Abstract
The hinge region of a mechanical bileaflet valve is implicated in blood damage and initiation of thrombus formation. Detailed fluid dynamic analysis in the complex geometry of the hinge region during the closing phase of the bileaflet valve is the focus of this study to understand the effect of fluid-induced stresses on the activation of platelets. A fixed-grid Cartesian mesh flow solver is used to simulate the blood flow through a two-dimensional geometry of the hinge region of a bi-leaflet mechanical valve. Use of local mesh refinement algorithm provides mesh adaptation based on the gradients of flow in the constricted geometry of the hinge. Leaflet motion is specified from the fluid-structure interaction analysis of the leaflet dynamics during the closing phase from a previous study which focused on the fluid mechanics at the gap between the leaflet edges and the valve housing. A Lagrangian particle tracking method is used to model and track the platelets and to compute the magnitude of the shear stress on the platelets as they pass through the hinge region. Results show that there is a boundary layer separation in the gaps between the leaflet ear and the constricted hinge geometry. Separated shear layers roll up into vortical structures that lead to high residence times combined with exposure to high shear stresses for particles in the hinge region. Particles are preferentially entrained into this re-circulation zone, presenting the possibility of platelet activation, aggregation, and initiation of thrombi.
Keywords: Bi-leaflet mechanical heart valve, fluid mechanics in the hinge geometry, Local mesh refinement, Platelet activation
Introduction
More than 250,000 prosthetic heart valves are being implanted in patients with valve failure every year [1, 2]. Although research has been carried out towards improvement in design, problems associated with prosthetic heart valves have not been completely eliminated. Thrombus formation and thromboembolism in the vicinity of the mechanical valve (MHV) has been a major problem with the operation of the mechanical heart valves and patients implanted with MHV need to be on long-term anticoagulant therapy. The incidence of major embolism in the absence of antithrombotic therapy is 4 per 100 patient years with the risk being twice in mitral position compared to that in the aortic position [3]. The reason may be that mitral position is the harshest environment where the valve resists a flow with the maximum pressure that the left ventricle can generate. The bi-leaflet mechanical valve, when compared to other mechanical heart valves exhibits superior bulk flow hemodynamics especially in smaller sizes, a larger orifice area and fewer regions of flow stasis. But the problems associated with the implantation of mechanical heart valves such as hemolysis, platelet activation and thromboembolic events are a major concern for the implantation of bi-leaflet heart valves as well.
The flow through the mechanical heart valve, especially during closure, presents large pressure transients across the leaflets. The resulting high pressure gradient across the leaflet and the local flow dynamics may initiate platelet activation leading to aggregation and initiation of thrombi. Direct mechanical trauma by impact with the valve housing and other support structures such as the hinges and local flow-induced stresses could also contribute to the deleterious effects on the blood components [1].
Exposure to fluid shear stress is seen to activate blood platelets without any exogenous agonist [4]. Blood cells are damaged when they are exposed to high shear stress and this may lead to platelet activation. It was observed that platelets get activated when the shear stress exceeds 10 Pa [5] and the activation can be further enhanced by the presence of foreign surfaces such as the mechanical valve structures. It has also been shown that platelets tends to get activated when exposed to a high shear stress for a small duration of time and a low shear stress for a longer duration of time [6, 7]. Hence the time of exposure to shear stress must also be considered to be major factor for the activation of platelets. Though the shear stress may not be the only factor inducing the activation, it would decrease the threshold of activation to a greater extent. Other experiments have also shown the relationship between the shear stress and its exposure time [6–9]. Bluestein et al. [6], based on experiments on platelet deposition in stenosis models, formulated a platelet activation parameter as the integral of shear stress and time. They suggested that shear and elongational stresses in the throat region of a model of arterial stenosis increase the activation potential of platelets. In addition, they also pointed out that flow separation and re-circulation downstream from the throat of the stenosis is conducive to the activation of platelets. Thus any disturbances in the normal blood flow (for example flow separation, recirculation, turbulence) coupled with an earlier exposure to high shear stress could be a major factor for platelet activation.
In the bi-leaflet valves thrombus formation is observed predominantly in the hinge region and on the valve housing [10, 11]. It is hypothesized that the local flow conditions contribute to the thrombus formation. All the studies on bi-leaflet valves have reported flow separations and vortex shedding at the valve leaflet edge [12, 13]. The high velocity leakage flow generates regions of high shear stress where platelets could get activated. The activated platelets have high residence time in the valve vicinity when they get caught in the regions of stagnant flow of recirculation. In this region having low velocity magnitudes, there is a greater chance for the activated platelets sticking to each other for longer periods of time thus forming aggregates [14]. Aggregated platelets could get deposited in the hinge region of a mechanical valve initiating the formation of thrombi that are detrimental to the proper functioning of the valve.
The combination of high-shear stress and large residence times that are observed in the leakage flows between valve leaflets and the housing in the closure phase of the valve [9] can result in platelet activation. It has been shown experimentally that the sequence of high-shear stress followed by recirculation may also exist in the flow through the valve hinge. Examination of flow through the hinge is rather difficult, both from an experimental as well as computational standpoint since the hinge is embedded within the valve housing and the clearances in the hinge region are of very small dimensions (order of hundred microns). The geometry is complex and details of flow are difficult to measure, visualize and compute. Furthermore, as the valve rotates the geometry of the hinge continually changes. These aspects render investigation of the fluid mechanics and platelet transport through the hinge a rather challenging task.
Despite these challenges, various studies have been conducted in understanding the flow through the hinge region. Gross et al. [15] conducted a detailed study to understand the flow structures in the hinge region in order to investigate the fluid mechanical factors involved in the presence of thrombus in the hinge region of the Medtronic Parallel Valve. Velocity and Reynolds stress measurements in the hinge region were complemented by the computational analysis for the hinge geometry of a 27 mm Parallel Valve. They reported velocity magnitudes of 2.55 m/s during the reversed flow phase in the hinge pocket and the flow was marked by regions of vortical flow and stagnation. They reported the presence of “Reynolds shear stresses” in the leakage jet of about 700 dynes/cm2. The study also suggested that the highest levels of unsteadiness in the hinge regions are generated in the backflow jet. Simon et al. [16] compared the hinge flow fields of St. Jude and Carbomedics bi-leaflet mechanical heart valves under aortic and mitral conditions experimentally in a pulsatile flow loop employing a three-component laser velocimetry. Velocity measurements exhibited common features such as forward flow patterns and leakage jets for the two valves even though the magnitudes of velocities and shear stress were different. The difference in the magnitudes of the velocity and turbulent stresses were attributed to the local differences in the hinge geometry between the two valves. Vortical structures were observed throughout the forward and the reverse flow in the hinge region when the Medtronic parallel valve’s hinge region was computationally and experimentally simulated and compared with the hinge areas of the St. Jude Medical and On-X valves by Gao et al. [17]. It was predicted that these vortices are responsible for the high thrombogenic potential of Medtronic parallel valve, where as the SJM valve and the On-X valve did not present any stagnation or vortices indicating that these two valves ran a risk free environment for mechanically induced thrombus. All these studies uniformly predict high values of shear stress coupled with reverse flow and stagnation in the hinge region which could play an important role in thrombus development and these results suggest that the geometry of the hinge region may be a critical valve design parameter. However, due to the limitations posed by the complex hinge geometry details on flow features in the complex geometry of the hinge gap and the transport of platelets through this complex geometry and flow field are not revealed by the above experimental studies.
A further point to note concerns the relevance of the quoted Reynolds shear stresses in the flow in the vicinity of the hinge gap to platelet activation is not clear for two reasons: 1) It is not readily apparent that flow through the narrow hinge gap is turbulent, since the local Reynolds numbers do not exceed order of 100, the length scale of the gap is rather small and the geometry of the hinge gap is rather complex, so that concepts from turbulence in isotropic, homogeneous systems may not readily apply, and 2) Reynolds shear stresses are the time-averaged behavior of the flow field and may be adequate to describe characteristics of turbulent flow at large length and time scales, but inadequate to represent the stresses experienced on the surface of individual blood cells as commented by Quinlan [18]. Quinlan and Dooley [19] developed a simplified mathematical model for flow of suspended blood cells in plasma and analyzed both laminar and turbulent regimes. For the turbulent regime, root mean square stresses were estimated on the cells and it was observed that these values fall under an order of magnitude less that Reynolds shear stress values. They also suggested that there is very little relationship between Reynolds shear stress and blood cell damage. This was ascertained by studies conducted by Ge et al. [20] on the effect of Reynolds and viscous stresses on blood elements and Dasi et al. [21] on the mechanical forces experienced by the blood cells as the pass downstream of a 23 mm St Jude medical regent valve. 3-D numerical study excluding the hinge region and experiments using DPIV measurements were conducted and viscous shear stress and Reynolds shear stress were calculated to illuminate the difference between the both. It was observed that the Reynolds shear stress mainly occurred due to cycle to cycle variations and the resultant vortex shedding downstream. It was suggested the length scale of these vortices were several magnitudes higher than an individual blood cell and hence, the resulting RSS cannot be responsible for blood cell damage. Ellis et al. [22] reported on an in vitro study of the hinge and near field forward flow dynamics of the St. Jude Medical Regent bileaflet valve and the study observed two dominant recirculation regions near the hinge during the forward flow. The measurements also revealed a strong back flow jet with velocity magnitudes of 0.72 m/s and Reynolds shear stresses of about 2600 dynes/cm2. Though Reynolds shear stress were calculated, the above studies observed development of recirculation regions which could be important for activation of platelets as explained later, and not a major player for blood cell damage. Thus, there is at present, insufficient clarity into the mechanisms and flow structures that lead to damage to blood components for flow through the hinge.
This computational study will focus on the flow through the hinge region and its effect on the platelet activation due to high shear stress and subsequent region of deposition due to high residence time in these areas during the closure of valve. The focus will be on the flow dynamics through the small gap within the hinge region of a typical bi-leaflet heart valve which pivots the leaflets to rotate to valve closure under mitral flow condition. The geometry of the hinge region as well as the range of motion of the leaflet ears interacting with the valve housing were specified to correspond with nominal dimensions of a bileaflet valve that was employed in our previous studies on the analysis during the closing phase of the valve in the mitral position [9]. Both static and dynamic simulations are performed to compare the results solving the two-dimensional Navier–Stokes equations in non-dimensional form using an Eulerian Level set based sharp interface Cartesian grid method [23]. Platelets are modeled as point particles in a Lagrangian particle tracking algorithm. The time history of platelet exposure to shear stress is tracked to demarcate areas of high likelihood of platelet activation and deposition in the hinge region. The impact of modification of the leaflet ear geometry is also investigated to study the impact of such design changes on the flow and its influence on the activation of platelets.
Methods
The two-dimensional Navier-Stokes equations in the non-dimensional form are solved:
| (1) |
| (2) |
In the above equation Re = ρVD/μ is the Reynolds number where ρ, V, D, μ are fluid density, velocity, length scale and fluid viscosity respectively. The equations are solved by an Eulerian level-set based sharp interface Cartesian grid method that has been described in detail and validated with several test problems previously [23–26]. Complex flow calculations such as this can be very expensive if a uniform mesh is used in the entire domain, and this computational study uses a local mesh refining algorithm [27, 28] that has been designed to work with the above method to allow fast flow computation with desirable solution accuracy in the region of interest. The mesh is refined or coarsened depending on the gradient and the curvature of the velocity field automatically without any user intervention.
Platelets are modeled as point particles by the Lagrangian particle tracking algorithm [29] with one way coupling. This means that the particles are influenced by the flow, but the particles are assumed not to affect the flow field. Dilute particulate flow is assumed and particle-particle interactions are neglected. All particles are assumed to be spheres of the same diameter (2 μm) and carrier fluid is assumed to be incompressible and Newtonian. Field variables such as particle concentration, fluid velocities, particle velocities and activation parameter are computed for each particle. A particle momentum equation in the non-dimensional form is solved to calculate particle velocity as
| (3) |
In the above equation v⃗ is the non-dimensional particle velocity vector, f⃗ is the total non-dimensional force on the particle including the drag and lift forces [29].
Simulation Conditions
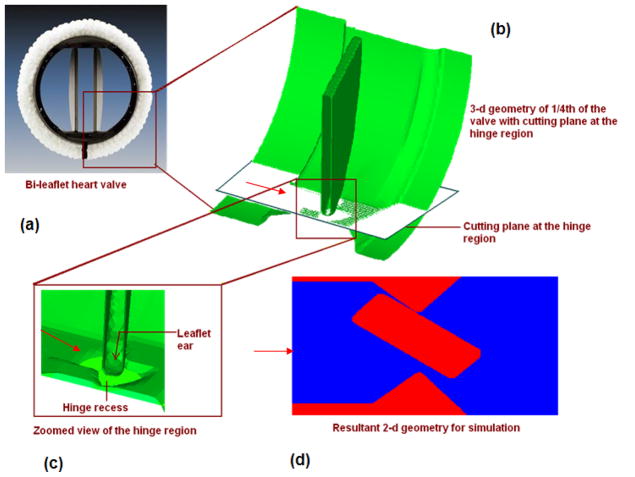
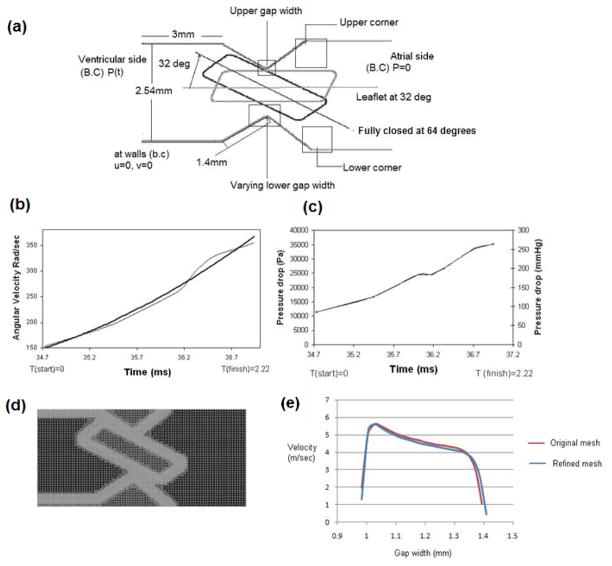
In order to analyze the fluid mechanics in the hinge region during the valve closure phase, a 2D model was employed in this initial analysis. The details of generating the 2D geometry for the analysis from a typical bileaflet valve are shown in Figure 1. Taking advantage of the symmetry planes of a typical bi-leaflet valve shown in Figure 1(a), a three-dimensional geometry of one-quarter section of the valve is shown in Figure 1(b). The two-dimensional geometry of the hinge region for the simulation was obtained by making a planar cut in the hinge region that includes the leaflet ear that interacts with the hinge recess as shown in Figure 1(c). The direction of flow across the hinge region during the valve closure phase is indicated in the schematic of the geometry (Figure 1(d)) and the leaflet ear is assumed to slide and pivot against the top edge of the hinge as it rotates towards the fully closed position. The detailed dimensions of the hinge region for the typical bi-leaflet valve geometry employed in this study are shown in Figure 2(a). The leaflets for this valve traverse about 64 degrees from the fully open to the fully closed position. However, our earlier analysis [9] of the fluid dynamics in the gap region between the leaflet edge and valve housing during the valve closure phase indicated that significant fluid motion occurs only during the latter half of valve closure. Hence in the dynamic analysis for flow in the hinge region in the present study, we restricted the analysis during the final 32 degrees of leaflet motion that occurs in approximately 2.22 ms towards the end of the closing phase. The position of the leaflet ear at 32 degrees and the fully closed positions are indicated in this figure. The motion of the leaflet ear will be the same as the motion of the leaflet during valve closing that was computed in our earlier studies and the rotational speed of the leaflet ear was specified based on the calculations performed in [9]. The pressure difference between the ventricular and atrial sides of the leaflet in the leaflet pivot region that was computed from the earlier study was also employed as the time-dependent pressure boundary condition for the analysis as shown in Figure 2(a). The data on the leaflet angular velocity as well as the pressure difference across the hinge region employed in this analysis during the last 2.22 ms of valve closure are shown in Figures 2(b) and (c) respectively. No-slip condition was assumed at the walls. The fluid was assumed to be incompressible with a density of 1050 kg/m3 and a viscosity coefficient of 0.0035 Kgm−1 s−1, representative of the human blood at 37 °C. We employed a fixed Cartesian grid in the flow solver and incorporated a local mesh refinement algorithm in order to obtain accurate solutions in regions with high velocity and vorticity gradients. The level of mesh refinement employed in this study to ensure accurate results were based on the results from our earlier study and a figure depicting the local mesh refinement in the geometry is shown in Figure 2(d). Grid refinement study was conducted by the reducing the base mesh size to half its original value and it was observed that the computed velocity magnitudes of both the studies had a percentage difference of less than 0.5%. Figure 2(e) shows the comparison of velocity plotted at the lower gap width, at time 1.34 ms. Based on these results, we employed the base mesh for the analysis described in this work.
Figure 1.
a) Photograph of a bi-leaflet valve; b) Three dimensional model of 1/4th of a bi-leaflet valve with a cutting plane from which the two dimensional model was extracted; c) Zoomed in view of the hinge region; and d) Resultant two dimensional model that was used in this study
Figure 2.
a) Schematic of the hinge geometry used in the analysis with dimensions and the leaflet ear position at 32 degrees and the fully closed position at 63.8 degrees. Also indicated are the applied boundary conditions; b) Angular velocity of the leaflet starting at 32 degrees moving towards closure; c) Time varying pressure drop at the vicinity of the hinge region specified as pressure boundary condition; d) Plot of the Cartesian grid with local mesh refinement on the hinge geometry. The mesh is adapted according to the gradients of flow for efficiency and accuracy; and e) Comparison of velocity profiles at an instant in the lower gap width with the base mesh and the finer mesh density.
Platelet Activation
Exposure to high shear and entrapment of platelets in the re-circulation regions with high residence time in the vicinity of the valve structures are expected to be conducive to platelet deposition and thrombus formation [30, 31]. A dynamic platelet activation model that includes the effects of shear stress and exposure time is employed [31]. The potential for activation of platelets is quantified in terms of an activation parameter computed as:
| (5) |
Since the platelets are transported in Lagrangian fashion the limit T on the integral represents the residence time of each particle in the flow domain. The shear stress acting on the particle is computed at the particle location from the underlying grid via bilinear interpolation. This parameter incorporates, for each particle, the history of shear stress and residence time encountered by the particle as it traverses the flow domain.
Results
Static analysis
Initially a static analysis was performed to study the vortex shedding from the trailing edge of the leaflet with the leaflet ear in the fully closed position and the pressure difference across the hinge at closure as the specified boundary condition. This static analysis provides a baseline to separate out the effects of the flow generated by the applied pressure differential across the hinge and the motion of the hinge itself. In particular, we are interested in the differences in flow structure and activation parameter obtained in the constricted hinge geometry with and without motion of the leaflet ear in the hinge.
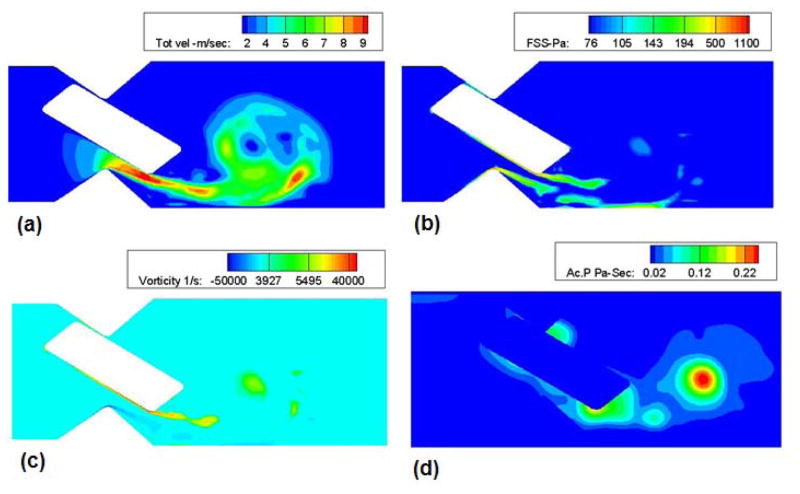
Plots of the velocity, shear stress, vorticity distribution and the activation parameter in the region of interest are shown in Figure 3. As time proceeds the anti-clockwise vortex from the trailing edge of the leaflet ear starts to roll up and sheds towards the distal side of the hinge region. Due to the fact that the leaflet ear rests against the top surface of the hinge gap, the flow is predominantly through the lower gap of the hinge region and the high values of velocity magnitude occur there. This leads to higher shear rates and hence enhanced shear layer instability and roll up at the lower surface of the leaflet ear The vortex that is formed in the hinge pocket advects at low velocity downstream of the leaflet ear. The axial velocity in the vortex core is less that 1 m/sec, much lower than the jet velocity of about 10 m/sec. This implies that the vortex remains in the hinge pocket for an extended period of time. The maximum (local) Reynolds number (computed based on the maximum velocity in the gap and gap thickness as velocity and length scales) was found to be approximately 600 at the lower gap width and the corresponding shear stress was about 900 Pa. Therefore, despite the rather high jet velocities the flow through the hinge remains laminar and the present two-dimensional laminar computations face no difficulties in computing flows through the specified geometry provided the local mesh refinement algorithm [9] is employed to resolve the flow through the narrow gaps. The computed activation parameter distribution is shown in Figure 3(d) and had a maximum value of 0.24 in the vortex core.
Figure 3.
a) Velocity; b) Shear stress; c) Vorticity contour plots in the hinge region based on the static analysis with the leaflet ear in the fully closed position; and d) Platelet activation parameter calculated during static analysis. Jet-like flow and high bulk shear stresses through the gap-width as well as the development of strong vortices that are advected away from the leaflet edge can be observed.
Dynamic Simulation
Flow through the hinge and Activation of platelets
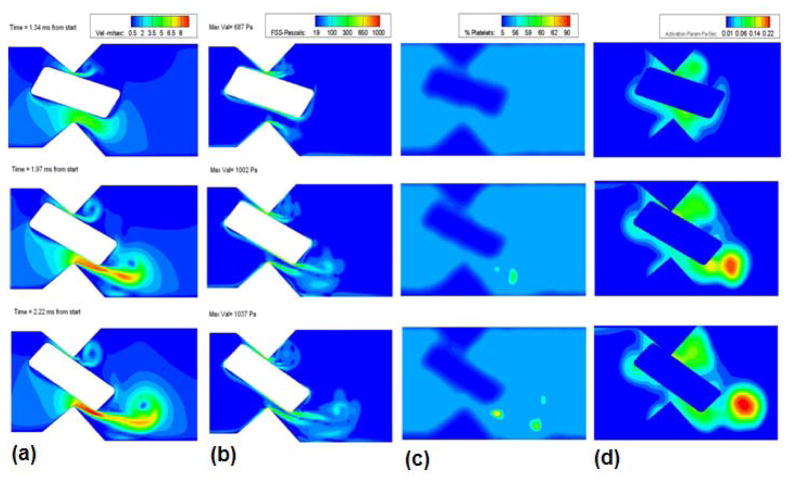
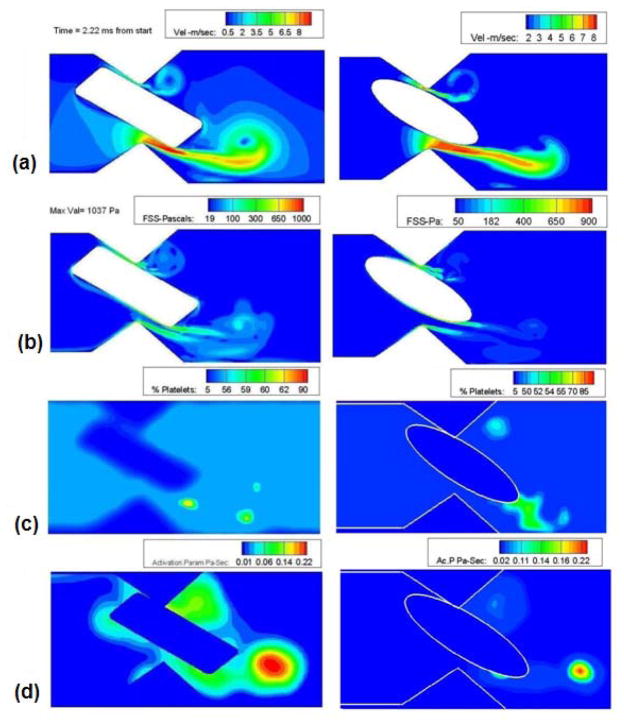
From the previous study [9] it was observed that the leaflet rotation proceeds in two phases, the first being a slow phase, during which the leaflet rotates from a nearly vertical position to a position that places it at an angle of 32° from the vertical. This displacement takes approximately 35 milliseconds. During this period the angular velocity of the valve is rather small. Following this slow phase, which may be approximated in the hinge flow as a quasi-static condition for the hinge, the leaflet rotates from 32° from the vertical to the closure position where it assumes an angle of approximately 64 ° from the vertical; this second phase of rotation is rapid and occurs in 2.22ms. For the fully dynamic hinge simulation this second phase is of interest and hence the dynamic simulation starts at 35 ms (considered as the starting point (T=0)) for the present simulation and ends at T= 2.22 milliseconds when the valve completely closes shut. As the motion of the leaflet ear that rests in the hinge gap is driven by the motion of the leaflet itself, this motion is imposed from previous computation of the fluid-structure interaction of the leaflet in the mitral position subjected to physiological transvalvular pressure. In Figure 4, the velocity, wall shear stress, and particle concentration plots at various leaflet rotational positions are shown. Comparing the magnitudes of the flow quantities with those shown in Figure 3, it can be observed that the static simulation quite adequately captures the final flow conditions observed in Figure 4. This indicates that the flow through the hinge is nearly quasi-steady with respect to leaflet rotation and the rather fast transient pressure rise dictates the flow features observed in the hinge pocket. This offers simplifications to solutions of the flow through the hinge in that simulation of hinge flow can be performed without including leaflet ear rotation. In 3-dimensional calculations this can be a valuable and reasonable first approximation for hinge flow computations.
Figure 4.
Plots of a) Velocity (m/sec); b) Fluid shear stress (Pa); c) Concentration; and d) Simulated platelet activation (Pa-sec) at various stages of the closing phase in the dynamics analysis of flow in the hinge region.
The maximum leakage jet velocity regions vary with time as the valve rotates. Initially the jet velocity was higher at the upper gap width as there was no obstruction to the flow. But as the valve starts to close, owing to the rotation of the leaflet the bulk of the flow is diverted through the lower gap width and there is very little flow through the upper gap width. The jet becomes stronger with time and presents a maximum velocity magnitude of 9.8 m/sec at the lower gap width at the time of closure. We can also observe from the velocity contours shown in the figure 4(a) that the center of the vortex that is shed from the leaflet ear as the jet issues through the gap has a low velocity magnitude in the order of 1 m/sec at the time of closure. Particles (modeled platelets) that are trapped in the boundary layer formed on the leaflet ear surface are carried into the shear layer which rolls up downstream into a vortical structure. The particles that are entrained by this vortex are carried in the axial direction at the low velocity of advection of the vortex center as opposed to being flushed through the domain at the high velocity of the leakage jet, thus increasing the time of residence in the hinge pocket.
Figure 4(b) shows the fluid shear stress magnitude at various closing angles. The shear stress magnitude is observed to increase as the valve moves towards closure, reaching a maximum value of 1040 Pa. The maximum shear stress is experienced at the trailing edge of the leaflet ear at the lower gap width as can be seen from figure 4(b). This is where the boundary layer that was attached to the leaflet ear leaves the trailing edge of the ear. The platelets that were entrained in the boundary layer are carried into the shear layer that subsequently rolls up into the vortex and the concentration of the platelets in the vortex core will increase as observed in Figure 4(c). The activation parameter is calculated for every particle seeded in the domain and is shown in Figure 4(d) from which it is evident that platelet activation increases with time and peaks at the later stages of closure. The activated platelets are seen to be drawn into the core of the shed vortex. The origin of these activated platelets was in the boundary layer on the solid surface, where relatively slow moving and high shear fluid entrains platelets. The trailing edge vortex is advected at a relatively low velocity downstream, so that the platelets with high activation parameter values may remain in the hinge region for an extended period of time. The platelets that are carried by the high velocity leakage jet are seen to have low activation parameter values. This result is revealing and counter-intuitive because it is natural to think that platelets passing through the narrow gaps are carried at high velocities in the leakage jets and therefore activated platelets would exist in the jet. However, due to the transient nature of the flow through the gap viscous effects do not penetrate from the solid surfaces into the jet. Thus shear stresses are predominantly concentrated in the rather thin boundary layers that adhere to the walls, as shown in Figure 4(b). Therefore, activated platelets are most likely to be found in the shear layers leaving the leaflet ear and to be trapped in the rolled-up vortex core, rather than in the high-velocity leakage jet. Note that similar behavior was noted in previous work, in the case of the leakage jet flow between the leaflet and housing during valve closure. The computed activation parameter magnitudes for unsteady flow were comparable to those for steady flow (Figure 3(d)).
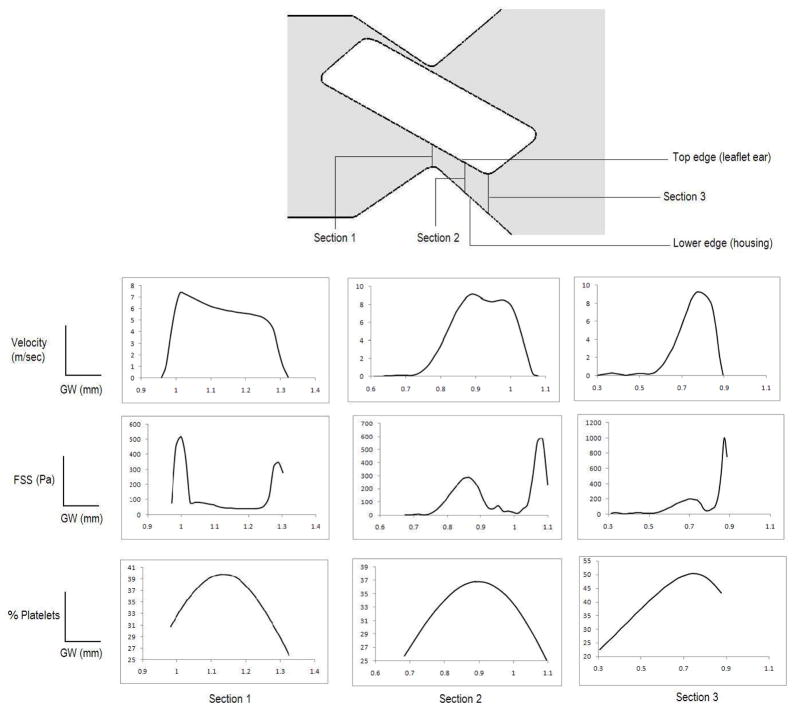
It is instructive to analyze the flow in the gap region to delineate the region within the hinge gap where platelets are most likely be activated and to understand the precise nature of the mechanics in the hinge gap that impacts on activation. To this end, the velocity profiles, shear stress distribution and the platelet concentration at three cross-sections in the gap width with the leaflet in the fully closed position are plotted in Figure 5. It can be observed that, as the fluid moves through the gap width, the velocity gradients and hence the shear stresses are larger on the boundary of the jet emanating from the gap on the leaflet ear side. This is due to the fact that a boundary layer is formed on the walls and large shears are experienced by particles in these boundary layers. The platelets that are within this high shear layer will be more likely activated and subsequently get trapped in the rolled-up shear layers in the distal hinge pocket as shown in Figures 4. Initially, i.e. at section 1 the shear stresses and platelet concentration are highest in regions that do not overlap. This is because the particles that flow through the jet are carried in the core of the jet that issues through the constriction. At the subsequent sections (2 and 3) however the velocity profiles shift in such a way that the platelet concentration becomes high in the region of high jet velocity and is coincident with the region of high shear. The shear layers on the leaflet ear and housing surface have the most significant impact on the predilection towards activation in the hinge pocket. An important effect that is revealed in the profiles at section 3 in Figure 5 is that as the jet exits the proximal side of the constriction the velocity magnitudes, shear stresses and platelet concentration concomitantly increase towards the leaflet ear surface. This coincidence of the region of the highest shear and concentration implies that platelets find themselves increasingly drawn into the shear layer and subjected to high shears as the jet exits through the constriction. Further downstream this shear layer rolls up into a vortex, trapping the platelets within its core.
Figure 5.
The velocity, fluid shear stress (FSS), and platelet concentration distribution at cross-sections 1–3 within the gap width with the rectangular geometry of the leaflet ear. The horizontal axis indicates the distance from the valve housing (lower) edge to the leaflet ear (top) edge in reference to the figure within the gap width (GW) in the schematic of the geometry (top panel).
It is interesting to compare the potential for activation in the flow through the hinge gap during closure with that of the previously quantified activation parameter for leakage flows between the valve leaflet and housing. In the present case of flow through the hinge, at the time of closure the activation parameter reached a value of 0.23 at the lower corner and 0.14 in the upper corner of the hinge region. In our previous study on the leaflet edge an activation parameter of 0.21 was computed at the end of the closure phase. This indicates that, at least within the limitations of the present two-dimensional computations, the activation parameter for the leaflet edge leakage flow and that for leakage through the hinge are comparable. However, there are some subtle yet significant differences in the activation behavior for flow through these two potentially risky areas of the valve. Table 1 shows the comparison of velocity, shear stress and platelet activation experienced at the leaflet edge and that of the hinge region. We can observe that although a similar platelet activation parameter was obtained for the two cases, the velocity and shear stress at the leaflet edge is far more than that experienced at the hinge region. This could be due to difference in residence time of platelets in both the regions. It could be concluded that the residence time of the platelets in the hinge region is more than that of the leaflet edge as platelet activation parameter includes both the effect of exposure to shear stress and residence time. As shear stress is less in the hinge region, a higher residence time compared to the leaflet edge should be reason for making the platelet activation parameter similar to that calculated at the leaflet edge.
Table 1.
shows the comparison of leakage velocity, fluid shear stress and activation parameter that was calculated at the hinge and at the leaflet edge.
| At closure | Max. Velocity (Gap width) m/sec | Shear stress Pa | Activation Param Pa-Sec |
|---|---|---|---|
| Hinge | 7 | 1037 | 0.23 |
| Leaflet edge | 20 | 3228 | 0.21 |
Dependence of activation on hinge geometry – isolating the key effects
Since the primary flow field characteristics that determine the influence of the hinge flow on activation parameters are the shear stress experienced by the platelets and the time of exposure to high values of shear stress, it is natural to ask how and if specific features of the hinge geometry are responsible for the damage to platelets. Maintaining the same transvalvular pressures as in the previous cases (and as dictated by the conditions at the overall valve scale) one potential change to the hinge geometry is the shape of the leaflet ear. With this in view, the impact of replacing the rectangular leaflet ear geometry shown in Figure 2 with a more streamlined shape (such as an ellipse of the same length along the major axis) was examined. Flow through this modified hinge geometry was simulated with all other conditions remaining the same as with the original geometry. The ellipsoidal leaflet ear replaced the original leaflet ear and was placed in the hinge recess as shown in Figure 6. The major axis of the ellipsoidal geometry matched with the length of the leaflet ear and the minor axis was equal to the thickness of the leaflet ear. The leaflet ear was made to rotate with the same angular velocity for the same duration and boundary conditions.
Figure 6.
Flow field with contours of existing geometry and the ellipsoidal geometry: a) Velocity contour showing the leakage jet from the hinge region; b) Shear stress contour during the late stage of valve closure where the shear stress reaches the maximum value; c) Particle concentration; and d) Activation parameter at the time of closure.
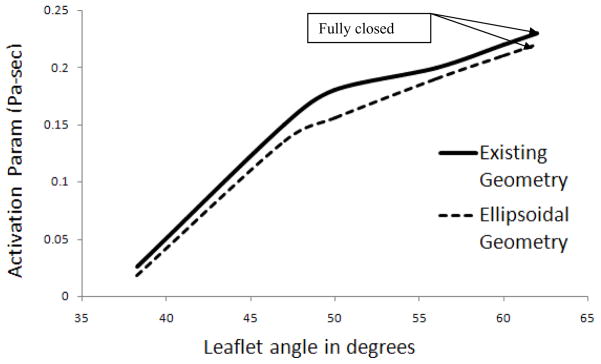
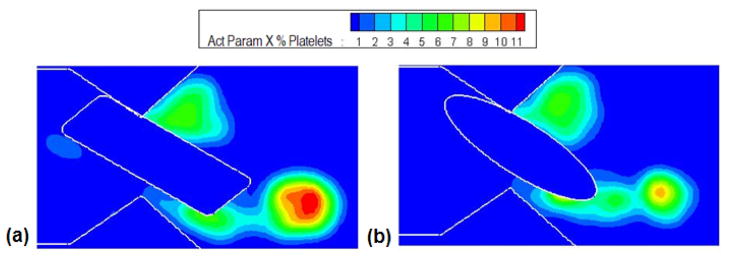
Figure 6 shows the end stage dynamics of both the existing geometry and the ellipsoidal geometry for comparison. The leakage velocity for the ellipsoidal geometry (Figure 6(a)) reached a maximum of 9 m/sec and in the axial velocity of the vortex core was observed to be around 1.5 m/sec compared to a velocity of 1 m/sec with the rectangular geometry. The maximum shear stress recorded during the simulation was around 980 Pa (Figure 6(b)) which differs only marginally from that for the original geometry (1030 Pa). It was also observed that the shear stress level was essentially unchanged when compared with the previous case during all the stages of closure. Figure 6(c) shows the concentration of the particles at the near closure stage of the leaflet ear for both the leaflet ears. It was observed that there is relatively high concentration in the vortices especially near the trailing edge of the leaflet during the end stages of valve closure. Figure 6(d) shows the platelet activation parameter for both elliptical and the original geometry. The maximum activation parameter was calculated to be 0.23 Pa-sec at the fully closed position for both the cases. For both cases this maximum activation parameter is observed to coincide with the vortex core downstream of the leaflet ear. The computed maximum activation parameter as a function of angular position of the leaflet is shown in Figure 7 and similar trend in the increase of the activation parameter as the leaflet moves towards the closed position is observed in both cases. Therefore, the shape of the leaflet ear appears to have little impact on the maximum activation parameter attained by the platelets flowing through the hinge gap. However, the maximum activation parameter is only one indication of the predilection to thrombus initiation. It is also of interest to quantify what fraction of platelets experience these high activation parameter levels. To examine this Figure 8 shows the cross-correlation between the activation parameter and the platelet concentration by plotting contours of the product of the two quantities. This plot shows that while the two geometries produce essentially the same level of activation, the number of particles that experience high levels of activation is lower in the case of the more streamlined ellipsoidal geometry of the hinge. This difference can be explained from the fluid flow characteristics in the gap. The same level of maximum activation is achieved in both cases due to the fact that the maximum shear is experienced in the boundary layer on the leaflet ear surface. The shears in the boundary layer are of similar magnitude for both geometries as this value depends primarily on the jet core velocity which is nearly the same as seen from Figure 6(a). On the other hand, downstream of the gap, the flow diverges to a greater extent for the ellipsoidal geometry than for the rectangular one. The shear layers and the vortex rollup in the case of the ellipsoidal geometry are therefore weaker than that for the rectangular geometry. This leads to fewer particles being entrained into the vortex downstream of the constriction and hence fewer platelets that experience high levels of shear.
Figure 7.
Activation parameter during various stages of valve closure of the two geometries. Thick line shows the activation parameter of the existing geometry and the dashed line shows that of the ellipsoidal geometry
Figure 8.
The product of activation parameter and the concentration of platelets at the end stage of closure for: (a) Existing geometry and (b) Elliptical geometry. It can be inferred that the number of platelets getting trapped in the region of high activation is less in the elliptical geometry than the existing geometry.
Discussion and conclusion
This study examined the flow structures through and within the hinge region of a mechanical heart valve and its implication on the platelets under physiological mitral conditions focusing on the closure phase of the valve. This was done computationally using a fixed Cartesian mesh and local mesh refinement. Angular velocity and inlet pressure data were obtained from a previous study [9] which focused on flow through the overall bileaflet geometry. This study enabled visualization of the flow dynamics within and through the gap regions of the hinge. Detailed interrogation such as this is limited with experimental studies because of the complex geometry formed by the pivoting of the leaflet and the hinge where visual access is not possible. Previous experiments [16] have compared the hinge flow fields of two bi-leaflet valves under aortic and mitral conditions in a pulsatile flow loop and laser Doppler velocimetry. These studies reported flow separations and presence of re-circulation region in the vicinity of the hinge as was observed in the present study. Most of the observations of flow structures were concerned with the flow patterns that emerged from the hinge pocket and exited into the region distal to the valve. Limited information was available on the flow details inside the hinge pocket and, to our knowledge, no previous investigations have been reported on particle dynamics in the hinge pocket. The present study focused on the leakage flow between the gap regions between the leaflet ear and the housing and its effect on the platelets during the end stages of closure. Both static and dynamic analysis was done; the dynamic study predicted marginally higher fluid shear stress compared to steady flow analysis. However, due to the short transients imposed by the transvalvular pressure rise the differences between the static and dynamic leaflet ear cases were not significant.
In the present paper, the dynamics of the platelets was tracked by a Lagrangian particle tracking method with a one way coupling. A shear stress based platelet activation model was used to predict the likely sites of platelet activation which could initiate thrombus deposition. High velocity and shear stress magnitudes were recorded but the flow remained in the laminar regime during the entire simulation. Flow in the hinge pocket was observed to be highly dynamic because of the shear layer formation and roll-up and the flow did not wash the vortex that was shed downstream of the leaflet ear. This vortex had a low advection velocity and therefore a high residence time in the hinge pocket. In their experiments, Gross et al. [15] observed a similar flow pattern in their study of the hinge region where the recirculation zone did not get washed with the flow. Platelets caught in such a vortical structure are likely to remain in the vicinity of the hinge. High shear stresses experienced by the platelets and high residence time in this region make this region highly vulnerable to thrombus formation. In our simulation we observed that platelets are subjected to high shear stress (more than 500 Pa for the last 2 ms of valve closure and the shear stress rose to a value of 1100 Pa at complete closure. These values of shear stress and exposure time are in the range in which platelet activation and lysis were observed in the experiments reported in the literature [32–34]. Comparison of the activation parameter obtained for flow through the hinge and that for the flow through leakage gaps between the leaflet and the valve housing showed that the tendency to activate is comparable for the two flows. However the environments faced by the platelets in the two cases are quite different. The velocity magnitude and consequently the shear stress in the leakage jet through the hinge gap is smaller than that for the leakage flow for the leaflet-housing gap but the residence time is larger. Therefore the activation parameter which is a product of the shear stress and residence time is comparable in the two cases. Therefore, from the present computation and previous work [9], it appears that both the hinge region and the leaflet-housing leakage region present nearly equal risk for platelet activation.
The results for the original leaflet ear geometry were compared to a new ellipsoidal hinge cross section (i.e. a more streamlined shape) and it was observed that the maximum activation parameter did not change significantly. The shear stress and the maximum leakage jet velocity were nearly identical in both the cases. However the number of platelets that experienced the higher levels of activation were reduced by the change in the geometry to the more streamlined ellipsoidal one. This indicates that the factors that are critical to blood damage and platelet activation in the hinge are the presence of strong gradients in the boundary layer that is attached to the leaflet ear surface, the subsequent separation of this boundary layer and the formation of a trailing edge vortex downstream of the leaflet ear. Platelets are entrained into the boundary layer, subject to high shears and then transported into the vortex core. This combination of high-shear environment experienced on the surface of the prosthesis and the resulting recirculating, long residence time fluid presents the potential for thrombus formation.
Biomechanical relevance and limitations of the study
In the present study the flow through the hinge geometry is calculated in a two-dimensional setting. Three-dimensional analysis of flow past mechanical heart valves and the relative importance of turbulent shear and viscous stresses in the aortic root has been reported in the literature [20, 21]. The hinge geometry in the bi-leaflet valve is also highly three-dimensional with the recessed region in the valve housing in which the leaflet ears interact with the butterfly mechanism. The blood flows through small gap width between the leaflet ears and the hinge housing during the valve function and the fluid dynamics can be anticipated to be highly three-dimensional. To our knowledge, no attempts have been reported to date to simulate the 3D flow dynamics in the hinge region in detail and its relationship to platelet activation and initiation of thrombus deposition. In this initial attempt, we have employed a highly resolved 2D analysis in order to delineate the flows through the narrow gaps with relatively high fluid velocities and gradients of velocity. We also employed the particle dynamic analysis in order to compute the shear stress-time history of platelets passing through this region and subsequently getting trapped in the vortical flow downstream for a significant duration. The suggested basic mechanism of platelets being subjected to relatively high shear stresses in the gap and subsequently being trapped in the vortical flow downstream agree with the experimental findings of the presence of vortical flows downstream to the hinge reported in the literature [15, 16]. We anticipate that extending the studies for a 3D analysis will result in confirming this mechanism for platelet activation in a complex 3D flow field. It has also been shown [16] that the leakage flow velocity magnitudes and therefore the stress environment experienced by blood elements in the hinge depend on the transvalvular pressure difference across the hinge during the opening and closing phases. For this purpose we have supplied pressure data from our previous simulations of the closure phase of the bileaflet valve in the mitral position [9]. In addition, we were able to compare the potential for platelet activation and the shear history experienced by the platelets through the hinge with those experienced by the platelets as they traverse the leakage gap between the valve leaflet and the housing as calculated in Krishnan et al [9]. Employing the 2D analysis, we were also able to analyze the effect of modifying the leaflet ear geometry from a rectangular shape to a streamlined elliptical geometry. Our analysis suggested that the change from rectangular to the elliptical geometry did not affect the computed activation parameter. However, our computations revealed that streamlining reduced the number of particles being trapped in the vortex roll up. Such parametric studies towards an optimized design can be performed more readily with a 2D analysis before performing a detailed 3D analysis on a prototype design.
Our platelet model is solely based on shear stress time integral experienced by the platelets that passes through the gap widths of the hinge region. Studies have suggested that a specific relationship exists for the activation of platelets in arterial flows [35–37]. Platelets get activated beyond a minimum shear stress as in the shear stress time integral model. Apart from shear stress, there are many other factors which could influence the process of platelet activation, such as the presence of foreign surfaces such as a mechanical valve leaflet, agonist synthesis and release by activated platelets and concentration, platelet-phospholipid dependent thrombin generation, and thrombin inhibition by heparin etc. These complex biochemical effects are not included in the present model, which must therefore be viewed as phenomenological. The current particle tracking algorithm assumes a dilute flow with low platelet loading in blood. It neglects the effect of platelet loading on blood flow as well as interaction of platelets with other platelets and red blood cells. The interaction of the larger and far more numerous bi-concave shaped red blood cells and platelets needs to be analyzed to study the precise activation mechanism of the platelets due to the fluid induced stresses. This requires multi-scale modeling that will incorporate the effects of the particulate nature of blood, including cell-cell and cell-surface interactions. Efforts are underway to develop a model that would simulate the effects of interaction of red blood cells and its effect on the flow as well as platelet- platelet and RBC- Platelet interaction through the hinge region.
Acknowledgments
Partial support of this work by a grant from the National Heart, Lung, and Blood Institute (NIH: HL 071814) and the Iowa Department of Economic Development are gratefully acknowledged.
References
- 1.Yoganathan AP, Chandran KB, Sotiropoulos F. Flow in prosthetic heart valves: state-of-the-art and future directions. Ann Biomed Eng. 2005;33(12):1689–1694. doi: 10.1007/s10439-005-8759-z. [DOI] [PubMed] [Google Scholar]
- 2.Yoganathan AP, He Z, Casey Jones S. Fluid mechanics of heart valves. Annu Rev Biomed Eng. 2004;6:331–362. doi: 10.1146/annurev.bioeng.6.040803.140111. [DOI] [PubMed] [Google Scholar]
- 3.Cannegieter SC, Rosendaal FR, Briet E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation. 1994;89(2):635–641. doi: 10.1161/01.cir.89.2.635. [DOI] [PubMed] [Google Scholar]
- 4.Einav S, Bluestein D. Dynamics of blood flow and platelet transport in pathological vessels. Ann N Y Acad Sci. 2004;1015:351–366. doi: 10.1196/annals.1302.031. [DOI] [PubMed] [Google Scholar]
- 5.Hellums JD. 1993 Whitaker Lecture: biorheology in thrombosis research. Ann Biomed Eng. 1994;22(5):445–455. doi: 10.1007/BF02367081. [DOI] [PubMed] [Google Scholar]
- 6.Bluestein D, Niu L, Schoephoerster RT, Dewanjee MK. Fluid mechanics of arterial stenosis: relationship to the development of mural thrombus. Ann Biomed Eng. 1997;25(2):344–356. doi: 10.1007/BF02648048. [DOI] [PubMed] [Google Scholar]
- 7.Jesty J, Yin W, Perrotta P, Bluestein D. Platelet activation in a circulating flow loop: combined effects of shear stress and exposure time. Platelets. 2003;14(3):143–149. doi: 10.1080/0953710031000092839. [DOI] [PubMed] [Google Scholar]
- 8.Ramstack JM, Zuckerman L, Mockros LF. Shear-induced activation of platelets. J Biomech. 1979;12(2):113–125. doi: 10.1016/0021-9290(79)90150-7. [DOI] [PubMed] [Google Scholar]
- 9.Krishnan S, Udaykumar HS, Marshall JS, Chandran KB. Two-dimensional dynamic simulation of platelet activation during mechanical heart valve closure. Ann Biomed Eng. 2006;34(10):1519–1534. doi: 10.1007/s10439-006-9194-5. [DOI] [PubMed] [Google Scholar]
- 10.Ellis JT, Healy TM, Fontaine AA, Saxena R, Yoganathan AP. Velocity measurements and flow patterns within the hinge region of a Medtronic Parallel bileaflet mechanical valve with clear housing. J Heart Valve Dis. 1996;5(6):591–599. [PubMed] [Google Scholar]
- 11.Healy TM, Fontaine AA, Ellis JT, Walton SP, Yoganathan AP. Visualization of the hinge flow in a 5 : 1 scaled model of the medtronic parallel bileaflet heart valve prosthesis. Experiments in Fluids. 1998;25(5–6):512–518. [Google Scholar]
- 12.Bodnar E, Grunkemeier GL, Gabbay S. Heart valve replacement: A statistical review of 35 years results - Discussion. Heart Valve Disease. 1999;8:470–471. [PubMed] [Google Scholar]
- 13.Manning KB, Kini V, Fontaine AA, Deutsch S, Tarbell JM. Regurgitant Flow Field Characteristics of the St. Jude Bileaflet Mechanical Heart Valve under Physiologic Pulsatile Flow Using Particle Image Velocimetry. Artif Organs. 2003;27(9):840–846. doi: 10.1046/j.1525-1594.2003.07194.x. [DOI] [PubMed] [Google Scholar]
- 14.Woo YR, Yoganathan AP. Pulsatile flow velocity and shear stress measurements on the St. Jude bileaflet valve prosthesis. Scand J Thorac Cardiovasc Surg. 1986;20(1):15–28. doi: 10.3109/14017438609105910. [DOI] [PubMed] [Google Scholar]
- 15.Gross JM, Shu MC, Dai FF, Ellis J, Yoganathan AP. A microstructural flow analysis within a bileaflet mechanical heart valve hinge. J Heart Valve Dis. 1996;5(6):581–590. [PubMed] [Google Scholar]
- 16.Simon HA, Leo HL, Carberry J, Yoganathan AP. Comparison of the hinge flow fields of two bileaflet mechanical heart valves under aortic and mitral conditions. Ann Biomed Eng. 2004;32(12):1607–1617. doi: 10.1007/s10439-004-7814-5. [DOI] [PubMed] [Google Scholar]
- 17.Gao ZB, Hosein N, Dai FF, Hwang NH. Pressure and flow fields in the hinge region of bileaflet mechanical heart valves. J Heart Valve Dis. 1999;8(2):197–205. [PubMed] [Google Scholar]
- 18.Quinlan NJ. Comment on “Prosthetic heart valves’ mechanical loading of red blood cells in patients with hereditary membrane defects” by Grigioni et al., Journal of Biomechanics 38: 1557–1565. J Biomechanics. 2006;39(13):2542. doi: 10.1016/j.jbiomech.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Quinlan NJ, Dooley PN. Models of flow-induced loading on blood cells in laminar and turbulent flow, with application to cardiovascular device flow. Ann Biomed Eng. 2007;35(8):1347–1356. doi: 10.1007/s10439-007-9308-8. [DOI] [PubMed] [Google Scholar]
- 20.Ge L, Dasi LP, Sotiropoulos F, Yoganathan AP. Characterization of hemodynamic forces induced by mechanical heart valves: Reynolds vs viscous shear stresses. Ann Biomed Eng. 2008;36(2):276–297. doi: 10.1007/s10439-007-9411-x. [DOI] [PubMed] [Google Scholar]
- 21.Dasi LP, Ge L, Simon HA, Sotiropoulos F, Yoganathan AP. Vorticity dynamics of a bileaflet mechanical valve in an asymmetric aorta. Physics of Fluids. 2007;19(067105):1–17. [Google Scholar]
- 22.Ellis JT, Travis BR, Yoganathan AP. An in vitro study of the hinge and near-field forward flow dynamics of the St. Jude Medical Regent bileaflet mechanical heart valve. Ann Biomed Eng. 2000;28(5):524–532. doi: 10.1114/1.297. [DOI] [PubMed] [Google Scholar]
- 23.Marella S, Krishnan S, Liu H, Udaykumar HS. Sharp interface Cartesian grid Method I: An easily implemented technique for 3D moving boundary computations. J Computational Physics. 2005;210(1–31) [Google Scholar]
- 24.Liu H, Krishnan S, Marella S, Udaykumar HS. Sharp interface Cartesian grid method II: A technique for simulating droplet interactions with surfaces of arbitrary shape. J Computational Physics. 2005;210:32–54. [Google Scholar]
- 25.Udaykumar H, Mittal R, Rampunggoon P, Khanna A. A fixed grid sharp interfae method for flows in the presence of moving embedded solid boundaries. Computational Physics. 2001;174:1–36. [Google Scholar]
- 26.Udaykumar HS, Marella S, Krishnan S. Sharp-interface simulation of dendritic growth with convection: Benchmarks. Int J Heat and Mass Transfer. 2003;46(2615–2627) [Google Scholar]
- 27.Greaves D. A quadtree adaptive method for simulating fluid flows with moving interfaces. J Computational Physics. 2004;194:35–56. [Google Scholar]
- 28.Greaves D. Simulations of interfaces and free surface flows in a viscous fluid using adaptive quadtree grids. Int J Numerical Methods in Fluids. 2004;44:1093–1117. [Google Scholar]
- 29.Chen H, Marshall JS. A Lagrangian vorticity method for two-phase particulate flows with two-way coupling. J Computational Physics. 1999;148:169–198. [Google Scholar]
- 30.Lai YG, Chandran KB, Lemmon J. A numerical simulation of mechanical heart valve closure fluid dynamics. J Biomech. 2002;35(7):881–892. doi: 10.1016/s0021-9290(02)00056-8. [DOI] [PubMed] [Google Scholar]
- 31.Bluestein D, Yin W, Affeld K, Jesty J. Flow-induced platelet activation in mechanical heart valves. J Heart Valve Dis. 2004;13(3):501–508. [PubMed] [Google Scholar]
- 32.Colantoini G, Hellums JD, Maoke JL, Alfrey CP., Jr The response of human platelets to shear stress at short exposure times. Trans Am Soc Art Int Org. 1977;23:626–631. doi: 10.1097/00002480-197700230-00169. [DOI] [PubMed] [Google Scholar]
- 33.Brown C, Leverett L, Lewis C, Alfrey C, Hellums J. Morphological, Biochemical, and Functional Changes in Human Platelets Subjected to Shear Stress. Journal of Laboratory and Clinical Medicine. 1975;86(3) [PubMed] [Google Scholar]
- 34.Anderson GH, Hellums JD, Moake JL, Alfrey CP., Jr Platelet lysis and aggregation in shear fields. Blood Cells. 1978;4(3):499–511. [PubMed] [Google Scholar]
- 35.Weston MW, Goldstein S, Epting RE, 2nd, He S, Mauldin JM, Yoganathan AP. Establishing a protocol to quantify leaflet fibroblast responses to physiologic flow through a viable heart valve. Asaio J. 1997;43(5):M377–382. [PubMed] [Google Scholar]
- 36.Giddens DP, Yoganathan AP, Schoen FJ. Prosthetic Cardiac Valves. Cardiovasc Pathol. 1993;2(S167–S177) [Google Scholar]
- 37.Tambasco M, Steinman DA. Path-dependent hemodynamics of the stenosed carotid bifurcation. Ann Biomed Eng. 2003;31(9):1054–1065. doi: 10.1114/1.1603257. [DOI] [PubMed] [Google Scholar]