Abstract
A series of dicyanomethylenedihydrofuran (DCDHF) fluorophores with different hydrophilic groups were synthesized and their photophysical properties and water solubilities were measured. Significant water solubility was achieved without compromising desirable photophysical properties, permitting applications of these fluorophores in biological systems.
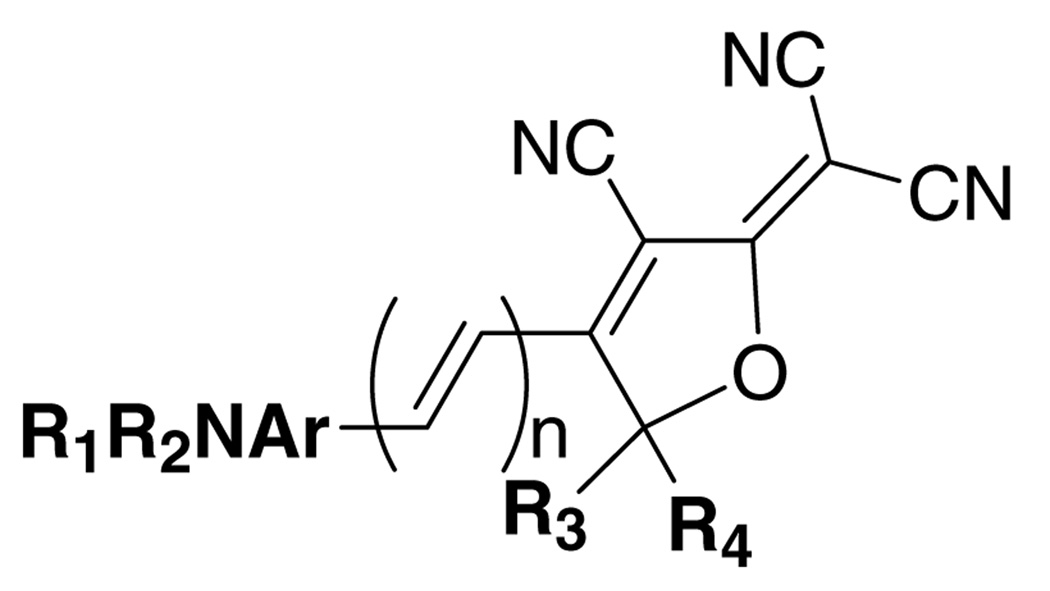
Dicyanomethylenedihydrofuran (DCDHF) chromophores are useful not only as photorefractive materials1–4 but also can be imaged at the single-molecule level with additional beneficial properties such as viscosity-dependent fluorescence, strong solvatochromism, significant ground-state dipole moment and moderate hyperpolarizability.5–11 From a synthetic point of view, this family of fluorophores also has a high degree of flexibility in all of the donor, acceptor and conjugated core substructures (Fig. 1).
Figure 1.
Generic structure of the DCDHF fluorophores.
Most of the previously described DCDHF fluorophores are soluble in organic solvents but are not soluble in aqueous media. For example, DCDHF-61 (1: R1 = R2 = n-hexyl, R3=R4 = methyl; n = 0 and Ar = phenyl) and DCDHF-2-V10 (2: R1=R2 = ethyl; R3 = R4 = methyl; n = 1 and Ar = phenyl) have less than 0.001 ppm water solubility. With alkyl substituents at the various R positions, such molecules have an amphiphilic motif and label cell membranes easily.11 In order to prevent membrane binding and enable applications in the cellular cytosol, it is useful to develop water soluble forms.12 To explore the water solubility potential of these fluorophores, we modified the donor component with a variety of hydrophilic groups (alcohol, carboxylic acid, and sulfonic acid), and their water solubility and photophysics in aqueous environments were determined.
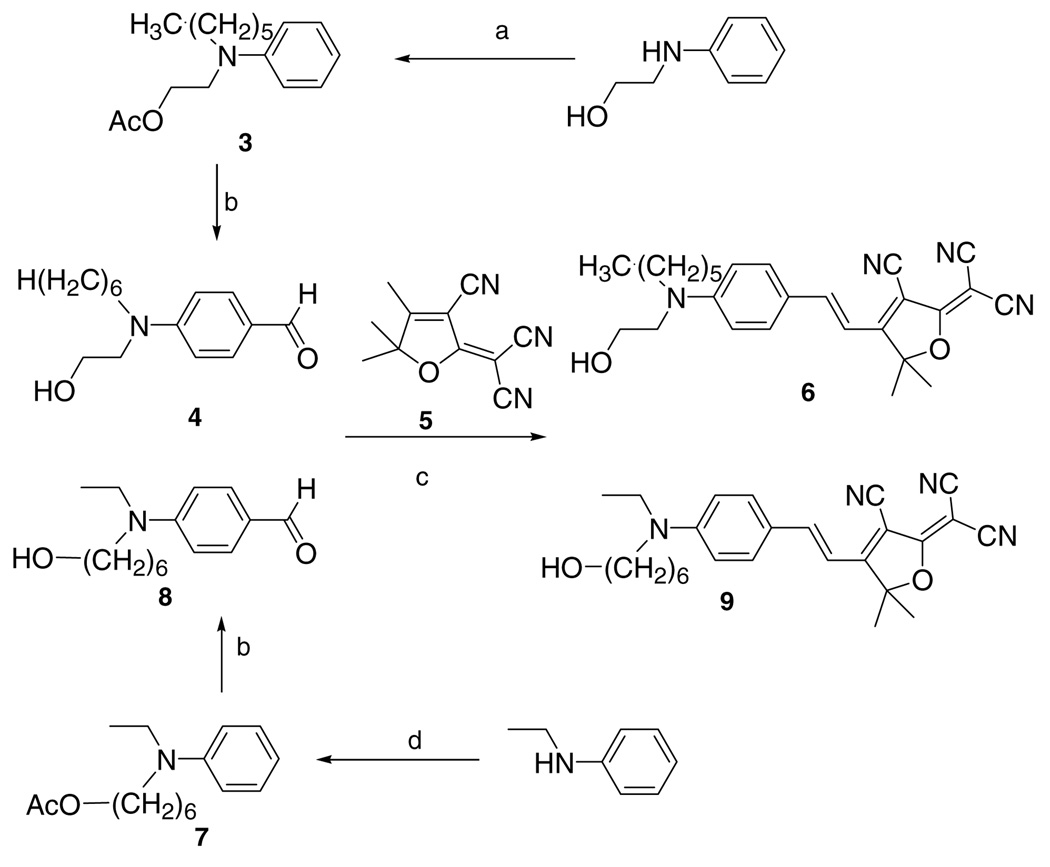
Fluorophores 6 and 9 (alcohols) were first synthesized as shown in Scheme 1. Intermediate 5 was made by condensation of 3-hydroxy-3-methyl-2-butanone with malononitrile.13 Alkylation of 2-(anilino)ethanol, protection of the alcohol group by esterification (3), Vilsmeier reaction, and deprotection gave the key aldehyde precursor 4, which was subsequently condensed with intermediate 5 to give fluorophore 6 with one free alcohol group terminating the short alkyl chain. Alternatively, alkylation of N-ethylaniline with 6-chloro-1-hexanol, protection of the alcohol group by esterification (7), Vilsmeier reaction and deprotection gives the key aldehyde precursor 8, which was also condensed with intermediate 5 to give the desired fluorophore 9 with one free alcohol group terminating the longer chain. Measurements (Table 1) show that both of these two mono-alcohol terminated fluorophores still have very low water solubility (around 0.01 ppm) with fluorophore 6 slightly higher than fluorophore 9 (Scheme 1).
Scheme 1.
Synthesis of DCDHF-V dyes with a single primary alcohol group in the amine donor tail. Reagents and conditions: (a) 1-iodohexane, K2CO3, DMF and then Ac2O, Py; (b) POCl3, DMF and HCl/methanol reflux; (c) Py, acetic acid; (d) 6-chloro-1-hexanol; NaOH, KI, DMF and Ac2O, Py.
Table 1.
Photophysical properties and water solubility measurements of different DCDHF (I) and DCDHF-V (II) chromophores
| λmax | λem | ΦF (water) |
εmax (Lcm−1 mol−1) |
Solubility in water14 (ppm) |
||
|---|---|---|---|---|---|---|
| I | 1 | 470a | 546 | 0.12 | 89,900 | <0.001 |
| 12 | 498 | 529 | 0.001 | 15,100 | 0.40 | |
| 14 | 496a | 531 | 0.002 | 44,200 | 1.40 | |
| 15 | 493 | 528 | 0.001 | 64,700 | 1.50 × 102 | |
| 16 | 500 | 526 | 0.001 | 37,300 | 3.80 | |
| 17 | 489 | 529 | 0.002 | 31,500 | 2.80 × 103 | |
| 19 | 500 | 517 | 0.001 | 79,300 | >2 × 104 | |
| 21 | 496b | — | — | 65,800 | >2 × 104 | |
| 26 | 477 | 517 | 0.001 | 57,300 | 7.30 × 102 | |
| 27 | 480 | 520 | 0.004 | 42,700 | 4.70 × 102 | |
| II | 2 | 560a | 642 | 0.002 | 13,500 | <0.001 |
| 6 | 535a | 646 | 0.002 | 77,900 | ~0.05 | |
| 9 | 587b | — | — | 87,200 | ~0.01 | |
| 24 | 585 | 639 | 0.01 | 37,000 | >2 × 104 |
A small volume of ethanol stock solution added to the cuvette of water.
Aggregation was observed in water.
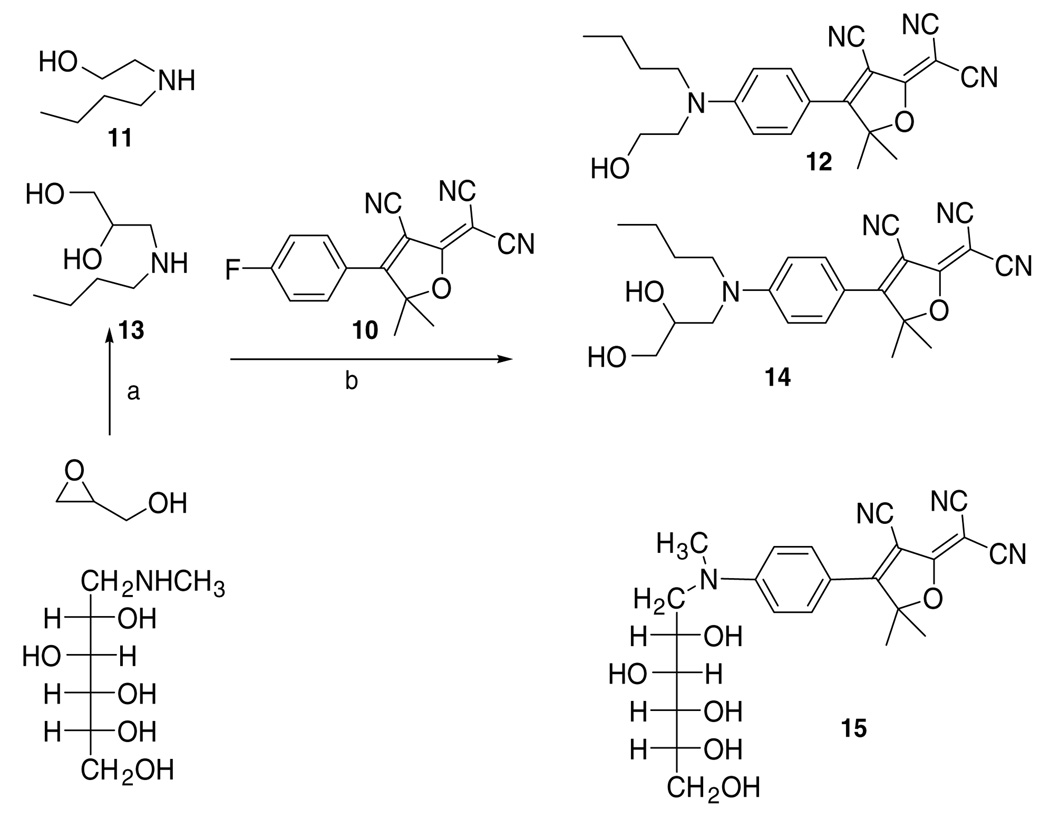
Although these two fluorophores show very low water solubility, it is improved compared with fluorophores without any alcohol group at all. Therefore, we designed fluorophore 12 to increase water solubility by removing the vinyl linkage. The synthesis of the key intermediate 10 has been reported before.1 Commercially available 2-(n-butylamino)ethanol 11 was chosen as a nucleophile to prepare fluorophore 12.15 Measurements show a solubility of 0.40 ppm for 12, which is better than the dyes with the vinyl linkage but still insufficient for our applications. Next, we decided to increase the number of the free alcohols to improve water solubility and so fluorophores 14 and 15 were subsequently synthesized. Glycidol undergoes nucleophilic attack with n-butylamine to give secondary amine 13, which then undergoes the same nucleophilic aromatic substitution on 10 to give fluorophore 14.15 The same reaction occurs between N-methyl-d-glutamine and intermediate 10 to give multiply hydroxylated fluorophore 15.15 Measurements (Table 1) show that the solubility increases almost 10 times with an increase of one free alcohol group, and increases another 30 times with an increase of another three free alcohol groups (two and five total alcohol groups, respectively, in 14 and 15) (Scheme 2).
Scheme 2.
Synthesis of DCDHF dyes with alcohol functionalization in the amine donor tail. (a) n-Butylamine, reflux; (b) pyridine.
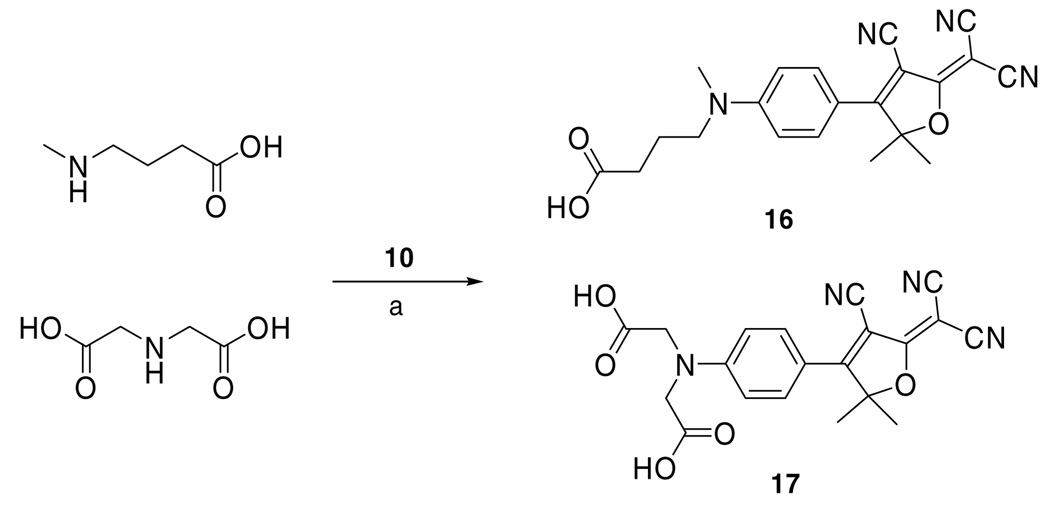
Thus far, we had been able to increase the water solubility of the DCDHF fluorophores from less than 0.001 ppm to 150 ppm. Clearly, addition of hydroxyl groups alone is not a particularly effective means to enhance solubility so we switched our attention to other functional groups beginning with carboxylic acid groups. N-Methylbutyric acid and iminodiacetic acid reacted with 10 to give fluorophores 16 and 17, respectively. 15 Fluorophore 16 with a single carboxylic acid has only 3.80 ppm water solubility, which is comparable to fluorophore 14 with two free alcohol groups. However, and to our surprise, fluorophore 17 with two carboxylic acids was found to have 2830 ppm water solubility, which is at least 20 times higher than that compared with fluorophore 15, the one with five free alcohol groups (Scheme 3).
Scheme 3.
Synthesis of DCDHF dyes with carboxylic acid functionalization in the amine donor tail. (a) Pyridine.
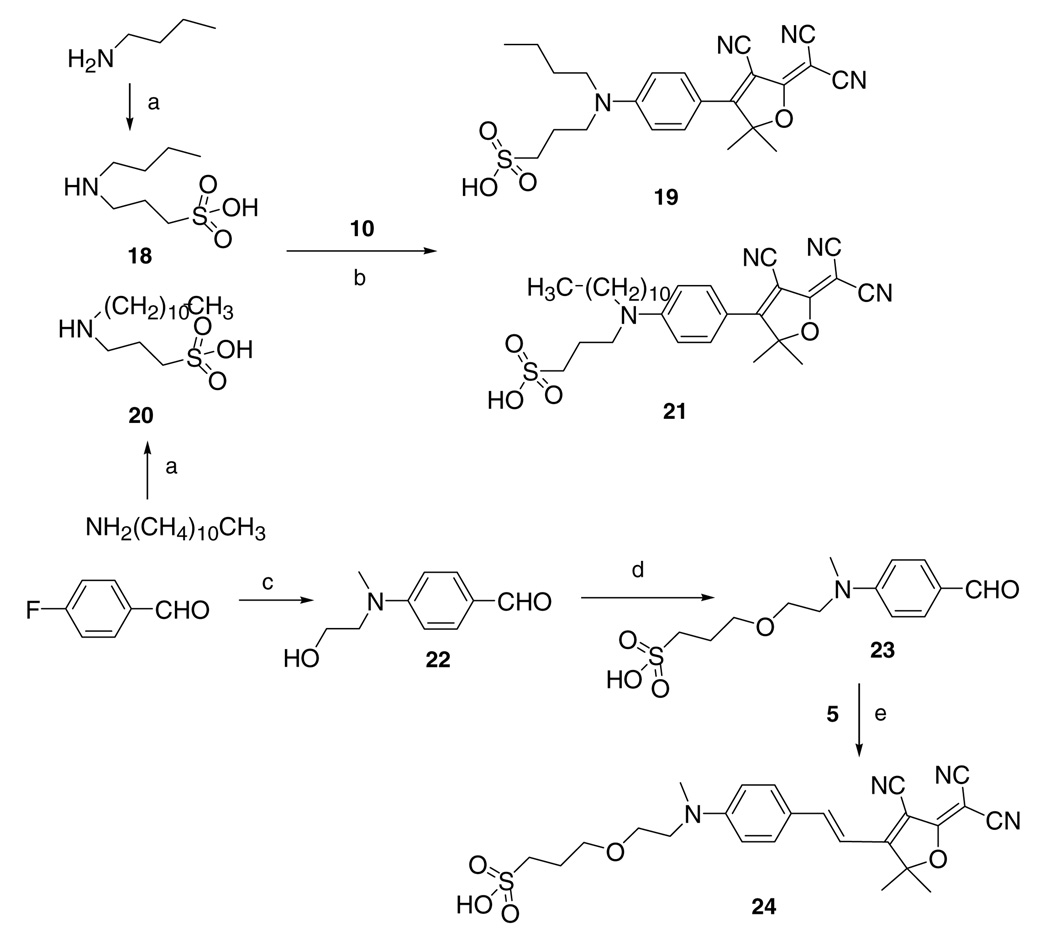
Although fluorophore 17 already has enhanced water solubility, we still wanted to push further, and so a sulfonic acid group was introduced next into the system. In this synthesis, 1,3-propanesultone underwent attack from either n-butylamine or dodecylamine to give precursors 18 and 20, respectively, which underwent the same reaction with aryl fluoride intermediate 10 to give fluorophores 19 and 21.16 Measurements show that these sulfonic acid substituted fluorophores have significant water solubilities (larger than 2 × 104 ppm). The influence of this single sulfonic acid is also so large that we decided to add the vinyl linkage back again so that we could also assay the group of DCDHF-V fluorophores containing the vinyl linkage. Fluorophore 24 was synthesized by nucleophilic aromatic substitution of 4-fluorobenzaldehyde with 2-(methylamino)ethanol. The alcohol was then deprotonated and reacted with a sultone to attach a sulfonic acid to the benzylaldehyde substrate. Finally, the aldehyde was condensed with intermediate 5 to provide the desired vinyl DCDHF-V fluorophores functionalized with sulfonic acid (Scheme 4). Here again measurements indicate water solubility larger than 2 × 104.
Scheme 4.
DCDHF and DCDHF-V dyes functionalized with sulfonic acids. Reagents and conditions: (a) 1,3-propanesultone, reflux; (b) pyridine; (c) DMF, K2CO3, 2-(methylamino)ethanol, 130 °C; (d) NaH, DMF, 1,3-propanesultone; (e) pyridine, acetic acid.
With these highly water soluble fluorophores available, our concerns about whether or not the presence of these hydrophilic groups will affect the photophysical properties of these fluorophores could be evaluated. To this end, we compared the fluorophores bearing different hydrophilic groups with their original low polarity analogs 1 and 2. Table 1 shows that within groups I (DCDHFs) and II (DCDHF-Vs) the absorption and emission wavelengths are quite similar. The differences for fluorophores 1, 2, 6 from their counterparts can be attributed to the addition of ethanol to the aqueous solution. A few other fluorophores in Table 1 (i.e., 17, 19, and 27) exhibit somewhat blue-shifted absorption or emission relative to their counterparts. Given the amphiphilic character of these molecules, the anomalous photophysics may be due to the formation of aggregates or micelles in water at the relatively high concentrations required for bulk measurements. However, at the nano-molar concentrations actually used for single-molecule experiments, such effects are unlikely. For instance, although the fluorophores used in our earlier studies were not soluble in water at bulk concentrations, single molecules were observed diffusing in the cell membrane at the nanomolar regime.11 As such, this means that while we increased the water solubility of this family of fluorophores, the desirable spectroscopic properties were essentially unchanged.
This outcome is also confirmed by independent viscosity dependence experiments. Previously, in a mixed solvent of glycerol and methanol, the quantum yield of fluorophore 1 was found to grow with the increase of the glycerol content.7 Here, two identical solutions, each of 17 and 24 in water, were prepared. For each pair, the sample on the right was frozen while the other sample remained at room temperature (Fig. 2). Under illumination with a UV lamp, the liquid sample (left of each pair) shows limited fluorescence while the frozen sample (right of each pair) is strongly emissive. Therefore, the viscosity dependence of the emission is maintained after the addition of dicarboxylic acid and sulfonic acid. This suggests that our DCDHF fluorophores have the potential for application in a cellular environment beyond lipid-analog membrane probes.
Figure 2.
Fluorophores 17 and 24 dissolved in liquid water (vials l) and in solid ice (vials s) under irradiation with a UV lamp (a filter was used to block the scattered light). The fluorescence is significantly enhanced in the ice samples responding to the dramatic enhancement in viscosity.
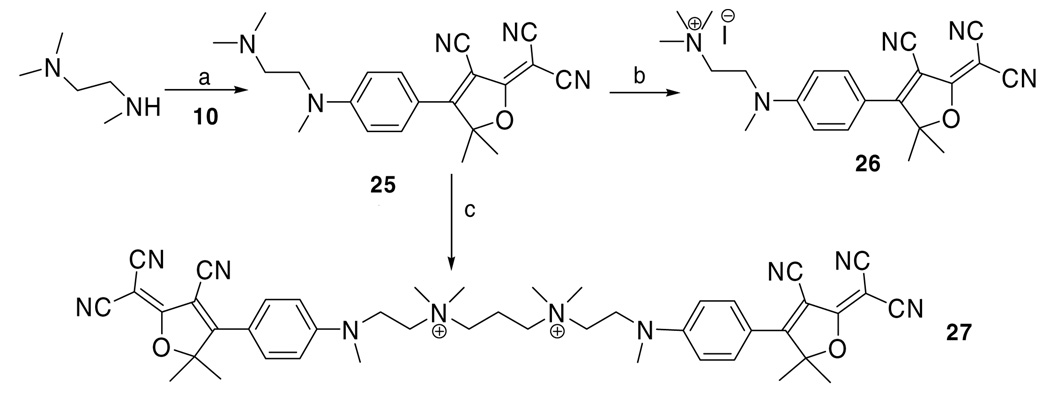
All the examples of solubilized DCDHF fluorophores described thus far are anionic. We have also briefly examined some cationic derivatives. The secondary amine in N,N,N′-trimethylethylenediamine reacts with 10 to give fluorophore 25, which next underwent alkylation at the trialkylamine end with methyl iodide to give monocationic fluorophore 26. In a similar fashion, alkylation of 25 with 1,3-diiodopropane gives dicationic dimer fluorophore 27 (Scheme 5). Although 26 and 27 are not as water soluble (730 ppm, 470 ppm respectively) as fluorophores 19, 21 and 24, they demonstrate still another option to functionalize and solubilize this family of DCDHF fluorophores.
Scheme 5.
The preparation of water solubilized DCDHF dyes, which are functionalized with quaternary amines. Reagents and conditions: (a) pyridine; (b) 1-iodomethane, CH2Cl2; (c) 1,3-diiodopropane, CH2Cl2.
In conclusion, we have synthesized a series of DCDHF fluorophores with a range of functional groups and modified their water solubility without compromising their photophysical properties. These results suggest that this class of fluorophores possess strong potential for a broad range of bio-labeling applications. The methods applied here to solubilize the DCDHF fluorophores should also be applicable in other cases, where water solubilization is required.
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.tetlet. 2007.03.026.
Acknowledgments
Support from DOE (DG-FG02-04ER63777), NIH (1P20HG003638-01) and the Ohio Board of Regents is acknowledged.
References and notes
- 1.Gubler U, He M, Wright D, Roh Y, Twieg R, Moerner WE. Adv. Mater. 2002;14:313–317. [Google Scholar]
- 2.Ostroverkhova O, He M, Twieg RJ, Moerner WE. ChemPhysChem. 2003;4:732–744. doi: 10.1002/cphc.200200633. [DOI] [PubMed] [Google Scholar]
- 3.You W, Hou ZJ, Yu LP. Adv. Mater. 2004;16:356. [Google Scholar]
- 4.Ostroverkhova O, Moerner WE, He M, Twieg RJ. Appl. Phys. Lett. 2003;82:3602–3604. [Google Scholar]
- 5.Schuck PJ, Willets KA, Fromm DP, Twieg RJ, Moerner WE. Chem. Phys. 2005;318:7–11. [Google Scholar]
- 6.Willets KA, Callis PR, Moerner WE. J. Phys. Chem. B. 2004;108:10465–10473. [Google Scholar]
- 7.Willets KA, Nishimura SY, Schuck PJ, Twieg RJ, Moerner WE. Acc. Chem. Res. 2005;38:549–556. doi: 10.1021/ar0401294. [DOI] [PubMed] [Google Scholar]
- 8.Willets KA, Ostroverkhova O, He M, Twieg RJ, Moerner WE. J. Am. Chem. Soc. 2003;125:1174–1175. doi: 10.1021/ja029100q. [DOI] [PubMed] [Google Scholar]
- 9.Willets KA, Ostroverkhova O, Hess S, He M, Twieg RJ, Moerner WE. Proc. SPIE; 2003. pp. 150–157. [Google Scholar]
- 10.Wang H, Lu Z, Lord SJ, Willets KA, Bunge S, Moerner WE, Twieg RJ. Tetrahedron. 2007;63:103–114. [Google Scholar]
- 11.Nishimura SY, Lord SJ, Klein LO, Willets KA, He M, Lu ZK, Twieg RJ, Moerner WE. J. Phys. Chem. B. 2006;110:8151–8157. doi: 10.1021/jp0574145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jose J, Burgess K. J. Org. Chem. 2006;71:7835–7839. doi: 10.1021/jo061369v. [DOI] [PubMed] [Google Scholar]
- 13.Melikian G, Rouessac FP, Alexandre C. Synth. Commun. 1995;25:3045–3051. [Google Scholar]
- 14.The water solubilities of the DCDHF chromophores were determined from UV-vis data. A standard solution of known concentration was prepared by dissolving about 1 mg of the compound in methanol/H2O (3/7, 10 ml) for fluorophores 6, 9, 12, 14–16, or in methanol/H2O (2/8, 10 ml) for fluorophores 17, 19, 21, 24, 26, 27. The saturated solutions were prepared by suspending about 2 mg or more fluorophores in H2O (10 ml) and then stirring at 60 °C for 2 h, followed by equilibration at room temperature for 12 h. These samples were passed through a syringe filter (2 µm). The absorption could be measured from the UV-vis spectra and the absorption coefficients could be calculated from standard solutions, which were used to determine the concentration of the substrate in the pure H2O sample. The results reported are averages of duplicate runs. Benbow JW, Bernberg EL, Korda A, Mead JR. Antimicrob. Agents Chemother. 1998;42:339–343. doi: 10.1128/aac.42.2.339.
- 15.General methods to obtain fluorophores 12, 14–17, 25: the starting disubstituted amine was mixed with precursor 10 in dry pyridine. The reaction mixture was then stirred at room temperature or heated to 40 °C overnight depending on the substrate. Pyridine was then distilled out under vacuum and the residue was purified via flash chromatography.
- 16.General methods to obtain fluorophores 19, 21: the starting disubstituted amine was mixed with precursor 10 in anhydrous DMF with 2 equiv i-Pr2NEt. The reaction mixture was then stirred at 40 °C overnight. Hydrochloric acid (10%) was added and the suspension was filtered to give the product as a yellow solid.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.tetlet. 2007.03.026.