Abstract
The store-operated, calcium release-activated calcium current ICRAC is activated by the depletion of inositol 1,4,5-trisphosphate (IP3)-sensitive stores. The significantly different dose-response relationships of IP3-mediated Ca2+ release and CRAC channel activation indicate that ICRAC is activated by a functionally, and possibly physically, distinct sub-compartment of the endoplasmic reticulum (ER), the so-called CRAC store. Vertebrate genomes contain three IP3-receptor (IP3R) genes and most cells express at least two subtypes, but the functional relevance of various IP3R subtypes with respect to store-operated Ca2+ entry is completely unknown. We here demonstrate in avian B cells (chicken DT40) that IP3R type II and type III participate in IP3-induced activation of ICRAC, but IP3R type I does not. This suggests that the expression pattern of IP3R contributes to the formation of specialized CRAC stores in B cells.
Keywords: ICRAC, IP3 receptor, Ca2+ store heterogeneity
Introduction
Calcium release from intracellular Ca2+ stores by inositol 1,4,5-trisphosphate (IP3) represents an important mechanism for calcium (Ca2+) influx, since in many cell types, store depletion results in activation of store-operated CRAC channels in the plasma membrane. Interestingly, however, a significant discordance exists between IP3-induced Ca2+ release and ICRAC activation. While IP3 causes considerable Ca2+ release in the nanomolar range, CRAC channel activation occurs only at micromolar IP3 concentrations [1]. Such differing response thresholds may arise from a number of circumstances, including different complements of IP3R subtypes with different affinities for IP3, store heterogeneity with separate compartments containing specific molecular components, and/or differential localization and activity of enzymes involved in IP3 metabolism with different parts of the endoplasmic reticulum (ER) experiencing different IP3 concentrations [2-6]. This concept of store-heterogeneity is contentious, with some studies favoring the concept, whereas others propose a continuous calcium store [7, 8]. Some of these discrepancies may be due to the cell under investigation, possibly linked to the utilization of store-operated calcium entry as a primary mechanism for signaling [9].
It seems clear by now that the ER is not the only organelle involved in Ca2+ storage. Mitochondria, Golgi, nucleus and lysosomes - all have been implicated in agonist-induced Ca2+ release [10]. Most recently, peroxisomes have been ascribed a role in Ca2+ storage [11], further contributing to cellular Ca2+ store heterogeneity. Clearly, store heterogeneity would also be critically influenced by the complement of calcium release channels within the respective organelle. In the ER for example, the activation of the dual Ca2+-influx and Ca2+-release channel TRPV1 (Transient Receptor Potential Vallinoid type 1) by capsaicin can mobilize substantial amounts of Ca2+ from thapsigargin-insensitive intracellular stores without resulting in ICRAC activation [12]. Thus, there is evidence to suggest the presence of a functionally and morphologically separate CRAC store that contains IP3 receptors and thapsigargin-sensitive Ca2+ pumps. Heterogeneity also exists within IP3-sensitive Ca2+ stores, as they respond to IP3 with different sensitivities and complex release kinetics [13]. IP3-sensitive receptors in vertebrates are encoded by three different genes, expressed as IP3R type I, II, and III, which can form homo- or heterotetrameric channel complexes [14-20]. The question therefore arises whether IP3R subtype composition contributes to the formation of a distinct CRAC store.
Methods
Cell Culture
All DT40 chicken B cell lines were cultured in RPMI 1640 (Life Technologies) supplemented with 10% fetal bovine serum, 5 % chicken serum, penicillin, streptomycin, and glutamine. DT40 cells expressing all three types of InsP3 receptors, the triple InsP3 receptor knock-out cell line and the cell lines expressing type I, type II or type III InsP3 receptor were a gift of Dr. Kurosaki [21].
Electrophysiology
Patch-clamp experiments were performed in the tight-seal whole-cell configuration at 21-25 °C. High-resolution current recordings were acquired using the EPC-9 (HEKA). Voltage ramps of 50 ms duration spanning a range of -150 to +100 mV were delivered from a holding potential of 0 mV at a rate of 0.5 Hz over a period of 300 sec. Liquid junction potential was 10 mV. Currents were filtered at 2.9 kHz and digitized at 100 μs intervals. Extracting the current amplitude at -130 mV from individual ramp current records assessed the low-resolution temporal development of currents. Where applicable, statistical errors of averaged data are given as means ± S.E.M. with n determinations. Standard external solutions were as follows (in mM): 120 NaCl, 2.8 KCl, 2 MgCl2, 20 CaCl2, 10 HEPES, 11 glucose, pH 7.2 with NaOH, 300 mosm. In some experiments 2 μM ionomycin in external solution containing no CaCl2 was applied for 2 seconds. Standard internal solutions were as follows (in mM): 120 Cs-glutamate, 8 NaCl, 10 Cs·BAPTA, 3 MgCl2, 4 CaCl2, 10 HEPES, pH 7.2 with CsOH, 300 mOsm. IP3 concentration was adjusted as indicated. [Ca2+]i was buffered to 150 nM free [Ca2+]i using 10 mM Cs·BAPTA and 4 mM CaCl2 as calculated with WebMaxC (http://www.stanford.edu/~cpatton/webmaxcS.htm). All chemicals were purchased from Sigma-Aldrich Co.
In combined patch-clamp and balanced Fura-2 experiments, cells were preloaded with 5 μM Fura-2-AM for 30 minutes. In subsequent whole-cell patch clamp experiments 200 μM Fura-2 was added to the standard internal solution in addition to 10 μM IP3.
Results
To identify whether differential expression of IP3R occurs in CRAC stores, we used wild-type DT40 chicken B-lymphocytes expressing all three IP3R types as well as genetically altered DT40 cells that lack all IP3R or express only one of the three IP3R subtypes [21, 22]. We first assessed the sensitivity of ICRAC to intracellular IP3 in wild-type (wt) DT40 cells using whole-cell patch-clamp recording. Experiments were performed in a standard extracellular NaCl-based saline containing 20 mM CaCl2 and cells were perfused with a standard Cs-based intracellular solution, where intracellular free Ca2+ ([Ca2+]i) was clamped to 150 nM using a mixture of 10 mM Cs-BAPTA and 4 mM CaCl2. At this [Ca2+]i, activation of ICRAC by passive store depletion is prevented and optimal sensitivity of IP3Rs to IP3 is ensured [23-26]. Data were acquired using a voltage ramp of 50 ms length that spanned from -150 mV to +100 mV and was applied every 2 s. Adding 1 μM IP3 to the pipette solution caused the development of a current that plateaued at 200 s (Fig. 1A) and exhibited the inward rectification typical for ICRAC ([27]; Fig. 1B). A further increase in IP3 concentrations beyond 1 μM did not result in larger currents and reducing IP3 to 500 nM was insufficient to activate CRAC currents (Fig. 1C). This all-or-none behavior of ICRAC in response to IP3 is consistent with previous investigations performed in mast cells [1, 28]. To obtain the activation time constant, the averaged current development of CRAC was fitted with a single exponential function. The activation time constants for all concentrations of IP3 that activated ICRAC were ~60 s. The average current amplitudes measured at 200 s into the experiment were plotted against their respective IP3 concentrations and fitted with a dose-response curve. The resulting fit rendered a half-maximal effective concentration (EC50) of 670 nM with a Hill coefficient fixed to 12. The EC50 did not change significantly when increasing the values for the Hill coefficient between 12 and 30, thus the Hill coefficient value is unlikely to reflect any mechanistic process in this case but allows a phenomenological description of the data. These data show that IP3-induced activation of ICRAC in wild-type DT40 cells proceeds in a highly non-linear fashion, indicating that similar to RBL mast cells [1, 28], B cells might express a functionally distinct CRAC store. This was further explored in DT40 cells with individual IP3R subtype expression.
Figure 1. Non-linear activation of ICRAC by IP3 in wild-type DT40 B cells.
(A) Average CRAC currents induced by 1 μM IP3 in wild type DT40 cells expressing all three types of IP3 receptors (n = 5). Error bars indicated S.E.M. Currents were acquired using a 50 ms voltage ramp spanning from -150 mV to +100 mV applied at 2 s intervals. Current sizes were extracted at -130 mV, normalized to cell size, averaged and plotted versus time. Currents were leak-corrected by subtracting averages of the first 1-3 ramp measurements after whole-cell establishment from subsequent current records. The intracellular calcium concentration was clamped to 150 nM (10 mM BAPTA and 4 mM CaCl2). (B) Average current-voltage (I/V) relationship of CRAC currents extracted from representative DT40 cells shown in panel A at 300 s into the experiment (n = 3). (C) Average time-course of CRAC currents induced by 500 nM IP3 (filled squares; n = 3), 2 μM IP3 (open circles; n = 5) and 5 μM IP3 (filled circles; n = 5). The calcium concentration was clamped to 150 nM (10 mM BAPTA and 4 mM CaCl2) to prevent passive depletion of intracellular calcium stores. Data were fitted using the single exponential function Inorm (t) = Itotal · exp(-t/π) + Amplitude. (D) Average ICRAC amplitude assessed at 200 s and plotted against IP3 concentration. A dose-response fit to the data yielded a EC50 of 670 nM and a Hill coefficient of 12.
DT40 cells lacking all three types of IP3 receptors (T0) fail to respond to IP3-producing stimuli, but exhibit store-operated Ca2+ entry when emptying stores with thapsigargin [29]. It is reasonable to assume that in the absence of any IP3 receptors, store-operated recruitment of ICRAC via the second messenger IP3 should be abolished, provided that the functionality of CRAC currents is not affected by the absence of IP3Rs. To test this, we perfused T0 cells with IP3, and neither 1 μM nor 20 μM IP3 were able to activate any measurable CRAC currents (Fig. 2A). To ascertain that store-operated calcium influx via CRAC channels was functional in T0 cells, we perfused T0 DT40 cells with 1 μM IP3, which failed to activate ICRAC, and induced IP3-independent store-depletion by applying the Ca2+ ionophore ionomycin. A brief exposure of cells to 2 μM ionomycin for 2 s applied from the outside of the cell in a Ca2+-free solution reliably activated ICRAC with a time constant of 22 s (Fig. 2A). Here, CRAC current density reached 1.7 pA/pF and showed an I/V relationship typical for ICRAC (Fig. 2B). These data indicate that the absence of IP3Rs only prevents IP3-induced activation of ICRAC, but does not prevent its recruitment by IP3-independent store-depletion, consistent with observations made with thapsigargin [29].
Figure 2. Ionomycin but not IP3 activates ICRAC in the absence of IP>3 receptors.
(A) Average CRAC currents in T0 DT40 cells in response to 1 μM IP3 (open squares, n = 5), 20 μM IP3 (filled squares, n = 3) and 1 μM IP3 plus stimulation for 2 s with 2 μM ionomycin in Ca2+-free saline as indicated by the arrow (filled circles, n = 5). [Ca2+]i was clamped to 150 nM. Data were analyzed as in Fig. 1A. (B) Average I/V curves of ICRAC extracted from representative T0 cells at 300 s and obtained after application of ionomycin (n = 3). (C) Average Ca2+ release responses evoked by perfusion of wild type (black, n = 5), T1 (red, n = 4), T2 (green, n = 4) and T3 (blue, n = 4) DT40 with 10 μM IP3 in combined patch- and balanced Fura-2 experiments (see methods). Baseline of traces was adjusted to T2 DT40 for clarity (50 nM to 130 nM). Arrow indicates time of whole-cell break-in. Cells were kept in regular Ca2+-containing solution but were superfused with a Ca2+-free saline before whole-cell break-in and throughout the experiment.
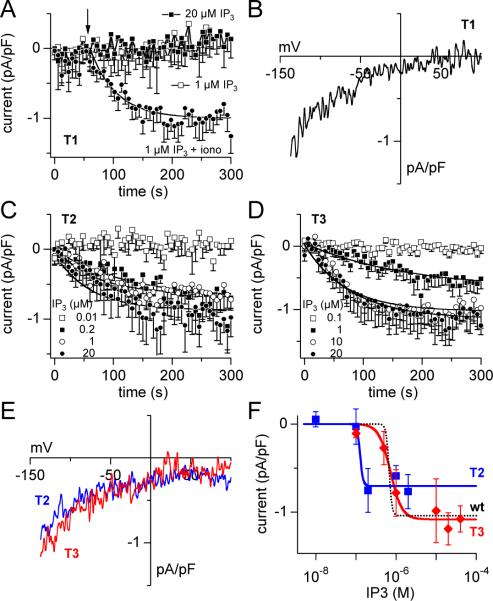
To determine whether all IP3Rs or only specific subtypes are coupled to ICRAC activation we studied DT40 cells in which all but one specific IP3R subtype were knocked out [21], resulting in cells expressing either IP3R type I (T1), type II (T2) or type III (T3). To confirm that IP3-induced Ca2+-release occurs in these cells, we conducted combined patch- and balanced Fura-2 experiments. Fura-2-AM loaded wild-type, T1, T2 or T3 DT40 cells were subsequently perfused with 10 μM IP3 and 200 μM Fura-2 after whole-cell break-in in the absence of extracellular Ca2+. Consistent with previous observations [22], T1, T2 and T3 cells were capable of causing Ca2+ release that was almost identical to wild type DT40 expressing all three IP3R subtypes (Fig. 2C). These cells were then tested for IP3-induced activation of CRAC channels. At 1 μM IP3, which causes maximal activation of ICRAC in wt DT40, T1 DT40 cells did not activate measurable CRAC currents and cells remained unresponsive even when increasing the intracellular IP3 concentration to 20 μM (Fig. 3A). However, similar to T0 DT40 cells (Fig. 2), challenging T1 DT40 cells with ionomycin activated inwardly rectifying currents with a time constant of 51 s (Fig. 3A) and the typical current-voltage relationship of ICRAC (Fig. 3B). These data suggest that, although T1 DT40 cells can release Ca2+ from intracellular stores [22], this IP3-sensitive store does not appear to couple to CRAC channel activation.
Figure 3. IP3 receptor type I does not couple to ICRAC activation.
(A) Average CRAC currents in T1 DT40 cells perfused with 1 μM IP3 (open squares, n = 6), 20 μM IP3 (filled squares, n = 3) or 1 μM IP3 plus stimulation for 2 s with 2 μM ionomycin in Ca2+-free saline as indicated by the arrow (closed circles, n = 4). Intracellular calcium concentration was clamped to 150 nM. Data were analyzed as in Fig. 1A. (B) Average I/V curves of ICRAC extracted from representative T1 cells at 300 s and obtained after application of ionomycin (n = 4). (C) Average time-course of ICRAC in T2 DT40 cells perfused with 10 nM IP3 (open squares, n = 3), 200 nM IP3 (filled squares, n = 5), 1 μM IP3 (open circles, n = 5) or 20 μM IP3 (filled circles, n = 8). [Ca2+]i was clamped to 150 nM. The data were fitted with a single exponential function as in Fig. 1C. (D) Average time-course of ICRAC in T3 DT40 cells perfused with 100 nM IP3 (open squares, n = 4), 1 μM IP3 (filled squares, n = 10), 10 μM IP3 (open circles, n = 7) and 20 μM IP3 (filled circles, n = 8) in the presence of 150 nM [Ca2+]i. Data analysis was performed as in Fig. 1A and data fit as in Fig. 1C. (E) Average I/V relationships of CRAC currents induced with 1 μM IP3 and extracted at 300 s from representative T2 (blue, n = 3) and T3 cells (red, n = 3). (F) Average ICRAC amplitude of T2 (blue) and T3 cells (red) assessed at 200 s and plotted against IP3 concentration. Dose-response fits yielded EC50 values of 130 nM (Hill = 12) for T2 cells and 720 nM (Hill = 3) for T3 cells. For comparison purposes, the dashed curve represents the dose-response fit to ICRAC recruitment in wild type DT40 cells shown in Fig. 1D.
In marked contrast to T1 DT40, challenging T2 DT40 with 1 μM intracellular IP3 did cause ICRAC activation with a time constant comparable to wt DT40 cells (66 s) and characteristic I/V relationship (Fig. 3E). However, the overall current size was significantly reduced compared to wt cells (0.6 pA/pF compared to 1.1 pA/pF, respectively; Fig. 3C). To see whether smaller current amplitudes were due to incomplete CRAC recruitment at 1 μM IP3, we completed a dose-response curve for IP3 in T2 DT40 cells. Interestingly, while 20 μM IP3 did not cause larger CRAC currents than 1 μM IP3, full activation of ICRAC was already achieved at 200 nM of IP3 (Fig. 3C). At 10 nM IP3, no measurable CRAC currents could be observed in T2 DT40 cells. Plotting and fitting the current amplitudes measured at 200 s into the experiment versus the respective IP3 concentrations resulted in a dose-response curve with an EC50 of 130 nM that was not significantly affected when varying Hill coefficient values between 12 and 30. Thus, ICRAC activation in both wt DT40 and T2 DT40 was highly non-linear, with T2 DT40 being about 5-fold more sensitive to intracellular IP3. These results establish that Type II IP3R is located in a compartment that upon depletion can activate CRAC channels. The reduced amplitude of the response, however, suggests that some of the CRAC channels are not recruited, raising the possibility that the remaining CRAC channels are controlled by another compartment that lacks Type II IP3R, but possibly contains the Type III subtype.
We tested the above hypothesis in T3 DT40 cells by establishing a dose-response curve of IP3-induced ICRAC activation. Here, current recruitment at 1 μM IP3 was significantly slower than either in wt or T2 cells, with an estimated time constantof ~150 s. Increasing intracellular IP3 concentration to 10 μM gave rise to larger CRAC currents, but could not be further augmented by 20 μM IP3 (Fig. 3D). In addition, 10 μM and 20 μM IP3 caused faster ICRAC recruitment with identical time constants (□ = 58 s) that approached the kinetics of wt and T2 cells. The current voltage relationships of IP3-activated currents in T3 DT40 cells were typical for CRAC currents (Fig. 3E). A dose-response fit to these data yielded an EC50 for ICRAC activation of 720 nM (Fig. 3F), similar to wt DT40 (670 nM). The Hill coefficient obtained in T3 DT40 cells was 3, reflecting a more graded recruitment of CRAC channels compared to wt (Fig. 3F, dashed black curve) or type II expressing DT40 (Fig. 3F, blue curve). Taken together, these results demonstrate that the Type III IP3R can recruit the entire population of CRAC channels, suggesting that it is expressed in all CRAC-competent compartments and may co-localize with the Type II subtype in subset of stores.
Discussion
Our results show that IP3R type II and type III participate in IP3-induced activation of ICRAC in DT40 B cells, but IP3R type I does not. This suggests that the expression pattern of IP3R contributes to the formation of specialized CRAC stores in B cells.
DT40 cells express all three IP3R isoforms. Based on Northern analysis, the Type I receptor far outnumbers the other two types, with Type III being expressed at the lowest level [22]. However, maximal release rates was comparable within a factor of two among DT40 clones expressing different IP3R, suggesting that expression levels of functional channel proteins are very similar for all three subtypes. Measurements of cytosolic Ca2+ signals in intact cells expressing individual IP3R isoforms and stimulated through B cell receptors (BCR) reveal distinct [Ca2+]i signals in the form of monophasic transients (Types I and III) or repetitive oscillations (Type II). Moreover, measurements of luminal Ca2+ levels of intracellular stores in permeabilized cells and functional characterization in planar lipid bilayers have established that individual IP3 receptor isoforms have different sensitivities to IP3, with Type II being most sensitive and Type III being least sensitive [22, 30]. Our data are entirely consistent with these findings for T2 and T3 DT40 cells with apparent EC50's of 130 nM and 720 nM, respectively. While T1 DT40 cells do not develop CRAC currents in response to IP3 and hence elude the functional assay employed here, these cells do produce IP3-induced calcium release (Fig. 2C and [22]). This confirms that all IP3 receptors can release Ca2+ from stores, but that they may give rise to distinct Ca2+ signals, possibly shaped by differential sensitivity to IP3, different Ca2+ release and uptake activities of multiple stores, and/or specific regulatory feedback mechanisms exerted by ATP and or [Ca2+]i itself [22, 30].
The data presented in the present study have important implications for a number of cell biological questions regarding the expression of IP3R isoforms and Ca2+ store heterogeneity. They demonstrate IP3 receptor heterogeneity in that the most abundantly expressed Type I receptor does not couple to store-operated CRAC channels, whereas the Type III receptor, which is the least-abundantly expressed and least IP3-sensitive isoform, can fully account for CRAC currents in DT40 cells. Since both isoforms give rise to very similar Ca2+ signals [22] and have similar rates of release, it would seem that the bulk of Ca2+ release and store depletion may not be the determinant factor for mediating CRAC current activation. Instead, the release of Ca2+ from and the depletion of a specialized store that may not contribute significantly to the overall Ca2+ release response appears to be responsible for store-operated Ca2+ entry. This is entirely compatible with previous work in mast cells demonstrating that low concentrations of IP3 release almost all Ca2+ from intracellular stores without activating CRAC [1]. However, a subsequent increase in IP3 concentration can trigger CRAC currents without significant additional Ca2+ release. The relevance of type II and III IP3R subtypes for Ca2+ release and Ca2+ entry has recently been demonstrated for pancreatic acinar cells [31]. Acinar cells isolated from knockout mice lacking these two IP3Rs cannot respond to IP3-coupled receptor stimulation via carbachol. This loss in phenotype is consistent with our result that IP3R type II and III are required for CRAC activation and Ca2+ influx in DT40 cells. However, the complete absence of agonist-induced Ca2+ release observed in acinar cells from double knockout mice would prevent CRAC activation by itself, especially since ionomycin-stimulation confirms intact Ca2+ storage in these mice [31]. Nevertheless, the involvement of IP3R type II and III in the differentiation of granule cell precursors after postnatal day 12 implicates that receptor-initiated Ca2+ signaling is fundamentally perturbed in the double knockout mice [32].
The specialized store that mediates the IP3-dependent activation of CRAC remains to be identified. It could be a subcompartment of the ER or a completely separate store with distinct molecular markers, although it would have to harbor IP3 receptors of Type III as well as thapsigargin-sensitive SERCA isoforms, since activation of the former and inhibition of the latter can activate CRAC. The identification of such a small store in DT40 cells expressing native IP3 receptors poses a significant challenge, as it would require the combination of immunofluorescent labeling of IP3 receptor isoforms with highly specific antibodies for chicken IP3R, fluorimetric determination of Ca2+ release, and electrophysiological recording of CRAC currents. However, multiphoton excitation imaging of heterologously expressed and epitope-tagged IP3R Type III in DT40 cells lacking all three isoforms has revealed that this isoform is expressed throughout the ER, but also in some small non-ER areas just underneath or in the plasma membrane [33]. If the overex-pressed protein distributes identically as the native protein, these areas might represent IP3 receptors within the specialized CRAC store and future work may provide insights into its nature.
In summary, our data establish heterogeneity of the ER calcium store in B cells and the presence of at least a specialized CRAC store that is characterized by the lack of Type I IP3R, but specifically expresses IP3R Type III. The IP3R Type II appears to have partial access to the CRAC store, since it can recruit partial ICRAC even at lower concentrations of IP3 than either wt or T3 cells. While this confirms the notion that IP3R subtypes have different sensitivities to IP3, it raises the question why wt DT40 cells do not activate ICRAC at the low concentrations that are effective in T2 cells. Since ICRAC activation by IP3 in wild-type cells represents a mixture of the non-linear recruitment seen with type II receptor involvement, but the overall IP3-sensitivity observed for type III receptors, it is tempting to speculate that these two receptor types form heteromeric channels [19, 20]. The exclusion of receptor type I from CRAC stores leading to the exclusive heteromerization of only two receptor subtypes within this substore, affords both heterogeneity and fine-tuning of IP3-induced calcium signaling.
Acknowledgments
We thank T. Kurosaki for generously providing genetically modified DT40 cell lines. We thank M. Bellinger for technical assistance. Supported in part by DFG grant PE 1478/1-1 (CP), NIH grant R01-AI050200, R01-GM080555 (RP) and R01-GM070634 (AF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parekh AB, Fleig A, Penner R. The store-operated calcium current I(CRAC): nonlinear activation by InsP3 and dissociation from calcium release. Cell. 1997;89:973–80. doi: 10.1016/s0092-8674(00)80282-2. [DOI] [PubMed] [Google Scholar]
- 2.Glitsch MD, Parekh AB. Ca2+ store dynamics determines the pattern of activation of the store-operated Ca2+ current I(CRAC) in response to InsP3 in rat basophilic leukaemia cells. J Physiol. 2000;523(Pt 2):283–90. doi: 10.1111/j.1469-7793.2000.t01-2-00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golovina VA, Blaustein MP. Spatially and functionally distinct Ca2+ stores in sarcoplasmic and endoplasmic reticulum. Science. 1997;275:1643–8. doi: 10.1126/science.275.5306.1643. [DOI] [PubMed] [Google Scholar]
- 4.Hermosura MC, Takeuchi H, Fleig A, Riley AM, Potter BV, Hirata M, Penner R. InsP4 facilitates store-operated calcium influx by inhibition of InsP3 5-phosphatase. Nature. 2000;408:735–40. doi: 10.1038/35047115. [DOI] [PubMed] [Google Scholar]
- 5.Hirose K, Iino M. Heterogeneity of channel density in inositol-1,4,5-trisphosphate-sensitive Ca2+ stores. Nature. 1994;372:791–4. doi: 10.1038/372791a0. [DOI] [PubMed] [Google Scholar]
- 6.Rooney E, Meldolesi J. The endoplasmic reticulum in PC12 cells. Evidence for a mosaic of domains differently specialized in Ca2+ handling. J Biol Chem. 1996;271:29304–11. doi: 10.1074/jbc.271.46.29304. [DOI] [PubMed] [Google Scholar]
- 7.Mogami H, Nakano K, Tepikin AV, Petersen OH. Ca2+ flow via tunnels in polarized cells: recharging of apical Ca2+ stores by focal Ca2+ entry through basal membrane patch. Cell. 1997;88:49–55. doi: 10.1016/s0092-8674(00)81857-7. [DOI] [PubMed] [Google Scholar]
- 8.Park MK, Petersen OH, Tepikin AV. The endoplasmic reticulum as one continuous Ca(2+) pool: visualization of rapid Ca(2+) movements and equilibration. Embo J. 2000;19:5729–39. doi: 10.1093/emboj/19.21.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 10.Michelangeli F, Ogunbayo OA, Wootton LL. A plethora of interacting organellar Ca2+ stores. Curr Opin Cell Biol. 2005;17:135–40. doi: 10.1016/j.ceb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Lasorsa FM, Pinton P, Palmieri L, Scarcia P, Rottensteiner H, Rizzuto R, Palmieri F. Peroxisomes as novel players in cell calcium homeostasis. J Biol Chem. 2008;283:15300–8. doi: 10.1074/jbc.M800648200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner H, Fleig A, Stokes A, Kinet JP, Penner R. Discrimination of intracellular calcium store subcompartments using TRPV1 (transient receptor potential channel, vanilloid subfamily member 1) release channel activity. Biochem J. 2003;371:341–50. doi: 10.1042/BJ20021381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meldolesi J, Pozzan T. The heterogeneity of ER Ca2+ stores has a key role in nonmuscle cell signaling and function. J Cell Biol. 1998;142:1395–8. doi: 10.1083/jcb.142.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujino I, Yamada N, Miyawaki A, Hasegawa M, Furuichi T, Mikoshiba K. Differential expression of type 2 and type 3 inositol 1,4,5-trisphosphate receptor mRNAs in various mouse tissues: in situ hybridization study. Cell Tissue Res. 1995;280:201–10. doi: 10.1007/BF00307790. [DOI] [PubMed] [Google Scholar]
- 15.Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda N, Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989;342:32–8. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- 16.Joseph SK. The inositol triphosphate receptor family. Cell Signal. 1996;8:1–7. doi: 10.1016/0898-6568(95)02012-8. [DOI] [PubMed] [Google Scholar]
- 17.Joseph SK, Lin C, Pierson S, Thomas AP, Maranto AR. Heteroligomers of type-I and type-III inositol trisphosphate receptors in WB rat liver epithelial cells. J Biol Chem. 1995;270:23310–6. doi: 10.1074/jbc.270.40.23310. [DOI] [PubMed] [Google Scholar]
- 18.Mignery GA, Sudhof TC, Takei K, De Camilli P. Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature. 1989;342:192–5. doi: 10.1038/342192a0. [DOI] [PubMed] [Google Scholar]
- 19.Monkawa T, Miyawaki A, Sugiyama T, Yoneshima H, Yamamoto-Hino M, Furuichi T, Saruta T, Hasegawa M, Mikoshiba K. Heterotetrameric complex formation of inositol 1,4,5-trisphosphate receptor subunits. J Biol Chem. 1995;270:14700–4. doi: 10.1074/jbc.270.24.14700. [DOI] [PubMed] [Google Scholar]
- 20.Wojcikiewicz RJ, He Y. Type I, II and III inositol 1,4,5-trisphosphate receptor coimmunoprecipitation as evidence for the existence of heterotetrameric receptor complexes. Biochem Biophys Res Commun. 1995;213:334–41. doi: 10.1006/bbrc.1995.2134. [DOI] [PubMed] [Google Scholar]
- 21.Sugawara H, Kurosaki M, Takata M, Kurosaki T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. Embo J. 1997;16:3078–88. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyakawa T, Maeda A, Yamazawa T, Hirose K, Kurosaki T, Iino M. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. Embo J. 1999;18:1303–8. doi: 10.1093/emboj/18.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagar RE, Burgstahler AD, Nathanson MH, Ehrlich BE. Type III InsP3 receptor channel stays open in the presence of increased calcium. Nature. 1998;396:81–4. doi: 10.1038/23954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mak DO, McBride S, Foskett JK. Regulation by Ca2+ and inositol 1,4,5-trisphosphate (InsP3) of single recombinant type 3 InsP3 receptor channels. Ca2+ activation uniquely distinguishes types 1 and 3 insp3 receptors. J Gen Physiol. 2001;117:435–46. doi: 10.1085/jgp.117.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swatton JE, Morris SA, Cardy TJ, Taylor CW. Type 3 inositol trisphosphate receptors in RINm5F cells are biphasically regulated by cytosolic Ca2+ and mediate quantal Ca2+ mobilization. Biochem J. 1999;344(Pt 1):55–60. [PMC free article] [PubMed] [Google Scholar]
- 27.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–6. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 28.Chang WC, Di Capite J, Nelson C, Parekh AB. All-or-none activation of CRAC channels by agonist elicits graded responses in populations of mast cells. J Immunol. 2007;179:5255–63. doi: 10.4049/jimmunol.179.8.5255. [DOI] [PubMed] [Google Scholar]
- 29.Broad LM, Braun FJ, Lievremont JP, Bird GS, Kurosaki T, Putney JW., Jr. Role of the phospholipase C-inositol 1,4,5-trisphosphate pathway in calcium release-activated calcium current and capacitative calcium entry. J Biol Chem. 2001;276:15945–52. doi: 10.1074/jbc.M011571200. [DOI] [PubMed] [Google Scholar]
- 30.Tu H, Wang Z, Nosyreva E, De Smedt H, Bezprozvanny I. Functional characterization of mammalian inositol 1,4,5-trisphosphate receptor isoforms. Biophys J. 2005;88:1046–55. doi: 10.1529/biophysj.104.049593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Futatsugi A, Nakamura T, Yamada MK, Ebisui E, Nakamura K, Uchida K, Kitaguchi T, Takahashi-Iwanaga H, Noda T, Aruga J, Mikoshiba K. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science. 2005;309:2232–4. doi: 10.1126/science.1114110. [DOI] [PubMed] [Google Scholar]
- 32.Futatsugi A, Ebisui E, Mikoshiba K. Type 2 and type 3 inositol 1,4,5-trisphosphate (IP3) receptors promote the differentiation of granule cell precursors in the postnatal cerebellum. J Neurochem. 2008;105:1153–64. doi: 10.1111/j.1471-4159.2008.05221.x. [DOI] [PubMed] [Google Scholar]
- 33.Morita T, Tanimura A, Nezu A, Kurosaki T, Tojyo Y. Functional analysis of the green fluorescent protein-tagged inositol 1,4,5-trisphosphate receptor type 3 in Ca(2+) release and entry in DT40 B lymphocytes. Biochem J. 2004;382:793–801. doi: 10.1042/BJ20031970. [DOI] [PMC free article] [PubMed] [Google Scholar]