular and brain injury along with the stages of injury progression, and to eventually evaluate and develop therapeutic treatments to brain disorders in HHcy.
Acknowledgments
Grants: The study is supported in part by American Heart Association Postdoctoral training grant (to Karni S. Moshal) and NIH Grants HL-71010 and NS-51568 (to Suresh C. Tyagi)
5. References
- Bar-Or D, Curtis CG, Sullivan A, Rael LT, Thomas GW, Craun M, Bar-or R, Maclean KN, Kraus JP. Plasma albumin cysteinylation is regulated by cystathionine beta-synthase. Biochem Biophys Res Commun. 2004;325L:1449–55. doi: 10.1016/j.bbrc.2004.10.191. [DOI] [PubMed] [Google Scholar]
- Bendeck MP, Zempo N, Clowes AW, Galardy RE, Reidy MA. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res. 1994;75:539–45. doi: 10.1161/01.res.75.3.539. [DOI] [PubMed] [Google Scholar]
- Bigg HF, Shi YE, Liu YE, Steffensen B. Overall CM. Specific, high affinity binding of tissue inhibitor of metalloproteinases-4 (TIMP-4) to the COOH-terminal hemopexin-like domain of human gelatinase A: TIMP-4 binds progelatinase A and the COOH-terminal domain in a similar manner to TIMP-2. J Biol Chem. 1997;272:15496–00. doi: 10.1074/jbc.272.24.15496. [DOI] [PubMed] [Google Scholar]
- Dollery CM, McEwan JR, Wang M, Sang QA, Liu YE, Shi YE. TIMP-4 is regulated by vascular injury in rats. Circ Res. 1999;84:498–04. doi: 10.1161/01.res.84.5.498. [DOI] [PubMed] [Google Scholar]
- Durand P, Prost M, Loreau N, Lussier-Cacan S, Blache D. Impaired homocysteine metabolism and atherothrombotic disease. Lab Invest. 2001;81:645–72. doi: 10.1038/labinvest.3780275. [DOI] [PubMed] [Google Scholar]
- Erdo SL. Peripheral GABAergic mechanism. Trends Pharmacol Sci. 1985;5:141–58. [Google Scholar]
- Folstein M, Liu T, Peter I, Buel J, Arsenault L, Scott T, Qiu WW. The homocysteine hypothesis of depression. Am J Psychiatry. 2007;164:861–67. doi: 10.1176/ajp.2007.164.6.861. [DOI] [PubMed] [Google Scholar]
- Griffiths R, Williams DC, O'Neill C, Dewhurst IC, Ekuwem CE, Sinclair CD. Synergistic inhibition of (3H) muscimol binding to calf brain synaptic membranes in the presence of L-homocysteine and pyridoxal 5-phosphate: a possible mechanism for homocysteine-induced seizures. Eur J Biochem. 1983;137:467–78. doi: 10.1111/j.1432-1033.1983.tb07850.x. [DOI] [PubMed] [Google Scholar]
- Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, Persidsky Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain dysfunction. J Neurochem. 2007;101:566–76. doi: 10.1111/j.1471-4159.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Wen G, Lawton MT, Boudreau NJ, Bollen AW, Yang GY, Barbaro NM, Higashida RT, Dowd CF, Halbach VV, Young WL. Abnormal expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in brain arteriovenous malformations. Stroke. 2003;34:925–31. doi: 10.1161/01.STR.0000061888.71524.DF. [DOI] [PubMed] [Google Scholar]
- Herzlich BC, Lichstein E, Schulhoff N, Weinstock M, Pagala M, Ravindran K, Namba T, Nieto FJ, Stabler SP, Allen RH, Malinow MR. Relationship between homocyst(e)ine, vitamin B-12 and cardiac disease in the elderly: association between vitamin B-12 deficiency and decreased left ventricular ejection fraction. J Nutr. 1996;126:1249–53. doi: 10.1093/jn/126.suppl_4.1249S. [DOI] [PubMed] [Google Scholar]
- Holven KB, Halvorsen B, Schulz H, Aukrust P, Ose L, Nenseter MS. Expression of matrix metalloproteinase-9 in mononuclear cells of hyperhomocysteinaemic subjects. Eur J Clin Invest. 2003;33:555–60. doi: 10.1046/j.1365-2362.2003.01189.x. [DOI] [PubMed] [Google Scholar]
- Kieseier BC, Seifert T, Giovannoni G, Hartung HP. Matrix metalloproteinases in inflammatory demyelination: targets for treatment. Neurology. 1999;53:20–5. doi: 10.1212/wnl.53.1.20. [DOI] [PubMed] [Google Scholar]
- Korshunov VA, Mohan A,M, Georger MA, Berk BC. Axl, a receptor tyrosine kinase, mediates flow-induced vascular remodeling. Circ Res. 2006;98:1446–52. doi: 10.1161/01.RES.0000223322.16149.9a. [DOI] [PubMed] [Google Scholar]
- Kumar M, Tyagi N, Moshal KS, Sen U, Pushpakumar SB, Vacek T, Lominadze D, Tyagi SC. Brain Research. 2008. GABAA receptor agonist mitigates homocysteine-induced cerbrovascular remodeling in knockout mice. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini R, Malucelli BE, Palermo-Neto J. Reduction of acute inflammation in rats by diazepam: role of peripheral benzodaiazepine receptors and corticosterone. Immunopharmacology and Immunotoxicology. 2001;23:253–65. doi: 10.1081/iph-100103864. [DOI] [PubMed] [Google Scholar]
- Lentz SR. Homocysteine and vascular dysfunction. Life Sci. 1997;61:1205–15. doi: 10.1016/s0024-3205(97)00392-5. [DOI] [PubMed] [Google Scholar]
- Lenz B, Bleich S, Beutler S, Schlierf B, Schwager K, Reulbach U, Kornhuber J, Bonsch D. Homocysteine regulates expression of HERP by DNA methylation involving the AARE and CREB binding sites. Exp Cell Res. 2006;312:4049–55. doi: 10.1016/j.yexcr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Liu YE, Wang M, Greene J, Su J, Ullrich S, Li H, Sheng S, Alexander P, Sang QA, Shi YE. Preparation and characterization of recombination TIMP-4. J Biol Chem. 1997;272:20479–83. doi: 10.1074/jbc.272.33.20479. [DOI] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, mechanism and challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–15. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Lominadze D, Roberts AM, Tyagi N, Moshal KS, Tyagi SC. Homocysteine causes cerebrovascular leakage in mice. Am J Physiol Heart Circ Physiol. 2006;290:1206–13. doi: 10.1152/ajpheart.00376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLellan CL, Silasi G, Poon CC, Edmundson CL, Buist R, Peeling J, Colbourne F. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. Journal of Cerebral Blood Flow & Metabolism. 2007;28:516–25. doi: 10.1038/sj.jcbfm.9600548. [DOI] [PubMed] [Google Scholar]
- Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006;11:1696–01. doi: 10.2741/1915. [DOI] [PubMed] [Google Scholar]
- Malinow MR. Plasma homocyst(e)ine: a risk factor for arterial occlusive disease. J Nutr. 1996;126:1238–43. doi: 10.1093/jn/126.suppl_4.1238S. [DOI] [PubMed] [Google Scholar]
- McCully KS. Vascular pathology of homocysteinemia: Implications for the pathogenesis of atherosclerosis. Am J Pathol. 1969;56:111–28. [PMC free article] [PubMed] [Google Scholar]
- Moshal KS, Metreveli N, Frank I, Tyagi SC. Mitochondrial MMP Activation, Dysfunction and Arrhythmogenesis in Hyperhomocysteinemia. Curr Vasc Pharmacol. 2008;6:84–92. doi: 10.2174/157016108783955301. [DOI] [PubMed] [Google Scholar]
- Raymond J, Lebel V, Ogoudikpe C, Metcalfe-Chagnon M, Robledo O. Recanalization of arterial thrombus, and inhibition with β-radiation in a new murine carotid occlusion model: mRNA expression of angiopoietins, metalloproteinases, and their inhibitor. J Vas Surg. 2004;40:1190–98. doi: 10.1016/j.jvs.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- Sen U, Tyagi N, Kumar M, Moshal KS, Rodriguez WE, Tyagi SC. Cystathionine-beta-synthase gene transfer and 3-deazaadenosine ameliorate inflammatory response in endothelial cells. Am J Physiol Cell Physiol. 2007;293:1779–87. doi: 10.1152/ajpcell.00207.2007. [DOI] [PubMed] [Google Scholar]
- Seshadri S. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Eng J Med. 2002;346:476–83. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- Shapiro SS, Wilk MB. An analysis of variance test for normality complete samples. Biometrika. 1965;52:591–11. [Google Scholar]
- Shastry S, Tyagi N, Moshal KS, Lominadze D, Hayden MR, Tyagi SC. GABA receptors ameliorate Hcy-mediated integrin shedding and constrictive collagen remodeling in microvascular endothelial cells. Cell Biochem Biophys. 2006;45:157–65. doi: 10.1385/CBB:45:2:157. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi N, Moshal KS, Tyagi SC, Lominadze D. gamma-Aminbuturic Acid A Receptor mitigates Homocysteine-Induced Endothelial Cell Permeability. Endothelium. 2007;14:315–23. doi: 10.1080/10623320701746164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, Tyagi SC. Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circ Physiol. 2005;289:H2649–56. doi: 10.1152/ajpheart.00548.2005. [DOI] [PubMed] [Google Scholar]
- Vine N, Powell JT. Metalloproteinases in degenerative aortic disease. Clin Sci (Colch) 1991;81:233–39. doi: 10.1042/cs0810233. [DOI] [PubMed] [Google Scholar]
- Wald DS. Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. Brit Med J. 2002;325:1202–06. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]


