Abstract
Osteopontin is a noncollagenous, phosphorylated extracellular glycoprotein, expressed in mineralized and nonmineralized tissues, organs and body fluids. The protein contains an RGD tripeptide cell-binding motif, and is subjected to a variety of posttranslational modifications that play important roles in its multiple biological functions, such as bone remodeling and inhibition of pathological calcification. In this study, we have expressed bovine osteopontin in a prokaryotic system and identified the seven amino acid residues phosphorylated in vitro by CKII.
Keywords: osteopontin phosphorylation, casein kinase II
OPN cDNA sequences have been determined and patterns of expression characterized in several species. OPN contains an arginine-glycine-aspartate (RGD) cell-binding tripeptide [Oldberg et al., 1986] and regions with an elevated level of aspartic acid. There are tissue specific differences of OPN not only in protein expression levels but also in the extent of phosphorylation. Osteopontin in the mineralized extracellular matrix of bone tissue [Prince et al., 1987] and milk [Sorensen et al., 1995] is highly phosphorylated and rat kidney cells secrete both phosphorylated and non-phosphorylated forms of the protein [Singh et al., 1990]. A number of experiments have reported as many as 10 residues of phosphorylated amino acid residues in chicken bone and as many as 28 phosphoresidues in bovine milk osteopontin [Sorensen et al., 1995; Salih et al., 1996a]. Recent analysis of OPN-deficient mice also showed impaired angiogenesis, early callus formation, and late stage remodeling in fracture healing [Duvall et al., 2007]. Several in vivo biological functions of OPN have been postulated [Chen et al., 1993; Weber and Cantor, 1996; Oates et al., 1997; Liaw et al., 1998; Ashkar et al., 2000; Rittling and Chambers, 2004; Duvall et al., 2007; Mori et al., 2007]. For example, the pathological calcification of arteries and cartilage in mice in which matrix gla protein has been deleted [Luo et al., 1997] increases when OPN is also deleted [Speer et al., 2002]. OPN-deficient mice also develop pathological calcification in vascular smooth muscle cells [Ohri et al., 2005]. The inhibition of these instances of pathological calcification has been attributed to the presence of the casein kinase II (CKII) phosphorylated residues in OPN [Jono et al., 2000].
The binding of Ca ions by osteopontin [Chen et al., 1992] may explain its inhibition of apatite crystal nucleation when added to metastable solutions of Ca2+ and inorganic phosphate [Hunter et al., 1996; Pampena et al., 2004].
Since CKII has been identified as a major kinase synthesized in bone during organ and cell culture [Mikuni-Takagaki and Glimcher, 1990; Salih et al., 1996a; Sfeir and Veis, 1996; Wang et al., 1998], we used CKII to phosphorylate recombinant OPN and to identify the specific phosphorylated residues that may play a role in some of these biological functions. We note, however, that no significant changes are observed in the extent of bone calcification or growth or shape when OPN is deleted.
MATERIALS AND METHODS
Generation of OPN Constructs
A bovine OPN cDNA clone-OP12, kindly provided by Larry W. Fisher [Kerr et al., 1991], was used as a PCR template to generate the full-length fragment under the same chemical and thermal profiles described previously [Saad et al., 2005]. The bvOPN F4: 5′ cc gga tcc atg ctt cca gtt aaa ccg acc ag 3′ and bvOPN R3: 5′ gg ctc gag tca att gac ctc aga aga ggc ac 3′ primers were used to generate the full-length fragment. The PCR product was digested with BamHI and XhoI, gel purified and ligated to pGEX-4T-1 expression vector. The OPN construct from L17 to N278 was termed pGEX-4T-1-bvOPN.
Selection of Recombinant Clones
A fraction of the ligation reaction was used to transform DH5α bacterial competent cells. The plasmid DNA was extracted using a micro-extraction procedure described elsewhere [Saad et al., 1997]. Recombinant clones were selected by PCR using primers originating from the vector sequences. To assess the integrity and the correct reading frame of the cDNA sequences, several recombinant clones were sequenced.
Expression and Purification of the Recombinant Protein
Clones displaying integrate cDNA sequence and correct reading frame were selected for protein expression in BL21 bacterial cells (Invitrogen) under protocols described elsewhere [Saad et al., 2005].
Thrombin Cleavage
Thrombin (Amersham) was mixed with aliquots of the beads-GST-fusion protein at a ratio of 2–4 U/mg in 1× PBS. The mixture was rotated at room temperature for 3 h and centrifuged to collect the supernatant, which contains the released protein. The protein concentration was determined, and a portion of the recombinant protein was fractionated on a 10% SDS-PAGE, and stained by Stains-all.
Phosphorylation of OPN by CKII
One hundred micrograms of rbvOPN were phosphorylated by [32P] ATP using 100 ng of recombinant CKII expressed in Sf9 insect cells (Upstate Biotechnology) in 0.5 ml of 0.1 M KH2PO4/Na2HPO4 buffer (pH 7.4) containing 5 mM MgCl2 and 1 mM EGTA for 1 h at room temperature. The tryptic peptide mapping of the 32P-labled peptides was as described previously [Saad et al., 2005].
Identification of the Phosphorylation Sites
N-terminal sequencing was carried out by Edman degradation using an automated model 477A sequenator (Applied Biosystems), under the same conditions described previously [Carr et al., 1987]. The sequencing conditions for the identification of the 32P-labeled peptides and the specific sites of phosphorylation were as described previously [Salih et al., 1997].
RESULTS
Expression and Purification of the Recombinant Protein
OPN cDNA was inserted in frame into the pGEX-4T-1 expression vector and expressed in BL21 bacterial cells as a GST fusion protein under the control of the IPTG inducible tac promoter [Smith and Johnson, 1988]. After IPTG induction, the bacterial pellet was resuspended in 1× BPS and sonicated to disrupt the cell wall. Then, the GST fusion protein was purified by binding to glutathione agarose (beads) in solution. On 10% SDS-PAGE, a single protein band was seen that corresponds to the full-length OPN (Fig. 1, lane B).
Fig. 1.
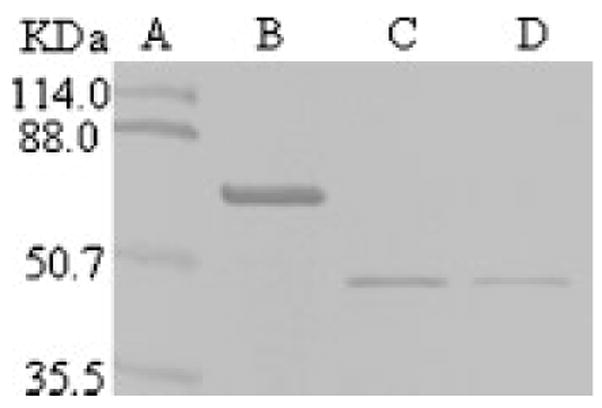
Expression, purification and thrombin cleavage of GST fused bovine osteopontin. Lane A: Prestained protein marker. Lane B: GST fused OPN, no thrombin. Lane C: The released recombinant OPN at 4 U thrombin per mg of GST fused protein. Lane D: The released recombinant OPN at 2 U thrombin per mg of GST fused protein. A portion of the recombinant protein was mixed with protein loading dye, fractionated onto 10% SDS-PAGE, and stained with Stains-all.
Thrombin Cleavage of GST Fused Protein
The pGEX expression vector built-in thrombin cleavage site was used to cleave the recombinant protein from the glutathione S-transferase (GST). As little as 2 units’ thrombin per milligram of GST-OPN released the fused protein that runs as a single band on 10% SDS-PAGE (Fig. 1, Lanes C and D). The N-terminal amino acid sequencing of the thrombin cleavage product led to a single sequence, GSMLPVKP. This sequence contains three exogenous residues (GSM). The rest of the sequence (LPVKP) is identical to the bovine OPN amino acid sequence derived from the cDNA [Kerr et al., 1991] starting at L17. We previously reported that thrombin cleavage of OPN generates two fragments [Salih et al., 1996b]. Most likely the susceptibility of this site to thrombin was generated by the use of an excessive amount of thrombin and the higher temperature used to digest OPN.
Identification of Phosphorylated Residues
Figure 2 shows a tryptic peptide map of recombinant bovine OPN phosphorylated by [32P] ATP and CKII. Five 32P-labeled peptides were observed within the RP-HPLC profile using a C18 column. Each of the radiolabeled fractions was N-terminally sequenced using a solid-phase sequencing approach. Five 32P-labeled peptides were identified: residues 78–87 (SPNESPPEQTDD), 170–181 (SNVQSPDATPEED), 186–195 (IESPEEMHDAP), 250–259 (LDLDHKSPEED), and 266–276 (ISPHELDSPASSE). Six phosphoserines and one phosphothreonine were identified within the five peptides.
Fig. 2.
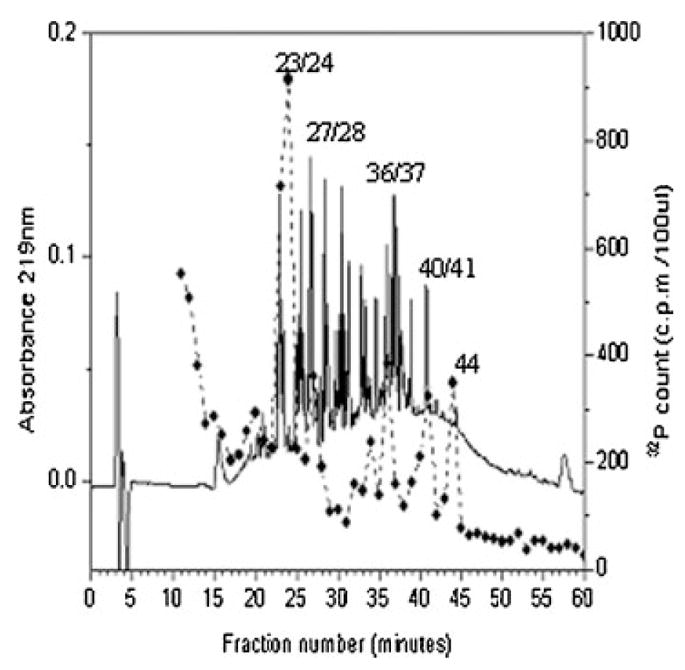
The phosphorylated tryptic peptides of recombinant bovine osteopontin. The peptides were separated by RP-HPLC on a Vydac C18 column (25 cm × 0.46 cm) using a linear gradient from H2O and 0.1% (v/v) trifluoroacetic acid to 60% CH3CN and 0.055% (v/v) trifluoroacetic acid over 80 min at a flow rate of 1 ml/min. Absorbance at 219 nm was recorded continuously. Fractions of 1 ml were collected, and aliquots were counted for 32P radioactivity. The phosphorylated tryptic peptides are numbered.
DISCUSSION
We have identified the specific positions of six serine and one threonine phosphorylated residues in five 32P-labeled peptides of osteopontin expressed in a prokaryotic system. The seven phosphorylated amino acid residues (S78, S81, T178, S188, S256, S267 and S272) were identified within five peptides, with amino acid sequence motifs identical to the primary (SXXE/D/SP) or the secondary (SXE/D) consensus sequences of CKII [Litchfield, 2003; Meggio and Pinna, 2003]. Although bovine OPN contains 12 potential phosphorylation sites for CKII, only seven of them were identified in the present work. However, as has been pointed out [Meggio et al., 1994], the presence of consensus amino acid sequences for CKII in a protein does not assure phosphorylation. Although there are more than 300 physiological substrates of CKII identified in vitro [Meggio and Pinna, 2003], in vivo phosphorylation of some of them is not yet documented. The phosphoresidues identified in this study are known to be phosphorylated in vivo [Sorensen et al., 1995].
Bovine milk OPN contains 27 phosphoserines and one phosphothreonine [Sorensen et al., 1995]. Neame and Butler [1996] identified 11 phosphorylated residues in OPN of rat bone. Recent analysis of rat bone OPN indicates that it contains 29 phosphorylated residues, of which ten were located in CKII consensus sequences, and 17 appear in sequences homologous to bovine milk OPN [Keykhosravani et al., 2005]. Native OPN of human milk contains 36 phosphorylated residues. Twenty-nine of them were located in the consensus sequence for the mammary gland casein kinase (MGCK), whilst six residues were located in CKII consensus sequence, and one was not in any of MGCK or CKII consensus sequences [Christensen et al., 2005]. OPN from cultured chicken osteoblasts contains nine phosphoserines and one phospho-threonine [Salih et al., 1996a].
Conservation of phosphorylation sequences in OPN suggests that phosphorylation plays a fundamental role in many of its biological functions, including bone remodeling [Reinholt et al., 1990]. In fact, phosphorylation of recombinant osteopontin by CKII (which contains no phosphorylated amino acid residues when expressed in a prokaryotic system) increases its ability to support osteoclast adhesion and cell attachment which is dependent on an RGD sequence [Katayama et al., 1998], and inhibits vascular calcification to the same extent as the native phosphorylated osteopontin [Jono et al., 2000].
Acknowledgments
The authors would like to acknowledge Lila Graham for critical reading of the manuscript and Rudolf Flückiger for helping with RP-HPLC profiling. This study was supported by NIH grants AG014701 to Melvin J. Glimcher and AG17969 to Erdjan Salih. Fawzy A. Saad is recipient of a National Research Service Award, 5 T32 AR7112-27.
Grant sponsor: NIH; Grant numbers: AG014701, AG17969, AR7112.
References
- Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H. Eta-1 (osteopontin): An early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- Carr C, McCount D, Cohen JB. The 43-kilodalton protein of Torpedo nicotinic postsynaptic membranes: Purification and determination of primary structure. Biochemistry. 1987;26:7090–7102. doi: 10.1021/bi00396a034. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bal BS, Gorski JP. Calcium and collagen binding properties of osteopontin, bone sialoprotein, and bone acidic glycoprotein-75 from bone. J Biol Chem. 1992;267:24871–24878. [PubMed] [Google Scholar]
- Chen J, Singh K, Mukherjee BB, Sodek J. Developmental expression of osteopontin (OPN) mRNA in rat tissues: Evidence for a role for OPN in bone formation and resorption. Matrix. 1993;13:113–123. doi: 10.1016/s0934-8832(11)80070-3. [DOI] [PubMed] [Google Scholar]
- Christensen B, Nielsen MS, Haselmann KF, Petersen TE, Sorensen ES. Posttranslationally modified residues of native human osteopontin are located in clusters. Identification of thirty-six phosphorylation and five O-glycosylation sites and their biological implications. Biochem J. 2005;390:285–292. doi: 10.1042/BJ20050341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall CL, Taylor WR, Weiss D, Wojtowicz AM, Guldberg RE. Impaired angiogenesis, early callus formation, and late stage remodeling in fracture healing of osteopontin-deficient mice. J Bone Miner Res. 2007;22:286–297. doi: 10.1359/jbmr.061103. [DOI] [PubMed] [Google Scholar]
- Hunter GK, Hauschka PV, Poole AR, Rosenberg LC, Goldberg HA. Nucleation and inhibition of hydroxyapatite formation by mineralized tissue proteins. Biochem J. 1996;317:59–64. doi: 10.1042/bj3170059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jono S, Peinado C, Giachelli CM. Phosphorylation of osteopontin is required for inhibition of vascular smooth muscle cell calcification. J Biol Chem. 2000;275:20197–20203. doi: 10.1074/jbc.M909174199. [DOI] [PubMed] [Google Scholar]
- Katayama Y, House CM, Udagawa N, Kazama JJ, McFarland RJ, Martin TJ, Findlay DM. Casein kinase 2 phosphorylation of recombinant rat osteopontin enhances adhesion of osteoclasts but not osteoblasts. J Cell Physiol. 1998;176:179–187. doi: 10.1002/(SICI)1097-4652(199807)176:1<179::AID-JCP19>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Kerr JM, Fisher LW, Termine JD, Young MF. The cDNA cloning and RNA distribution of bovine osteopontin. Gene. 1991;108:237–243. doi: 10.1016/0378-1119(91)90439-i. [DOI] [PubMed] [Google Scholar]
- Keykhosravani M, Doherty-Kirby A, Zhang C, Brewer D, Goldberg HA, Hunter GK, Lajoie G. Comprehensive identification of post-translational modifications of rat bone osteopontin by mass spectrometry. Biochemistry. 2005;44:6990–7003. doi: 10.1021/bi050109p. [DOI] [PubMed] [Google Scholar]
- Liaw L, Birk DE, Ballas CB, Whitsitt JS, Davidson JM, Hogan BL. Altered wound healing in mice lacking a functional osteopontin gene (spp1) J Clin Invest. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield DW. Protein kinase C K2: Structure, regulation and role in cellular decisions of life and death. Biochem J. 2003;369:1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- Meggio F, Marin O, Pinna LA. Substrate specificity of protein kinase CK2. Cell Mol Biol Res. 1994;40:401–409. [PubMed] [Google Scholar]
- Mikuni-Takagaki Y, Glimcher MJ. Post-translational processing of chicken bone phosphoproteins. Identification of bone (phospho)protein kinase. Biochem J. 1990;268:593–597. doi: 10.1042/bj2680593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori N, Majima T, Iwasaki N, Kon S, Miyakawa K, Kimura C, Tanaka K, Denhardt DT, Rittling S, Minami A, Uede T. The role of osteopontin in tendon tissue remodeling after denervation-induced mechanical stress deprivation. Matrix Biol. 2007;26:42–53. doi: 10.1016/j.matbio.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Neame PJ, Butler WT. Posttranslational modification in rat bone osteopontin. Connect Tissue Res. 1996;35:145–150. doi: 10.3109/03008209609029185. [DOI] [PubMed] [Google Scholar]
- Oates AJ, Barraclough R, Rudland PS. The role of osteopontin in tumorigenesis and metastasis. Invasion Metastasis. 1997;17:1–15. [PubMed] [Google Scholar]
- Ohri R, Tung E, Rajachar R, Giachelli CM. Mitigation of ectopic calcification in osteopontin-deficient mice by exogenous osteopontin. Calcif Tissue Int. 2005;76:307–315. doi: 10.1007/s00223-004-0071-7. [DOI] [PubMed] [Google Scholar]
- Oldberg A, Franzen A, Heinegard D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. PNAS. 1986;83:8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampena DA, Robertson KA, Litvinova O, Lajoie G, Goldberg HA, Hunter GK. Inhibition of hydroxyapatite formation by osteopontin phosphopeptides. Biochem J. 2004;378:1083–1087. doi: 10.1042/BJ20031150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince CW, Oosawa T, Butler WT, Tomana M, Bhown AS, Bhown M, Schrohenloher RE. Isolation, characterization, and biosynthesis of a phosphorylated glycoprotein from rat bone. J Biol Chem. 1987;262:2900–2907. [PubMed] [Google Scholar]
- Reinholt FP, Hultenby K, Oldberg A, Heinegard D. Osteopontin—A possible anchor of osteoclasts to bone. Proc Natl Acad Sci. 1990;87:4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br J Cancer. 2004;90:1877–1881. doi: 10.1038/sj.bjc.6601839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad FA, Mostaciuolo ML, Trevisan CP, Tomelleri G, Angelini C, Abdel Salam E, Danieli GA. Novel mutations and polymorphisms in the human dystrophin gene detected by double-strand conformation analysis. Hum Mutat. 1997;9:188–190. doi: 10.1002/(SICI)1098-1004(1997)9:2<188::AID-HUMU15>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Saad FA, Salih E, Wunderlich L, Fluckiger R, Glimcher MJ. Prokaryotic expression of bone sialoprotein and identification of casein kinase II phosphorylation sites. Biochem Biophys Res Commun. 2005;333:443–447. doi: 10.1016/j.bbrc.2005.05.124. [DOI] [PubMed] [Google Scholar]
- Salih E, Ashkar S, Zhou HY, Gerstenfeld L, Glimcher MJ. Protein kinases of cultured chicken osteoblasts that phosphorylated extracellular bone proteins. Connect Tissue Res. 1996a;35:207–213. doi: 10.3109/03008209609029193. [DOI] [PubMed] [Google Scholar]
- Salih E, Zhou HY, Glimcher MJ. Phosphorylation of purified bovine bone sialoprotein and osteopontin by protein kinases. JBC. 1996b;271:16897–16905. doi: 10.1074/jbc.271.28.16897. [DOI] [PubMed] [Google Scholar]
- Salih E, Ashkar S, Gerstenfeld LC, Glimcher MJ. Identification of the phosphorylated sites of metabolically 32P-labeled osteopontin from cultured chicken osteoblasts. JBC. 1997;272:13966–13973. doi: 10.1074/jbc.272.21.13966. [DOI] [PubMed] [Google Scholar]
- Sfeir C, Veis A. The membrane associated kinases which phosphorylate bone and dentin extracellular matrix phosphoproteins are isoforms of cytosolic CKII. Connect Tissue Res. 1996;35:215–222. doi: 10.3109/03008209609029194. [DOI] [PubMed] [Google Scholar]
- Singh K, DeVouge MW, Mukherjee BB. Physiological properties and differential glycosylation of phosphorylated and nonphosphorylated forms of osteopontin secreted by normal rat kidney cells. J Biol Chem. 1990;265:18696–18701. [PubMed] [Google Scholar]
- Smith DB, Johnson KS. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Sorensen ES, Hojrup P, Petersen TE. Posttranslational modifications of bovine osteopontin: Identification of twenty-eight phosphorylation and three O-glycosylation sites. Protein Sci. 1995;4:2040–2049. doi: 10.1002/pro.5560041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer MY, McKee MD, Guldberg RE, Liaw L, Yang HY, Tung E, Karsenty G, Giachelli CM. Inactivation of the osteopontin gene enhances vascular calcification of matrix Gla protein-deficient mice: Evidence for osteopontin as an inducible inhibitor of vascular calcification in vivo. J Exp Med. 2002;196:1047–1055. doi: 10.1084/jem.20020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Glimcher MJ, Mah J, Zhou HY, Salih E. Expression of bone microsomal casein kinase II, bone sialoprotein, and osteopontin during the repair of calvarial defects. Bone. 1998;22:621–628. doi: 10.1016/s8756-3282(98)00057-x. [DOI] [PubMed] [Google Scholar]
- Weber GF, Cantor H. The immunology of Eta-1/osteopontin. Cytokine Growth Factor Rev. 1996;7:241–248. doi: 10.1016/s1359-6101(96)00030-5. [DOI] [PubMed] [Google Scholar]


