Abstract
Purpose
We investigated the effects of intrathecal application of GABAA and GABAB receptor agonists on detrusor overactivity in spinal cord injured rats.
Materials and Methods
Adult female Sprague-Dawley rats were used. At 4 weeks after Th9-10 spinal cord transection, awake cystometry and recordings of external urethral sphincter electromyogram were performed to examine the effect of intrathecal application of GABAA and GABAB agonists (muscimol and baclofen, respectively) or GABAA and GABAB antagonists (bicuculline and saclofen, respectively) at the level of L6-S1 spinal cord. Expression of glutamate decarboxylase 67 mRNA in the L6-S1 spinal cord and dorsal root ganglia was also assessed.
Results
Both muscimol and baclofen produced a dose-dependent inhibition of the number (51–73% decrease) and amplitude (35–93% decrease) of nonvoiding bladder contractions, and a decrease in micturition pressure. The effects of muscimol and baclofen were antagonized by bicuculline and saclofen, respectively. Bursting activity of external urethral sphincter electromyogram was inhibited corresponding to the inhibition of bladder activity by muscimol and baclofen. Glutamate decarboxylase 67 mRNA levels in the spinal cord and dorsal root ganglia was decreased (55% and 84%, respectively) after spinal cord transection.
Conclusions
These results indicate that GABAA and GABAB receptor activation in the spinal cord inhibits detrusor overactiivty. The reduction in glutamate decarboxylase 67 mRNA suggests hypofunction of GABAergic inhibitory mechanisms in the spinal cord. Therefore, stimulation of spinal GABAergic mechanisms could be effective for the treatment of detrusor overactivity after spinal cord injury.
Keywords: bladder, urethra, spinal cord injury, GABA, detrusor overactivity
INTRODUCTION
Micturition depends on the coordination between the bladder and EUS.1 However, SCI rostral to the lumbosacral level impairs voluntary and supraspinal control of voiding. SCI initially induces areflexic bladder and urinary retention, followed by the emergence of automatic micturition and eventually DO mediated by spinal reflex mechanisms. Bladder-sphincter coordination is impaired, leading to DSD.2 Thus, the organization of the micturition reflex shows marked changes after SCI.
It has been reported that glycine and opioids can inhibit bladder activity after SCI.3–4 GABA is another important inhibitory neurotransmitter in the central nervous system.5 GABA is synthesized from glutamate by GAD,5 and is known to have an important role in the inhibitory regulation of micturition in spinal intact rats.6 However, to our knowledge the role of GABAergic system in modulating the spinal micturition reflex after SCI is relatively unexplored although the GABAB receptor agonist baclofen administered intrathecally reduces DO in SCI humans with severe spasticity.7 Therefore, in the present study, we aimed to clarify the differential effects of GABAA and GABAB receptor activation in the spinal cord on bladder and EUS activity in SCI rats as well as changes in expression of GAD67 mRNA in L6-S1 spinal cord and DRG, where bladder afferent fibers originate following SCI.
MATERIALS AND METHODS
Animal model
A total of 28 adult female Sprague-Dawley rats weighing 232–270 g were used according to the experimental protocol approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Rats were anesthetized with 2% isoflurane and Th9 laminectomy was performed. To produce SCI, the dura was opened, complete transection of the Th9-Th10 spinal cord was performed with scissors and a sterile Surgifoam sponge (Ferrosan, Soeborg, Denmark) was placed between the cut ends of the spinal cord. The overlying muscle and skin were then sutured to close the wound. The animals were postoperatively treated with ampicillin (100 mg/kg, intramuscularly) for 5 days. The bladder of spinalized rats was emptied by abdominal compression twice a day until reflex voiding recovered, usually 10 to 14 days after spinalization.
Surgical procedure
Four weeks after spinal cord transection, under isoflurane anesthesia, a laminectomy was performed at the level of the 3rd lumbar vertebra, and a polyethylene catheter (PE-10, Clay-Adams, Parsippany, New Jersey) was implanted intrathecally at the level of the L6-S1 spinal cord through a small hole of the dura. The end of the catheter was heat sealed and placed subcutaneously.
One to two days after intrathecal catheter implantation, the animals were anesthetized with 2% isoflurane, and the abdomen was opened via midline laparotomy. The ureters were transected at the level of the aortic bifurcation, and the distal ends were ligated. A PE-90 catheter was inserted through the dome of the bladder to record intravesical pressure. The implanted intrathecal catheter was exteriorized through the skin incision for drug administration. After the abdomen was closed, the rats were placed in a restrainer (Braintree Scientific Inc., Braintree, Massachusetts) and were allowed to recover from anesthesia for 1 to 2 hours.
Cystometry and EUS-EMG before and after intrathecal injection of GABAA (n = 8) or GABAB agonists (n = 8)
The intravesical catheter was connected to a pressure transducer and an infusion pump through a three-way stopcock. Physiological saline at room temperature was infused at a rate of 0.08 ml/min to elicit repetitive bladder contractions. Two fine silver wire electrodes (Gauge wire, Astro-Med Inc., West Warwick, RI) were inserted into the EUS percutaneously through the lateral sides of external urethral meatus. After two micturition cycles were recorded before drug administration, muscimol (GABAA agonist; 0.001–1 μg) or (±)-baclofen (GABAB agonist; 0.001–1 μg) was administered cumulatively at 15–30 min intervals through the intrathecal catheter using a microsyringe (10 μl, Hamilton, Reno, Nevada).
Saline volume from the urethral meatus was collected and measured to determine voided volume. After each voiding, the infusion was stopped and residual volume was measured. Bladder capacity was calculated as the sum of voided volume and residual volume. Voiding efficiency was then calculated using a formula: voiding efficiency (%) = [(voided volume/bladder capacity) × 100]. The number and amplitude of NVCs were measured during 2 min prior to micturition. NVCs were defined as rhythmic intravesical pressure increases greater than 7 cm H2O from baseline pressure without release of fluid from the urethra, and MVP was also measured. In EMG recordings, the number of spikes, maximal and mean voltage of spikes during voiding bladder contractions was also evaluated.
Cystometry and EUS-EMG before and after intrathecal injection of GABAA (n = 4) or GABAB (n = 4) antagonist
In order to confirm the receptor subtype mediating the effect of agonists, (−)-bicuculline methobromide (GABAA antagonist; 0.01 μg) or saclofen (GABAB antagonists; 1 μg) were injected intrathecally 3 min prior to intrathecal administration of muscimol or baclofen (each 0.1 μg).
Administration of drugs
For intrathecal application, 1 μl of drug solution was given via the implanted intrathecal catheter and flushed by 10 μl saline. After the experiments, the position of the intrathecal catheter was checked in all animals, and the extent of dye distribution in the subarachnoid space was confirmed by injection of Evans blue flushed with 10 μl saline. Muscimol, (−)–bicuculline methobromide, and saclofen (Tocris Cookson Inc, Ellisville, Missouri) were dissolved in distilled water. (±)-Baclofen (Tocris Cookson Inc) was dissolved 1N NaOH.
Quantification of GAD67 mRNA
L6-S1 DRG and the spinal cord were rapidly removed in 4 spinal intact and 4 SCI rats. SCI rats were randomly selected from those used for cystometry measurements. Total RNA was extracted from the pooled DRG and spinal cord using TRIzol reagent (Invitrogen, Carlsbad, California). One and 5 μg of RNA (DRG and spinal cord, respectively) was reverse-transcribed into cDNA using Superscript II (Invitrogen). Primers for GAD67 8 and β-actin (Ambion, Austin, Texas) were used: GAD67 forward primer, 5′-GCTGGAAGGCATGGAAGGTTTTAA-3′; reverse primer, 5′-AATATCCCATCACCATCTTTATTTGACC-3′. The GAD67 and β-actin mRNA level was quantified with an MX3000P real-time PCR system (Stratagene, La Jolla, California) in a 25 μl volume using SYBR Green PCR Master Mix (QIAGEN, Valencia, California). Specificity of GAD67 in DRG and the spinal cord was confirmed by melting curve analysis. Quantification of the samples was achieved from the threshold cycle by interpolation from standard curve to calculate a copy number for GAD67, and ratio of GAD67 to β-actin mRNA was compared.
Statistical analysis
Data are expressed as the mean ± SE. Student’s t-test for unpaired data was used to compare changes in the GAD67 mRNA level between intact and SCI rats. Repeated measures ANOVA followed by Dunnett post-test was used to compare changes in bladder and EUS-EMG activity before and after cumulative drug injections. P values less than 0.05 were considered to be significant.
RESULTS
Changes in bladder and EUS-EMG activity after intrathecal injection of muscimol (0.001–1 μg) (n = 8)
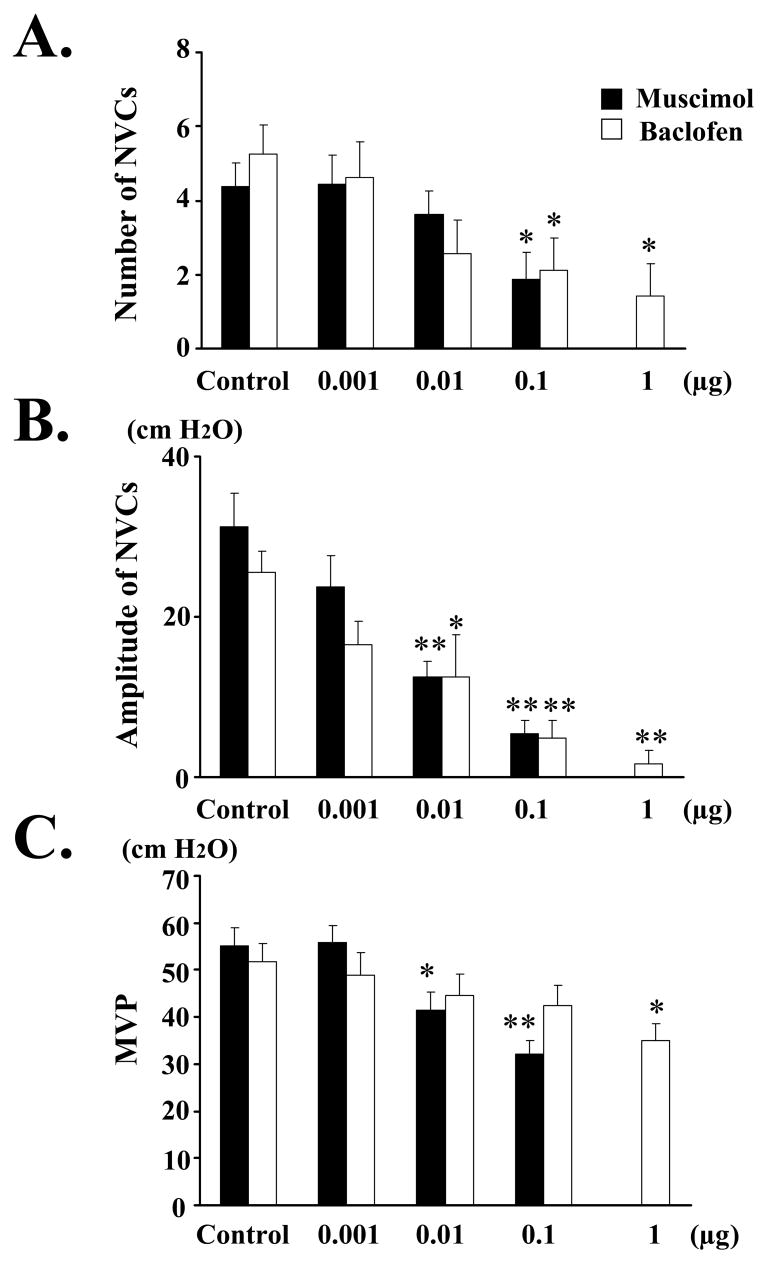
During awake cystometry, all 8 spinalized rats showed NVCs before large-amplitude voiding bladder contractions occurred (Figs. 1A, 5A). The number and amplitude of NVCs, and MVP were 4.38 ± 0.63, 31.3 ± 4.11 cm H2O, and 55.3 ± 3.81 cm H2O, respectively, before drug administration (Control). Voiding volume and voiding efficiency were decreased after 0.1 μg of muscimol (Table). Dribbling overflow incontinence was noted after 1 μg of muscimol in 7 out of 8 rats. Intrathecal application of 0.1 μg of muscimol significantly decreased the number of NVCs (Fig. 2A). The amplitude of NVCs was significantly reduced after intrathecal application of muscimol (0.01–0.1 μg) (Fig. 2B). MVP was significantly reduced after intrathecal application of muscimol (0.01–0.1 μg) (Fig. 2C).
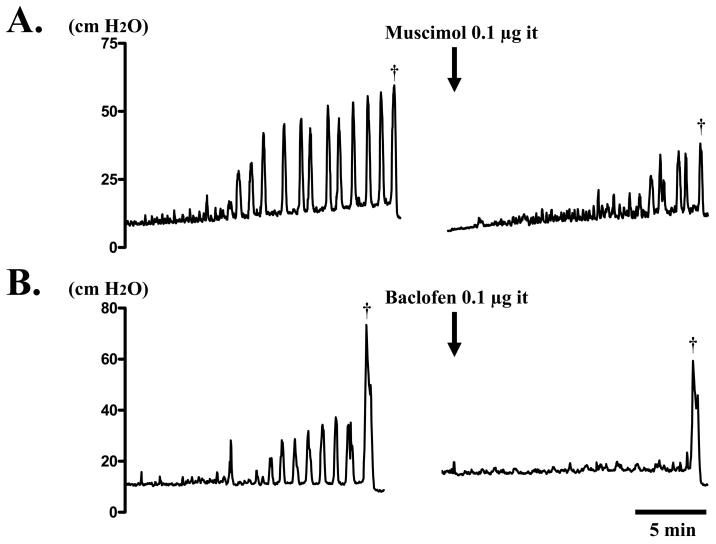
Fig. 1.
Continuous cystometry in a SCI rat before and after intrathecal (it) application of muscimol (A) or baclofen (B). A. Before voiding (dagger), many NVCs were observed. However, NVCs and voiding bladder contraction (dagger) were inhibited after intrathecal 0.l μg of muscimol. B. Before voiding (dagger), many NVCs were also observed. However, NVCs almost disappeared along with a small inhibition of voiding bladder contraction (dagger), after intrathecal 0.l μg of baclofen.
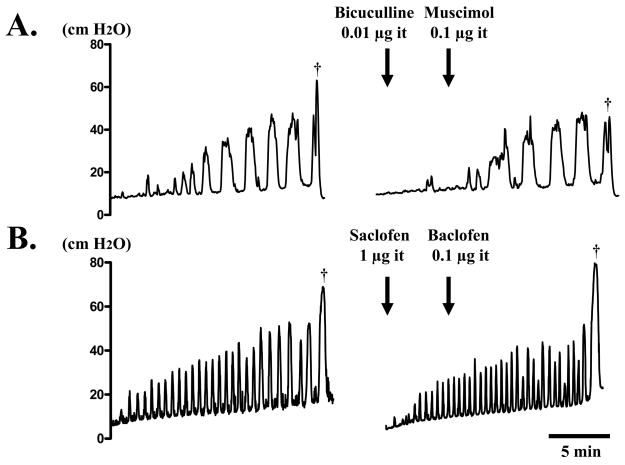
Fig. 5.
Continuous cystometry in a SCI rat before and after intrathecal (it) application of muscimol (A) or baclofen (B) following bicuculline or saclofen. A. Prior to voiding (dagger), many NVCs are shown. After intrathecal 0.l μg of muscimol following 0.01 μg of bicuculline, NVCs were not changed. B. Prior to voiding (dagger), many NVCs were also observed. After intrathecal 0.l μg of baclofen following 1 μg of saclofen, NVCs were not altered.
Table.
The Effects of intrathecal application of muscimol and baclofen in cystometric parameters in SCI rats
| Muscimol (n = 8) |
||||||
|---|---|---|---|---|---|---|
| Control | 0.001 | 0.01 | 0.1 | 1 (μg) | ||
| VV (ml) | 0.60 ± 0.17 | 0.53 ± 0.16 | 0.43 ± 0.16 | 0.20 ± 0.06* | – | |
| RV (ml) | 0.54 ± 0.21 | 0.63 ± 0.30 | 0.65 ± 0.30 | 1.17 ± 0.33 | – | |
| BC (ml) | 1.14 ± 0.38 | 1.11 ± 0.42 | 1.08 ± 0.34 | 1.37 ± 0.35 | – | |
| VE (%) | 56.4 ± 5.4 | 50.9 ± 8.0 | 44.2 ± 9.5 | 15.2 ± 4.0** | – | |
| Baclofen (n = 8) |
||||||
| Control | 0.001 | 0.01 | 0.1 | 1 (μg) | ||
|
| ||||||
| VV (ml) | 0.60 ± 0.14 | 0.53 ± 0.15 | 0.60 ± 0.15 | 0.52 ± 0.19 | 0.24 ± 0.04* | |
| RV (ml) | 0.25 ± 0.05 | 0.21 ± 0.03 | 0.28 ± 0.07 | 0.29 ± 0.08 | 0.61 ± 0.25 | |
| BC (ml) | 0.85 ± 0.15 | 0.74 ± 0.15 | 0.88 ± 0.15 | 0.81 ± 0.20 | 0.86 ± 0.25 | |
| VE (%) | 66.1 ± 7.7 | 65.4 ± 7.9 | 67.0 ± 9.4 | 59.9 ± 7.7 | 40.6 ± 11.3* | |
Values are the mean ± SE. Significant differences when compared with before application (control) are indicated by:
p < 0.05 and
p < 0.01.
VV: voided volume, RV: residual volume, BC: bladder capacity, VE: voiding efficiency
Fig. 2.
Effect of intrathecal application of muscimol (black bars; 0.001–0.1 μg) or baclofen (white bars; 0.001–1 μg) on the number (A) and amplitude (B) of NVCs, as well as the MVP (C) in SCI rats. Measurements after drugs were compared with the values before drug application (control). A: The number of NVCs was significantly decreased by intrathecal application of 0.1 μg of muscimol or baclofen (0.1–1 μg). B: The amplitude of NVCs was significantly and dose-dependently decreased after intrathecal application of muscimol (0.01–0.1 μg) or baclofen (0.01–1 μg). C: The MVP was significantly and dose-dependently decreased after intrathecal application of musimol (0.01–0.1 μg) or 1 μg of baclofen. Values are the mean ± standard error (SE). Significant difference from control (Cont): * p < 0.05, and ** p < 0.01. Increasing doses of drugs were administered at 15–30 min intervals.
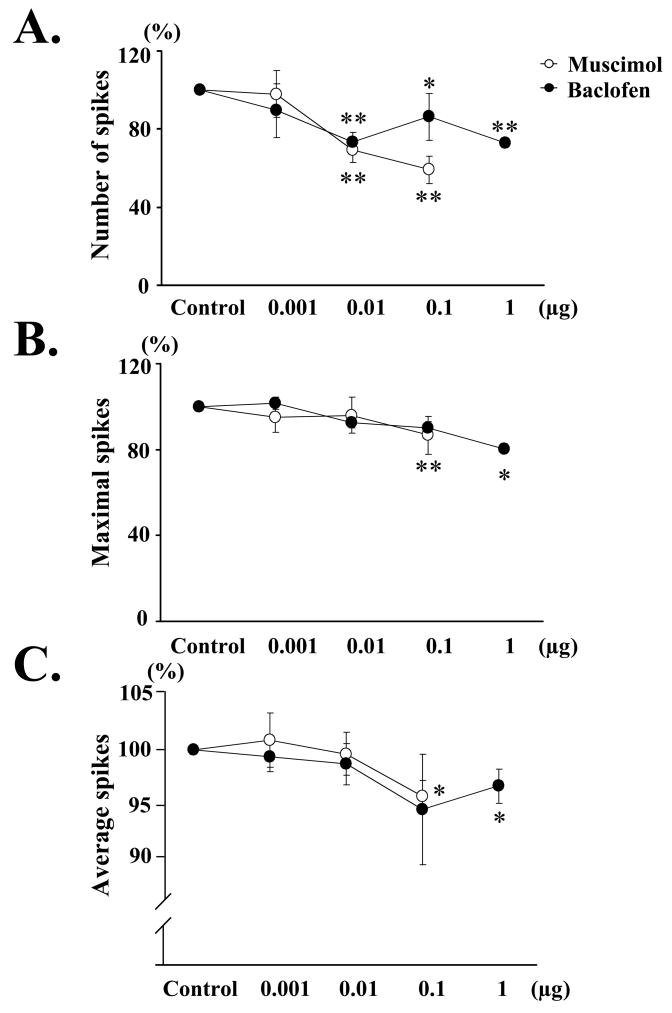
Intrathecal application of muscimol also decreased EUS-EMG activity (Fig. 3A). The number of spikes during voiding bladder contractions showed a significant decrease after intrathecal application of muscimol (0.01–0.1 μg) (Fig. 4A). The maximal and mean voltage of spikes during voiding bladder contractions was significantly decreased by 0.1 μg of muscimol (Fig. 4B and 4C).
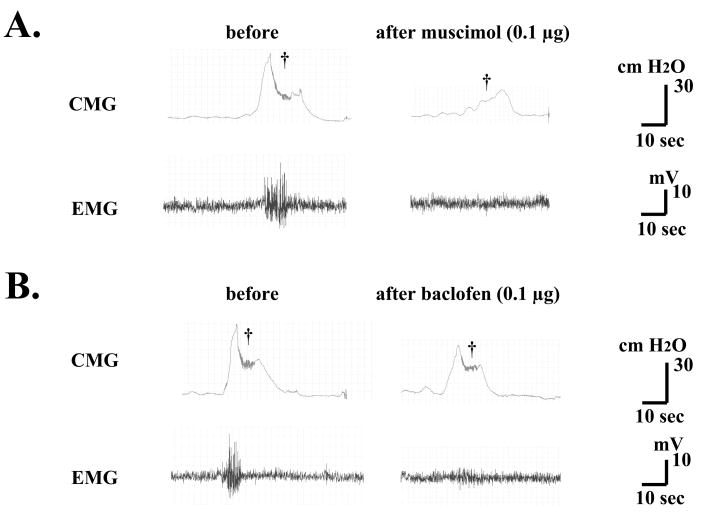
Fig. 3.
Continuous cystometry (CMG) and EMG in a SCI rat before and after intrathecal application of muscimol (A) or baclofen (B). A. Bursting activity of EUS-EMG and the bladder contractions during voiding (dagger) were inhibited by 0.1 μg of muscimol. B. Bursting activity of EUS-EMG during voiding (dagger) was also inhibited in association with a small inhibition of bladder activity by 0.1 μg of baclofen.
Fig. 4.
Effect of intrathecal application of muscimol (white circles; 0.001–0.1 μg) or baclofen (black circles; 0.001–1 μg) on the number (A), maximal (B), and average voltage (C) of EUS-EMG spikes during voiding bladder contractions, compared with the values before injection (control) in SCI rats. A: The number of spikes (expressed as % of control) during voiding bladder contractions was significantly and dose-dependently decreased by intrathecal application of muscimol (0.01–0.1 μg) or baclofen (0.01–1 μg). B: The maximal voltage of spikes during voiding bladder contractions was significantly decreased after intrathecal application of muscimol (0.1 μg) or baclofen (1 μg). C: The average voltage of spikes during voiding bladder contractions was significantly decreased after intrathecal application of muscimol (0.1 μg) or baclofen (1 μg). Values are the mean ± standard error (SE). Significant difference from control (Cont): * p < 0.05, and ** p < 0.01.
Changes in bladder and EUS-EMG activity after intrathecal injection of baclofen (0.001–1 μg) (n = 8)
During awake cystometry, all 8 spinalized rats showed NVCs before voiding bladder contractions occurred (Figs. 1B, 5B). The number and amplitude of NVCs, and MVP were 5.25 ± 0.77, 25.6 ± 2.60 cm H2O, and 51.9 ± 3.72 cm H2O, respectively, before drug administration (Control). Voiding volume and voiding efficiency were decreased after 1 μg of baclofen (Table). Dribbling overflow incontinence was noted after 1 μg of baclofen in 1 out of 8 rats. Intrathecal application of baclofen (0.1–1 μg) significantly decreased the number of NVCs (Fig. 2A). The amplitude of NVCs was significantly reduced after intrathecal application of baclofen (0.01–1 μg) (Fig. 2B). MVP was significantly reduced after 1 μg of baclofen (Fig. 2C).
Intrathecal application of baclofen also decreased EUS-EMG activity (Fig. 3B). The number of spikes during voiding bladder contractions showed a significant decrease after intrathecal application of baclofen (0.01–1 μg) (Fig. 4A). The maximal and mean voltage of spikes during voiding bladder contractions was significantly decreased by 1 μg of baclofen (Fig. 4B and 4C).
Changes in bladder and EUS-EMG activity after intrathecal application of GABA antagonists
The number and amplitude of NVCs, and MVP were 3.75 ± 0.25, 18.5 ± 3.47 cm H2O, and 52.4 ± 3.84 cm H2O, respectively, before drug administration (n = 4). When bicuculline (0.01 μg) was applied intrathecally prior to muscimol (0.1 μg), these cystometric parameters and EUS-EMG activity were not altered by bicuculline or subsequent administration of muscimol (Fig. 5A).
In another group, the number and amplitude of NVCs, and MVP were 3.75 ± 0.85, 20.1 ± 2.74 cm H2O, and 72.8 ± 2.09 cm H2O, respectively, before drug administration (n = 4). Intrathecal application of saclofen (1 μg) followed by baclofen (0.1 μg) did not affect these cystometric parameters and EUS-EMG activity (Fig. 5B).
Changes in the GAD67 mRNA level of L6-S1 DRG and the spinal cord after SCI
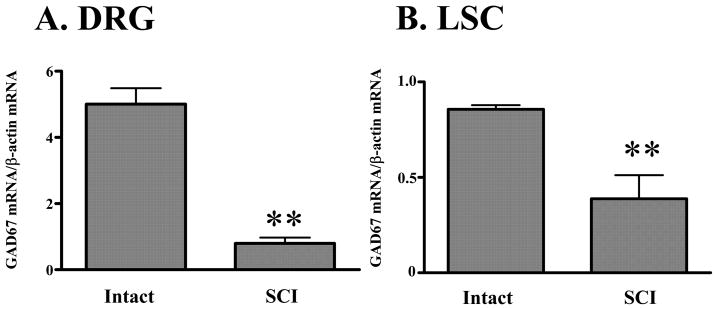
In L6-S1 DRG, the GAD67 mRNA/β-actin mRNA ratio in SCI rats (n = 4) was significantly (p < 0.01) lower (0.80 ± 0.18) compared with spinal intact rats (5.01 ± 0.48, n = 4) (Fig. 6A). In the spinal cord, the GAD67 mRNA/β-actin mRNA ratio was significantly (p < 0.01) reduced in SCI rats (n = 4) compared with spinal intact rats (n = 4) (0.39 ± 0.12 vs. 0.86 ± 0.02) (Fig. 6B).
Fig. 6.
GAD67 mRNA/β-actin mRNA ratio in the L6-S1 DRG (A) and spinal cord (LSC; B). GAD67 mRNA/β-actin mRNA ratio in the L6-S1 DRG (A) and LSC (B) was significantly decreased after SCI. Values are the mean ± SE. Significant differences between intact and SCI rats are indicated by: ** p < 0.01.
DISCUSSION
The results of the current study indicate that; (1) activation of GABAA and GABAB receptor inhibits DO as evidenced by a reduction in NVCs in SCI rats, (2) GABAB receptor activation preferentially reduces DO prior to inhibiting voiding contractions while GABAA receptor activation inhibits DO and voiding contraction at the same concentration, and (3) expression in GAD, a GABA synthesizing enzyme, is decreased in the spinal cord and DRG after SCI. In addition, since the effective doses of muscimol and baclofen (0.01–1 μg) to suppress bladder activity in this study using SCI rats seem to be lower than those (muscimol: 1 to 10 μg and baclofen: 0.1 to 5 μg) reported in normal rats,6 it is assumed that chronic SCI increases GABA receptor sensitivity in the spinal cord, possibly due to downregulation of the inhibitory GABAergic mechanism.
We found that the effect of muscimol on NVCs and MVP occurred at the same threshold dose (0.01 μg), while the effect of baclofen on NVCs started at a lower dose (0.01 μg) than that required to suppress MVP (1 μg). Dribbling overflow incontinence was noted in 7 of 8 rats after intrathecal application of muscimol (1 μg), while it was noted in only 1 of 8 rats after intrathecal application of baclofen (1 μg). Since decreased bladder contractility (i.e., a reduction in MVP) is assumed to reflect the suppression of bladder efferent pathways rather than afferent pathways,2 activation of spinal GABAA receptors by muscimol is likely to inhibit the efferent limb of the micturition reflex to reduce NVCs and MVP at the same concentration.
In contrast, since GABAB receptor activation inhibits NVCs before reducing MVP at lower doses (0.01–0.1 μg), spinal GABAB receptors could have a different role in the control of the micturition reflex in SCI. It is well known that 2 types of afferent fibers, namely Aδ and C-fibers, carry sensory information from the bladder to the spinal cord.9–10 In cats and rats showed that Aδ-fiber bladder afferents trigger the normal micturition via a long latency supraspinal pathway passing through the pons.2,10 In chronic SCI rats, desensitizing C-fiber afferents by systemic capsaicin administration suppressed NVCs without affecting the voiding reflex.11 This effect is similar to that seen in the current experiments following GABAB receptor activation by baclofen at lower doses (0.01–0.1 μg), suggesting that GABAB receptor activation initially inhibits DO by suppressing C-fiber bladder afferents prior to affecting MVP.
The current study also showed that GABA receptor activation reduced EUS-EMG activity during voiding bladder contractions. A decrease in EUS-EMG activity may be induced by (1) decreased bladder contraction pressure because EUS activity changes according to bladder activity, (2) inhibition of EUS motoneurons because these neurons receive GABAergic innervation,12 or (3) inhibition of C-fiber bladder afferents because a previous study indicated that hyperexcitability of C-fiber bladder afferents is also involved in DSD during voiding after SCI.13 Since a low dose of muscimol inhibited both NVCs and MVP to a similar extent, and a high dose induced dribbling overflow incontinence in most rats, the first and second possibilities are more likely to be involved in suppression of EUS-EMG activity during GABAA receptor activation. However, because baclofen predominantly inhibited C-fiber-mediated NVCs, especially at lower doses, the third mechanism may be more important in the suppression of EUS-EMG activity during GABAB receptor activation.
The current study shows that GAD67 mRNA level in the L6-S1 spinal cord and DRG was significantly decreased after SCI. Thus, hypofunction of the GABAergic inhibitory system might be responsible for the development of DO and/or DSD after SCI. There are many GABAergic interneurons in the ventral reticular formation of the medulla that project diffusely to the spinal cord.5 Thus, disruption of these spinal GABAgic pathways and/or other supraspinal projections to GABAergic interneurons in the sacral spinal cord may lead to downregulation of the spinal GABAergic inhibitory system. It is also reported that chronic SCI rats, which show DO, have lower glycine levels in the lumbosacral cord and serum compared with spinal intact rats.14 Intrathecal injection of strychnine (a glycine receptor antagonist) or bicuculline does not completely restore reflex bladder contractions that are abolished by rectal distension in spinal intact rats, but simultaneous intrathecal injection of small doses of both drugs does so.15 Therefore, the spinal inhibitory neurotransmitters such as glycine and GABA may have additive or synergistic inhibitory effects on bladder activity. These neurotransmitters may be lower in the central nervous system after SCI.
CONCLUSIONS
This study provides evidence that activation of GABAA and GABAB receptors in the spinal cord inhibits DO and EUS-EMG activity in SCI rats. A reduction in GAD67 mRNA in the spinal cord and DRG in SCI rats suggests hypofunction of the GABAergic system. Therefore, activation of the spinal GABAergic system may be a promising approach for the treatment of DO in patients with SCI.
Acknowledgments
This work was supported by NIH grants DK57267, DK68557 and HD39768.
Key of Definitions for Abbreviations
- GABA
Gamma-aminobutyric acid
- DO
detrusor overactivity
- SCI
spinal cord injury
- EUS-EMG
external urethral sphincter electromyogram
- GAD67
glutamate decarboxylase 67
- DRG
dorsal root ganglia
- DSD
detrusor-sphincter dyssynergia
- NVCs
nonvoiding bladder contractions
- MVP
Maximal voiding pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holstege G, Griffiths D, de Wall H, Dalm E. Anatomical and physiological observations on supraspinal control of bladder and urethral sphincter muscles in the cat. J Comp Neurol. 1986;250:449. doi: 10.1002/cne.902500404. [DOI] [PubMed] [Google Scholar]
- 2.de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res. 2006;152:59. doi: 10.1016/S0079-6123(05)52005-3. [DOI] [PubMed] [Google Scholar]
- 3.Miyazato M, Sugaya K, Nishijima S, Ashitomi K, Hatano T, Ogawa Y. Inhibitory effect of intrathecal glycine on the micturition reflex in normal and spinal cord injury rats. Exp Neurol. 2003;183:232. doi: 10.1016/s0014-4886(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 4.Herman RM, Wainberg MC, delGiudice PF, Willscher MK. The effect of a low dose of intrathecal morphine on impaired micturition reflexes in human subjects with spinal cord lesions. Anesthesiology. 1988;69:313. doi: 10.1097/00000542-198809000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro S. Neurotransmission by neurons that use serotonin, noradrenaline, glutamate, glycine, and γ-aminobutyric acid in the normal and injured spinal cord. Neurosurgery. 1997;40:168. doi: 10.1097/00006123-199701000-00037. [DOI] [PubMed] [Google Scholar]
- 6.Igawa Y, Mattiasson A, Andersson KE. Effects of GABA-receptor stimulation and blockade on micturition in normal rats and rats with bladder outflow obstruction. J Urol. 1993;150:537. doi: 10.1016/s0022-5347(17)35542-8. [DOI] [PubMed] [Google Scholar]
- 7.Steers WD, Meythaler JM, Haworth C, Herrell D, Park TS. Effects of acute bolus and chronic continuous intrathecal baclofen on genitourinary dysfunction due to spinal cord pathology. J Urol. 1992;148:1849. doi: 10.1016/s0022-5347(17)37048-9. [DOI] [PubMed] [Google Scholar]
- 8.Hayasaki H, Sohma Y, Kanbara K, Maemura K, Kubota T, Watanabe M. A local GABAergic system within rat trigeminal ganglion cells. Eur J Neurosci. 2006;23:745. doi: 10.1111/j.1460-9568.2006.04602.x. [DOI] [PubMed] [Google Scholar]
- 9.Keast JR, De Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol. 1992;319:615. doi: 10.1002/cne.903190411. [DOI] [PubMed] [Google Scholar]
- 10.Mallory B, Steers WD, De Groat WC. Electrophysiological study of micturition reflexes in rats. Am J Physiol. 1989;257:R410. doi: 10.1152/ajpregu.1989.257.2.R410. [DOI] [PubMed] [Google Scholar]
- 11.Cheng CL, Ma CP, de Groat WC. Effect of capsaicin on micturition and associated reflexes in chronic spinal rats. Brain Res. 1995;678:40. doi: 10.1016/0006-8993(95)00212-9. [DOI] [PubMed] [Google Scholar]
- 12.Blok BF, de Weerd H, Holstege G. The pontine micturition center projects to sacral cord GABA immunoreactive neurons in the cat. Neurosci Lett. 1997;233:109. doi: 10.1016/s0304-3940(97)00644-7. [DOI] [PubMed] [Google Scholar]
- 13.Seki S, Sasaki K, Igawa Y, Nishizawa O, Chancellor MB, De Groat WC, Yoshimura N. Suppression of detrusor-sphincter dyssynergia by immunoneutralization of nerve growth factor in lumbosacral spinal cord in spinal cord injured rats. J Urol. 2004;171:478. doi: 10.1097/01.ju.0000088340.26588.74. [DOI] [PubMed] [Google Scholar]
- 14.Miyazato M, Sugaya K, Nishijima S, Ashitomi K, Morozumi M, Ogawa Y. Dietary glycine inhibits bladder activity in normal rats and rats with spinal cord injury. J Urol. 2005;173:314. doi: 10.1097/01.ju.0000141579.91638.a3. [DOI] [PubMed] [Google Scholar]
- 15.Miyazato M, Sugaya K, Nishijima S, Ashitomi K, Ohyama C, Ogawa Y. Rectal distention inhibits bladder activity via glycinergic and GABAergic mechanisms in rats. J Urol. 2004;171:1353. doi: 10.1097/01.ju.0000099840.09816.22. [DOI] [PubMed] [Google Scholar]