Abstract
Objectives
Late-life depression is associated with alterations in regional cerebral blood flow (rCBF) and metabolism in a neural network that includes frontostriatal and limbic regions and the cerebellum. Prior studies suggest that clinical depression and subthreshold depressive symptoms (SDS) are associated with similar cognitive deficits and structural brain changes, but little is known about the relationship between SDS and patterns of brain activity. Additionally, the neural correlates of depression have not been fully explored in men and women separately. This study investigated cross-sectional and longitudinal relationships between SDS and rCBF in older men and women.
Methods
Sixty-one dementia-free older adults (35 men, 26 women), 56 years of age and older at baseline, from the neuroimaging substudy of the Baltimore Longitudinal Study of Aging participated. Participants underwent resting-state PET scans at baseline and at year 9 and completed the Center for Epidemiologic Studies Depression Scale annually.
Results
At eight-year follow-up, both men and women showed cross-sectional associations between mean depressive symptom scores and activity in primarily frontal and temporal regions and the cerebellum. Higher average depressive symptoms were associated with longitudinal rCBF decreases in frontal regions in both men and women, and in temporal regions in men.
Conclusion
Regions showing associations between activity and SDS were similar to those found in studies of clinical depression, providing support for the hypothesis that depressive syndromes exist on a continuum of severity. Sex differences in associations provide some evidence that the pathophysiology of depressive disorders differs between men and women.
Keywords: subthreshold depression, late-life depression, sex differences, positron emission tomography, aging, longitudinal studies
Key points.
Older adults with subthreshold depressive symptoms show a pattern of cerebral blood flow abnormalities that is similar to that observed in late-life major depression.
Patterns of activity differed somewhat for men and women, suggesting sex differences in the pathophysiology of depression.
Depressive symptoms at a subthreshold level are common in older adults and are associated with cognitive deficits (Bennett, et al., 2004, Chuan, et al., 2008, Elderkin-Thompson, et al., 2003, Ravdin, et al., 2003), longitudinal cognitive decline (Bassuk, et al., 1998, Chodosh, et al., 2007, Wilson, et al., 2004), and decreased regional brain volumes (Kumar, et al., 2000, Kumar, et al., 1998, Kumar, et al., 1997, Taki, et al., 2005). However, the relationship between subthreshold depressive symptoms (SDS) and regional brain function in the aging brain has not been studied, despite substantial evidence indicating alteration of frontostriatal, limbic and cerebellar metabolic activity in individuals with clinical depression (Alexopoulos, 2002, Drevets, 2000, Mayberg, 2003, Tekin and Cummings, 2002).
If depressive syndromes exist on a continuum of severity, as postulated by some researchers (Angst, et al., 2000, Geiselmann and Bauer, 2000, Goldberg, 2000), then SDS should be associated with alterations in functional activity in regions implicated in clinical depression. Indeed, a recent meta-analysis (Fitzgerald, et al., 2008) concluded that the most consistent alterations in regional activity in depressed individuals were in the anterior cingulate, dorsolateral, medial and inferior prefrontal cortex, insula, superior temporal gyrus, basal ganglia and cerebellum. In general, hypoactivity in many cortical regions and hyperactivity in many subcortical and limbic regions have been observed.
Some studies have shown sex differences in the cognitive and neural correlates of depressive syndromes (Dal Forno, et al., 2005, Fuhrer, et al., 2003, Taki, et al., 2005, Videbech, et al., 2002). Clinical depression is associated with greater decreases in frontal volumes in men compared to women (Lavretsky, et al., 2004), and depressive symptoms have been associated with an increased risk for dementia (Dal Forno, et al., 2005, Fuhrer, et al., 2003) and with reductions in hippocampal blood flow (Videbech, et al., 2002) in men, but not in women. However, the literature is lacking in studies that examine sex-specific longitudinal changes associated with depression and SDS.
In this study, we examined associations between SDS and regional cerebral blood flow (rCBF) in a sample of dementia-free older adults who were followed for 8 years. We identified cross-sectional correlations between an estimate of chronic depressive symptoms, measured over 8 years, and rCBF at the 9th visit (8-year follow-up) in men and women. To clarify the nature of these relationships, we distinguished between correlations that were unique to year 9 and those that were already present at baseline. Further, we examined associations between SDS and longitudinal blood flow changes in regions that showed significant correlations with SDS at year 9. Based on previous research, we expected to find cross-sectional and longitudinal associations between SDS and blood flow in frontal, temporal and cerebellar regions.
Methods
Participants
PET data were obtained from the neuroimaging substudy of the Baltimore Longitudinal Study of Aging (BLSA) (Resnick, et al., 2000) at the National Institute on Aging. This report includes PET evaluations at baseline and 8-year follow-up. Exclusionary criteria at initial evaluation included central nervous system disease, severe cardiovascular disease, severe pulmonary disease, and metastatic cancer. The current study included 61 participants (35 men, mean age = 69.31, SD = 6.53; 26 women, mean age = 68.84, SD = 6.95) who were free of dementia and mild cognitive impairment at baseline and all follow-up visits. Determination of dementia and mild cognitive impairment status was based on standard procedures (Kawas, et al., 2000). In brief, at each visit, participants who met screening criteria based on the Blessed Information Memory and Concentration Scale (BIMCS) (Blessed and Wilson, 1982) or a Clinical Dementia Rating (Morris, 1993) score of 0.5 or greater were selected for more comprehensive evaluations. Based on the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised criteria for dementia and the National Institute of Neurological and Communication Disorders-Alzheimer’s Disease and Related Disorders Association criteria for Alzheimer’s disease (McKhann, et al., 1984), diagnoses of mild cognitive impairment and dementia were made at consensus diagnostic conferences using neuropsychological diagnostic tests and clinical data. None of the remaining subjects reported a history of major psychiatric disorder. Only participants with PET scans at baseline and year 9 were included in the current sample. Although none of the participants reported a history of major depression, 9 participants reported taking antidepressant medication at baseline or year 9. As we would expect, these participants reported more depressive symptoms than did individuals who were not treated with antidepressant medication during the study interval (t(5) = 2.61, p < .05). As a result of this difference, as well as the known effects of antidepressants on blood flow (Davies, et al., 2003, Joe, et al., 2006, Nobler, et al., 2002, Smith, et al., 2002), antidepressant use was used as a covariate in our analyses. Demographic characteristics for the sample and information regarding antidepressant use are presented in Table 1. The study was approved by the local Institutional Review Boards and the National Institute on Aging Intramural Research Program, and all subjects gave written informed consent at each visit.
Table 1.
Sample Characteristics
| Men | Women | Total | |
|---|---|---|---|
| n | 35 | 26 | 61 |
| Baseline age (years) | 69.31 (6.53) | 68.84 (6.95) | 69.12 (6.66) |
| Education (years) | 16.43 (2.95) | 16.69 (2.38) | 16.54 (2.71) |
| Handedness (Right/Non-Right) | 34/1 | 24/2 | 58/3 |
| Average CES-D | 3.81 (3.61) | 4.66 (5.42) | 4.17 (4.48) |
| No. of CES-D measurements | 7.09 (1.74) | 7.04 (.92) | 7.06 (1.44) |
| Antidepressant use | |||
| Amitriptyline | 0 | 2 | 2 |
| Trazodone | 0 | 1 | 1 |
| Doxepin | 1 | 0 | 1 |
| Paroxetine | 2 | 0 | 2 |
| Fluoxetine | 2 | 0 | 2 |
| Sertraline | 0 | 1 | 1 |
Note. CES-D = Center for Epidemiologic Studies Depression Scale
Depressive Symptomatology
The Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff, 1977) served as a measure of depressive symptoms. This 20-item inventory assesses the frequency and severity of depressive symptoms experienced in the past week and is widely used in epidemiological and longitudinal studies. The CES-D has been validated in older community-dwelling adults (Beekman, et al., 1997, Haringsma, et al., 2004). For each participant, all CES-D scores during the study interval were averaged as a measure of chronic or persistent symptoms (mean number of CES-D measurements = 4.06, SD = 3.41). To avoid the inclusion of increases in scores which may be attributed to more transient life events, outlying scores (i.e., greater than two standard deviations above the subject mean) were excluded. Mean scores were used as continuous predictor variables in statistical analyses.
PET Scanning Conditions & Parameters
Participants underwent annual PET scanning sessions for nine years. Scans were acquired during rest, verbal recognition memory, and figural recognition memory condition at each imaging session. The current study focused on the rest condition at baseline and year 9. During rest, participants were instructed to keep their eyes open and focused on a computer screen covered by a black cloth.
PET measures of rCBF were obtained using [15O]water. For each scan, 75 mCi of [15O] water were injected as a bolus. Scans were performed on a GE 4096+ scanner, which provides 15 slices of 6.5mm thickness. A custom thermoplastic mask was made for each subject during the baseline scanning session to aid in head positioning. This mask was used in all subsequent years to control for head placement and image acquisition angle. Images were acquired for 60 seconds from the time the total radioactivity counts in the brain reached threshold level. A transmission scan acquired prior to the emission scans is used for attenuation correction.
Data Analysis
PET scans for each subject were realigned and spatially normalized into standard stereotactic space and smoothed to a full width at half maximum of 12, 12, and 12mm in the x, y, and z planes. rCBF values at each voxel were ratio adjusted to the mean global blood flow of 50 ml/100 g/min for each image to control for variability in global flow. Statistical Parametric Mapping (SPM2; Wellcome Department of Cognitive Neurology, London, England) was used for PET data analysis. Using multiple regression analyses, voxel by voxel comparisons determined associations between rCBF and CES-D scores. Significant effects for each contrast were based on the magnitude (p≤0.005) and spatial extent (>50 voxels) of activation. Baseline age and antidepressant use (yes, no) served as covariates.
To capture cross-sectional measures of the effects of SDS on rCBF, correlations were performed between the mean CES-D scores obtained during the study interval and rCBF at year 9. Brain function at year 9 was of primary interest because it provides the best measure of the cumulative effect of chronic depressive symptoms. Conjunction analyses (masking threshold of p≤0.05; magnitude p≤0.005; spatial extent >100mm3) were then performed to compare the patterns of correlation between mean CES-D and rCBF at year 9 and baseline. The conjunction analyses delineate associations that were unique to year 9 and those that were common to baseline and year 9. These analyses use a region-of-interest map based on areas that were significantly associated with CES-D scores at year 9 and determine whether those areas were also associated with mean CES-D scores at baseline.
Longitudinal change in rCBF was also correlated with mean CES-D scores. Difference images reflecting rCBF change over time from baseline to year 9 were created for each individual. The rCBF change was then correlated with mean CES-D scores using an analysis restricted to the regions showing significant associations between mean SDS and rCBF at year 9 (p <0.005 magnitude; >100mm3 spatial extent).
Results
The results presented here are focused on associations of mean CES-D scores with rCBF at year 9, which were of primary interest; however, correlations between mean CES-D scores and rCBF at baseline are presented in Table 2.
Table 2.
Regions showing significant correlations with CES-D scores at baseline
| Region | Side | Coordinate |
Cluster Size | t | p | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Women | |||||||
| Positive Correlations | |||||||
| Inferior Parietal Lobule (39) | L | -64 | -56 | 24 | 87 | 3.99 | <.001 |
| R | 66 | -58 | 26 | 130 | 3.39 | .001 | |
| Angular Gyrus (BA 39) | L | -48 | -70 | 38 | 134 | 3.55 | <.001 |
| Middle Occipital Gyrus (BA 19) | L | -24 | -100 | 16 | 107 | 3.36 | .001 |
| Negative Correlations | |||||||
| Cerebellum | R | 18 | -26 | -28 | 282 | 4.00 | <.001 |
| Inferior Frontal Gyrus (BA 46) | R | 32 | 34 | 8 | 135 | 3.84 | <.001 |
| Inferior Temporal Gyrus (BA 20) | R | 50 | -20 | -38 | 89 | 3.79 | <.001 |
| Orbitofrontal Gyrus (BA 47) | R | 32 | 30 | -26 | 107 | 3.53 | <.001 |
| Middle Occipital Gyrus (BA 19) | R | 48 | -66 | -10 | 147 | 3.50 | .001 |
| Insula | R | 36 | 6 | 4 | 95 | 3.44 | .001 |
| Inferior Parietal Lobule (BA 40) | R | 64 | -32 | 46 | 96 | 3.37 | .001 |
| Middle Temporal Gyrus (BA 21) | R | 54 | -46 | 0 | 52 | 3.04 | .002 |
| Men | |||||||
| Negative Correlations | |||||||
| Precentral Gyrus (BA 4) | L | -48 | -8 | 38 | 203 | 4.23 | <.001 |
| Inferior Frontal Gyrus (BA 47) | L | -24 | 18 | -14 | 211 | 3.77 | <.001 |
| Temporal Pole (BA 20) | R | 28 | 0 | -36 | 92 | 3.02 | .002 |
| Cerebellum | R | 62 | -58 | -24 | 109 | 3.59 | <.001 |
| Inferior Parietal Lobule (BA 40) | L | -42 | -36 | 34 | 96 | 3.13 | .001 |
Cross-sectional Associations between Mean CES-D and rCBF at Year 9
Results for cross-sectional associations between mean CES-D and rCBF at year 9 are presented in Table 3 and the upper half of the Figure.
Table 3.
Regions showing significant correlations with CES-D scores at year 9
| Region | Side | Coordinate |
Cluster Size | t | p | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Women | |||||||
| Positive Correlations | |||||||
| Inferior Occipital Gyrus (BA 18) | R | 26 | -102 | -8 | 535 | 4.28 | <.001 |
| Middle Occipital Gyrus (BA 19) | L | −20 | −92 | 14 | 170 | 3.07 | .002 |
| Superior Occipital Gyrus (BA 19) | L | -22 | -66 | 34 | 118 | 3.84 | <.001 |
| Cuneus (BA 18) | R | 26 | -70 | 16 | 57 | 3.11 | .002 |
| Fusiform Gyrus (BA 18) | L | -16 | -90 | -18 | 127 | 3.09 | .002 |
| Angular Gyrus (BA 39) | L | -56 | -66 | 30 | 173 | 3.60 | <.001 |
| Negative Correlations | |||||||
| Inferior Frontal Gyrus (BA 44/45) | L | -28 | 20 | 16 | 50 | 3.19 | .001 |
| Inferior Frontal Gyrus (BA 44) | R | 32 | 10 | 22 | 342 | 3.76 | <.001 |
| Inferior Frontal Gyrus (BA 46) | R | 32 | 32 | 12 | 487* | 4.67 | <.001 |
| Middle Frontal Gyrus (BA 9) | R | 32 | 42 | 32 | 487* | 3.88 | <.001 |
| Orbitofrontal Gyrus (BA 11) | R | 10 | 38 | 0 | 172 | 3.58 | .001 |
| Cingulate Gyrus (BA 32) | L | -12 | 44 | 14 | 64 | 3.49 | .001 |
| Temporal Pole (BA 38) | R | 48 | 22 | -32 | 439 | 3.81 | <.001 |
| Inferior Parietal Lobule (40) | R | 62 | -42 | 50 | 201 | 3.84 | <.001 |
| Cerebellum | L | -14 | -22 | -32 | 79 | 3.50 | .001 |
| R | 22 | -22 | -32 | 152 | 3.48 | .001 | |
| R | 42 | -28 | -36 | 107 | 3.11 | .002 | |
| Middle Occipital Gyrus (19) | R | 46 | -66 | -6 | 58 | 3.11 | .002 |
| Men | |||||||
| Positive Correlations | |||||||
| Cerebellum | L | -14 | -92 | -30 | 106 | 3.45 | .001 |
| R | 20 | -88 | -30 | 70 | 3.40 | .001 | |
| Negative Correlations | |||||||
| Inferior Frontal Gyrus (BA 47) | L | -18 | 8 | -24 | 1815† | 4.02 | <.001 |
| Medial Frontal Gyrus (BA 10) | L | -6 | 68 | -8 | 196 | 4.46 | <.001 |
| R | 14 | 66 | -4 | 292 | 3.66 | <.001 | |
| Middle Frontal Gyrus (BA 46) | L | -52 | 30 | 20 | 328 | 4.28 | <.001 |
| Precentral Gyrus (BA 6) | L | -48 | -8 | 38 | 110 | 3.11 | .001 |
| Insula | L | -46 | -14 | 12 | 1815 | 3.28 | <.001 |
| Inferior Temporal Gyrus (BA 20) | L | -54 | -24 | -16 | 128 | 3.11 | .001 |
| R | 62 | -12 | -22 | 772 | 4.42 | <.001 | |
| Middle Temporal Gyrus (BA 21) | L | -54 | 14 | -30 | 146 | 4.23 | <.001 |
| Superior Temporal Gyrus (BA 22) | L | -50 | 2 | 2 | 1815† | 3.59 | <.001 |
| Inferior Parietal Lobule (BA 40) | L | -60 | -28 | 38 | 761 | 4.15 | <.001 |
| Superior Occipital Gyrus (BA 19) | L | -34 | -78 | 34 | 244 | 3.93 | <.001 |
Regions contained within the same cluster.
Regions contained within the same cluster.
Figure.
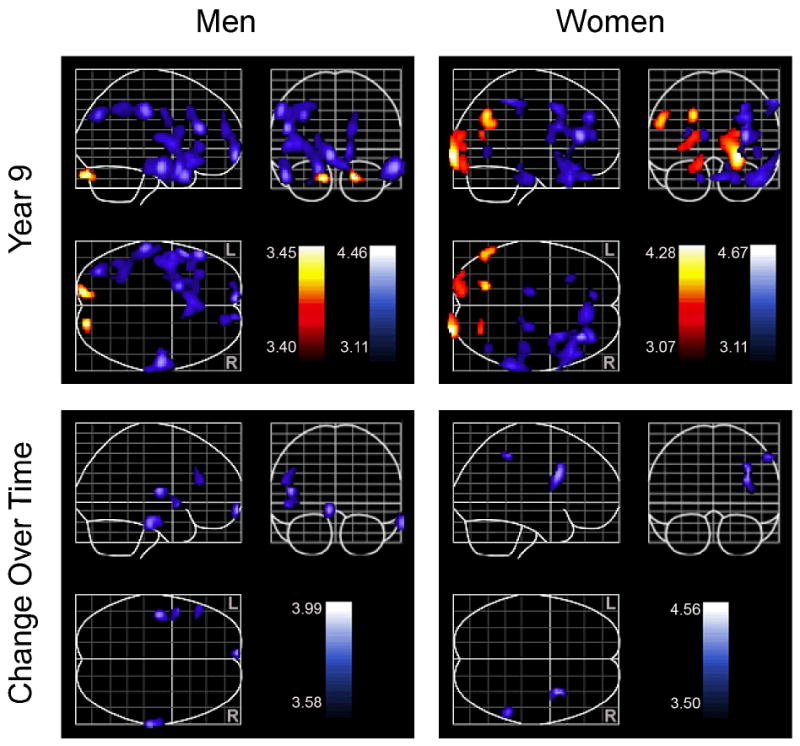
Cross-sectional (upper portion) and longitudinal (lower portion) associations between average Center for Epidemiologic Studies Depression Scale (CES-D) and blood flow. Cross-sectional associations are based on year 9 data. Positive associations between blood flow and CES-D scores are shown in red. Negative associations between blood flow and CES-D scores are shown in blue. Color bars represent the t-value of associations between blood flow and CES-D.
In women, mean CES-D scores were associated with increased blood blow in the occipital regions bilaterally, the left angular and fusiform gyri, and the right cuneus. Higher scores were associated with decreased blood flow in frontal regions, including the right orbitofrontal and left cingulate gyri, the inferior frontal gyri bilaterally, and the right middle frontal gyrus. Positive correlations between mean CES-D scores and blood flow were also found in the right temporal pole, inferior parietal lobule, middle occipital gyrus, and in both cerebellar hemispheres.
Men showed increased rCBF in both cerebellar hemispheres at year 9 as a function of higher mean depressive symptoms. Depressive symptoms were associated with decreased blood flow in frontal regions, including the medial frontal gyri bilaterally, left middle frontal and inferior frontal gyri, and left precentral gyrus, as well as temporal regions, including the inferior temporal gyri bilaterally, and left middle and superior temporal gyri. Higher mean CES-D scores were also associated with decreased rCBF in the left insula, left inferior parietal lobule, and the left superior occipital gyrus.
Year 9 correlations compared to baseline
Results for this section are presented in Tables 4 and 5. Most regions showing significant associations with CES-D scores at year 9 also showed significant associations at baseline. For most of these regions, the peak area of activity shifted between baseline and year 9, although the Brodmann areas remained the same. In contrast, unique associations were found between average depressive symptoms and decreased rCBF at year 9 in the left cingulate gyrus and right inferior frontal gyrus in women and in the right medial frontal gyrus as well as the left middle frontal, inferior temporal, and superior occipital gyri in men.
Table 4.
Results of conjunction analyses showing regions correlated with CES-D scores at both year 9 and year 1
| Region | Side | Coordinate |
Cluster Size | t | p | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Women | |||||||
| Positive Correlations | |||||||
| Inferior Occipital Gyrus (BA 18)* | R | 26 | -102 | -8 | 295 | 4.28 | <.001 |
| Middle Occipital Gyrus (19) | L | -20 | -92 | 14 | 119 | 3.07 | .002 |
| Superior Occipital Gyrus (BA 19)* | L | -22 | -66 | 34 | 76 | 3.84 | <.001 |
| Fusiform Gyrus (BA 18)* | L | -16 | -90 | -18 | 121 | 3.09 | .002 |
| Angular Gyrus (BA 39) | L | -56 | -66 | 30 | 160 | 3.60 | <.001 |
| Negative Correlations | |||||||
| Inferior Frontal Gyrus (BA 46) | R | 32 | 32 | 12 | 289 | 4.67 | <.001 |
| Middle Frontal Gyrus (BA 9)* | R | 30 | 8 | 42 | 125 | 3.72 | <.001 |
| Orbitofrontal Gyrus (BA 11) | R | 10 | 38 | 0 | 117 | 3.58 | .001 |
| Temporal Pole (BA 38)* | R | 48 | 22 | -32 | 344 | 3.81 | <.001 |
| Inferior Parietal Lobule (40) | R | 62 | -42 | 50 | 143 | 3.84 | <.001 |
| Insula | R | 40 | 2 | 10 | 58 | 3.27 | .001 |
| Cerebellum | L | -14 | -22 | -32 | 79 | 3.50 | .001 |
| R | 22 | -22 | -32 | 152 | 3.48 | .001 | |
| R | 42 | -28 | -36 | 65 | 3.11 | .002 | |
| Middle Occipital Gyrus (19) | R | 46 | -66 | -6 | 58 | 3.11 | .002 |
| Men | |||||||
| Positive Correlations | |||||||
| Cerebellum* | L | -14 | -92 | -30 | 106 | 3.45 | .001 |
| R | 20 | -88 | -30 | 61 | 3.40 | .001 | |
| Negative Correlations | |||||||
| Inferior Frontal Gyrus (BA 47) | L | -18 | 8 | -24 | 553 | 4.02 | <.001 |
| Medial Frontal Gyrus (BA 10)* | L | -12 | 70 | 0 | 78 | 3.27 | .001 |
| R | 14 | 66 | -4 | 57 | 3.47 | <.001 | |
| Precentral Gyrus (BA 6) | L | -48 | -8 | 38 | 107 | 3.11 | .001 |
| Inferior Temporal Gyrus (BA 20)* | R | 62 | -12 | -22 | 289 | 4.42 | <.001 |
| Middle Temporal Gyrus (BA 21)* | L | -54 | 14 | -30 | 82 | 4.23 | <.001 |
| Superior Temporal Gyrus (BA 22)* | L | -52 | 2 | 2 | 145 | 3.56 | <.001 |
| Inferior Parietal Lobule (BA 40) | L | -60 | -28 | 38 | 580 | 4.15 | <.001 |
Regions were significant in the conjunction analysis but not in the baseline analysis alone because of the increased power due to the use of a region-of-interest map and restricted search in the conjunction analysis. These regions reached significance at baseline when the statistical threshold was lowered.
Table 5.
Results of conjunction analysis showing regions correlated with CES-D scores at year 9 but not year 1
| Region | Side | Coordinate |
Cluster Size | t | p | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Women | |||||||
| Negative Correlations | |||||||
| Cingulate Gyrus (BA 32) | L | -12 | 44 | 14 | 64 | 3.49 | .001 |
| Inferior Frontal Gyrus (BA 44) | R | 32 | 8 | 22 | 130 | 3.64 | .000 |
| Men | |||||||
| Negative Correlations | |||||||
| Medial Frontal Gyrus (BA 11) | R | 8 | 18 | -20 | 439 | 3.51 | .000 |
| Middle Frontal Gyrus (BA 46) | L | -52 | 30 | 20 | 328 | 4.28 | .000 |
| Inferior Temporal Gyrus (BA 20) | L | -18 | 6 | -22 | 160 | 3.78 | .000 |
| -54 | -24 | -16 | 128 | 3.11 | .001 | ||
| Superior Occipital Gyrus (BA 19) | L | -34 | -78 | 34 | 220 | 3.08 | .000 |
Note: Some regions were significant in both the conjunction analysis showing common associations between year 9 and baseline and in the conjunction analysis showing unique associations at year 9. In these regions, the peak area of activity shifted from baseline to year 9. Because the Brodmann areas for these regions were identical at baseline and year 9, activity in those regions are considered to be common to baseline and year 9 and are not included in this table.
Association between mean CES-D and Longitudinal rCBF Change
Associations between mean CES-D and longitudinal rCBF change are presented in Table 6 and the lower half of the Figure. Higher mean depressive symptoms in women were associated with longitudinal decreases in blood flow in the right inferior frontal gyrus and inferior parietal lobule. In men, higher average CES-D scores were associated with decreased rCBF in the left middle and medial frontal gyri, the left superior temporal gyrus and insula, and the right inferior temporal gyrus over time.
Table 6.
Regions showing significant correlations with CES-D scores and longitudinal changes in blood flow from year 1 to year 9
| Region | Side | Coordinate |
Cluster Size | t | p | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Women | |||||||
| Negative Correlations | |||||||
| Inferior Frontal Gyrus (BA 44) | R | 36 | 8 | 28 | 75 | 3.99 | <.001 |
| Inferior Parietal Lobule (BA 40) | R | 56 | -44 | 50 | 54 | 3.58 | .001 |
| Men | |||||||
| Negative Correlations | |||||||
| Medial Frontal Gyrus (BA 10) | L | -6 | 68 | -8 | 58 | 4.56 | .000 |
| Middle Frontal Gyrus (BA 46) | L | -52 | 28 | 24 | 101 | 3.50 | .001 |
| Inferior Temporal Gyrus (BA 20) | R | 70 | -24 | -22 | 181 | 4.31 | .000 |
| Superior Temporal Gyrus (22) | L | -50 | 6 | -4 | 89 | 3.76 | .000 |
| Insula | L | -46 | -14 | 12 | 133 | 4.39 | .000 |
Discussion
These results reveal both cross-sectional and longitudinal associations of chronic SDS with rCBF in the aging brain. Consistent with our predictions, associations were primarily in frontal and temporal regions, as well as the cerebellum. These associations were found in both men and women; however, the regional patterns of activity differed for the two sexes.
Cross-sectional Findings
Cross-sectional analyses revealed reduced rCBF in association with increased SDS in frontal and temporal regions in both men and women. The pattern of decreased activity corresponds well with frontolimbic theories of depression (Alexopoulos, 2002, Drevets, 2000, Mayberg, 2003, Tekin and Cummings, 2002). These theories implicate a network of brain regions in the etiology of depression that primarily involves frontal, temporal, and limbic structures including the anterior cingulate and orbitofrontal cortices, amygdala, hippocampus, medial thalamus, striatum, and pallidum. Our finding of primarily frontal and temporal blood flow abnormalities associations with SDS in the absence of thalamic, striatal, and pallidal associations suggests that these cortical regions may be more vulnerable to the effects of subthreshold levels of depressive symptoms. Research that directly compares individuals with threshold and subthreshold depression will be important to evaluate that possibility.
Although both men and women showed associations between SDS and blood flow in frontal and temporal regions, there were sex-specific regional differences in the patterns of associations. Higher SDS were associated with reduced rCBF in orbitofrontal gyrus and cingulate gyrus in women, and reduced rCBF in inferior, medial and middle frontal regions in men. Decreases in activity were primarily bilateral and left-lateralized in men but primarily in the right hemisphere in women. Men and women also showed opposite patterns of associations in the cerebellum, with women showing decreased activity as a function of higher average depressive symptoms, while men with greater SDS showed greater activity in this region. The preponderance of evidence suggests that depression is associated with increased blood flow and metabolism in the cerebellum (Dunn, et al., 2002, Kim, et al., 2008, Kimbrell, et al., 2002, Liotti, et al., 2000, Milak, et al., 2005, Videbech, et al., 2002), although reduced cerebellar activity in major depression has been reported in some studies during cognitive performance (Bremner, et al., 2004, Elliott, et al., 1997). Although these prior studies did not examine sex differences explicitly, our findings suggest that sex may play a role in the relationship between depressive symptoms and cerebellar activity.
Cross-sectional findings in year 9 compared to baseline
Conjunction analyses were used to identify correlations between SDS and rCBF that were evident in year 9 and also observed at baseline, as well as those that were unique to year 9. Most regions showed similar patterns of associations between SDS and rCBF at baseline and at year 9 in both men and women, with similar Brodmann areas involved across timepoints.
The finding that activity in these regions at baseline correlated with the severity of depressive symptoms during the subsequent study interval has multiple possible interpretations. First, activity in these regions could represent a trait or dispositional characteristic of individuals who are at risk of experiencing elevated levels of depressive symptoms. Second, because this study provides a snapshot in time of participant’s depressive symptomatology and brain functioning, it is possible that individuals with higher levels of SDS during the study interval are also individuals who experienced elevated depressive symptoms prior to the start of the study. In that case, baseline findings could reflect the cumulative effect of SDS before the snapshot captured in this study. Longitudinal studies that provide detailed information about lifetime history of depression and depressive symptoms are needed to explore these possibilities.
Both men and women also showed unique associations between SDS and rCBF at year 9 that were not evident at baseline. Unique associations between SDS and decreased blood flow were observed in the left cingulate gyrus and right inferior frontal gyrus in women, and in the right medial frontal gyrus, and left middle frontal, inferior temporal, and superior occipital gyri in men. Again, these activation patterns are consistent with frontolimbic theories of depression. The finding that activity in these regions correlated with average CES-D scores at year 9 but not at baseline suggests that these associations may be related to the chronicity of SDS during the study interval.
Longitudinal Findings
Longitudinal analyses examined correlations between average CES-D scores and the differences in activity between baseline and year 9. Longitudinal associations were limited to decreased blood flow in the right inferior frontal gyrus and inferior parietal lobule in women and in multiple frontal and temporal regions in men. The specificity of these changes to frontal and temporal regions in our restricted analysis is consistent with our hypotheses and with previous research (Fitzgerald, et al., 2008, Tekin and Cummings, 2002). These longitudinal results provide more direct evidence for blood flow changes, rather than simply blood flow differences, in individuals with more chronic depressive symptoms. However, additional research is needed to determine whether these blood flow changes are the result of SDS or contribute to the development of SDS.
Sex Differences
Our finding of sex differences in the patterns of SDS associations with rCBF are consistent with numerous clinical (Beekman, et al., 1995, Ernst and Angst, 1992), cognitive (Dal Forno, et al., 2005, Fuhrer, et al., 2003) and neuroimaging (Lavretsky, et al., 2004, Videbech, et al., 2002) studies that point to disparate clinical manifestations, treatment response, and cognitive and neural correlates of depression in men and women. In our study, men and women showed similar associations between SDS and rCBF patterns, although some differences were observed. Men, but not women, showed the expected increase in blood flow to the cerebellum in association with higher SDS, and SDS were associated with reduced rCBF in a greater number of frontal and temporal regions in men than in women. Other studies have shown that men display more prototypic depression-related outcomes than women (Dal Forno, et al., 2005, Fuhrer, et al., 2003, Lavretsky, et al., 2004). Although there are some exceptions (e.g., Videbech, et al., 2002), our findings, as well as the work of others, provide evidence that men are more vulnerable to the effects of depressive syndromes (Beekman, et al., 1995, Piccinelli and Wilkinson, 2000).
Depressive symptoms may reflect different underlying pathology in men and women for various reasons. Since medical conditions such as cardiovascular disease are associated with depression (Alexopoulos, 2006) and are more common in men (Migeon, 2007), it is possible that older men with SDS are a more homogeneous group than are women with SDS, whose symptoms may reflect greater diversity of underlying pathology. Additionally, men are usually less willing to acknowledge experiencing depressive symptoms (Williams, et al., 1995); thus, symptoms may be more extreme among those men who actually report having them. Depressive symptoms may be overestimated in women, thus diluting effects observed in women because of a high number of false-positives. This would explain the more limited frontolimbic associations in women compared to men. Additional research is needed to elucidate sex differences in the correlates of depression and depressive symptoms. Of particular interest is a greater understanding of whether depressive disorders are fundamentally different in men and women, or if differences reflect differential reporting of symptoms.
It is also possible that the smaller sample size in women compared to men contributed to the differential correlations of rCBF with SDS in the current study. Another limitation of this study is the lack of availability of information regarding the age of onset of depressive symptoms, given the evidence of distinct etiologies and consequences of early and late onset depression. Additionally we did not have detailed information regarding antidepressant use (e.g., stability of medication dosage) in our sample. Both acute and chronic effects of antidepressant medication on rCBF have been documented (Davies, et al., 2003, Joe, et al., 2006, Nobler, et al., 2002, Smith, et al., 2002), and antidepressant use history may have impacted our results. However, the inclusion of antidepressant use as a covariate in our analyses minimized any potential confounding effects. Finally, the average of depressive symptoms scores over the study interval is a relatively rough estimate of chronic depressive symptoms and is an additional limitation of the study. These limitations should be considered in the context of the strengths of this study, which include the large sample size which allowed us to investigate associations between depressive symptoms and blood flow separately in men and women. In addition, the long follow-up period and prospective assessments provided a unique opportunity to examine both cross-sectional and longitudinal associations of SDS and rCBF in dementia-free older adults. To our knowledge, this is the first investigation to do so.
Conclusion
In summary, this study documented cross-sectional and longitudinal correlations between chronic SDS and blood flow in older men and women. Patterns of rCBF associations with SDS were similar to those found in studies of regional cerebral glucose metabolism and CBF in clinical depression, providing support for the hypothesis that depressive syndromes, including subthreshold levels of symptoms, exist on a continuum of severity. The neural correlates of SDS differed for men and women, with men showing more widespread fronto-temporal SDS-associated decreases in rCBF than women. The sex differences provide some evidence that the pathophysiology of depressive disorders differs between men and women. In addition, sex differences suggest that simply covarying for sex in depression research may obscure significant findings and limit our ability to determine differential correlates of mood disorders in men and women.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
The authors have no potential conflicts of interest to be disclosed.
References
- Alexopoulos GS. Frontostriatal and limbic dysfunction in late-life depression. Am J Geriatr Psychiatry. 2002;10:687–695. [PubMed] [Google Scholar]
- Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biol Psychiatry. 2006;60:1304–1305. doi: 10.1016/j.biopsych.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Angst J, Sellaro R, Merikangas KR. Depressive spectrum diagnoses. Compr Psychiatry. 2000;41:39–47. doi: 10.1016/s0010-440x(00)80007-3. [DOI] [PubMed] [Google Scholar]
- Bassuk SS, Berkman LF, Wypij D. Depressive symptomatology and incident cognitive decline in an elderly community sample. Arch Gen Psychiatry. 1998;55:1073–1081. doi: 10.1001/archpsyc.55.12.1073. [DOI] [PubMed] [Google Scholar]
- Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, et al. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol Med. 1997;27:231–235. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- Beekman AT, Kriegsman DM, Deeg DJ, van Tilburg W. The association of physical health and depressive symptoms in the older population: age and sex differences. Soc Psychiatry Psychiatr Epidemiol. 1995;30:32–38. doi: 10.1007/BF00784432. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Bienias JL, et al. Cerebral infarctions and the relationship of depression symptoms to level of cognitive functioning in older persons. Am J Geriatr Psychiatry. 2004;12:211–219. [PubMed] [Google Scholar]
- Blessed G, Wilson ID. The contemporary natural history of mental disorder in old age. Br J Psychiatry. 1982;141:59–67. doi: 10.1192/bjp.141.1.59. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Vaccarino V, et al. Deficits in hippocampal and anterior cingulate functioning during verbal declarative memory encoding in midlife major depression. Am J Psychiatry. 2004;161:637–645. doi: 10.1176/appi.ajp.161.4.637. [DOI] [PubMed] [Google Scholar]
- Chodosh J, Kado DM, Seeman TE, Karlamangla AS. Depressive symptoms as a predictor of cognitive decline: MacArthur Studies of Successful Aging. Am J Geriatr Psychiatry. 2007;15:406–415. doi: 10.1097/01.JGP.0b013e31802c0c63. [DOI] [PubMed] [Google Scholar]
- Chuan SK, Kumar R, Matthew N, Heok KE, et al. Subsyndromal depression in old age: clinical significance and impact in a multi-ethnic community sample of elderly Singaporeans. Int Psychogeriatr. 2008;20:188–200. doi: 10.1017/S1041610207006187. [DOI] [PubMed] [Google Scholar]
- Dal Forno G, Palermo MT, Donohue JE, Karagiozis H, et al. Depressive symptoms, sex, and risk for Alzheimer’s disease. Ann Neurol. 2005;57:381–387. doi: 10.1002/ana.20405. [DOI] [PubMed] [Google Scholar]
- Davies J, Lloyd KR, Jones IK, Barnes A, et al. Changes in regional cerebral blood flow with venlafaxine in the treatment of major depression. Am J Psychiatry. 2003;160:374–376. doi: 10.1176/appi.ajp.160.2.374. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Dunn RT, Kimbrell TA, Ketter TA, Frye MA, et al. Principal components of the Beck Depression Inventory and regional cerebral metabolism in unipolar and bipolar depression. Biol Psychiatry. 2002;51:387–399. doi: 10.1016/s0006-3223(01)01244-6. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Kumar A, Bilker WB, Dunkin JJ, et al. Neuropsychological deficits among patients with late-onset minor and major depression. Arch Clin Neuropsychol. 2003;18:529–549. doi: 10.1016/s0887-6177(03)00022-2. [DOI] [PubMed] [Google Scholar]
- Elliott R, Baker SC, Rogers RD, O’Leary DA, et al. Prefrontal dysfunction in depressed patients performing a complex planning task: a study using positron emission tomography. Psychol Med. 1997;27:931–942. doi: 10.1017/s0033291797005187. [DOI] [PubMed] [Google Scholar]
- Ernst C, Angst J. The Zurich Study. XII. Sex differences in depression. Evidence from longitudinal epidemiological data. Eur Arch Psychiatry Clin Neurosci. 1992;241:222–230. doi: 10.1007/BF02190257. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29:683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrer R, Dufouil C, Dartigues JF. Exploring sex differences in the relationship between depressive symptoms and dementia incidence: prospective results from the PAQUID Study. J Am Geriatr Soc. 2003;51:1055–1063. doi: 10.1046/j.1532-5415.2003.51352.x. [DOI] [PubMed] [Google Scholar]
- Geiselmann B, Bauer M. Subthreshold depression in the elderly: qualitative or quantitative distinction? Compr Psychiatry. 2000;41:32–38. doi: 10.1016/s0010-440x(00)80006-1. [DOI] [PubMed] [Google Scholar]
- Goldberg DD. Plato versus Aristotle: categorical and dimensional models for common mental disorders. Compr Psychiatry. 2000;41:8–13. doi: 10.1016/s0010-440x(00)80002-4. [DOI] [PubMed] [Google Scholar]
- Haringsma R, Engels GI, Beekman AT, Spinhoven P. The criterion validity of the Center for Epidemiological Studies Depression Scale (CES-D) in a sample of self-referred elders with depressive symptomatology. Int J Geriatr Psychiatry. 2004;19:558–563. doi: 10.1002/gps.1130. [DOI] [PubMed] [Google Scholar]
- Joe AY, Tielmann T, Bucerius J, Reinhardt MJ, et al. Response-dependent differences in regional cerebral blood flow changes with citalopram in treatment of major depression. J Nucl Med. 2006;47:1319–1325. [PubMed] [Google Scholar]
- Kawas C, Gray S, Brookmeyer R, Fozard J, et al. Age-specific incidence rates of Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54:2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Song SH, Kim JH, Kwak IS. Statistical parametric mapping analysis of the relationship between regional cerebral blood flow and symptom clusters of the depressive mood in patients with pre-dialytic chronic kidney disease. Ann Nucl Med. 2008;22:201–206. doi: 10.1007/s12149-007-0108-x. [DOI] [PubMed] [Google Scholar]
- Kimbrell TA, Ketter TA, George MS, Little JT, et al. Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. Biol Psychiatry. 2002;51:237–252. doi: 10.1016/s0006-3223(01)01216-1. [DOI] [PubMed] [Google Scholar]
- Kumar A, Bilker W, Lavretsky H, Gottlieb G. Volumetric asymmetries in late-onset mood disorders: an attenuation of frontal asymmetry with depression severity. Psychiatry Res. 2000;100:41–47. doi: 10.1016/s0925-4927(00)00067-6. [DOI] [PubMed] [Google Scholar]
- Kumar A, Jin Z, Bilker W, Udupa J, et al. Late-onset minor and major depression: early evidence for common neuroanatomical substrates detected by using MRI. Proc Natl Acad Sci U S A. 1998;95:7654–7658. doi: 10.1073/pnas.95.13.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Schweizer E, Jin Z, Miller D, et al. Neuroanatomical substrates of late-life minor depression. A quantitative magnetic resonance imaging study. Arch Neurol. 1997;54:613–617. doi: 10.1001/archneur.1997.00550170085018. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Kurbanyan K, Ballmaier M, Mintz J, et al. Sex differences in brain structure in geriatric depression. Am J Geriatr Psychiatry. 2004;12:653–657. doi: 10.1176/appi.ajgp.12.6.653. [DOI] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, Brannan SK, McGinnis S, et al. Differential limbic--cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biol Psychiatry. 2000;48:30–42. doi: 10.1016/s0006-3223(00)00874-x. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Positron emission tomography imaging in depression: a neural systems perspective. Neuroimaging Clin N Am. 2003;13:805–815. doi: 10.1016/s1052-5149(03)00104-7. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Migeon BR. Why females are mosaics, X-chromosome inactivation, and sex differences in disease. Gend Med. 2007;4:97–105. doi: 10.1016/s1550-8579(07)80024-6. [DOI] [PubMed] [Google Scholar]
- Milak MS, Parsey RV, Keilp J, Oquendo MA, et al. Neuroanatomic correlates of psychopathologic components of major depressive disorder. Arch Gen Psychiatry. 2005;62:397–408. doi: 10.1001/archpsyc.62.4.397. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Nobler MS, Olvet KR, Sackeim HA. Effects of medications on cerebral blood flow in late-life depression. Curr Psychiatry Rep. 2002;4:51–58. doi: 10.1007/s11920-002-0013-x. [DOI] [PubMed] [Google Scholar]
- Piccinelli M, Wilkinson G. Gender differences in depression. Critical review. Br J Psychiatry. 2000;177:486–492. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ravdin LD, Katzen HL, Agrawal P, Relkin NR. Letter and semantic fluency in older adults: effects of mild depressive symptoms and age-stratified normative data. Clin Neuropsychol. 2003;17:195–202. doi: 10.1076/clin.17.2.195.16500. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Goldszal AF, Davatzikos C, Golski S, et al. One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000;10:464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- Smith GS, Kramer E, Hermann CR, Goldberg S, et al. Acute and chronic effects of citalopram on cerebral glucose metabolism in geriatric depression. Am J Geriatr Psychiatry. 2002;10:715–723. [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Awata S, Inoue K, et al. Male elderly subthreshold depression patients have smaller volume of medial part of prefrontal cortex and precentral gyrus compared with age-matched normal subjects: a voxel-based morphometry. J Affect Disord. 2005;88:313–320. doi: 10.1016/j.jad.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. 2002;53:647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B, Pedersen TH, Hartvig H, et al. The Danish PET/depression project: clinical symptoms and cerebral blood flow. A regions-of-interest analysis. Acta Psychiatr Scand. 2002;106:35–44. doi: 10.1034/j.1600-0447.2002.02245.x. [DOI] [PubMed] [Google Scholar]
- Williams JB, Spitzer RL, Linzer M, Kroenke K, et al. Gender differences in depression in primary care. Am J Obstet Gynecol. 1995;173:654–659. doi: 10.1016/0002-9378(95)90298-8. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Mendes De Leon CF, Bennett DA, Bienias JL, et al. Depressive symptoms and cognitive decline in a community population of older persons. J Neurol Neurosurg Psychiatry. 2004;75:126–129. [PMC free article] [PubMed] [Google Scholar]


