Abstract
Introduction
Cerebral vasoconstriction is associated with increased cytosolic Ca2+ concentration in vascular smooth muscle, presumably due to Ca2+ influx and Ca2+ release from intracellular stores. We tested the hypothesis that dantrolene (a blocker of Ca2+-induced Ca2+ release from the ryanodine receptor channel on the sarco-endoplasmic reticulum) would potentiate the action of nimodipine (a voltage-dependent L-type Ca2+ channel blocker, considered standard therapy for SAH) in inhibiting the vasoconstriction of isolated cerebral arteries.
Method
Sprague-Dawley rat basilar and femoral arteries were analyzed for ryanodine receptor expression by immunofluorescence and PCR. Vasoconstriction of basilar artery ex vivo was measured in a wire myograph while exposed to serotonin (5-HT) or endothelin-1 (ET-1) in the presence or absence of dantrolene (10–100μM) and/or nimodipine (30nM). Femoral artery was examined for comparison.
Results
Basilar and femoral arteries express only the ryanodine receptor 3 (RyR3) isoform. In both basilar and femoral arteries dantrolene significantly inhibited the constriction to 5-HT, whereas it poorly affected the constriction to ET-1. The inhibitory effect of dantrolene on 5-HT was substantially increased by nimodipine, inducing a 10-fold increase in the 50% effective concentration of 5-HT and a 46% reduction in maximum basilar constriction. In femoral artery, dantrolene modestly affected constriction to phenylephrine and there was no interaction with nimodipine.
Conclusion
Dantrolene has synergistic effects with nimodipine against 5-HT-induced vasoconstriction in isolated cerebral arteries. Dantrolene-nimodipine interaction will require testing in a pathophysiological model but might provide treatment for reducing SAH-related vasospasm or other 5-HT-related vasospastic syndromes, such as Call-Fleming syndrome.
Index Entries: dantrolene, nimodipine, vasospasm, ryanodine receptor, calcium, serotonin, endothelin-1, phenylephrine, basilar artery, sarco-endoplasmic reticulum
Introduction
Dantrolene is an FDA approved drug for the treatment of malignant hyperthermia. It inhibits the ryanodine receptor Ca2+ channel (RyR) located on the sarco-endoplasmic reticulum, thereby inhibiting Ca2+ release from the large Ca2+ stores of the sarco-endoplasmic reticulum into the cytosol. 1 The ryanodine channel is expressed in 3 isoforms: RyR1, RyR2, and RyR3. 2 RyR1 isoform is predominantly found in skeletal muscle, RyR2 in myocardium, and RyR3 in neurons, smooth muscle cells and inflammatory cells.2 Dantrolene blocks RyR1 and RyR3 isoforms, but not RyR2,3 consistent with its lack of significant cardiac effect when used in vivo.1
Understanding cerebral artery physiology is imperative to understanding pathophysiology such as cerebral vasospasm following subarachnoid hemorrhage (SAH) is a frequent cause of secondary brain ischemia.4 The pathogenesis of vasospasm is incompletely understood, but it is associated with maintenance of tone due to persistent elevation of cytosolic Ca2+ concentration in vascular smooth muscle5 and involves multiple mediators, including 5-hydroxytryptamine (serotonin, 5-HT) and endothelin-1 (ET-1).6–8 Nimodipine, a 1,4-dihydropyridine blocker of voltage-dependent L-type Ca2+-channel (VDCC), is currently indicated after SAH and modestly improves outcomes, but the outcome remains generally poor9 and despite the ability to induce vasorelaxation it is not clear whether this or a neuroprotective effect is responsible for improved outcomes.
VDCC blockers (also referred to as calcium antagonists) relax vascular smooth muscle, by inhibiting Ca2+ influx, a pathway shared by different vasoconstrictor agonists.10 Because Ca2+-induced Ca2+ release (CICR) is a recognized mechanism of excitation-contraction coupling in vascular smooth muscle,11, 12 including cerebral arteries,13, 14 we hypothesized that dantrolene i) might inhibit cerebrovascular constriction to agonists and; ii) might potentiate the action of nimodipine, because the two drugs moderate sequentially linked physiological steps. Nimodipine inhibits Ca2+-influx resulting in lower cytosolic Ca2+ concentrations, thereby reducing sarco-endoplasmic reticulum Ca2+ release, which in turn is blocked by direct inhibition of RyR3 channel by dantrolene, eventually leading to reduce smooth muscle contraction.
To our knowledge, dantrolene has not been investigated as a treatment for any cerebral vasospasm syndrome, while nimodipine is now standard treatment for SAH.9, 15 Another potential advantage of dantrolene is its potential for neuroprotection (same can be noted for nimodipine), as demonstrated in in vitro and in vivo models of injury.16
In this physiology study we challenged ex vivo basilar arteries, isolated from rats, with serotonin (5-HT) or endothelin-1 (ET-1), in a wire myograph in the presence or absence of dantrolene and/or nimodipine. To determine whether the effects of dantrolene and nimodipine were specific for the preparation and/or for the agonist, we applied the same experimental protocol to rat femoral arteries for comparison. Our data support the hypothesis that the association dantrolene+nimodipine has a synergistic inhibitory effects on vasoconstriction induced by 5-HT in the cerebrovascular bed.
Methods
The subcommittee for research and animal care at Massachusetts General Hospital approved animal use. Rats (Sprague-Dawley, male, 200–300 g) were sedated with inhaled chloroform followed by decapitation. A total of 73 rats were used for this study.
Reverse transcription and Polymerase Chain Reaction (PCR)
Cerebral or femoral arteries were removed and cleaned to remove surrounding adventitial tissue (n=2). Cortex, striatal muscle and cardiac muscle were also removed as positive controls. All tissue was homogenized in RNA lysis buffer and processed as recommended by Ilustra RNAspin Mini kit (GE Healthcare; Piscataway, New Jersey, USA). RNA was eluted with 40 μl of water. cDNA was made with 18ul of RNA as directed using Superscript III kit (Invitrogen; Carlsbad, California, USA). Total cDNA was measured with a BioPhotometer (Eppendorf; Westbury, New York, USA). A total of 1 μg of cDNA in a 20μl PowerSybr reaction (Applied Biosystems; Foster City, California, USA) was used in real-time PCR analysis on a 7500 Real-Time PCR System (Applied Biosytems). Cycling parameters for all genes were 50°C for 10min, 95°C for 10min and 40 cycles of 95°C for 15s and 60°C for 1min. Sequence for the following genes were:
RyR1: forward 5′ GAAGGTTCTGGACAAACACGGG 3′, reverse 5′ TCGCTCTTGTTGTAGAATTTGCGG 3′,
RyR2: forward 5′ GAATCAGTGAGTTACTGGGCATGG 3′, reverse 5′ TTGATCTCTGAGTTCTCCAAAAGC 3′
RyR3: forward 5′ ACTGGGTATATGGCACCAACACT 3′, reverse 5′ CCACACAGACCAGAGAGATGACA 3′
18s rRNA (endogenous control for cDNA loading, Applied Biosystems, #4310893E). Specificity of amplicon verified by dissociation curves and 2% agarose gel electrophoresis. Agarose gel imaged with Typhoon 9410 (GE Healthcare) with Sybr Green, (image settings: 488nm laser, 520nm filter, 500V photomultiplier tube, normal sensitivity, 50μm resolution).
Immunoflourescence
Basilar artery was removed as described above (n=2). It was post-fixed in 4% paraformaldehyde for 24 hours at 4°C followed by treatment for cryopreservation in 30% sucrose at 4°C. Sections were cut on a cryostat at 20μm thick. Sections were blocked in 10% normal donkey serum (Vector Laboratories, Burlingame, California, USA) and 0.3% Triton X-100 for 30 minutes at room temperature (RT). Slides were then incubated in same solution as above with mouse anti-RyR3 at 1:100 dilution (Developmental Studies Hybridoma Bank at University of Iowa) overnight at 4°C. After washing, slides were incubated with donkey anti-mouse biotinylated antibody (Vector Laboratories) at 1:250 dilution in PBS and 2% normal donkey serum for 2 hours at RT. After washing, slides were incubated with Alexa546 streptavidin at a 1:500 dilution for 1 hour at RT. Nuclei were stained with Sytox Green (Invitrogen). Specificity of staining was confirmed by above protocol but only the secondary antibody was eliminated. Images were taken with a confocal Zeiss LSM5 system. Images were taken with 100x oil objective 2μm thick z-stack for 5 sections and projected into a single image.
Measurement of contractile tone in isolated vessels
Basilar and femoral arteries were removed and immersed in physiological solution (composition, mM: NaCl, 118; KCl, 4.6; NaHCO3, 25; MgSO4, 1.2; KH2PO4, 1.2; CaCl2, 1.25; glucose, 10; EDTA, 0.025; pH 7.4 at 37 °C). Arteries were cleaned of surrounding tissue, cut into segments (1.5 – 2 mm long), threaded onto 40 μm stainless steel wires and mounted in a isometric myograph (610M, Danish Myo Technology, Aarhus, Denmark). After mounting, each preparation was equilibrated, unstretched, for 30 min, in physiological solution, maintained at 37°C and aerated with a gas mixture of 95% O2 - 5% CO2. Then, the normalized passive resting force and the corresponding diameter were determined for each preparation from its own length-pressure curve, according to Mulvany and Halpern.17 Contractile responses were recorded into a computer, by using a data acquisition and recording software (Myodaq and Myodata, Danish Myo Technology). After tension normalization and 30-min equilibration in physiological solution, the preparations were stimulated with isotonic depolarizing 100 mM KCl solution, in which part of NaCl had been replaced by equimolar amount of KCl. After washout, the preparations were incubated with or without dantrolene, nimodipine or both, for 30-min; then the preparations were exposed to vasoconstrictor agonists (5-hydroxytryptamine, 5-HT, 1 nM – 10 μM; phenylephrine, PE, 10 nM – 100 μM; endothelin-1, ET-1 10 pM – 100 nM). The amount of vasoconstriction induced by each drug was normalized to the maximum contraction induced by high KCl for each individual preparation.
5-HT, PE, ET-1, dantrolene and nimodipine were from Sigma (St Louis, MO); millimolar stock solution were prepared in H2O for 5-HT, PE, ET-1, and further diluted as required; dantrolene was dissolved in dimethylsulfoxyde as a 3 mM stock solution, nimodipine was dissolved in ethanol as a 0.1 mM stock solution; experiments with nimodipine were performed by protecting the organ chamber from light, to avoid drug photoinactivation; final concentrations of dimethylsulfoxyde and ethanol in the organ chamber did not exceed 0.33 and 0.03%, respectively. Nimodipine final concentration of 30 nM for all experiments was chosen based on a low-end drug concentration of oral dosed nimodipine in humans.18
Data was graphed as a percentage of high KCl-induced vasoconstriction against a log molar concentration of drug. Each set of data points was curve-fitted by a non-linear regression, best-fit, sigmoidal dose-response curve with no constraints, with the use of GraphPad Prism version 4.0c for Macintosh (GraphPad Software, San Diego, CA). Each curve is represented by 6 or greater individual preparations from 3 or more rats. Analysis is performed on the average of the number of preparations, as the preparation of the segments generated greater variability than individual normal rat basilar or femoral artery. Whole curves were compared by analysis of variance (ANOVA), with significance set at p<0.05. Those curves with differences were compared by ANOVA or t-test for logEC50 (concentration producing 50% of maximum contraction) and maximum contraction (Emax), with significance set at p<0.05.
Results
Ryanodine Receptor Expression
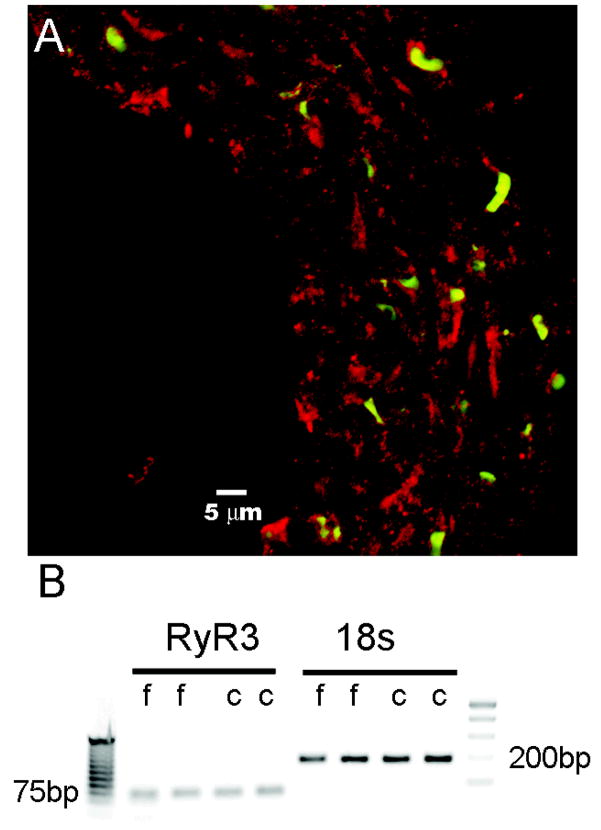
Cerebral and femoral vessels were isolated and analyzed for expression of the ryanodine receptor channel. Evidence of ryanodine receptor expression throughout the smooth muscle wall was obtained in basilar artery (Figure 1A) and in femoral artery (not shown). However, because this antibody does not discriminate among the three ryanodine isoforms, RyR1, RyR2, or RyR3, we further investigated the expression of each isoform at the gene level. Neither cerebral nor femoral vessel smooth muscle cells expressed mRNA for RyR1 or RyR2 despite expression noted in our positive controls of skeletal muscle and cardiac muscle, respectively (data not shown). Both cerebral and femoral smooth muscles cells expressed RyR3 at relatively the same levels (4.66×10−6 and 3.91×10−6 per 18s rRNA), as measured by real-time PCR (Figure 1B).
Figure 1.
Ryanodine Receptor Expression. A, cross-section of rat basilar artery immunostained for ryanodine receptor (red) and a nuclear counterstain (green). B, Agarose gel electrophoresis of PCR products from 2 samples of femoral (f) and cerebral (c) arteries. The left panel shows a 70 bp PCR fragment of the RyR3 sequence and the corresponding loading control of 18s rRNA in the right panel. Closest ladder bands to amplicon fragments are labeled 75 bp and 200bp, respectively.
Vasoconstriction Studies
Basilar Artery
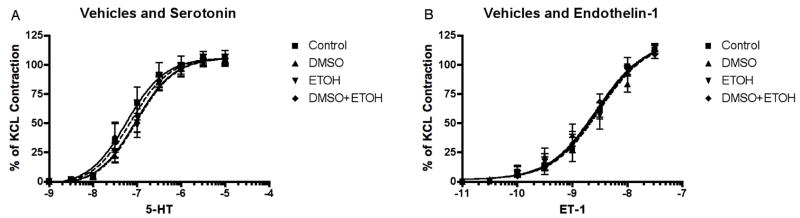
Our goal was to test the effect of dantrolene and nimodipine on the normal physiological contractile response to the potent vasoconstrictors, serotonin (5-HT) and endothelin-1 (ET-1). These two agents have been chosen because they cause reproducible contraction of cerebral arteries ex vivo8, 19–21 and, most importantly, because they have both been implicated in the pathogenesis of cerebrovascular vasospasm. 6–8, 22, 23 The solvents for dantrolene and nimodipine are DMSO and ethanol (ETOH), respectively. We tested whether these solvents effect the normal contractile response, at final maximum concentration of 0.33% (DMSO) and 0.03% ETOH. Figure 2 shows that neither solvent nor the combination of the two had a significant effect for either 5-HT or ET-1, at the maximum solvent doses used for dantrolene and nimodipine.
Figure 2.
Vehicle effect on concentration-response curves. A, Cumulative serotonin (5-hydroxytryptamine, 5-HT) concentration-response curve of basilar artery constriction normalized to 100 mM KCl induced-contraction, control arterial segments are compared to segments (n=5) incubated with 0.33% DMSO, 0.03% ethanol (ETOH) or both (n=6, for all vehicle groups), there are no statistical differences among curves. B, Cumulative endothelin-1 (ET-1) concentration-response curve of basilar artery constriction normalized to 100 mM KCl induced-contraction, control arterial segments (n=5) are compared to segments incubated with 0.33% DMSO, 0.03% ethanol (ETOH) or both (n=6, for all vehicle groups), there are no statistical differences among curves.
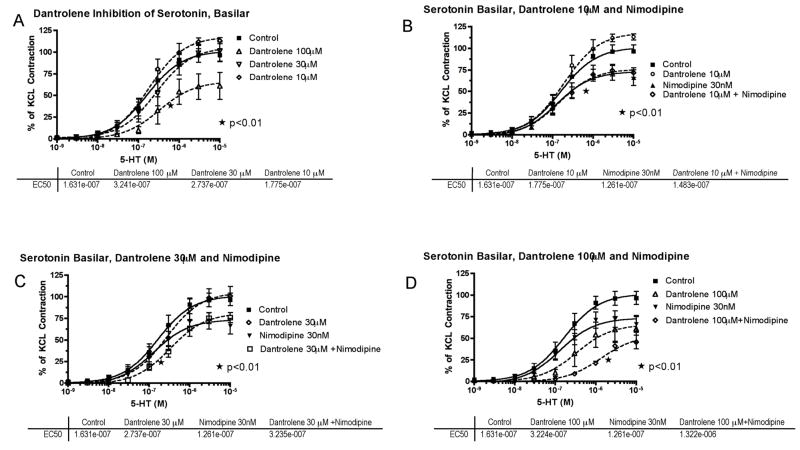
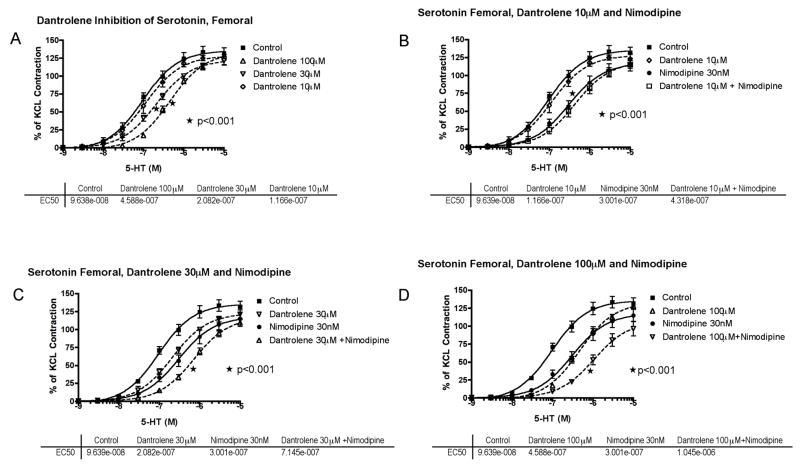
Next, isolated basilar artery was challenged with cumulative concentrations of 5-HT (1nM – 10μM) or ET-1 (10pM – 30nM). The addition of either 5-HT (Figure 3) or ET-1 (Figure 4) caused a concentration-dependent increase in contractile tone, reaching approximately 100%–120% of the contraction previously induced in the same preparations by high K+ (100 mM). Preincubation with 10 or 30 μM dantrolene did not significantly affect the contractile response to 5-HT, whereas 100 μM dantrolene produced a significant inhibition (Figure 3A); both the 50% effective concentration (EC50) and the maximum contraction to 5-HT (Emax) were significantly changed (p<0.01).
Figure 3.
Serotonin (5-hydroxytryptamine, 5-HT) concentration-response curves in basilar artery. A, Cumulative concentration-response curve of basilar artery constriction normalized to 100 mM KCl induced-contraction, control arterial segments are compared to segments incubated with increasing concentration of dantrolene (10–100μM) (*EC50 and Emax compared to control). B, The effect of dantrolene (10μM) plus nimodipine (30nM) (*EC50 and Emax of nimodipine compared to control). C, The effect of dantrolene (30μM) plus nimodipine (30nM) (*EC50 of dantrolene+nimodipine compared to nimodipine or dantrolene alone). D, The effect of dantrolene (100μM) plus nimodipine (30nM) (*EC50 and Emax of dantrolene+nimodipine compared to nimodipine or dantrolene alone). The effective concentration of 5-HT that gave 50% contraction (EC50) for each treatment is listed below the respective graph.
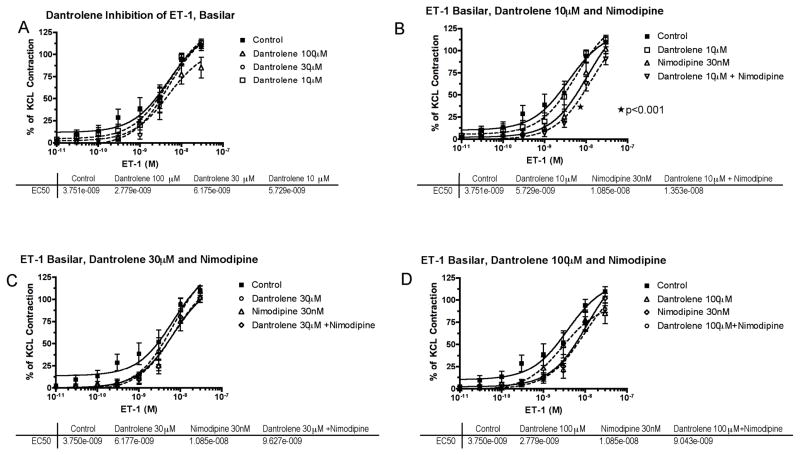
Figure 4.
Endothelin-1 (ET-1) concentration-response curves in basilar artery. A, Cumulative concentration-response curve of basilar artery constriction normalized to 100 mM KCl induced-contraction, control arterial segments are compared to segments incubated with increasing concentration of dantrolene (10–100μM). B, The effect of dantrolene (10μM) plus nimodipine (30nM) (*EC50 of nimodipine compared to control). C, The effect of dantrolene (30μM) plus nimodipine (30nM) shows no interaction. D, The effect of dantrolene (100μM) plus nimodipine (30nM) shows no interaction. The effective concentration of ET-1 that gave 50% contraction (EC50) for each treatment is listed below the respective graph.
In contrast to dantrolene, preincubation with nimodipine (30nM) alone did not affect the EC50 of 5-HT, but significantly reduced Emax by 27% (p<0.01, Figure 3B).
The effect of adding low concentrations of dantrolene (10 or 30 μM) with nimodipine (30 nM) was studied on the effect of 5-HT-induced constriction. The maximal inhibition was not greater than with nimodipine (30 nM) alone (Figure 3B, C); however, dantrolene (30μM) did reduce the EC50, p=0.01 (Figure 3C). The highest dantrolene dose (100μM) with nimodipine (30nM) caused a further 10-fold shift to the right in the EC50 of 5-HT and a further reduction of the maximal contraction to 46% (p<0.01; Figure 3D).
When tested on ET-1, the effects of dantrolene and nimodipine, either individually or in combination, were barely detectable (Figure 4).
Femoral Artery
To determine whether the effects of dantrolene and nimodipine on 5-HT constriction were specific to the basilar artery, we investigated the response to phenylephrine (PE), 5-HT and ET-1 in femoral artery. Rat basilar artery is known to not constrict in response to alpha adrenergic stimulation.24, 25
5-HT (1nM – 10μM), PE (10nM – 100μM) or ET-1 (100pM – 100nM) induced concentration-dependent increase in contractile tone of isolated femoral artery. The potency (EC50) of 5-HT was similar to that estimated in basilar artery, but the efficacy (Emax) was higher, reaching 120–140% of the contraction previously induced in the same preparations by high K+ (100 mM) (Figure 5). In contrast, ET-1 was about 10-fold less potent in femoral than in basilar artery (P<0.01) (data not shown).
Figure 5.
Serotonin (5-hydroxytryptamine, 5-HT) concentration-response curves in femoral artery. A, Cumulative concentration-response curve of basilar artery constriction normalized to 100 mM KCl induced-contraction, control arterial segments are compared to segments incubated with increasing concentration of dantrolene from 10–100μM (*EC50 of 30 and 100 μM compared to control). B, The effect of dantrolene (10μM) plus nimodipine (30nM) (*EC50 of nimodipine compared to control). C, The effect of dantrolene (30μM) plus nimodipine (30nM) (*EC50 of dantrolene+nimodipine compared to nimodipine or dantrolene alone). D, The effect of dantrolene (100μM) plus nimodipine (30nM) (*EC50 and Emax of dantrolene+nimodipine compared to nimodipine or dantrolene alone). The effective concentration of 5-HT that gave 50% contraction (EC50) for each treatment is listed below the respective graph.
Preincubation with dantrolene, 30 and 100 μM but not 10 μM, produced a significant (p<0.001) inhibition of the contractile response to 5-HT, with a parallel rightward shift of the concentration-constriction curve, without a significant change of Emax (Figure 5).
Preincubation with nimodipine alone (30nM) produced a parallel rightward shift in the response to 5-HT (P< 0.01) similar in amplitude to that observed with dantrolene 100 μM (Figure 5).
Dantrolene 30 and 100 μM + nimodipine (30 nM) together produced an additive inhibitory effect on the contractile response to 5-HT (Figure 5).
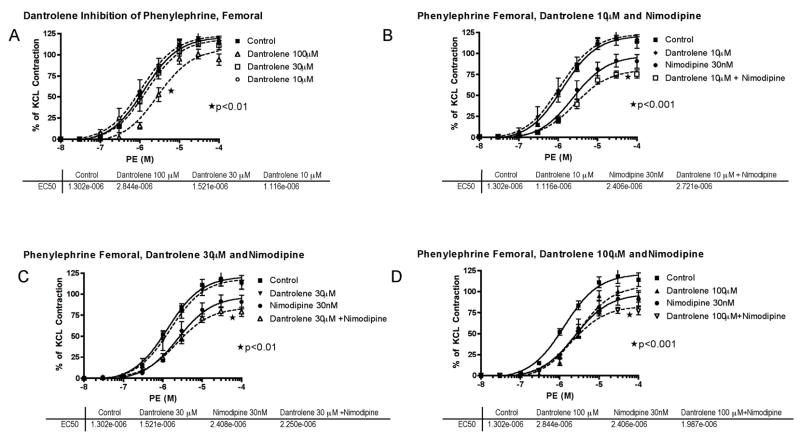
When tested individually on PE-induced constriction, 100μM dantrolene or 30 nM nimodipine produced a modest inhibition (Figure 6); dantrolene (at all concentrations tested) + nimodipine (30nM) did not produce an inhibitory effect stronger than nimodipine (30nM) alone, except for a further slight depression of Emax (Figure 6).
Figure 6.
Phenylephrine (PE) concentration-response curves in femoral artery. A, Cumulative concentration-response curve of basilar artery constriction normalized to 100 mM KCl induced-contraction, control arterial segments are compared to segments incubated with increasing concentration of dantrolene from 10–100μM (*EC50 of 100μM compared to control). B, The effect of dantrolene (10μM) plus nimodipine (30nM) (*EC50 and Emax of nimodipine compared to control; *Emax of dantrolene+nimodipine compared to nimodipine or dantrolene alone). C, The effect of dantrolene (30μM) plus nimodipine (30nM) (*Emax of dantrolene+nimodipine compared to nimodipine or dantrolene alone). D, The effect of dantrolene (100μM) plus nimodipine (30nM) (*Emax of dantrolene+nimodipine compared to nimodipine or dantrolene alone). The effective concentration of PE that gave 50% contraction (EC50) for each treatment is listed below the respective graph.
As for basilar artery, the effects of dantrolene and nimodipine, either individually or in combination, on ET-1 induced constriction of the femoral artery, were barely detectable (data not shown).
Discussion
The main finding of this study is that dantrolene + nimodipine exerts a synergistic inhibition on 5-HT-induced vasoconstriction. This synergy was observed in either basilar or femoral artery, though with pharmacological differences (non-parallel shift of the concentration-contraction curve in basilar with a decrease in Emax versus a parallel shift in femoral without change in Emax) that remain to be elucidated. In vascular smooth muscle cells, 5-HT potentially stimulates 5-HT1B/D receptors coupled to Gi (adenylate cyclase inhibition and cAMP decrease) and/or 5-HT2A coupled to Gq (phospholipase C (PLC) stimulation with IP3 and DAG generation).21, 26, 27 The vasoconstrictive effect of 5-HT in the basilar is mediated only by 5-HT1B/D, whereas in the femoral artery it is mediated by both 5-HT1B/D and 5-HT2A;26, 28, 29 this difference might explain why Emax is variably altered in the two vascular beds. If so, the mechanism of this difference is unclear.
5-HT exerts multiple effects on membrane cation channels, leading to membrane depolarization and subsequent activation of VDCC.30, 31 Following 5-HT stimulation of rat basilar artery we therefore would expect an increase in cytosolic Ca2+ concentration due to opening of VDCC, IP3-induced Ca 2+ release from intracellular stores and Ca2+-induced Ca2+ release (CICR) through RyR. Nimodipine blocks Ca2+ influx through VDCC.10 Dantrolene blocks CICR by binding to RyR3, although it does not directly block IP3-induced-Ca2+ release in smooth muscle, it may block CICR due to cytosolic increases in Ca2+ from IP3.32 Worthy of note, we found that smooth muscle cells in rat basilar and femoral arteries express only the RyR3 isoform, which is an isoform inhibited by dantrolene.3 Albeit excitation-contraction coupling following 5-HT has not yet been studied in detail in rat basilar artery, we speculate that simultaneous inhibition of Ca2+ influx via nimodipine and of CICR via dantrolene may affect sequential steps leading to synergistic inhibition of vasoconstriction. Our data show, not only that such an inhibitory synergism occurs, but also that it is most pronounced in the cerebral vasculature.
Interestingly, dantrolene had no effect on ET-1 vasoconstriction and a modest inhibition on PE-induced vasoconstriction; and there was no potentiation of either vasoconstrictor with nimodipine. The reason for this peculiar sensitivity of 5-HT-induced vasoconstriction, relative to ET-1 and PE is at present unclear. Ca2+-independent modulation of the contractile apparatus may differ among various vasoactive agents.33 ET-1 induces vasoconstriction through its ETA receptor. ETAR is a G-protein-coupled receptor, which modulates Gq and G12.34, 35 ET-1 vasoconstriction is dependent on Ca2+; however it does not activate VDCC. It mobilizes Ca2+ through non-voltage dependent channels such as store-operated Ca2+ channels (SOCCs) and nonselective cation channels (NSCCs) each regulated by Gq and G12, respectively.34 Furthermore, ET-1 can increase myosin phosphorylation and the force of contraction through activation of the rho-rho kinase pathway in vascular smooth muscle and this mechanism is independent of cytosolic Ca2+ concentration;36, 37 the activation of this pathway by ET-1 may be particularly effective.38, 39 PE activates vasoconstriction through alpha-1 adrenergic receptors, which are G-protein-coupled receptors that modulate Gq, which in turns activates PLC and IP3 mediated Ca2+ release. This mechanism is similar to 5-HT2A receptors, which play a role in mediating the vasoconstrictive properties of 5-HT in the femoral artery, however there was no potentiation of dantrolene with nimodipine in response to PE in contrast to the EC50 for 5-HT in the femoral artery. Possible explanations for this difference, although unexplored, are potential differential regulation of cyclic ADP ribose (cADPR) and/or nicotinic acid adenine dinucleotide phosphate (NAADP) by 5-HT receptors. Both cADPR and NAADP are secondary signaling molecules that can stimulate ryanodine receptor Ca2+ release and potentiate CICR.40–42
The therapeutic significance of VDCC blockade has been well established for decades, because selective drugs, i.e. calcium antagonists, have been available.43 In contrast, the therapeutic significance of RyR blockade is largely unknown, because, except for dantrolene, available RyR ligands are therapeutically unusable due to toxicity, non-specific blockade of RyR1, 2 or 3, or pharmacologically unsuitable (irreversible binding to RyR, opening/blocking mixed activity of RyR).44 Furthermore, mutant mice lacking RyR3 do not show a dramatic vascular phenotype,45 except for a modest increase in myogenic tone,46 suggesting that pharmacological blockade of these channels might not produce detectable physiologic changes in vascular tone under most circumstances, despite ex vivo evidence that RYR regulates arterial diameter.47 Activation of RyR located in proximity to the plasma membrane by extracellular Ca2+ influx through VDCC produces CICR (Ca2+sparks), which in turn modulates plasma membrane excitability (e.g., hyperpolarization) through activation of Ca2+-dependent K+ channels; hyperpolarization then produces vasodilatation, by decreasing the probability of opening of VDCC.48 It therefore appears that regulation of K+ channels expression and function may profoundly affect the impact of CICR on vascular tone. Consistent with this view is evidence that vascular Ca2+-dependent K+ channels with reduced function are associated with greater depolarization and increased vascular tone.49 At variance with CICR occurring close to plasma membrane, CICR from RyR at SR locations further away from the plasma membrane could propagate Ca2+ waves that induce vasoconstriction.50
Although efficacy in a pathophysiological model was not tested in this paper, it is important to note that for SAH in animal models and humans, 5-HT, a potent vasoconstrictor, is released from activated platelets. After SAH evidence supports an upregulation of 5-HT1B receptors and enhanced vasoconstriction in response to 5-HT with significant reductions in cerebral blood flow.51–53 Other drug treatments, particularly calcium channel blockers, have been proposed to prevent and/or treat the vasospasm of reversible cerebral vasoconstrictive syndromes (e.g., Call-Fleming)54 and SAH55 (despite lack of evidence of vasospastic relaxation in humans). However, one potential limitation these drugs are systemic vasodilatation that may lead to hypotension and further reduce cerebral perfusion pressure and cerebral blood flow.56 Dantrolene at IV doses of 2.5mg/kg (10μM), lower than the most effective dose tested here, does not appear to cause hypotension, even in the presence of nimodipine and may have a vasorelaxation effect in humans.57
In conclusion, dantrolene has synergistic effects with nimodipine against 5-HT-induced vasoconstriction in isolated cerebral arteries. The association dantrolene-nimodipine might be a promising treatment for reducing SAH-related vasospasm or other serotonin-related vasospastic syndromes, such as Call-Fleming syndrome, but requires testing in a pathophysiological model.
Acknowledgments
Dr. John Sims is supported by NIH 1 K08 NS049241-01A2.
Footnotes
DISCLOSURES: None of the authors have any conflict of interest.
Bibliography
- 1.Krause T, Gerbershagen MU, Fiege M, Weisshorn R, Wappler F. Dantrolene--a review of its pharmacology, therapeutic use and new developments. Anaesthesia. 2004;59(4):364–73. doi: 10.1111/j.1365-2044.2004.03658.x. [DOI] [PubMed] [Google Scholar]
- 2.Endo M. Calcium ion as a second messenger with special reference to excitation-contraction coupling. J Pharmacol Sci. 2006;100(5):519–24. doi: 10.1254/jphs.cpj06004x. [DOI] [PubMed] [Google Scholar]
- 3.Zhao F, Li P, Chen SR, Louis CF, Fruen BR. Dantrolene inhibition of ryanodine receptor Ca2+ release channels. Molecular mechanism and isoform selectivity. The Journal of biological chemistry. 2001;276(17):13810–6. doi: 10.1074/jbc.M006104200. [DOI] [PubMed] [Google Scholar]
- 4.Dorsch NW. Cerebral arterial spasm--a clinical review. Br J Neurosurg. 1995;9(3):403–12. doi: 10.1080/02688699550041403. [DOI] [PubMed] [Google Scholar]
- 5.Tani E, Matsumoto T. Continuous elevation of intracellular Ca2+ is essential for the development of cerebral vasospasm. Curr Vasc Pharmacol. 2004;2(1):13–21. doi: 10.2174/1570161043476492. [DOI] [PubMed] [Google Scholar]
- 6.Beg SS, Hansen-Schwartz JA, Vikman PJ, Xu CB, Edvinsson LI. Protein kinase C inhibition prevents upregulation of vascular ET(B) and 5-HT(1B) receptors and reverses cerebral blood flow reduction after subarachnoid haemorrhage in rats. J Cereb Blood Flow Metab. 2007;27(1):21–32. doi: 10.1038/sj.jcbfm.9600313. [DOI] [PubMed] [Google Scholar]
- 7.Ansar S, Svendgaard NA, Edvinsson L. Neurokinin-1 receptor antagonism in a rat model of subarachnoid hemorrhage: prevention of upregulation of contractile ETB and 5-HT1B receptors and cerebral blood flow reduction. J Neurosurg. 2007;106(5):881–6. doi: 10.3171/jns.2007.106.5.881. [DOI] [PubMed] [Google Scholar]
- 8.Alafaci C, Jansen I, Arbab MA, Shiokawa Y, Svendgaard NA, Edvinsson L. Enhanced vasoconstrictor effect of endothelin in cerebral arteries from rats with subarachnoid haemorrhage. Acta Physiol Scand. 1990;138(3):317–9. doi: 10.1111/j.1748-1716.1990.tb08852.x. [DOI] [PubMed] [Google Scholar]
- 9.Dorhout Mees SM, Rinkel GJ, Feigin VL, et al. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2007;3:CD000277. doi: 10.1002/14651858.CD000277.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godfraind T, Miller R, Wibo M. Calcium antagonism and calcium entry blockade. Pharmacol Rev. 1986;38(4):321–416. [PubMed] [Google Scholar]
- 11.Zhang AY, Li PL. Vascular physiology of a Ca2+ mobilizing second messenger - cyclic ADP-ribose. Journal of cellular and molecular medicine. 2006;10(2):407–22. doi: 10.1111/j.1582-4934.2006.tb00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pacaud P, Loirand G. Release of Ca2+ by noradrenaline and ATP from the same Ca2+ store sensitive to both InsP3 and Ca2+ in rat portal vein myocytes. J Physiol. 1995;484 (Pt 3):549–55. doi: 10.1113/jphysiol.1995.sp020685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamishima T, McCarron JG. Regulation of the cytosolic Ca2+ concentration by Ca2+ stores in single smooth muscle cells from rat cerebral arteries. J Physiol. 1997;501 (Pt 3):497–508. doi: 10.1111/j.1469-7793.1997.497bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asano M, Kuwako M, Nomura Y, et al. Possible mechanism of the potent vasoconstrictor responses to ryanodine in dog cerebral arteries. Eur J Pharmacol. 1996;311(1):53–60. doi: 10.1016/0014-2999(96)00408-6. [DOI] [PubMed] [Google Scholar]
- 15.Loch Macdonald R. Management of cerebral vasospasm. Neurosurg Rev. 2006;29(3):179–93. doi: 10.1007/s10143-005-0013-5. [DOI] [PubMed] [Google Scholar]
- 16.Muehlschlegel S, Sims JR. Dantrolene: Mechanisms of Neuroprotection and Possible Clinical Applications in the Neurointensive Care Unit. Neurocritical care. 2008 doi: 10.1007/s12028-008-9133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41(1):19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- 18.Ramsch KD, Ahr G, Tettenborn D, Auer LM. Overview on pharmacokinetics of nimodipine in healthy volunteers and in patients with subarachnoid hemorrhage. Neurochirurgia. 1985;28 (Suppl 1):74–8. doi: 10.1055/s-2008-1054107. [DOI] [PubMed] [Google Scholar]
- 19.Schilling L, Vatter H, Mursch K, Ehrenreich H, Schmiedek P. Characterization of the contractile and relaxant action of the endothelin-1 precursor, big endothelin-1, in the isolated rat basilar artery. Peptides. 2000;21(1):91–9. doi: 10.1016/s0196-9781(99)00179-5. [DOI] [PubMed] [Google Scholar]
- 20.Salomone S, Morel N, Godfraind T. Role of nitric oxide in the contractile response to 5-hydroxytryptamine of the basilar artery from Wistar Kyoto and stroke-prone rats. Br J Pharmacol. 1997;121(6):1051–8. doi: 10.1038/sj.bjp.0701227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishimura Y. Characterization of 5-hydroxytryptamine receptors mediating contractions in basilar arteries from stroke-prone spontaneously hypertensive rats. Br J Pharmacol. 1996;117(6):1325–33. doi: 10.1111/j.1476-5381.1996.tb16732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackowski A, Crockard A, Burnstock G, Lincoln J. Alterations in serotonin and neuropeptide Y content of cerebrovascular sympathetic nerves following experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab. 1989;9(3):271–9. doi: 10.1038/jcbfm.1989.45. [DOI] [PubMed] [Google Scholar]
- 23.Cambj-Sapunar L, Yu M, Harder DR, Roman RJ. Contribution of 5-hydroxytryptamine1B receptors and 20-hydroxyeiscosatetraenoic acid to fall in cerebral blood flow after subarachnoid hemorrhage. Stroke. 2003;34(5):1269–75. doi: 10.1161/01.STR.0000065829.45234.69. [DOI] [PubMed] [Google Scholar]
- 24.Chang JY, Hardebo JE, Owman C. Differential vasomotor action of noradrenaline, serotonin, and histamine in isolated basilar artery from rat and guinea-pig. Acta Physiol Scand. 1988;132(1):91–102. doi: 10.1111/j.1748-1716.1988.tb08302.x. [DOI] [PubMed] [Google Scholar]
- 25.Kitazono T, Faraci FM, Heistad DD. Effect of norepinephrine on rat basilar artery in vivo. The American journal of physiology. 1993;264(1 Pt 2):H178–82. doi: 10.1152/ajpheart.1993.264.1.H178. [DOI] [PubMed] [Google Scholar]
- 26.Kaumann AJ, Levy FO. 5-hydroxytryptamine receptors in the human cardiovascular system. Pharmacology & therapeutics. 2006;111(3):674–706. doi: 10.1016/j.pharmthera.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Villalon CM, Centurion D. Cardiovascular responses produced by 5-hydroxytriptamine:a pharmacological update on the receptors/mechanisms involved and therapeutic implications. Naunyn-Schmiedeberg’s archives of pharmacology. 2007;376(1–2):45–63. doi: 10.1007/s00210-007-0179-1. [DOI] [PubMed] [Google Scholar]
- 28.Parsons AA, Whalley ET. Evidence for the presence of 5-HT1-like receptors in rabbit isolated basilar arteries. Eur J Pharmacol. 1989;174(2–3):189–96. doi: 10.1016/0014-2999(89)90311-7. [DOI] [PubMed] [Google Scholar]
- 29.Parsons AA, Whalley ET, Feniuk W, Connor HE, Humphrey PP. 5-HT1-like receptors mediate 5-hydroxytryptamine-induced contraction of human isolated basilar artery. Br J Pharmacol. 1989;96(2):434–40. doi: 10.1111/j.1476-5381.1989.tb11835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bae YM, Kim A, Kim J, et al. Serotonin depolarizes the membrane potential in rat mesenteric artery myocytes by decreasing voltage-gated K+ currents. Biochem Biophys Res Commun. 2006;347(2):468–76. doi: 10.1016/j.bbrc.2006.06.116. [DOI] [PubMed] [Google Scholar]
- 31.Bae YM, Kim A, Lee YJ, et al. Enhancement of receptor-operated cation current and TRPC6 expression in arterial smooth muscle cells of deoxycorticosterone acetate-salt hypertensive rats. J Hypertens. 2007;25(4):809–17. doi: 10.1097/HJH.0b013e3280148312. [DOI] [PubMed] [Google Scholar]
- 32.MacMillan D, Chalmers S, Muir TC, McCarron JG. IP3-mediated Ca2+ increases do not involve the ryanodine receptor, but ryanodine receptor antagonists reduce IP3-mediated Ca2+ increases in guinea-pig colonic smooth muscle cells. J Physiol. 2005;569(Pt 2):533–44. doi: 10.1113/jphysiol.2005.096529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakurada S, Okamoto H, Takuwa N, Sugimoto N, Takuwa Y. Rho activation in excitatory agonist-stimulated vascular smooth muscle. Am J Physiol Cell Physiol. 2001;281(2):C571–8. doi: 10.1152/ajpcell.2001.281.2.C571. [DOI] [PubMed] [Google Scholar]
- 34.Kawanabe Y, Nauli SM. Involvement of extracellular Ca2+ influx through voltage-independent Ca2+ channels in endothelin-1 function. Cellular signalling. 2005;17(8):911–6. doi: 10.1016/j.cellsig.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Kawanabe Y, Masaki T, Hashimoto N. Involvement of phospholipase C in endothelin 1-induced stimulation of Ca++ channels and basilar artery contraction in rabbits. J Neurosurg. 2006;105(2):288–93. doi: 10.3171/jns.2006.105.2.288. [DOI] [PubMed] [Google Scholar]
- 36.Evans AM, Cobban HJ, Nixon GF. ET(A) receptors are the primary mediators of myofilament calcium sensitization induced by ET-1 in rat pulmonary artery smooth muscle: a tyrosine kinase independent pathway. Br J Pharmacol. 1999;127(1):153–60. doi: 10.1038/sj.bjp.0702548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Somlyo AP, Somlyo AV. From pharmacomechanical coupling to G-proteins and myosin phosphatase. Acta Physiol Scand. 1998;164(4):437–48. doi: 10.1046/j.1365-201X.1998.00454.x. [DOI] [PubMed] [Google Scholar]
- 38.Miao L, Dai Y, Zhang J. Mechanism of RhoA/Rho kinase activation in endothelin-1- induced contraction in rabbit basilar artery. Am J Physiol Heart Circ Physiol. 2002;283(3):H983–9. doi: 10.1152/ajpheart.00141.2002. [DOI] [PubMed] [Google Scholar]
- 39.Scherer EQ, Herzog M, Wangemann P. Endothelin-1-induced vasospasms of spiral modiolar artery are mediated by rho-kinase-induced Ca(2+) sensitization of contractile apparatus and reversed by calcitonin gene-related Peptide. Stroke. 2002;33(12):2965–71. doi: 10.1161/01.str.0000043673.22993.fd. [DOI] [PubMed] [Google Scholar]
- 40.Churchill GC, Galione A. NAADP induces Ca2+ oscillations via a two-pool mechanism by priming IP3- and cADPR-sensitive Ca2+ stores. The EMBO journal. 2001;20(11):2666–71. doi: 10.1093/emboj/20.11.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higashida H. ADP-ribosyl cyclase coupled with receptors via G proteins. FEBS letters. 1997;418(3):355–6. doi: 10.1016/s0014-5793(97)01410-5. [DOI] [PubMed] [Google Scholar]
- 42.Wilson HL, Dipp M, Thomas JM, Lad C, Galione A, Evans AM. Adp-ribosyl cyclase and cyclic ADP-ribose hydrolase act as a redox sensor. a primary role for cyclic ADP-ribose in hypoxic pulmonary vasoconstriction. The Journal of biological chemistry. 2001;276(14):11180–8. doi: 10.1074/jbc.M004849200. [DOI] [PubMed] [Google Scholar]
- 43.Godfraind T. Calcium-channel modulators for cardiovascular disease. Expert Opin Emerg Drugs. 2006;11(1):49–73. doi: 10.1517/14728214.11.1.49. [DOI] [PubMed] [Google Scholar]
- 44.Laporte R, Hui A, Laher I. Pharmacological modulation of sarcoplasmic reticulum function in smooth muscle. Pharmacol Rev. 2004;56(4):439–513. doi: 10.1124/pr.56.4.1. [DOI] [PubMed] [Google Scholar]
- 45.Takeshima H, Ikemoto T, Nishi M, et al. Generation and characterization of mutant mice lacking ryanodine receptor type 3. The Journal of biological chemistry. 1996;271(33):19649–52. doi: 10.1074/jbc.271.33.19649. [DOI] [PubMed] [Google Scholar]
- 46.Lohn M, Jessner W, Furstenau M, et al. Regulation of calcium sparks and spontaneous transient outward currents by RyR3 in arterial vascular smooth muscle cells. Circ Res. 2001;89(11):1051–7. doi: 10.1161/hh2301.100250. [DOI] [PubMed] [Google Scholar]
- 47.Knot HJ, Standen NB, Nelson MT. Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels. J Physiol. 1998;508 (Pt 1):211–21. doi: 10.1111/j.1469-7793.1998.211br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perez GJ, Bonev AD, Patlak JB, Nelson MT. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J Gen Physiol. 1999;113(2):229–38. doi: 10.1085/jgp.113.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amberg GC, Bonev AD, Rossow CF, Nelson MT, Santana LF. Modulation of the molecular composition of large conductance, Ca(2+) activated K(+) channels in vascular smooth muscle during hypertension. J Clin Invest. 2003;112(5):717–24. doi: 10.1172/JCI18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collier ML, Ji G, Wang Y, Kotlikoff MI. Calcium-induced calcium release in smooth muscle: loose coupling between the action potential and calcium release. J Gen Physiol. 2000;115(5):653–62. doi: 10.1085/jgp.115.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hansen-Schwartz J, Ansar S, Edvinsson L. Cerebral vasoconstriction after subarachnoid hemorrhage--role of changes in vascular receptor phenotype. Front Biosci. 2008;13:2160–4. doi: 10.2741/2831. [DOI] [PubMed] [Google Scholar]
- 52.Mendelow AD, McCalden TA, Hattingh J, Coull A, Rosendorff C, Eidelman BH. Cerebrovascular reactivity and metabolism after subarachnoid hemorrhage in baboons. Stroke. 1981;12(1):58–65. doi: 10.1161/01.str.12.1.58. [DOI] [PubMed] [Google Scholar]
- 53.Sahlin C, Owman C, Chang JY, Delgado T, Salford LG, Svendgaard NA. Changes in contractile response and effect of a calcium antagonist, nimodipine, in isolated intracranial arteries of baboon following experimental subarachnoid hemorrhage. Brain research bulletin. 1990;24(3):355–61. doi: 10.1016/0361-9230(90)90089-i. [DOI] [PubMed] [Google Scholar]
- 54.Calabrese LH, Dodick DW, Schwedt TJ, Singhal AB. Narrative review: reversible cerebral vasoconstriction syndromes. Annals of internal medicine. 2007;146(1):34–44. doi: 10.7326/0003-4819-146-1-200701020-00007. [DOI] [PubMed] [Google Scholar]
- 55.Weyer GW, Nolan CP, Macdonald RL. Evidence-based cerebral vasospasm management. Neurosurgical focus. 2006;21(3):E8. doi: 10.3171/foc.2006.21.3.8. [DOI] [PubMed] [Google Scholar]
- 56.Dodick DW. Reversible segmental cerebral vasoconstriction (Call-Fleming syndrome): the role of calcium antagonists. Cephalalgia. 2003;23(3):163–5. doi: 10.1046/j.1468-2982.2003.00506.x. [DOI] [PubMed] [Google Scholar]
- 57.Muehlschlegel S, Rordorf G, Bodock M, Sims JR. Dantrolene Mediates Vasorelaxation in Cerebral Vasoconstriction: A Case Series. Neurocritical care. 2008 doi: 10.1007/s12028-008-9132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]