Abstract
The mandibular glands of queen honeybees produce a pheromone that modulates many aspects of worker honeybee physiology and behavior and is critical for colony social organization. The exact chemical blend produced by the queen differs between virgin and mated, laying queens. Here, we investigate the role of mating and reproductive state on queen pheromone production and worker responses. Virgin queens, naturally mated queens, and queens instrumentally inseminated with either semen or saline were collected 2 days after mating or insemination. Naturally mated queens had the most activated ovaries and the most distinct chemical profile in their mandibular glands. Instrumentally inseminated queens were intermediate between virgins and naturally mated queens for both ovary activation and chemical profiles. There were no significant differences between semen- and saline-inseminated queens. Workers were preferentially attracted to the mandibular gland extracts from queens with significantly more activated ovaries. These studies suggest that the queen pheromone blend is modulated by the reproductive status of the queens, and workers can detect these subtle differences and are more responsive to queens with higher reproductive potential. Furthermore, it appears as if insemination substance does not strongly affect physiological characteristics of honeybee queens 2 days after insemination, suggesting that the insemination process or volume is responsible for stimulating these early postmating changes in honeybee queens.
Keywords: behavior, caste, chemical communication, pheromone, reproduction, social insect
Pheromones are chemicals that elicit behavioral and physiological responses in conspecific individuals or groups (reviewed in Wyatt 2003). Typically, both the chemical composition of a pheromone and the responses of other individuals to it are thought to be highly stereotyped, so that any deviation in composition can affect the system of communication. In the case of sex pheromones, any reduction in the efficacy of chemical communication can reduce fitness for both the males and females, which leads to stabilizing selection and can promote speciation (Cardé and Baker 1984). However, for pheromones involved in more dynamic and plastic processes, such as communication within a social insect colony, there may be more latitude for variability and modulation.
In honeybees (Apis mellifera L.), the queen produces a pheromone that controls many aspects of worker behavior and physiology (Slessor et al. 2005; Le Conte and Hefetz 2008). The main source of this pheromone is the mandibular glands, which contain 5 well-characterized compounds (called queen mandibular pheromone, or QMP; reviewed in Slessor et al. 2005). QMP elicits the retinue response, in which workers are attracted to the pheromone and lick and antennate it (Slessor et al. 1988), though 4 other compounds are required to produce a retinue response equivalent to that of a live queen in worker bees (Keeling et al. 2003). QMP also inhibits ovary development (Hoover et al. 2003), the rearing of new queens (Winston et al. 1989), and the transition from nursing to foraging behavior in adult workers (Pankiw et al. 1998). The Dufour's gland of honeybee queens also appears to produce a pheromonal signal that attracts workers at short distances (Katzav-Gozansky et al. 2002).
It has long been debated whether this communication system functions as a means of queen control or simply as a signal for queen fecundity (Seeley 1989; Keller and Nonacs 1993; Gadagkar 1997; Le Conte and Hefetz 2008). Although it is important to note that these hypotheses are not mutually exclusive, several different assumptions are made with each model. Under the strict “control” model, both the queen and the group benefit because resources are funneled toward supporting reproduction of the highly fecund queen. However, this system is detrimental to the fitness of individual workers because they are inhibited from reproducing. Under the “signal” model, queen pheromone signals to the workers that their queen is viable and fecund. If the queen's fecundity decreases, then the pheromone composition is hypothesized to change and workers may rear replacement queens and/or initiate their own reproduction. In this scenario, the pheromone is an honest signal of the queen's quality and the workers benefit by cooperating with her, because the queen has much greater fecundity than individual workers and thus can produce a greater number of new reproductives and a larger workforce. Thus, by rearing the offspring of the queen, the workers improve their inclusive fitness.
There are data to support both models. There are several examples of modified worker responses to queen pheromone, which would suggest that some workers may be attempting to “escape” queen control. In “anarchistic” strains of bees, for example, workers can activate their ovaries even in the presence of a queen and her pheromones. It has been suggested that this is due to a modification of pheromone perception rather than pheromone production (Hoover et al. 2005). Moreover, in Cape bees (Apis mellifera capensis), workers with high reproductive potential are less attracted to the queen, potentially reducing their exposure to the queen pheromone (Moritz et al. 2002). In unselected European honeybees (Apis mellifera ligustica), there is also variation in worker responses to queen pheromone, such that workers with high reproductive potential are less attracted to a lure containing queen pheromone (Kocher et al. submitted).
There is also evidence that pheromone production can be modified based on reproductive status. The chemical composition of the queen mandibular glands changes after natural mating and instrumental insemination (Plettner et al. 1993; Al-Qarni et al. 2005). Recently, it has been demonstrated that insemination quantity modifies both mandibular and Dufour's gland composition as well as its attractiveness to workers (Richard et al. 2007, in preparation). It has also been demonstrated that ovary activation is initiated in queen bees that have started (but not completed) the mating process and that such reproductive development is correlated with changes in mandibular gland composition (Kocher et al. 2008). However, thus far, no study has examined if the workers' ability to perceive pheromonal differences is correlated with queen ovary development.
In this study, we examined the chemical composition of queen mandibular gland extracts associated with 4 reproductive states: virgins, instrumentally inseminated with saline or semen, and naturally mated. We collected all queens 2 days after insemination and assessed them for ovary activation, sperm storage, and mandibular gland composition. We then examined differences in worker attraction to the different gland extracts associated with each reproductive state. If queen pheromone acts as an honest signal of fecundity, we expect that workers would discriminate between extracts from the different reproductive states and prefer queens with the greatest amount of ovary activation.
MATERIALS AND METHODS
Field methods
Virgin honeybee queens (Apis mellifera carnica) were reared at the NCSU Lake Wheeler HoneyBee Research Facility in Raleigh, NC. All experimental subjects were daughters of a single queen instrumentally inseminated with semen from a single drone (Glenn Apiaries, Fallbrook, CA) and therefore their average coefficient of relatedness was 0.75. Queens were produced by grafting larvae into queenless colonies for 7 days (Laidlaw and Page 1997) before being transferred to an incubator set at brood-nest temperature (34 °C and ∼50% relative humidity). Two days prior to their expected emergence, the queen cells were placed into individual nucleus colonies each with approximately 1000 workers and 3 frames of brood, honey, and empty comb. Two days after emergence, surviving queens were randomly assigned to 1 of 4 treatment groups (virgin, saline-inseminated, semen-inseminated, or naturally mated), marked with individual numbered tags (BetterBee, Greenwich, NY) and returned to their respective colonies. Colony entrances were modified with specialized runways so that individuals attempting to fly could be identified and their flight behavior regulated (see Tarpy and Page 2000; Kocher et al. 2008) On the 7th day after emergence, queens in the instrumental insemination groups were anesthetized with CO2 for 4.0 min during their insemination procedure (Laidlaw 1987). All queens were inseminated with either 10 μl of saline (Williams and Harbo 1982) or 10 μl of mixed semen pooled from groups of approximately 10 brother drones. Queens in the naturally mated group were allowed to take multiple orientation flights but only one mating flight; once a queen returned to her colony with a mating sign (the endophallus/reproductive organs of the last male to mate), she was no longer allowed to fly and was confined to the colony. Note that honeybee queens are highly polyandrous and will mate with an average of 12 males over the course of multiple mating flights (Tarpy et al. 2004). After mating, queens store a proportion of each mates’ sperm in their spermathecae (Oldroyd et al. 1998) in order to fertilize their eggs over their life span. We limited the naturally mated queens to a single mating flight to more closely match the instrumental insemination procedure, in which queens were inseminated only once. Two days after the date that a given queen was mated/inseminated, she was collected on dry ice and stored at −80 °C for processing. Inseminated queens were collected 2 days after insemination with age-matched virgins, and naturally mated queens were collected 2 days after a successful mating flight. There was at most a 2-day difference in collection times between individual queens. Queen heads were removed and partially lyophilized to facilitate dissection (Grozinger et al. 2003) after which their mandibular glands and brains were dissected out on dry ice and stored for future processing. In total, there were 18 virgin queens, 17 saline-inseminated queens, 20 semen-inseminated queens, and 18 naturally mated queens.
Spermathecae and ovary dissections and analysis
Analysis of ovary activation was performed as in Kocher et al. (2008). Dissections were performed on ice in RNAlater solution (Qiagen, Valencia, CA). Pictures were taken of each ovary, and an ovary development score was assigned according to the following scale (as in Kocher et al. 2008): 1—no development; 2—larger ovaries, but ovarioles not clearly visible; 3—visible ovarioles, but no eggs; and 4—fully developed, large ovarioles, and mature eggs. The spermathecae were then removed and stored in 100 μl Kiev buffer for sperm counting (0.3 g D+ glucose, 0.41 g potassium chloride, 0.21 g sodium bicarbonate, 2.43 g sodium citrate per 100 ml deionized, and sterile water). Data analysis was performed in JMP 7.0 (SAS, Cary, NC).
Sperm counting
The spermathecae of the naturally mated and semen-inseminated queens were ruptured and the contents were diluted in 10 ml of Kiev buffer and immediately counted on a hemocytometer (Tarpy and Page 2000). Counts were performed 5 times each, and an average value was obtained. A 2-tailed t-test was conducted in JMP 7.0 to determine significant differences.
Pheromone analysis
Pheromone analysis was performed as described in (Richard et al. 2007). The mandibular glands were dissected and immersed in 50 μl diethyl ether containing 0.4 μg/μl of undec-10-enoic acid (as an internal standard) for minimum of 24 h. A 5-μl portion (ca. 0.1 bee equivalents) of each extract was placed in individual small glass inserts and the solvent gently evaporated. The residue was silylated overnight at room temperature in the insert with 10 μl of neat bistrimethylsilyltrifluoroacetamide (Keeling et al. 2003). The derivatized sample was diluted with hexane (100 μl) and a 2-μl portion was analyzed using gas chromatography on an HP 5890 equipped with a capillary column (30 m × 0.25 mm ID, 0.5-μm film thickness) DB-5 (5% diphenyl–95% dimethylsiloxane) column (J&W scientific, Folsom, CA) in splitless mode. Helium was used as the carrier gas at a head pressure of 18 psi (flow rate = 1.3 ml/min). The GC temperature was held at 100 °C for 1 min and then increased at 5 °C/min to 200 °C (5 min), followed by an increase of 10 °C/min to 250 °C (15 min). Injector and flame ionization detector temperatures were both set at 250 °C. We extracted the mandibular glands of 18 virgin queens, 17 saline-inseminated queens, 20 semen-inseminated queens, and 18 naturally mated queens. Selected samples were analyzed by GC–MS using a Hewlett-Packard (San Fernando, CA) model 6890GC coupled to a model 5973A mass selective detector with an electron impact ion source. Mass spectra were compared with the Wiley Registry of Mass Spectral Data and NIST 05 Mass Spectral Library for compound identification.
To examine differences in the chemical profiles between the sample groups based on the relative proportion of the chemical compounds, a discriminant analysis was employed using all the chemical compounds (Statistica 6.0. StatSoft Inc, Tulsa, OK). Significant differences in the quantities or relative proportions of individual compounds between virgin and the naturally mated queens were determined using a Mann–Whitney test. Because these 2 groups of queens were found to be most different in the discriminant analysis, it was hypothesized that if any statistically significant differences in the levels of individual compounds existed, they would most likely be detected between these 2 groups of queens.
Behavioral assays
We compared worker responses with mandibular gland extracts of virgin, saline-inseminated, semen-inseminated, and naturally mated queens. Ten queen mandibular gland extracts were randomly selected from the extracts prepared for the GC analysis. The pooled extracts were evaporated and resuspended into 140 μl of hexane such that 7 μl of extract was approximately equivalent to 0.05 queen equivalents (Qeq) of QMP.
Brood frames were collected from a single colony and incubated at 33 °C. Emerging day-old workers were then collected and 30 bees were placed into Plexiglas cages (10 × 10 × 7 cm). Bees were given 1 ml of water, 1 ml of 50% sucrose, and approximately 5 ml of a honey–pollen mixture (45% honey, 45% pollen, 10% water by weight). Food was changed every other day. The cages were kept in a 33 °C incubator with ∼40% relative humidity, and manipulations and observations were performed under red light to negate any potential behavioral effects. Cages were maintained for 5 days prior to the preference assays. Every day, 0.1 Qeq of fresh synthetic QMP (Pherotech, Vancouver, Canada) diluted in isopropanol/1% water was placed on a microscope coverslip and allowed to evaporate before being placed in the cage; QMP has significant effects on worker physiology (Grozinger et al. 2003; Hoover et al. 2003; Fischer and Grozinger 2008), and thus, QMP exposure was included to more closely approximate natural conditions in the colony. This experiment was repeated twice (Trial 1: August 2007 and Trial 2: September 2007).
On the fifth day of the experiment, workers were presented with 2 slides containing equal quantities of extract (0.05 Qeq) from 2 groups, with choices as follows:
1. Virgin and solvent control (Trial 1: n = 5 and Trial 2: n = 5)
2. Virgin and saline queen (Trial 1: n = 5 and Trial 2: n = 5)
3. Saline and semen queen (Trial 1: n = 5 and Trial 2: n = 5)
4. Semen and mated (Trial 1: n = 5 and Trial 2: n = 5)
5. Virgin and mated (Trial 1: n = 5 and Trial 2: n = 5)
6. Saline and mated (Trial 1: n = 5 and Trial 2: n = 5)
The number of workers antennating and licking each slide was counted every 5.0 min for 25 min after slide presentation. Data for each choice test were analyzed with a repeated-measures analysis of variance (ANOVA) using proc MIXED (SAS, Cary, NC).
RESULTS
Sperm counts
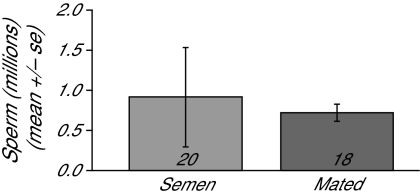
There was no significant difference in the number of stored sperm between naturally mated and semen-inseminated queens (Figure 1; one-way ANOVA, F = 0.10, df = 20, P = 0.75). We also confirmed that no sperm was present in the spermathecae of the virgin or saline-inseminated queens.
Figure 1.
Quantities of stored sperm. Spermathecae of all queens were dissected and the number of stored sperm counted, as described in the Materials and Methods. There were no significant differences in the number of sperm found in the semen-inseminated (denoted as “semen” in the graph below) and naturally mated (“mated”) queens. No sperm was found in the virgin or saline-inseminated group. The number of queens in each group is shown in the bottom of the bar. Statistical analysis: 2-tailed t-test, P = 0.75.
Ovary activation
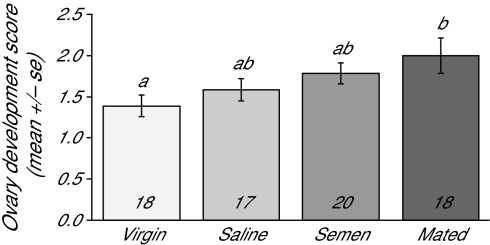
There were significant differences in ovary activation among groups (Figure 2; one-way ANOVA, F = 2.99, df = 59, P = 0.038). Naturally mated queens had the most activated ovaries, virgins had the least-developed ovaries, and saline- and semen-inseminated queens were intermediate.
Figure 2.
Ovary activation. Abdomens of queens were dissected and the amount of ovary activation was assessed, as described in the Materials and Methods section and in Kocher et al. (2008). There were significant differences in ovary activation among the groups (One-way ANOVA, P = 0.0385), with post hoc pairwise comparisons with Tukey adjustments revealing that naturally mated queens (denoted “mated” in the graph below) had the highest ovary activation scores, virgin queens had the lowest, and 2 inseminated groups (“saline” and “semen”) had intermediate scores. The number of queens is shown in the bottom of the bars, and different letters denote significant differences (Tukey honest significant difference technique, P < 0.05).
Pheromone analysis
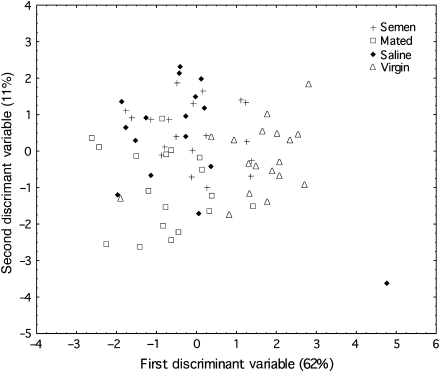
Relative proportions of the chemicals present in the mandibular glands of the individual queens were determined using gas chromatography (see Supplementary Figure 1 for an example of the chromatograms), and 29 peaks were included in the subsequent analyses. A stepwise discriminant analysis revealed 2 canonical axes (Figure 3), with the first differentiating virgin and mated (both naturally mated and inseminated) queens (62%) and the second differentiating saline and semen queens (11%). There were significant differences in the relative proportions of the chemical compounds present in the queen mandibular gland across the 4 sample groups (F(24,160) = 3.07, P < 10−4). Mahalanobis distance (MD) of virgin queen chemical profiles was significantly different from saline-, semen-inseminated, and mated queen chemical profiles (All MD > 3.40 and P‘s < 0.01). The saline-inseminated queen chemical profiles were not significantly separated from semen-inseminated queens chemical profiles (MD: 1.07, P = 0.44;). However, saline- and semen-inseminated queen chemical profiles were significantly different from the naturally mated queen chemical profiles (respectively, MD = 2.84, P = 0.02; MD = 2.94, P = 0.01).
Figure 3.
Mandibular gland chemical profiles. The relative proportions of the chemical compounds identified in the mandibular glands for each individual queen were used in a discriminant analysis. There were significant differences in the relative proportions of the chemical compounds present in the queen mandibular gland across the 4 groups of queens (F(24,180) = 6.64. P < 10−4).
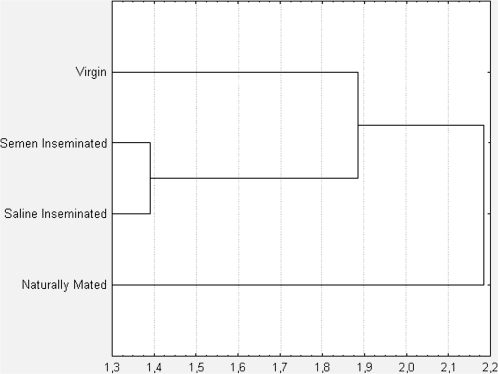
The mean relative proportion of each chemical identified in the mandibular gland extracts for each of the 4 groups of queens (virgin, saline-inseminated, semen-inseminated, and naturally mated) was calculated. The average values for all the identified chemicals were used to produce a dendrogram using Euclidean distance for the 4 groups of queens. The saline- and semen-inseminated queens group together in the same clade as virgins, whereas naturally mated queens are the outgroup (Figure 4). These results are consistent with the observed behavioral data (see Figure 5).
Figure 4.
Clustering of queen sample groups using mandibular gland chemical profiles. The mean relative proportion of each chemical identified in the mandibular glands extract for each of the 4 groups of queens (virgin, saline-inseminated, semen-inseminated, and naturally mated) was calculated. The average values for all the identified chemicals were used to produce a dendrogram using Euclidean distance.
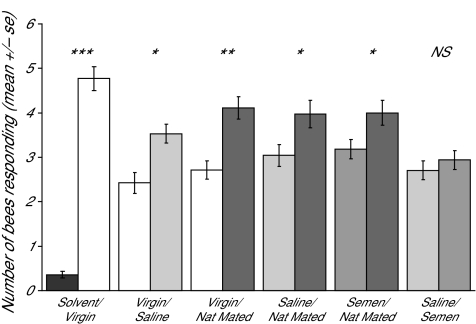
Figure 5.
Assay for worker preference for queen mandibular gland extracts. In order to determine if workers were differentially attracted to the mandibular gland extracts of the 4 different types of queens, we conducted a preference assay using the retinue response, as in Richard et al. (2007). One-day-old worker bees were reared in cages (30 bees/cage) in the presence of synthetic QMP until they were 5 days old. They were then presented with 2 coverslips with mandibular gland extracts from 2 different types of queens (0.05 Qeq of extract, the extracts were mixtures from 10 different queens; see Materials and Methods). The retinue response was monitored for each type of extract. Two trials were performed, each trial had 5 cages/preference assay. Significant differences in retinue response for each choice test were calculated using a repeated-measures ANOVA using proc MIXED (SAS, Cary, NC). Significant differences are denoted as follows: *P < 0.05, **P < 0.005, and ***P < 0.0005.
Analysis of the relative proportions and quantities of the identified QMP components ((E)-9-keto-2-decenoic acid, 9-ODA; (RE)-(−)- and (S,E)-(+)-9-hydroxy-2-decenoic acid, ±9-HDA; methyl p-hydroxybenzoate, HOB) in the mandibular glands of these queens revealed that there were no differences in the quantities or relative proportions of these compounds between the virgins and the naturally mated queens (Tables 1 and 2). Note that 4-hydroxy-3-methyoxyphenylethanol (HVA) was not detected in our samples, and previous studies have found that HVA levels are undetectable or at very low levels in virgin and newly inseminated queens (Slessor et al. 1990; Al-Qarni et al. 2005; Richard et al. 2007). A stepwise discriminant analysis using only these QMP components found no significant differences between the 4 groups (F(6, 136) = 1.77, P < 10.80), and all the MDs were not significant (P > 0.05). However, a stepwise discriminant analysis using all the non-QMP compounds produced similar results as when all the compounds were used for the analysis (data not shown). These results suggest that differences in the proportions of the non-QMP components are important for producing the overall differences in the chemical blend.
Table 1.
Quantity of QMP components (mean ± standard deviation, SD, in μg)
| QMP | Virgin | Saline | Semen | Mated | Virgin versus mated |
|
| U | P | |||||
| HOB | 3.41 ± 0.49 | 2.92 ± 0.46 | 2.74 ± 0.28 | 4.2 ± 0.53 | 118 | 0.16 |
| 9-ODA | 75.65 ± 8.93 | 60.34 ± 8.16 | 61.84 ± 6.58 | 91.19 ± 9.5 | 130 | 0.31 |
| 9-HDA (+/−) | 14.31 ± 1.39 | 10.2 ± 1.26 | 11.05 ± 1.29 | 15.64 ± 1.84 | 117 | 0.15 |
The quantities of the identified QMP compounds in the mandibular gland extracts of virgin, saline-inseminated, semen-inseminated, and naturally mated queens are shown. Statistical comparisons of the quantities of each compound between virgins the naturally mated queens were performed using a Mann–Whitney test.
Table 2.
Relative proportions of QMP components (mean ± SD)
| QMP | Virgin | Saline | Semen | Mated | Virgin versus mated |
|
| U | P | |||||
| HOB | 1.98 ± 0.15 | 2.15 ± 0.13 | 2.01 ± 0.08 | 2.12 ± 0.10 | 143 | 0.55 |
| 9-ODA | 44.54 ± 2.07 | 45.42 ± 0.84 | 45 ± 0.86 | 47.34 ± 1.38 | 159 | 0.92 |
| 9-HDA (+/−) | 9.22 ± 1.03 | 7.93 ± 0.49 | 8.15 ± 0.29 | 8.03 ± 0.31 | 152 | 0.75 |
The relative proportions (mean ± SD) of the identified QMP compounds in the mandibular gland extracts of virgin, saline-inseminated, semen-inseminated, and naturally mated queens are shown. Statistical comparisons of the proportions of each compound between virgins and naturally mated queens were performed using a Mann–Whitney test.
Statistical analysis of the relative proportions of the individual compounds present in the mandibular glands revealed significant differences in the proportions of 3 chemicals between the virgins and the naturally mated queens. Two of these chemicals could not be identified, whereas the third is an alkane (Supplementary Table 1; P < 0.01 Mann–Whitney). One of these chemicals (Unknown 5) was also found to be at significantly different relative proportions in comparisons of queens inseminated with semen from a single drone versus multiple drones (Richard et al. 2007), making this chemical a good candidate for future chemical identification studies. However, this chemical was present in low quantities in both studies and as such was not possible to identify by mass spectroscopy in either study. Additional studies using different methods of chemical analysis will be necessary to identify this compound. Note, however, that it is more likely that overall changes of several compounds in the pheromone blend, rather than changes in an individual compound, are responsible for eliciting altered behavioral responses in workers.
Worker preference
A greater number of workers responded to virgin queens relative to the solvent control (Figure 5; repeated-measures ANOVA, F = 289.24, df = 6, P < 0.0001), saline-inseminated compared with virgin (F = 15.97,df = 6, P = 0.007), and naturally mated queen extracts compared with virgins (F = 21.79, df = 6, P = 0.003). Workers also preferentially responded to naturally mated extracts compared with saline-inseminated (F = 6.03, df = 6, P = 0.049), as well as preferring naturally mated queens to semen-inseminated queens (F = 6.02, df = 6, P = 0.049). There was no significant difference in the number of workers responding to saline- or semen-inseminated queen extracts (F = 0.76, df = 6, P = 0.479).
DISCUSSION
We have demonstrated that differences in the mating process are associated with differences in ovary activation and pheromone composition and production. Furthermore, workers could discriminate between reproductive states and were more attracted to the extracts of queens with higher levels of ovary activation. These results support a model in which queen pheromone composition is linked to her reproductive status and fecundity, such that queen pheromone serves as an honest signal to the workers. However, it is important to note that other studies (Moritz et al. 2002; Hoover et al. 2003; Kocher et al. submitted) indicate that workers modulate their responses to queen pheromone, supporting a model in which workers may be attempting to escape queen control. Thus, both models may be operating in this system, and queen-worker chemical communication in honeybees appears to be a complex process.
Previous studies have suggested that pheromone production in honeybees is linked to reproductive status, but the direct link between changes in reproductive status and behavioral consequences has not been demonstrated. For example, reproductive workers produce queen-like blends of Dufour's glands extracts (Malka et al. 2007, 2008), dominant workers (which are more likely to ultimately become reproductive workers) produce queen-like blends of the mandibular glands (Moritz et al. 2002; Malka et al. 2007, 2008), and mated, laying queens produce different mandibular gland blends compared with virgin queens (Plettner et al. 1993; Kocher et al. 2008; Strauss et al. 2008). Furthermore, naturally mated, laying queens produce different mandibular gland blends compared with instrumentally inseminated, laying queens (Al-Qarni et al. 2005). We have also previously demonstrated that insemination quantity modulates mandibular and Dufour's gland composition (Richard et al. 2007, in preparation), and queens collected during the transition from virgin to laying also have different pheromone blends (Kocher et al. 2008). This current study provides a crucial link between ovarian status, pheromone production, and worker responses. However, it is important to note that these particular queens were analyzed only 2 days after insemination or mating (and differed at most by 2 days in age) in order to accentuate differences in ovary activation, and thus, although these differences represent a link between ovary activation and worker preference, a definitive link between variation in queen fecundity and worker preference still needs to be demonstrated. However, coupled with previous work demonstrating that there are significant differences in the pheromonal blends of naturally mated queens compared with instrumentally inseminated queens at 1 and 2 weeks after the initiation of egg laying, and that workers prefer the extracts from the naturally mated queens (Al-Qarni et al. 2005), it appears that the observed differences in pheromone composition are stable over time and may function as an accurate indicator of queen fecundity in a natural colony. Furthermore, long-term studies comparing laying queens inseminated with low versus high volumes of semen in colonies demonstrate that these queens have significantly different longevity and that worker behavior and physiology is affected by queen insemination volume (Niño EL, Richard FJ, Tarpy DR, Grozinger CM, submitted). Studies to examine the chemical profiles of laying queens inseminated with low versus high volumes of semen are ongoing (Niño EL, Richard FJ, Tarpy DR, Grozinger CM, unpublished results).
It is apparent that the chemical composition of the mandibular gland extract is quite complex and that subtle changes in the proportion or quantity of the chemical components can be detected by worker bees. Most research on queen pheromone has focused on characterizing quantities of compounds found in QMP. QMP consists of 5 components: 9-ODA, 9-HDA ((RE)-(−)- and (S,E)-(+)-), HOB, and HVA (Slessor et al. 1988). However, an additional 4 components have been identified that improve the retinue response (Keeling et al. 2003), and there are several more compounds that are present but have not yet been characterized in terms of behavioral responses by workers. The levels of QMP and associated compounds are affected by mating status and age (Plettner et al. 1993, 1997). Previous studies have sought to determine if specific compounds are associated with reproductive state and fecundity by comparing virgin queens, queens treated with CO2 (which stimulates ovary development and laying of unfertilized drone eggs), and naturally mated, egg-laying queens (Strauss et al. 2008). In that study, there were no overall differences in the proportions of the 5 QMP compounds or 2 additional compounds (10-HDA: 10-hydroxy-2-decenoic acid and 10-HDAA: 10-hydroxydecanoic acid) between naturally mated and drone-laying queens, although there were significant differences in the levels of some individual compounds (i.e., 9-ODA levels were lower and 10-HDA levels were higher in naturally mated queens). Al-Qarni (2005) found that naturally mated queens had significantly higher levels of 9-ODA than instrumentally inseminated queens. In our studies, we found no significant differences in the relative proportions or quantities of these specific compounds, but when all of the components of the mandibular glands were considered, the samples separated into a distinct hierarchy with virgin and naturally mated queen extracts most distant and the inseminated queen extracts most similar to each other and clustering near the virgin extract chemical profiles. Similarly, in previous studies comparing differentially inseminated queens (Richard et al. 2007) or virgin, partially mated, and laying queens (Kocher et al. 2008), there was also little variation in the QMP components but there was significant overall variation in the proportions of the blend when the majority of compounds were considered. Our results suggest that the mandibular gland composition is quite complex, subtle changes in proportion or quantities may have significant behavioral consequences, and other components in addition to the 5 main QMP chemicals play a significant role in regulating worker behavior. Furthermore, several other pheromone-producing glands exist in queens, such as Dufour's gland (reviewed in Katzav-Gozansky et al. 2002) and tergal glands (Wossler and Crewe 1999a, 1999b), and there are also significant differences in volatile chemical production between virgin and mated queens (Gilley et al. 2006). Thus, fully understanding how queen reproductive state alters chemical communication between queens and workers will require a complete analysis of all chemicals produced by the queen.
Although workers clearly could distinguish between these chemical extracts in our assay, it remains to be determined if this has consequences for worker behavior and physiology in a functioning colony. In this study, we monitored the responses of workers to different queen pheromone extracts using a retinue-response choice assay. The underlying assumption is that the stronger attraction of the workers for a particular pheromone extract indicates that this is a higher “quality” extract. However, the retinue response is only one of many behaviors regulated by queen pheromone; more important to colony and individual fitness are the effects of queen pheromone on inhibiting worker reproduction and inhibiting the rearing of new queens (Winston et al. 1989; Hoover et al. 2003). Previous studies have demonstrated that a 9-component queen pheromone blend that produces a stronger retinue response than QMP was not more effective at inhibiting ovary development in caged workers (Hoover et al. 2003). Thus, improved retinue response may not necessarily be correlated with improved inhibition of worker reproduction. Obviously, to fully demonstrate that the pheromone extracts produced by differentially inseminated queens have different behavioral and physiological consequences, it will be necessary to demonstrate that both worker reproduction and queen rearing are differentially inhibited by live queens in full colonies. However, the retinue response serves as a useful assay to probe for differences in the biological activity of these pheromone blends.
It is also apparent from our results that instrumental insemination and natural mating have different effects on queens. The naturally mated queens had the most activated ovaries and the most divergent pheromone blend, whereas the instrumentally inseminated queens were more similar to virgins. The saline- and semen-inseminated queens were not significantly different in terms of their pheromone profiles, ovary activation, or worker responses. These results suggest that instrumental insemination is not a perfect mimic of natural mating, and that instrumentally inseminated queens may transition to the fully mated state more slowly. Indeed, previous studies have found that instrumentally inseminated queens take longer to initiate egg-laying than naturally mated queens (Kaftanoglu and Peng 1982; Al-Qarni et al. 2003), and have significantly different pheromonal blends after the initiation of egg-laying (Al-Qarni et al. 2005). It is somewhat surprising that there are no strong differences between saline- and semen-inseminated queens, but previous studies have demonstrated that both insemination volume and substance (i.e., seminal proteins or sperm) can affect postmating responses in female insects (Nijhout 1984; Ringo 1996; Klowden 2006; Wolfner 2007). Our studies suggest that at least for the early postmating changes, insemination volume may be the dominant regulatory factor, or other aspects of the insemination procedure (such as anesthetization with carbon dioxide or physical manipulation of the oviducts) may cause changes which obscure any differences caused by insemination substance.
These studies and others suggest that pheromonal communication in honeybees involves modulation of both the production of the signal and response of the receiver. In this particular case, queen pheromone appears to be acting as a signal for workers to monitor queen fecundity, but it remains to be determined if variation in these pheromone blends also alters other aspects of worker behavior and physiology. However, other studies suggest that workers can modulate how they respond to the pheromone (Moritz et al. 2002; Hoover et al. 2005; Kocher et al., submitted), which would be more consistent with a queen-control model. Thus, pheromonal communication in honeybees and other social insects may have evolved as an intricate dialog between the queen and workers, rather following a simple signal-receiver model.
SUPPLEMENTARY MATERIAL
Supplementary material can be found at http://www.beheco.oxfordjournals.org/.
FUNDING
US Department of Agriculture, National Research Initiative (to C.M.G. and D.R.T., 2006-35607-16625); National Institute of Health Training Grant (to S.D.K); Conseil regional d'Indre et Loire and the W.M. Keck Center for Behavioral Biology (postdoctoral training fellowships to F.J.R).
Supplementary Material
Acknowledgments
We would like to thank Joe Flowers and Jennifer Keller for their excellent beekeeping expertise, Kelly Hutcherson for assistance with field collections, Coby Schal and Michael Roe for access to GC and GC/MS instruments, and Satoshi Nojima and Kevin Donohue for their assistance with the chemical analyses. We would like to thank members of the Grozinger and Tarpy research groups for helpful discussions and Andrew Barron for critical reading of the manuscript.
References
- Al-Qarni AS, Phelan PL, Smith BH, Cobey SW. The influence of mating type and oviposition period on mandibular pheromone levels in Apis mellifera L. honeybee queens. Saudi J Biol Sci. 2005;12:39–47. [Google Scholar]
- Al-Qarni AS, Smith BH, Cobey SW. Performance evaluation of naturally mated and instrumentally inseminated honeybee (Apis mellifera L.) queens in field colonies. Pakistan J Biol Sci. 2003;6:1476–1481. [Google Scholar]
- Cardé RT, Baker TC. Sexual communication with pheromones. In: Bell WJ, Cardé RT, editors. Chemical ecology of insects. London: Chapman and Hall; 1984. pp. 355–383. [Google Scholar]
- Fischer P, Grozinger CM. Pheromonal regulation of starvation resistance in honey bee workers (Apis mellifera) Naturwissenschaften. 2008;95(8):723–729. doi: 10.1007/s00114-008-0378-8. [DOI] [PubMed] [Google Scholar]
- Gadagkar R. The evolution of communication and the communication of evolution: the case of the honey bee queen pheromone. In: Lehrer M, editor. Orientation and communication in Arthropods. Basel (Switzerland): Birkhauser Verlag; 1997. pp. 375–395. [Google Scholar]
- Gilley DC, Degrandi-Hoffman G, Hooper JE. Volatile compounds emitted by live European honey bee (Apis mellifera L.) queens. J Insect Physiol. 2006;52(5):520–527. doi: 10.1016/j.jinsphys.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Grozinger CM, Sharabash NM, Whitfield CW, Robinson GE. Pheromone-mediated gene expression in the honey bee brain. Proc Natl Acad Sci USA. 2003;100(Suppl 2):14519–14525. doi: 10.1073/pnas.2335884100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover SE, Keeling CI, Winston ML, Slessor KN. The effect of queen pheromones on worker honey bee ovary development. Naturwissenschaften. 2003;90:477–480. doi: 10.1007/s00114-003-0462-z. [DOI] [PubMed] [Google Scholar]
- Hoover SE, Winston ML, Oldroyd BP. Retinue attraction and ovary activation: responses of wild type and anarchistic honey bees (Apis mellifera) to queen and brood pheromones. Behav Ecol Sociobiol. 2005;59:278–284. [Google Scholar]
- Kaftanoglu O, Peng YS. Effects of insemination on the initiation of oviposition in the queen honey bee. J Apic Res. 1982;21:3–6. [Google Scholar]
- Katzav-Gozansky T, Soroker V, Hefetz A. Honeybees Dufour's gland—idiosyncrasy of a new queen signal. Apidologie. 2002;33:525–537. [Google Scholar]
- Keeling CI, Slessor KN, Higo HA, Winston ML. New components of the honey bee (Apis mellifera L.) queen retinue pheromone. Proc Natl Acad Sci USA. 2003;100:4486–4491. doi: 10.1073/pnas.0836984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller L, Nonacs P. The role of queen pheromones in social insects: queen control or queen signal? Anim Behav. 1993;45:787–794. [Google Scholar]
- Klowden MJ. Switchover to the mated state by spermathecal activation in female Anopheles gambiae mosquitoes. J Insect Physiol. 2006;52:679–684. doi: 10.1016/j.jinsphys.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Kocher SD, Richard FJ, Tarpy DR, Grozinger CM. Genomic analysis of post-mating changes in the honey bee queen (Apis mellifera) BMC Genomics. 2008;9:232. doi: 10.1186/1471-2164-9-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidlaw JHH. Instrumental insemination of honeybee queens: its origin and development. Bee World. 1987;68(1):17–38. [Google Scholar]
- Laidlaw JHH, Page RE., Jr . Queen rearing and bee breeding. Cheshire (CT): Wicwas; 1997. [Google Scholar]
- Le Conte Y, Hefetz A. Primer pheromones in social hymenoptera. Annu Rev Entomol. 2008;53:523–542. doi: 10.1146/annurev.ento.52.110405.091434. [DOI] [PubMed] [Google Scholar]
- Malka O, Shnieor S, Hefetz A, Katzav-Gozansky T. Reversible royalty in worker honeybees (Apis mellifera) under the queen influence. Behav Ecol Sociobiol. 2007;61:465–473. [Google Scholar]
- Malka O, Shnieor S, Katzav-Gozansky T, Hefetz A. Aggressive reproductive competition among hopelessly queenless honeybee workers triggered by pheromone signaling. Naturwissenschaften. 2008;95:553–559. doi: 10.1007/s00114-008-0358-z. [DOI] [PubMed] [Google Scholar]
- Moritz RFA, Crewe RM, Hepburn HR. Queen avoidance and mandibular gland secretion of honeybee workers (Apis mellifera L.) Insectes Soc. 2002;49:86–91. [Google Scholar]
- Nijhout HF. Abdominal stretch reception in Dipetalogaster–Maximus (Hemiptera, Reduviidae) J Insect Physiol. 1984;30:629–633. [Google Scholar]
- Oldroyd BP, Clifton MJ, Parker K, Wongsiri S, Rinderer TE, Crozier RH. Evolution of mating behaviour in the genus Apis and an estimate of mating frequency in Apis cerana (Hymenoptera: Apidae) Ann Entomol Soc Am. 1998;91:700–709. [Google Scholar]
- Pankiw T, Huang Z, Winston ML, Robinson GE. Queen mandibular gland pheromone influences worker honey bee (Apis mellifera L.) foraging ontogeny and juvenile hormone titers. J Insect Physiol. 1998;44:685–692. doi: 10.1016/s0022-1910(98)00040-7. [DOI] [PubMed] [Google Scholar]
- Plettner E, Otis GW, Wimalaratne PDC, Winston ML, Slessor KN, Pankiw T, Punchihewa PWK. Species- and caste-determined mandibular gland signals in honeybees (Apis) J Chem Ecol. 1997;23:363–377. [Google Scholar]
- Plettner E, Slessor KN, Winston ML, Robinson GE, Page RE., Jr Mandibular gland components and ovarian development as measures of caste differentiation in the honey bee (Apis mellifera L.) J Insect Physiol. 1993;39:235–240. [Google Scholar]
- Richard FJ, Tarpy DR, Grozinger CM. Effects of insemination quantity on honey bee queen physiology. PLoS ONE. 2007;2:e980. doi: 10.1371/journal.pone.0000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringo J. Sexual receptivity in insects. Annu Rev Entomol. 1996;41:473–494. doi: 10.1146/annurev.en.41.010196.002353. [DOI] [PubMed] [Google Scholar]
- Seeley TD. The honey bee colony as a superorganism. Am Sci. 1989;77:546–553. [Google Scholar]
- Slessor KN, Kaminski L-A, King GGS, Borden JH, Winston ML. Semiochemical basis of the retinue response to queen honey bees. Nature. 1988;332:354–356. [Google Scholar]
- Slessor KN, Kaminski L-A, King GGS, Winston ML. Semiochemicals of the honeybee queen mandibular glands. J Chem Ecol. 1990;16:851–860. doi: 10.1007/BF01016495. [DOI] [PubMed] [Google Scholar]
- Slessor KN, Winston ML, Le Conte Y. Pheromone communication in the honeybee (Apis mellifera L.) J Chem Ecol. 2005;31:2731–2745. doi: 10.1007/s10886-005-7623-9. [DOI] [PubMed] [Google Scholar]
- Strauss K, Scharpenberg H, Crewe RM, Glahn F, Foth H, Moritz RFA. The role of the queen mandibular gland pheromone in honeybees (Apis mellifera): honest signal or suppressive signal? Behav Ecol Sociobiol. 2008;62:1523–1531. [Google Scholar]
- Tarpy DR, Nielsen R, Nielsen DI. A scientific note on the revised estimates of effective paternity frequency inApis. Insect Soc. 2004;51: 203–204. [Google Scholar]
- Tarpy DR, Page RE. No behavioral control over mating frequency in queen honey bees (Apis mellifera L.): implications for the evolution of extreme polyandry. Am Nat. 2000;155:820–827. doi: 10.1086/303358. [DOI] [PubMed] [Google Scholar]
- Williams JL, Harbo JR. Bioassays for diluents of honey bee semen. Ann Entomol Soc Am. 1982;75:457–459. [Google Scholar]
- Winston ML, Slessor KN, Willis LG, Naumann K, Higo HA, Wyborn MH, Kaminski L-A. The influence of queen mandibular pheromones on worker attraction to swarm clusters and inhibition of queen rearing in the honey bee (Apis mellifera) Insectes Soc. 1989;36:15–27. [Google Scholar]
- Wolfner MF. S.P.E.R.M.” (seminal proteins (are) essential reproductive modulators): the view from Drosophila. Soc Reprod Fertil Suppl. 2007;65:183–199. [PubMed] [Google Scholar]
- Wossler TC, Crewe RM. Mass spectral identification of the tergal gland secretions of female castes of two African honey bee races (Apis mellifera) J Apic Res. 1999a;38:137–148. [Google Scholar]
- Wossler TC, Crewe RM. The releaser effects of the tergal gland secretion of queen honeybees (Apis mellifera) J Insect Behav. 1999b;12: 343–351. [Google Scholar]
- Wyatt TD. Pheromones and animal behavior: communication by taste and smell. Cambridge (United Kingdom): Cambridge University Press; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.