Summary
Fabry disease results from an X-linked mutation in the lysosomal α-galactosidase A (Gla). Defective Gla results in multi-organ accumulation of neutral glycosphingolipids (GSLs), especially in the vascular endothelium, the major accumulating GSL being globotriaosylceramide (Gb3). Excessive endothelial Gb3 accumulation is associated with increased thrombosis, atherogenesis, and endothelial dysfunction. The mechanism(s) by which endothelial dysfunction occurs, however, is unclear. The purpose of this study was to further characterize the vasculopathy associated with a murine model of Fabry disease.
Vascular reactivity was performed in vessels from wildtype (Gla +/0) and Gla knockout (Gla -/0) mice. Conscious blood pressure and heart rate were measured in the Gla +/0 and Gla -/0 mice by telemetry.
The present study demonstrates that vascular smooth muscle (VSM) contractions were blunted in the Gla -/0 mice to phenylephrine and serotonin, but not to U46619. Endothelium-dependent contraction and receptor-mediated endothelium-dependent relaxation to acetylcholine were significantly attenuated in vessels from Gla -/0 mice. However, receptor-independent endothelium-dependent relaxation to the calcium ionophore, ionomycin, remained intact in vessels from Gla -/0 mice. Furthermore, VSM reactivity was normal in the aortas from Gla -/0 mice in the absence of endothelium. These changes in vascular function were observed without changes in whole-animal blood pressure or heart rate.
These results suggest that the vasculopathy associated with Fabry disease is localized to the endothelium despite the accumulation of GSLs throughout the vasculature.
Keywords: α-galactosidase A, endothelium, Fabry disease, globotriaosylceramide
Introduction
Fabry disease, a rare lysosomal storage disorder, results from a deficiency in the lysosomal hydrolase, α-galactosidase A (Gla) (1, 2). The multi-organ accumulation of neutral glycosphingolipids (GSLs) with α-galactosyl linkages, primarily globotriaosylceramide (Gb3), is the consequence of deficient lysosomal Gla activity (3, 4). Because the disease is a recessive, X-linked disorder, hemizygous males are more severely affected by the disease, and premature mortality is the result of the development of renal insufficiency and end-stage renal disease, as well as cardiovascular and cerebrovascular complications (5, 6).
The basis for these cardio- and cerebrovascular complications associated with Fabry disease may be derived, in part, from endothelial dysfunction associated with Gb3 accumulation in vascular endothelium. To date, previous studies have demonstrated Gb3 accumulation in both vascular smooth muscle and endothelium in the vasculature of Gla knockout (Gla -/0) mice (7, 8), a murine model for Fabry disease (9). The increased vascular Gb3 accumulation has been associated with an increased propensity for thrombosis (7, 10), as well as increased atherogenesis (11), both of which are associated with endothelial dysfunction.
Excess endothelial Gb3 is believed to contribute to endothelial dysfunction associated with Fabry disease (12), and Heare and colleagues demonstrated that blunted endothelium-dependent relaxation is associated with increased vascular Gb3 accumulation in older (19 months old) Gla -/0 mice (13). The purpose of this study was to further characterize the vasculopathy associated with a murine model of Fabry disease. Reactivity was performed in isolated vessels from wildtype (Gla +/0) and Gla -/0 mice. Our results demonstrate that vascular contractility to phenylephrine and serotonin, but not to U46619, were blunted. Endothelium-dependent contraction and relaxation to acetylcholine also were attenuated, while receptor-independent endothelium-dependent relaxation remained intact in Gla -/0 mice. Furthermore, vascular reactivity was normalized in aortas from Gla -/0 mice after endothelium removal. These changes in vascular function were evident despite insignificant changes in consciously-measured blood pressure and heart rate. These results suggest that vasculopathy in this model of Fabry disease is localized to the endothelium despite the accumulation of GSLs throughout the vasculature. More importantly, these observations suggest that the endothelial defect may stem from excess GSLs affecting receptor coupling, thereby extending our understanding of how excess endothelial GSL accumulation may have a functional impact on vascular function and pharmacology.
Methods
Mice
Male C57Bl/6 mice (wildtype; 12 - 20 weeks old) were from Charles River (Wilmington, MA) or Jackson Laboratories (Bar Harbor, ME). Male Gla knockout (Gla -/0) mice (9) were bred from mice provided by Drs. Ashok Kulkarni and Roscoe Brady (National Institutes of Health, Bethesda, MD). These mice were backcrossed at least five generations to the C57Bl/6 strain. All mice were maintained on normal chow in specific pathogen-free facilities.
The procedures performed in mice were in accordance with guidelines of the University of Michigan Committee on the use and care of animals. The University of Michigan Unit for Laboratory Animal Medicine provided veterinary care. The University of Michigan is accredited by the American Association of laboratory Animal Care. The animal care and use program conformed to the standards in “The Guide for the Care and Use of laboratory Animals,” Department of Health, Education, and Welfare Publication No. (NIH) 86-23.
Direct Blood Pressure and Heart Rate Measurements with Radiotelemetry
Blood pressure and heart rate were measured in wildtype and Gla -/0 mice as previously described (14). Briefly, the left common carotid artery was cannulated with the catheter of a telemetric blood pressure transducer (model TAP20-C10; Data Sciences International, St. Paul, MN), securing the device body in the abdominal cavity. Diastolic, systolic and mean arterial pressures, as well as heart rate, were collected every 10 minutes continuously for 3 weeks, beginning immediately after implantation. At the end of 3 weeks, 3 consecutive 28-hour periods were averaged from each mouse, and a group mean was calculated. Rate-pressure product (mVO2), an indicator of myocardial oxygen consumption, was calculated from systolic pressure × heart rate.
Isometric force measurements
Vascular rings (2-3 mm in length) with or without endothelium were mounted in a myograph system (Danish Myo Technology A/S, Aarhus, Denmark) and bathed with warmed (37°C), aerated (95% O2/5% CO2) physiological salt solution (PSS, mmol/L: NaCl 130, KCl 4.7, KHPO4 1.18, MgSO4 1.17, CaCl2 1.6, NaHCO3 14.9, dextrose 5.5, CaNa2 EDTA 0.03). Carotid rings were denuded of endothelium using a human hair, and thoracic aortic rings were denuded of endothelium by perfusing the rings with 100 μL of 0.1% triton in PBS (15). Carotid rings were set at 250 mg passive tension while thoracic aortic rings were set at 700mg passive tension. These passive tensions were chosen based on previous studies in the carotid artery (16) and thoracic aorta (17). Arterial preparations were equilibrated for 1 hour with washes every 20 minutes. Prior to experimental protocols, rings were subjected to a wake-up protocol consisting of 2 consecutive contractions with KPSS (mmol/L: NaCl 14.7, KCl 100, KHPO4 1.18, MgSO4 1.17, CaCl2 1.6, NaHCO3 14.9, dextrose 5.5, CaNa2 EDTA 0.03) and phenylephrine (PE; 10-6 mol/L) with washes in between each KPSS contraction. After the PE contraction reached a plateau in the thoracic aortas, endothelial integrity was tested with 10-5 mol/L acetylcholine (Ach). The carotid rings were not exposed to Ach during the wake up protocol, because previous exposure of the arterial preparations to Ach or SNP desensitizes the preparations to endothelium-dependent contractions (18).
Experimental protocols
Endothelium-dependent contraction
The carotid arteries were used to evaluate endothelium-dependent contractions since aortas do not have as robust an endothelium-dependent contraction as carotids (19). All equilibration and reactivity in the carotid rings (KPSS and Ach-induced contractions) were performed in the presence of 3 × 10-4 mol/L Nω-nitro-l-arginine (LNNA). After the wake-up protocol was performed, the PE contraction was washed out. Because of the biphasic nature of the Ach-mediated contraction, only a single concentration of Ach (10-5 mol/L) was used on the arterial preparations (19). A total of 10 mice were used for reactivity in the carotid arteries.
Vascular smooth muscle contractility
The thoracic aortas were used to evaluate vascular smooth muscle reactivity in endothelium intact rings in the presence or absence of 3 × 10-4 mol/L LNNA, or endothelium-denuded rings. After the wake-up protocol, cumulative concentrations of PE (10-9 mol/L to 10-5 mol/L), serotonin (5HT; 10-9 mol/L to 10-5 mol/L), or the PGH2/TxA2 (TP) receptor agonist, U46619 (10-11 mol/L to 3 × 10-7 mol/L) were added to the bath to establish a concentration-response curve. Contractions to PE, 5HT or U46619 were expressed as a percent of the second KPSS contraction. 5HT and U46619 reactivity were performed in a group of aortas different from those used for PE.
Endothelium-dependent and endothelium-independent relaxation
In rings of thoracic aorta in which a PE concentration response was performed, the PE contraction was washed out, and the PE EC80 was calculated for each individual ring. The individual PE EC80 values were used to contract the appropriate rings and allowed to reach a stable plateau. Ach (10-10 mol/L to 10-5 mol/L), ionomycin (10-10 mol/L to 3 × 10-7 mol/L) or sodium nitroprusside (SNP; 10-11 mol/L to 10-6 mol/L) was added cumulatively to the bath to examine endothelium-dependent (Ach and ionomycin) or – independent (SNP) relaxation. Ach, ionomycin, and SNP relaxation were expressed as a percent of the PE EC80 contraction. Ach and SNP reactivity were performed in the same rings, while ionomycin reactivity was performed in separate rings that did not receive Ach or SNP. Separate reactivity to all agonists also was performed in the presence of LNNA (10-4 mol/L). PE, Ach, and SNP reactivity were also performed again in separate rings denuded of endothelium.
Chemicals
PE, 5HT, Ach, SNP, LNNA, triton and all salts for PSS were purchased from Sigma Chemical Co. (St. Louis, MO). U46619 was purchased from Cayman Chemical (Ann Arbor, MI). Ionomycin was purchased from Calbiochem (La Jolla, CA).
Data and Statistical Analysis
Agonist EC50 values were calculated with a nonlinear regression analysis with the algorithm [effect = maximum response/1 + (EC50/agonist concentration)] in the computer program GraphPad Prism (San Diego, CA). Hill slope values were also derived from GraphPad Prism. The PE EC80 values were calculated from the equation, logEC50 = logECF − (1/HillSlope) * log (F/[100-F]), where F = 80. Data were expressed as mean ± SEM. Blood pressures, heart-rate, and rate-pressure products were analyzed using two-way ANOVA. Concentration-response data were analyzed using two-way ANOVA to compare the concentration-response curves between groups. The Bonferonni post hoc test was used to assess differences at individual points on the concentration-response curves if the two-way ANOVA comparison between curves was p < 0.05. One-way ANOVA was used to evaluate differences between groups in endothelium-dependent contractions. Differences in KPSS contractions, EC50, and Emax between 2 groups were analyzed by student t-test. A p < 0.05 was considered statistically significant.
Results
Blood pressure and heart rate in wildtype (WT) and Gla knockout (Gla -/0) mice
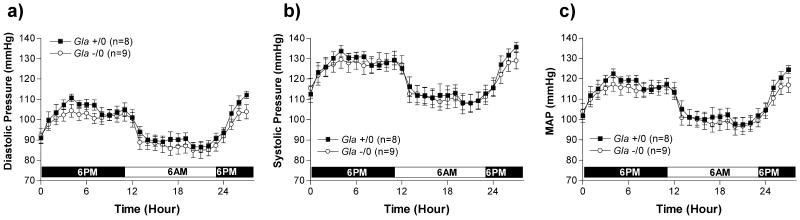
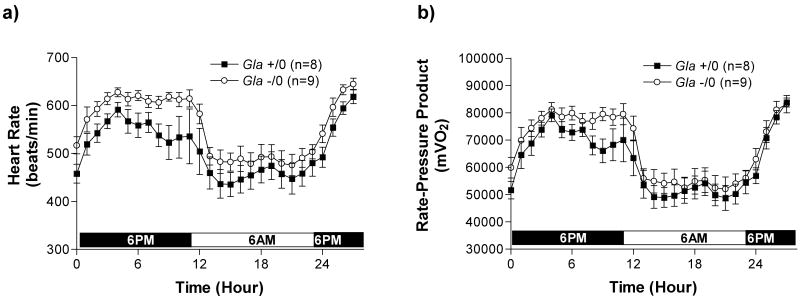
Blood pressure and heart rate measured in WT and Gla -/0 are illustrated in Figure 1 and Figure 2. Diastolic (Figure 1a), systolic (Figure 1b) and mean arterial pressure (Figure 1c) fluctuated due to circadian rhythms but did not differ between the 2 groups over the 28 hour period measured, suggesting the Gla -/0 mice were not hypertensive. Heart rate (Figure 2) also fluctuated through the 28 hour period due to circadian rhythms. The heart rates in Gla -/0 mice were higher, but not statistically significant, compared to WT mice during the dark cycle. The rate-pressure product (mVO2), a calculated index of myocardial oxygen consumption (Figure 2b) was also higher, but not significantly different in the Gla -/0 mice compared to WT mice during the dark cycle, suggesting the hearts in Gla -/0 mice may be working harder to maintain perfusion pressure.
Figure 1.
a) Conscious diastolic, b) systolic, and c) mean arterial, blood pressures (mmHg) measured during a 28-hour light-dark cycle by telemetry in wildtype (Gla +/0) or Gla knockout (Gla -/0) mice. p>0.05 by two-way ANOVA.
Figure 2.
a) Heart rate (beats/min), and calculated myocardial oxygen consumption (mVO2) or b) Rate-Pressure Product obtained from measurements derived from a telemetric blood pressure transducer. p>0.05 by two-way ANOVA.
Vascular contractility with phenylephrine, serotonin, and U46619
Phenylephrine (PE)
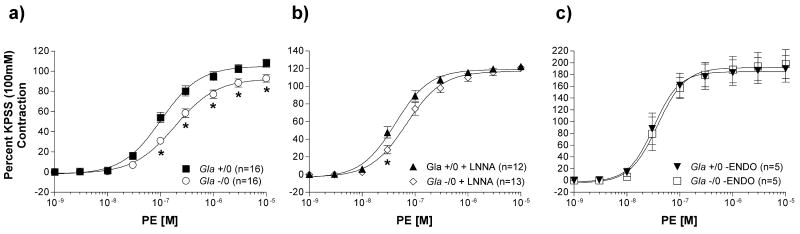
The vascular contraction mediated by 100 mmol/L KPSS was equivalent in aortas from wildtype (Gla +/0) and Gla knockout (Gla -/0) mice (1708 ± 107 mg; n=16 vs. 1496 ± 93 mg; n=16, respectively; p > 0.05). KPSS contractions, in the presence of Nω-nitro-l-arginine (LNNA), were 2078 ± 99 mg (n=12) for Gla +/0 versus 2056 ± 93 mg (n=13) for Gla -/0 (p > 0.05). After endothelial denudation, KPSS contractions were 1065 ± 123 mg (n=5) for Gla +/0 versus 1007 ± 118 mg (n=5) for Gla -/0 (p > 0.05).
PE caused a concentration-dependent contraction in isolated endothelium-intact aortic rings from both Gla +/0 and Gla -/0 mice (figure 3). PE contractility in untreated, endothelium-intact vessels (figure 3a) was approximately 2-fold less sensitive in aortas from Gla -/0 mice compared to Gla +/0 (Table 1). In addition, maximal contraction (Emax) to PE in aortas from Gla -/0 mice was significantly less (Emax = 92.9 ± 3.7 %) compared to that in aortas from Gla +/0 mice (Emax = 107.9 ± 4.0 %; p < 0.05). PE contractility in the presence of LNNA (figure 3b) was still less sensitive (∼1.6-fold less) in aortas from Gla -/0 mice compared to Gla +/0 mice (Table 2) while maximal contraction to PE were not different in the presence of LNNA (Emax = 122.5 ± 3.1 % for Gla +/0 vs. 121.1 ± 3.6 % for Gla -/0; p > 0.05). Figure 3c illustrates PE reactivity in endothelium-denuded aortas from Gla +/0 and Gla -/0 mice. The PE-induced contractions were equivalent in aortas from Gla +/0 and Gla -/0 mice as demonstrated by similar log EC50 values (Table 3) as well as equivalent Emax values (189.9 ± 22.6 % vs. 198.0 ± 24.8 %, respectively; p > 0.05).
Figure 3.
Phenylephrine (PE)-mediated vascular contraction in endothelium-intact mouse aortic rings from wildtype (Gla +/0) or Gla knockout (Gla -/0) mice a) alone without any pharmacological intervention, b) in the presence of 10-4 mol/L Nω-nitro-l-arginine (LNNA), or c) with endothelium denuded. Data are expressed as a percentage of the contraction elicited by a 100 mmol/L KCl-containing physiological salt solution. * = p<0.05 compared to Gla +/0 by two-way ANOVA followed by Bonferonni post hoc test.
Table 1.
Potency of agonists in vascular reactivity of thoracic aortas with endothelium intact from wildtype (Gla +/0) or Gla knockout (Gla -/0) mice. Data are reported as mean ± SEM for the number of animals in parentheses. The EC50 values are expressed as the −log EC50 for each agonist. * = p<0.05 compared to Gla +/0 by the Student t test.
| Agonist | -log EC50 [M] | |
|---|---|---|
| Gla +/0 (+ENDO) | Gla -/0 (+ENDO) | |
| PE | 6.99 ± 0.05 (16) | 6.72 ± 0.05 (16) * |
| 5HT | 7.08 ± 0.03 (8) | 6.89 ± 0.04 (8) * |
| U46619 | 8.77 ± 0.02 (8) | 8.70 ± 0.02 (8) * |
| Ach | 7.43 ± 0.05 (7) | 7.37 ± 0.10 (7) |
| Ionomycin | 7.98 ± 0.03 (6) | 8.01 ± 0.02 (6) |
| SNP | 8.27 ± 0.10 (7) | 7.93 ± 0.05 (7) * |
Table 2.
Potency of agonists in vascular reactivity of thoracic aortas with endothelium intact, in the presence of 3 × 10-4 mol/L Nω-nitro-l-arginine (LNNA) from wildtype (Gla +/0) or Gla knockout (Gla -/0) mice. Data are reported as mean ± SEM for the number of animals in parentheses. The EC50 values are expressed as the −log EC50 for each agonist. * = p<0.05 compared to Gla +/0 by the Student t test.
| Agonist | -log EC50 [M] | |
|---|---|---|
| Gla +/0 (+LNNA) | Gla -/0 (+LNNA) | |
| PE | 7.37 ± 0.04 (12) | 7.17 ± 0.05 (13) * |
| 5HT | 7.19 ± 0.02 (6) | 6.96 ± 0.04 (6) * |
| U46619 | 9.08 ± 0.03 (6) | 8.95 ± 0.03 (6) * |
| Ach | 6.57 ± 0.20 (7) | 6.76 ± 0.31 (7) |
| Ionomycin | 8.01 ± 0.31 (5) | 7.77 ± 0.21 (6) |
| SNP | 9.07 ± 0.13 (5) | 9.11 ± 0.02 (5) * |
Table 3.
Potency of agonists in vascular reactivity of endothelium-denuded thoracic aortas from wildtype (Gla +/0) or Gla knockout (Gla -/0) mice. Data are reported as mean ± SEM for the number of animals in parentheses. The EC50 values are expressed as the −log EC50 for each agonist. * = p<0.05 compared to Gla +/0 by the Student t test.
| Agonist | -log EC50 [M] | |
|---|---|---|
| Gla +/0 (-ENDO) | Gla -/0 (-ENDO) | |
| PE | 7.49 ± 0.10 (5) | 7.42 ± 0.10 (5) |
| 5HT | 7.38 ± 0.04 (6) | 7.15 ± 0.05 (5) * |
| U46619 | 9.16 ± 0.04 (6) | 9.13 ± 0.02 (5) |
| Ach | 8.85 ± 0.95 (5) | 7.57 ± 0.86 (5) |
| Ionomycin | N.D. | N.D. |
| SNP | 9.07 ± 0.02 (5) | 9.11 ± 0.02 (5) * |
Serotonin (5HT)
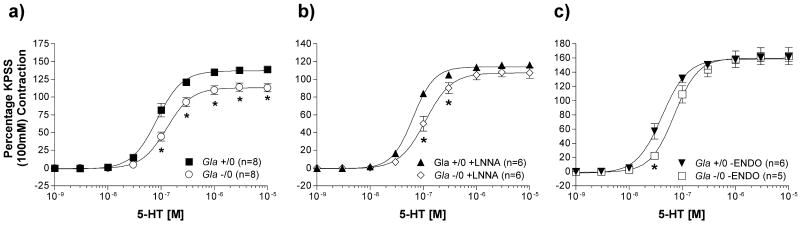
The vascular contraction mediated by 100 mmol/L KPSS for 5HT and U46619 reactivity did not differ between endothelium-intact rings from Gla +/0 and Gla -/0 mice (1225 ± 109 mg; n=8 vs. 1241 ± 101 mg; n=8, respectively; p > 0.05). KPSS contractions, in the presence of LNNA or endothelium denudation (-ENDO), also did not differ between Gla +/0 and Gla -/0 (LNNA: Gla +/0 = 1414 ± 60 mg; n=6 vs. Gla -/0 = 1455 ± 73 mg; n=6, p > 0.05; -ENDO: Gla +/0 = 1064 ± 76 mg; n=6 vs. Gla -/0 = 1043 ± 82 mg; n=5, p > 0.05).
5HT contractility occurred in a concentration-dependent manner, as illustrated in figure 4. Similar to PE, 5HT contractility in endothelium-intact aortic rings (figure 4a) from Gla -/0 mice were significantly less sensitive to the contractility in aortas from Gla +/0 (Table 1). Maximal contraction (Emax) to 5HT in aortas from Gla -/0 mice also was significantly less (Emax = 119.4 ± 5.7 %) compared to that in aortas from Gla +/0 mice (Emax = 138.3 ± 2.9 %; p < 0.05). In the presence of LNNA (figure 4b), the difference in Emax was no longer significant (Gla +/0 + LNNA Emax = 116.3 ± 1.7 % vs. Gla -/0 + LNNA Emax = 108.1 ± 5.3 %; p > 0.05), but the difference in sensitivity was maintained (Table 2). Similarly, when endothelium was removed (figure 4c), 5HT Emax was no longer significant (Gla +/0 -ENDO Emax = 161.1 ± 5.9 % vs. Gla -/0 -ENDO Emax = 162.8 ± 12.2 %; p > 0.05), but unlike PE, the 5HT log EC50 in Gla -/0 was still less sensitive than the 5HT log EC50 in Gla +/0 (Table 3).
Figure 4.
Serotonin (5HT)-mediated vascular contraction in endothelium-intact mouse aortic rings from wildtype (Gla +/0) or Gla knockout (Gla -/0) mice a) alone without any pharmacological intervention, b) in the presence of 10-4 mol/L Nω-nitro-l-arginine (LNNA), or c) with endothelium denuded. Data are expressed as a percentage of the contraction elicited by a 100 mmol/L KCl-containing physiological salt solution. * = p<0.05 compared to Gla +/0 by two-way ANOVA followed by Bonferonni post hoc test.
The TP receptor agonist, U46619
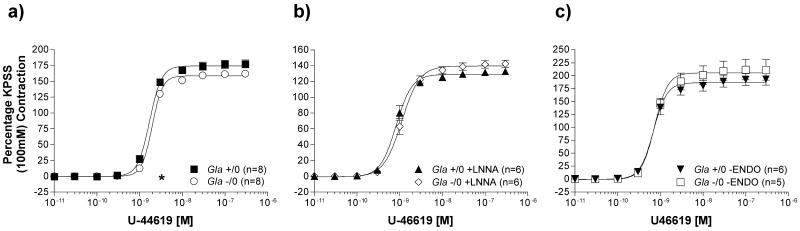
The 3rd vasopressor, U46619, also caused concentration-dependent contractions in the aortic rings from both Gla +/0 and Gla -/0 mice (figure 5). U46619 U46619 Emax contractility, however, did not differ between Gla +/0 and Gla -/0, regardless of whether endothelium was present (figure 5a: Gla +/0 Emax = 177.9 ± 5.3 % vs. Gla -/0 Emax = 169.4 ± 7.0 %; p > 0.05), LNNA was present (figure 5b: Gla +/0 + LNNA Emax = 132.7 ± 4.0 % vs. Gla -/0 + LNNA Emax = 142.4 ± 4.7 %; p > 0.05) or endothelium was absent (figure 5c: Gla +/0 -ENDO Emax = 193.0 ± 11.1 % vs. Gla -/0 + LNNA Emax = 211.5 ± 19.9 %; p > 0.05). The EC50 for Gla -/0 with endothelium was minimally different, yet still statistically significant, compared to Gla +/0 with endothelium (Table 1). Likewise was observed when U46619 contractility was performed in the presence of LNNA (Table 2). Removing the endothelium, however, resulted in similar EC50 values for both Gla +/0 and Gla -/0 (Table 3).
Figure 5.
The thromboxane A2/prostaglandin H2 (TP) receptor agonist, U46619, mediated vascular contraction in endothelium-intact mouse aortic rings from wildtype (Gla +/0) or Gla knockout (Gla -/0) mice a) alone without any pharmacological intervention, b) in the presence of 10-4 mol/L Nω-nitro-l-arginine (LNNA), or c) with endothelium denuded. Data are expressed as a percentage of the contraction elicited by a 100 mmol/L KCl-containing physiological salt solution. * = p<0.05 compared to Gla +/0 by two-way ANOVA followed by Bonferonni post hoc test.
Endothelium-dependent contraction with acetylcholine (Ach)
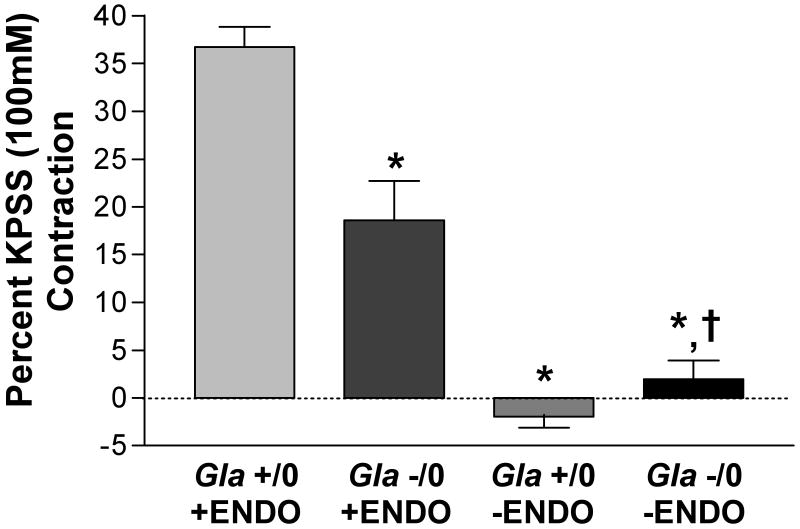
Endothelium-dependent contraction to Ach was examined in isolated carotid arteries from Gla +/0 and Gla -/0 mice (figure 6). The carotid arteries were used because they display a much more robust endothelium-dependent contraction to Ach compared to the aorta (19) KPSS contractions in endothelium-intact carotid rings from Gla +/0 and Gla -/0 mice were equivalent (Gla +/0 = 514 ± 56 mg, n=5; Gla -/0 = 490 ± 46 mg, n=5). In carotid arteries at baseline resting conditions, Ach (10-5 mol/L), in the presence of 3 × 10-4 mol/L LNNA, caused a contraction that was absent in the endothelium-denuded arteries. However, the endothelium-dependent contraction elicited by Ach was significantly less in the carotids from Gla -/0 mice (∼ 49% less) compared to Gla +/0 mice.
Figure 6.
Endothelium-dependent contraction mediated by 10-5 mol/L acetylcholine (Ach) in endothelium-intact and endothelium-denuded (-ENDO) mouse carotid artery rings from wildtype (Gla +/0) or Gla knockout (Gla -/0) mice in the presence of 3 × 10-4 mol/L Nω-nitro-l-arginine (LNNA). * = p<0.05 compared to Gla +/0 and † = p<0.05 compared to Gla +/0 –ENDO by one-way ANOVA; n=5 in each group.
Receptor-mediated endothelium-dependent relaxation with acetylcholine (Ach)
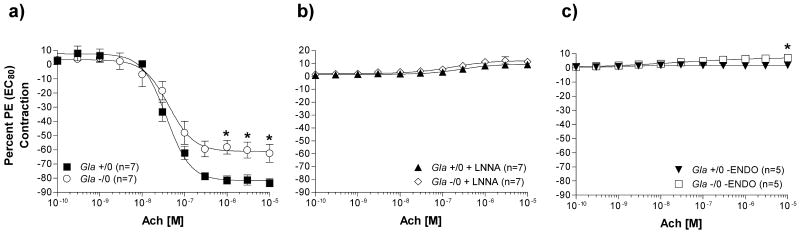
Endothelium-dependent relaxation to Ach also was examined in isolated aortas from Gla +/0 and Gla -/0 mice pre-contracted with a PE EC80 calculated for each ring after the PE concentration response. Aortic rings from Gla -/0 mice relaxed significantly less (Emax = 62.5 ± 6.3 %) compared to rings from Gla +/0 mice (Emax = 83.3 ± 2.9 %) (figure 7a). Ach responses are eNOS and endothelium dependent, since both LNNA (figure 7b) and endothelium denudation (figure 7c) prevented any Ach-mediated relaxation in the pre-contracted aortic rings from Gla +/0 and Gla -/0 mice.
Figure 7.
Acetylcholine (Ach)-mediated endothelium-dependent relaxation in endothelium-intact mouse aortic rings from wildtype (Gla +/0) or Gla knockout (Gla -/0) mice pre-contracted with an EC80 concentration of PE a) alone without any pharmacological intervention, b) in the presence of 10-4 mol/L Nω-nitro-l-arginine (LNNA), or c) with endothelium denuded. Data are expressed as a percentage of the contraction elicited by PE EC80. * = p<0.05 compared to Gla +/0 by two-way ANOVA followed by Bonferonni post hoc test.
Non receptor-mediated endothelium-dependent relaxation with ionomycin
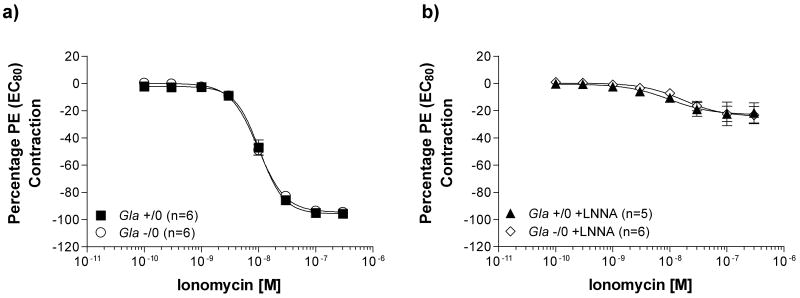
The calcium ionophore, ionomycin, was used to induce non-receptor mediated eNOS-dependent relaxations in endothelium-intact vessels pre-contracted with an EC80 of PE, as illustrated in figure 8. Ionomycin-induced relaxation (figure 8a) did not differ between vessels from Gla +/0 (log EC50 = -7.98 ± 0.03 mol/L and Emax = 95.5 ± 2.1 %) and Gla -/0 mice (log EC50 = -8.01 ± 0.02 mol/L and Emax = 94.5 ± 1.7 %). Ionomycin caused a slight relaxation in the pre-contracted endothelium-intact aortic rings from Gla +/0 and Gla -/0 mice incubated with LNNA (figure 8b), but no differences existed between the 2 groups in sensitivity or maximal response in the presence of LNNA.
Figure 8.
Ionomycin-induced eNOS-dependent relaxation in endothelium-intact mouse aortic rings from wildtype (Gla +/0) or Gla knockout (Gla -/0) mice pre-contracted with an EC80 concentration of PE a) alone without any pharmacological intervention or b) in the presence of 10-4 mol/L Nω-nitro-l-arginine (LNNA). Data are expressed as a percentage of the contraction elicited by PE EC80. p>0.05 by two-way ANOVA in all figures.
Endothelium-independent relaxation with sodium nitroprusside (SNP)
SNP mediated a concentration-dependent, endothelium-independent relaxation in isolated thoracic aortas from Gla +/0 and Gla -/0 mice. In the presence of endothelium, the SNP-induced relaxation was less sensitive in the vessels from Gla -/0 mice compared to vessels from Gla +/0 mice (Table 1). Vessels exposed to the NOS inhibitor, LNNA, were significantly more sensitive to SNP compared to their respective vessels with intact endothelium without LNNA (Table 2). Similarly, aortas denuded of endothelium (-ENDO) also displayed increased sensitivity to SNP, compared to their untreated, endothelium-intact counterparts (Table 3) and were similar in sensitivity to LNNA-treated vessels.
Discussion
Fabry disease has a complex cardiovascular phenotype. Premature mortality is more often the result of stroke and myocardial infarctions (20). Informative clinical studies in Fabry disease patients have documented both macrovascular and microvascular dysfunction, suggesting that the pathophysiology may be highly complex (21, 22). The α-galactosidase A knockout mouse (Gla -/0) provides a potentially useful tool to study these cardiovascular phenomenon. Indeed, our group has reported that these mice are more susceptible to oxidant induced thrombosis and accelerated atherogenesis (7, 11). In this study we used this model to ascertain the role of the endothelium in large vessel reactivity.
We report several novel observations in this Gla -/0 murine model of Fabry disease. First, abnormal vasopressor contractility to phenylephrine (PE) and serotonin (5HT) in the Gla -/0 aortas are less sensitive than that in the wildtype (Gla +/0) aortas but are normal to a thromboxane A2/prostaglandin H2 (TP) receptor agonist or when the endothelium is removed. Second, endothelium-dependent contraction is significantly less in the Gla -/0 carotid arteries compared to Gla +/0 carotid arteries. Third, impaired endothelium-dependent relaxation is not observed when a calcium ionophore is used to mediate endothelium-dependent relaxation. These observations are important because the defects observed in this murine model of Fabry disease demonstrate a complexity of the reactivity that can be attributed entirely to the endothelium, even though elevated Gb3 levels occur in the other vascular cell-types (7, 13).
In many vascular diseases such as hypertension and diabetes, vascular smooth muscle (VSM) vasopressor sensitivity is increased, while endothelium-dependent relaxation is diminished (23-26). Our data were surprising because abhorrent endothelium-dependent relaxation was observed but a concomitant increased sensitivity to vasopressor activity was absent, as illustrated by decreased sensitivity to PE or 5HT, in the presence of endothelium, while TP receptor –mediated VSM contraction with U46619 was similar in Gla -/0 mice compared to Gla +/0 mice.
How this anomaly developed in our mouse model of Fabry disease is unclear, but we speculate on several mechanisms that may partially explain our observations. If basal nitric oxide (NO) production is higher in the Gla -/0 mice, then VSM contraction would be inhibited. Additionally, if agonist-stimulated NO production is less in the Gla -/0 mice, for whatever reason, endothelium-dependent relaxation also would be inhibited or diminished. However, persistence in the decreased sensitivity to vasopressor in the presence of LNNA does not support that hypothesis, suggesting that the endothelium may be producing another factor to cause endothelium-dependent relaxation after stimulation with acetylcholine (Ach). Prostaglandin I2, which mediates endothelium-dependent relaxation by activation of cAMP in vascular smooth muscle (27), is a potential mechanism by which our anomalous reactivity may be occurring. However, whether cyclooxygenase-derived products have any role in the vascular dysfunction or are in some way regulated by glycosphingolipids is yet to be determined.
Alternatively, on a more cellular level, in cells subjected to pathologically increased levels of Gb3, excess Gb3 content may be present in compartments outside of the lysosome, including lipid rafts or caveolae. Recently, we reported that Gb3 and other globo series GSLs are concentrated higher in caveolae from Gla -/0 endothelial cells compared to Gla +/0 endothelial cells (28). These changes in GSLs increase as a function of age and are accompanied by corresponding decreases in cholesterol. Additionally, GSL concentrations in caveolae change dynamically after endothelial cells are exposed to recombinant α-galactosidase A or the glucosylceramide synthase inhibitor D-threo-ethylenedioxyphenyl-2-palmitoylamino-3-pyrillidino-propanol. Modulation of cellular GSL content regulates bradykinin-induced src kinase and phospholipase Cγ activation (29, 30). Conversely, increased Gb3 accumulation in the endothelial cells may inhibit receptor-induced signaling responsible for activation of endothelial nitric oxide synthase (eNOS). Our endothelium-dependent relaxation with the calcium ionophore, ionomycin, support this potential mechanism, since the ionophore relaxation is normal in the aortas from the Gla -/0 mice compared to the Gla +/0 mice, while the receptor-induced endothelium-dependent relaxation to Ach is attenuated in the aortas from the Gla -/0 mice.
Since these caveolar domains are enriched with and regulate eNOS, accumulated caveolar GSL associated with Fabry disease may conceivably alter eNOS activation by one of 2 mechanisms. First, eNOS and caveolin interactions are affected by excess Gb3 or a related sphingolipid or, alternatively, by secondary changes in other raft associated lipids. Second, receptor coupling to downstream mediators may be affected, since sphingolipid, as well as cholesterol, content in lipid rafts or caveolae can modulate the fluidity of these signaling “hotspots” (31). Our data appear to support the latter mechanism since eNOS activation independent of a receptor, is normal, suggesting that any interactions between eNOS and caveolin-1 are not affected by increased caveolar Gb3 content. Further studies are needed, however, to demonstrate that receptor coupling is affected by excess endothelial Gb3 in this mouse model of Fabry disease. Our endothelium-dependent contraction data and our endothelium-dependent relaxation data combined, however, are consistent with the hypothesis that receptor coupling is affected in this mouse model of Fabry disease, since both are attenuated in the Gla -/0 mice, especially since endothelium-dependent contractions, in the face of endothelial dysfunction, should be augmented.
Our data indicate that, whatever the mechanism might be, our changes in vascular function in the Gla -/0 mice are localized to the endothelium. This conclusion is supported by 2 main observations. First, most of the vascular contractility to vasopressor is close to normal after endothelium denudation. Second, endothelium-dependent contraction is significantly attenuated in the Gla -/0 mice, a phenomenon which is due to the paracrine release of a TP receptor agonist from the endothelium (32). Direct stimulation of VSM contraction with the TP receptor agonist, U46619, however, did not reveal any differences between Gla -/0 and Gla +/0.
In conclusion, we demonstrate that the vasculopathy associated with Gla -/0 mice occurs at a younger age than previously reported (13), and that the early vasculopathy are localized to the endothelium. Importantly, the vaculopathy reported here may be a result of impaired receptor signaling rather than impaired eNOS activity. These findings provide insight into how early vaculopathy may develop in Fabry disease, but they also suggest that glycosphingolipid metabolism may play a subtle, yet significant role in the regulation of receptor-mediated signaling.
Acknowledgments
This work was supported by NIH grant 5RO1DK055823-06. Some of this work was reported at the International Society of Nephrology Forefronts in Nephrology Conference on Endothelial Biology, in March, 2006.
Footnotes
Disclosures: None
References
- 1.Kint JA. The enzyme defect in Fabry's disease. Nature. 1970;227:1173. doi: 10.1038/2271173b0. [DOI] [PubMed] [Google Scholar]
- 2.Kint JA. Fabry's disease: alpha-galactosidase deficiency. Science. 1970;167:1268–9. doi: 10.1126/science.167.3922.1268. [DOI] [PubMed] [Google Scholar]
- 3.Sweeley CC, Klionsky B. Fabry's Disease: Classification as a Sphingolipidosis and Partial Characterization of a Novel Glycolipid. J Biol Chem. 1963;238:3148–50. [PubMed] [Google Scholar]
- 4.Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L. Enzymatic defect in Fabry's disease. Ceramidetrihexosidase deficiency. N Engl J Med. 1967;276:1163–7. doi: 10.1056/NEJM196705252762101. [DOI] [PubMed] [Google Scholar]
- 5.Desnick RJ, Ioannou YA, Eng CM. a-Galactosidase A Deficiency: Fabry Disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th. McGraw-Hill; New York: 2001. [Google Scholar]
- 6.Desnick RJ, Banikazemi M, Wasserstein M. Enzyme replacement therapy for Fabry disease, an inherited nephropathy. Clin Nephrol. 2002;57:1–8. doi: 10.5414/cnp57001. [DOI] [PubMed] [Google Scholar]
- 7.Eitzman DT, Bodary PF, Shen Y, et al. Fabry disease in mice is associated with age-dependent susceptibility to vascular thrombosis. J Am Soc Nephrol. 2003;14:298–302. doi: 10.1097/01.asn.0000043901.45141.d4. [DOI] [PubMed] [Google Scholar]
- 8.Shu L, Murphy HS, Cooling L, Shayman JA. An in vitro model of Fabry disease. J Am Soc Nephrol. 2005;16:2636–45. doi: 10.1681/ASN.2005040383. [DOI] [PubMed] [Google Scholar]
- 9.Ohshima T, Murray GJ, Swaim WD, et al. alpha-Galactosidase A deficient mice: a model of Fabry disease. Proc Natl Acad Sci U S A. 1997;94:2540–4. doi: 10.1073/pnas.94.6.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen Y, Bodary PF, Vargas FB, et al. Alpha-galactosidase A deficiency leads to increased tissue fibrin deposition and thrombosis in mice homozygous for the factor V Leiden mutation. Stroke. 2006;37:1106–8. doi: 10.1161/01.STR.0000206442.86238.39. [DOI] [PubMed] [Google Scholar]
- 11.Bodary PF, Shen Y, Vargas FB, et al. Alpha-galactosidase A deficiency accelerates atherosclerosis in mice with apolipoprotein E deficiency. Circulation. 2005;111:629–32. doi: 10.1161/01.CIR.0000154550.15963.80. [DOI] [PubMed] [Google Scholar]
- 12.Moore DF, Gelderman MP, Fuhrmann SR, Schiffmann R, Brady RO, Goldin E. Fabry disease and vascular risk factors: future strategies for patient-based studies and the knockout murine model. Acta Paediatr Suppl. 2006;95:69–71. doi: 10.1111/j.1651-2227.2006.tb02393.x. [DOI] [PubMed] [Google Scholar]
- 13.Heare T, Alp NJ, Priestman DA, et al. Severe endothelial dysfunction in the aorta of a mouse model of Fabry disease; partial prevention by N-butyldeoxynojirimycin treatment. J Inherit Metab Dis. 2007;30:79–87. doi: 10.1007/s10545-006-0473-y. [DOI] [PubMed] [Google Scholar]
- 14.Whitesall SE, Hoff JB, Vollmer AP, D'Alecy LG. Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. Am J Physiol Heart Circ Physiol. 2004;286:H2408–15. doi: 10.1152/ajpheart.01089.2003. [DOI] [PubMed] [Google Scholar]
- 15.Tang EH, Ku DD, Tipoe GL, Feletou M, Man RY, Vanhoutte PM. Endothelium-dependent contractions occur in the aorta of wild-type and COX2-/- knockout but not COX1-/- knockout mice. J Cardiovasc Pharmacol. 2005;46:761–5. doi: 10.1097/01.fjc.0000187174.67661.67. [DOI] [PubMed] [Google Scholar]
- 16.Faraci FM, Sigmund CD, Shesely EG, Maeda N, Heistad DD. Responses of carotid artery in mice deficient in expression of the gene for endothelial NO synthase. Am J Physiol. 1998;274:H564–70. doi: 10.1152/ajpheart.1998.274.2.H564. [DOI] [PubMed] [Google Scholar]
- 17.Park JL, Loberg RD, Duquaine D, et al. GLUT4 facilitative glucose transporter specifically and differentially contributes to agonist-induced vascular reactivity in mouse aorta. Arterioscler Thromb Vasc Biol. 2005;25:1596–602. doi: 10.1161/01.ATV.0000170137.41079.ab. [DOI] [PubMed] [Google Scholar]
- 18.Tang EH, Feletou M, Huang Y, Man RY, Vanhoutte PM. Acetylcholine and sodium nitroprusside cause long-term inhibition of EDCF-mediated contractions. Am J Physiol Heart Circ Physiol. 2005;289:H2434–40. doi: 10.1152/ajpheart.00568.2005. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Varadharaj S, Zhao X, Parinandi N, Flavahan NA, Zweier JL. Acetylcholine causes endothelium-dependent contraction of mouse arteries. Am J Physiol Heart Circ Physiol. 2005;289:H1027–32. doi: 10.1152/ajpheart.00226.2005. [DOI] [PubMed] [Google Scholar]
- 20.Shayman JA, Killen PD. Fabry Disease. In: Mount DB, Pollak MJ, editors. Molecular and Genetic Basis of Renal Disease. Ch. 13 Saunders/Elsevier; Philadelphia: 2008. [Google Scholar]
- 21.Elliott PM, Kindler H, Shah JS, et al. Coronary microvascular dysfunction in male patients with Anderson-Fabry disease and the effect of treatment with alpha galactosidase A. Heart. 2006;92:357–60. doi: 10.1136/hrt.2004.054015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore DF, Kaneski CR, Askari H, Schiffmann R. The cerebral vasculopathy of Fabry disease. J Neurol Sci. 2007;257:258–63. doi: 10.1016/j.jns.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 23.Guo Z, Su W, Allen S, et al. COX-2 up-regulation and vascular smooth muscle contractile hyperreactivity in spontaneous diabetic db/db mice. Cardiovasc Res. 2005;67:723–35. doi: 10.1016/j.cardiores.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Linder AE, Weber DS, Whitesall SE, D'Alecy LG, Webb RC. Altered vascular reactivity in mice made hypertensive by nitric oxide synthase inhibition. J Cardiovasc Pharmacol. 2005;46:438–44. doi: 10.1097/01.fjc.0000175879.14994.63. [DOI] [PubMed] [Google Scholar]
- 25.Pannirselvam M, Verma S, Anderson TJ, Triggle CR. Cellular basis of endothelial dysfunction in small mesenteric arteries from spontaneously diabetic (db/db -/-) mice: role of decreased tetrahydrobiopterin bioavailability. Br J Pharmacol. 2002;136:255–63. doi: 10.1038/sj.bjp.0704683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pannirselvam M, Wiehler WB, Anderson T, Triggle CR. Enhanced vascular reactivity of small mesenteric arteries from diabetic mice is associated with enhanced oxidative stress and cyclooxygenase products. Br J Pharmacol. 2005;144:953–60. doi: 10.1038/sj.bjp.0706121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shepherd JT, Katusic ZS. Endothelium-derived vasoactive factors: I. Endothelium-dependent relaxation. Hypertension. 1991;18:III76–85. doi: 10.1161/01.hyp.18.5_suppl.iii76. [DOI] [PubMed] [Google Scholar]
- 28.Shu L, Shayman JA. Caveolin-associated accumulation of globotriaosylceramide in the vascular endothelium of alpha-galactosidase A null mice. J Biol Chem. 2007;282:20960–7. doi: 10.1074/jbc.M702436200. [DOI] [PubMed] [Google Scholar]
- 29.Shu L, Lee L, Shayman JA. Regulation of phospholipase C-gamma activity by glycosphingolipids. J Biol Chem. 2002;277:18447–53. doi: 10.1074/jbc.M111363200. [DOI] [PubMed] [Google Scholar]
- 30.Shu L, Shayman JA. Src kinase mediates the regulation of phospholipase C-gamma activity by glycosphingolipids. J Biol Chem. 2003;278:31419–25. doi: 10.1074/jbc.M303783200. [DOI] [PubMed] [Google Scholar]
- 31.Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–4. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 32.Vanhoutte PM, Feletou M, Taddei S. Endothelium-dependent contractions in hypertension. Br J Pharmacol. 2005;144:449–58. doi: 10.1038/sj.bjp.0706042. [DOI] [PMC free article] [PubMed] [Google Scholar]