Abstract
AIM: To evaluate the proportion of successful complete cure en-bloc resections of large colorectal polyps achieved by endoscopic mucosal resection (EMR).
METHODS: Studies using the EMR technique to resect large colorectal polyps were selected. Successful complete cure en-bloc resection was defined as one piece margin-free polyp resection. Articles were searched for in Medline, Pubmed, and the Cochrane Control Trial Registry, among other sources.
RESULTS: An initial search identified 2620 reference articles, from which 429 relevant articles were selected and reviewed. Data was extracted from 25 studies (n = 5221) which met the inclusion criteria. All the studies used snares to perform EMR. Pooled proportion of en-bloc resections using a random effect model was 62.85% (95% CI: 51.50-73.52). The pooled proportion for complete cure en-bloc resections using a random effect model was 58.66% (95% CI: 47.14-69.71). With higher patient load (> 200 patients), this complete cure en-bloc resection rate improves from 44.19% (95% CI: 24.31-65.09) to 69.17% (95% CI: 51.11-84.61).
CONCLUSION: EMR is an effective technique for the resection of large colorectal polyps and offers an alternative to surgery.
Keywords: Meta-analysis, Systematic review, Polyps, Endoscopic mucosal resection, En-bloc resection
INTRODUCTION
The use of endoscopic mucosal resection (EMR), pioneered in Japan for the treatment of early gastric cancer, has expanded to include therapy of other early gastrointestinal malignancies and pre-cancerous lesions such as adenomas. At the same time, this technique has gained acceptance in Europe and in the US, especially for the treatment of Barrett’s esophagus with high grade dysplasia[1-3]. Several variations of the EMR technique have been devised such as inject-lift-cut, strip biopsy, suction cup (EMRC), and EMR with a ligating device.
Throughout the world, adenomas of the colorectum represent the single most important premalignant lesion of the GI tract. Large (> 2 cm) colorectal polyps have been found in 0.8%-5.2% of patients undergoing colonoscopies for different indications[4].
Large sessile and flat polyps represent a major technical challenge to conventional snare resection. Additional procedures and therapies such as Argon plasma coagulation are frequently needed to destroy remnant tissue after resection[5]. When these techniques are not used or possible, patients are frequently referred for surgical resection[6].
EMR has been shown to be useful in the removal of large colorectal sessile and flat lesions[7]. However, there are limits to the size of lesions which can be removed en-bloc with the various EMR techniques, with 1.5-2 cm generally being the upper limit[8].
En-bloc removal of large polyps is desirable as it facilitates thorough histological evaluation related to the completeness of resection, and is associated with a lower recurrence rate as compared to piecemeal removal[9-14].
MATERIALS AND METHODS
Study selection criteria
Studies using EMR technique to resect large (> 2 cm) colorectal polyps were selected. Successful cure en-bloc resection was defined as one piece removal with tumor-free vertical and lateral margins.
Data collection and extraction
Articles were searched for in Medline, Pubmed, Ovid journals, Japanese language literature, Cumulative Index for Nursing & Allied Health Literature, ACP journal club, DARE, International Pharmaceutical Abstracts, old Medline, Medline non-indexed citations, OVID Healthstar, and the Cochrane Controlled Trials Registry. The search terms used were EMR, endoscopic mucosal resection, colon polyps, lateral spreading tumors, large polyps, nonpolypoid colon lesions, flat colon polyps, and flat adenomas. Two authors (SP and YK) independently searched and extracted the data for revising into an abstracted form. Any differences were resolved by mutual agreement.
Quality of studies
Clinical trials with a control arm can be assessed for the quality of the study. A number of criteria have been used to assess the quality of a study (e.g. randomization, selection bias of the arms in the study, concealment of allocation, and blinding of outcome)[15,16]. There is no consensus regarding how to assess studies without a control arm. Hence, these criteria do not apply to studies without a control arm[16]. Therefore, for this meta-analysis and systematic review, studies were selected based on completeness of data and inclusion criteria.
Statistical methods
This meta-analysis was performed by calculating pooled proportions, i.e. pooled proportion of en-bloc resections and complete cure en-bloc resections. Firstly, the individual study proportions of successful resections were transformed into a quantity using Freeman-Tukey variant of the arcsine square root transformed proportion. The pooled proportion was calculated as the back-transform of the weighted mean of the transformed proportions, using inverse arcsine variance weights for the fixed effects model and DerSimonian-Laird weights for the random effects model[17,18]. Forrest plots were drawn to show the point estimates in each study in relation to the summary pooled estimate. The width of the point estimates in the Forrest plots indicated the assigned weight to that study. The heterogeneity among studies was tested using Cochran’s Q test based upon inverse variance weights[19]. If P value was > 0.10, the null hypothesis was rejected that the studies were heterogeneous. The effects of publication and selection bias on the summary estimates were tested by Begg-Mazumdar bias indicator[20]. Also, funnel plots were constructed to evaluate potential publication bias using the standard error and diagnostic odds ratio[21,22].
RESULTS
An initial search identified 2620 reference articles from which 429 relevant articles were selected and reviewed. Data was extracted from 25 studies (n = 5221) which met the inclusion criteria[23-46]. The search results are shown in Figure 1. All the studies used snare to perform EMR. Two studies used a strip biopsy technique[42,43]. The mean size of the polyps was 22.48 ± 4.52 mm. There were 3755 successful en-bloc resections. The study characteristics are shown in Table 1.
Figure 1.
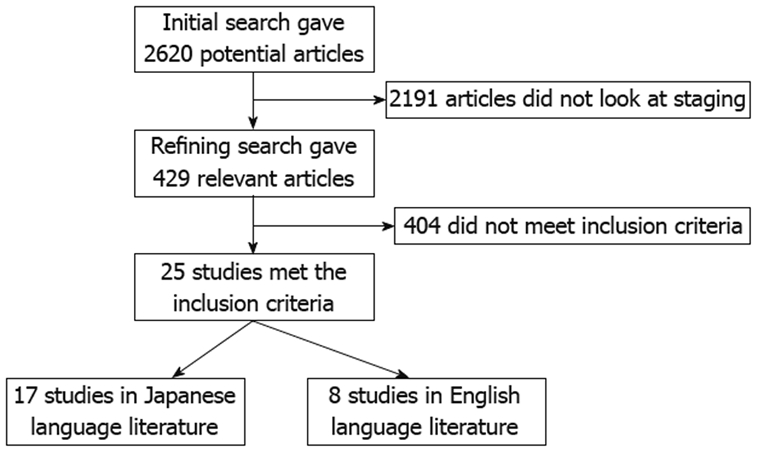
Search results.
Table 1.
Study characteristics
| Author, yr | Instrument used | n | Type of polyp | Technique | |
| 1 | Matsushita et al[23], 2003 | Snare | 935 | No information | EMR |
| 2 | Imai et al[24], 1999 | Snare | 30 | No information | EMR |
| 3 | Igarashi et al[25], 1999 | Snare | 884 | No information | EMR |
| 4 | Oka et al[26], 2005 | Snare | 410 | Lateral spreading tumor | EMR |
| 5 | Sano et al[27], 2004 | Snare | 392 | Lateral spreading tumor | EMR |
| 6 | Hotta et al[28], 2003 | Snare | 284 | Protrusion 68, flat 213, depressed 3 | EMR |
| 7 | Matsuda et al[29], 2006 | Snare | 154 | Is,Isp 33, LST-G 96, NG 25 | EMR |
| 8 | Yasumoto et al[30], 2005 | Snare | 240 | LST-G 180, NG 60 | EMR |
| 9 | Terai et al[31], 2003 | Snare | 223 | Lateral Spreading tumor | EMR |
| 10 | Nozaki et al[32], 2006 | Snare | 198 | Ip 3, Isp 34, Is 7, LST-G 85, NG 28 | EMR |
| 11 | Watari et al[33], 1998 | Snare | 186 | Lateral spreading tumor | EMR |
| 12 | Sugisaka et al[34], 2003 | Snare | 162 | No information | EMR |
| 13 | Matsunaga et al[35], 1999 | Snare | 134 | No information | EMR |
| 14 | Nomura et al[36], 2001 | Snare | 54 | No information | EMR |
| 15 | Kobayashi et al[37], 1999 | Snare | 131 | No information | EMR |
| 16 | Nakajima et al[38], 2006 | Snare | 52 | No information | EMR |
| 17 | Cho et al[39], 1999 | Snare | 34 | No information | EMR |
| 18 | Saito et al[40], 2001 | Snare | 170 | Lateral spreading tumor | EMR |
| 19 | Tanaka et al[13], 2001 | Snare with needle spike | 81 | Lateral spreading tumor | EMR |
| 20 | Ahmad et al[41], 2002 | Snare with suction | 41 | Colon and rectum | EMR |
| 21 | Hurlstone et al[42], 2004 | Strip technique of Karita | 80 | Rectal villous adenoma | EMR |
| 22 | Hurlstone et al[43], 2005 | Strip technique of Karita | 62 | Rectal villous adenoma | EMR |
| 23 | Su et al[44], 2005 | Snare with needle spike | 152 | Colonic nonpolypoid lesions | EMR |
| 24 | Uraoka et al[45], 2005 | Snare | 113 | Lateral spreading tumor | EMR |
| 25 | Kawamura et al[46], 1999 | Snare | 19 | Submucosal invasive colorectal cancers | EMR |
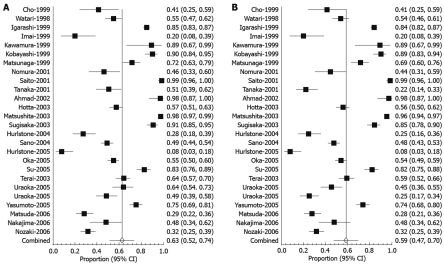
The pooled proportion of en-bloc resections using a random effect model was 62.85% (95% CI: 51.50-73.52). Forest plot in Figure 2A depicts the individual study proportion of successful en-bloc resections in relation to the pooled estimate. The pooled proportion for complete cure en-bloc resections using a random effect model was 58.66% (95% CI: 47.14-69.71). Figure 2B shows Forrest plot depicting the individual study successful cure en-bloc resections in relation to the pooled estimate. The fixed effect model was not used because of the heterogeneity of studies.
Figure 2.
Forrest plot showing successful en-bloc (A) and cure en-bloc (B) resection.
Subgroup analysis was carried out by grouping studies according to the study population. This was done because the expertise needed to perform procedures might have affected the outcome. Studies were categorized into three groups: < 100 patients, 100-200 patients and > 200 patients. The proportions for successful en-bloc and successful cure en-bloc resections are shown in Table 2.
Table 2.
Results based on study size
| Study size | No. of studies | Successful en-bloc resection (95% CI) | Successful cure en-bloc resection (95% CI) |
| < 100 patients | 9 | 48.07% (28.36-68.09) | 44.19% (24.31-65.09) |
| 100-200 patients | 9 | 68.93% (50.39-84.76) | 63.32% (43.50-81.04) |
| > 200 patients | 7 | 71.39% (52.24-87.20) | 69.17% (51.11-84.61) |
The publication bias calculated by Begg-Mazumdar bias indicator for successful cure en-bloc resections concluded that the Kendall’s tau b value was -0.19 (P = 0.17). The funnel plot in Figure 3 shows that there was no publication bias for successful cure en-bloc resections.
Figure 3.
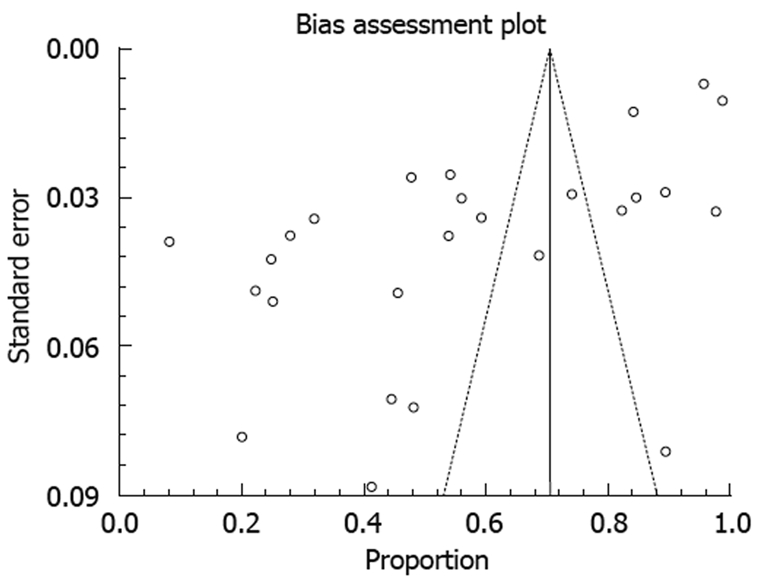
Funnel plot showing publication bias for successful cure en-bloc resection.
DISCUSSION
Some colorectal cancers develop from adenomas. The risk of high grade dysplasia and cancer increases with the size of the lesion. Endoscopic removal of large (> 2 cm) sessile and flat polyps represents a difficult challenge for conventional snare resection and they are frequently managed by piecemeal resection or surgically[6,47]. EMR was the definitive procedure in all the collated studies. The data for complications was not available for the majority of the studies, so this data was not collected. EMR is a technique that can be applied to sessile and flat lesions. Though initially used for the treatment of early gastric cancer in Japan, the technique has been expanded to the therapy of large colorectal neoplasms[7].
This meta-analysis revealed that en-bloc resection was achieved in 62.85% of lesions and tumor-free vertical and lateral margins were achieved in 58.6%. These results compare well to en-bloc resection rates achieved by conventional polypectomy snare, which have been reported to be between 7% and 34% for large sessile polyps[6,48].
Furthermore, our meta-analysis revealed that experience performing EMR plays an important role in achieving a better en-bloc resection and cure en-bloc tumor-free rate. Studies reporting more than 200 lesions removed reported a 71.39% en-bloc resection of lesions and tumor-free vertical and lateral margins in 69.17% of cases, while studies reporting less than a 100 lesions reported a 48.07% en-bloc removal and tumor-free vertical and lateral margins in 44.19% of cases. This indicates that experience in the technique of EMR increase the cure en-bloc rate.
In the present meta-analysis we searched the world literature which included articles published in Japanese language literature. We believe that our results are a reasonable reflection of the status of EMR in the therapy of large colorectal polyps.
EMR is an effective technique for resection of large colorectal polyps. The technique offers an alternative to surgery. This meta-analysis shows that the success rate for en-bloc margin-free resection is not high but improves with experience. Improvements in techniques and equipment are needed to increase complete cure en-bloc resection rates.
COMMENTS
Background
Endoscopic mucosal resection (EMR) has emerged as an alternative to surgery for the resection of large colorectal polyps. Complete cure with tumor-free lateral and vertical margins would prevent further therapy. Published data regarding successful en-bloc resection with tumor-free margins by EMR has been varied.
Innovations and breakthroughs
EMR has been shown to be useful in the removal of large colorectal sessile and flat lesions. However, there are limits to the size of lesions which can be removed en-bloc with the various EMR techniques, with 1.5-2 cm generally being the upper limit. En-bloc removal of large polyps is desirable as it facilitates thorough histological evaluation related to the completeness of resection, and is associated with a lower recurrence rate as compared to piecemeal removal.
Applications
EMR is an effective technique for resection of large colorectal polyps and offers an alternative to surgery. This meta-analysis shows that the success rate for en-bloc margin-free resection is not high but improves with experience. Improvements in techniques and equipment are needed to increase complete cure en-bloc resection.
Peer review
The authors evaluated the proportion of successful complete cure en-bloc resections of large colorectal polyps achieved by EMR. They found that EMR is an effective technique for resection of large colorectal polyps.This article is well written and easy to read.
Footnotes
Peer reviewer: Zvi Fireman, MD, Associate Professor of Medicine, Head, Gastroenterology Department, Hillel Yaffe Med Ctr, PO Box 169, 38100, Hadera, Israel
S- Editor Cheng JX L- Editor Logan S E- Editor Zheng XM
References
- 1.Kojima T, Parra-Blanco A, Takahashi H, Fujita R. Outcome of endoscopic mucosal resection for early gastric cancer: review of the Japanese literature. Gastrointest Endosc. 1998;48:550–554; discussion 554-555. doi: 10.1016/s0016-5107(98)70108-7. [DOI] [PubMed] [Google Scholar]
- 2.Gotoda T, Kondo H, Ono H, Saito Y, Yamaguchi H, Saito D, Yokota T. A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: report of two cases. Gastrointest Endosc. 1999;50:560–563. doi: 10.1016/s0016-5107(99)70084-2. [DOI] [PubMed] [Google Scholar]
- 3.Conio M, Cameron AJ, Chak A, Blanchi S, Filiberti R. Endoscopic treatment of high-grade dysplasia and early cancer in Barrett's oesophagus. Lancet Oncol. 2005;6:311–321. doi: 10.1016/S1470-2045(05)70167-4. [DOI] [PubMed] [Google Scholar]
- 4.Fukami N, Lee JH. Endoscopic treatment of large sessile and flat colorectal lesions. Curr Opin Gastroenterol. 2006;22:54–59. doi: 10.1097/01.mog.0000198075.59910.1f. [DOI] [PubMed] [Google Scholar]
- 5.Zlatanic J, Waye JD, Kim PS, Baiocco PJ, Gleim GW. Large sessile colonic adenomas: use of argon plasma coagulator to supplement piecemeal snare polypectomy. Gastrointest Endosc. 1999;49:731–735. doi: 10.1016/s0016-5107(99)70291-9. [DOI] [PubMed] [Google Scholar]
- 6.Church JM. Avoiding surgery in patients with colorectal polyps. Dis Colon Rectum. 2003;46:1513–1516. doi: 10.1007/s10350-004-6805-9. [DOI] [PubMed] [Google Scholar]
- 7.Jameel JK, Pillinger SH, Moncur P, Tsai HH, Duthie GS. Endoscopic mucosal resection (EMR) in the management of large colo-rectal polyps. Colorectal Dis. 2006;8:497–500. doi: 10.1111/j.1463-1318.2006.00966.x. [DOI] [PubMed] [Google Scholar]
- 8.Seewald S, Soehendra N. Perforation: part and parcel of endoscopic resection? Gastrointest Endosc. 2006;63:602–605. doi: 10.1016/j.gie.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe K, Ogata S, Kawazoe S, Watanabe K, Koyama T, Kajiwara T, Shimoda Y, Takase Y, Irie K, Mizuguchi M, et al. Clinical outcomes of EMR for gastric tumors: historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc. 2006;63:776–782. doi: 10.1016/j.gie.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 10.Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877–883. doi: 10.1016/j.gie.2006.03.932. [DOI] [PubMed] [Google Scholar]
- 11.Fujishiro M, Yahagi N, Nakamura M, Kakushima N, Kodashima S, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A, et al. Endoscopic submucosal dissection for rectal epithelial neoplasia. Endoscopy. 2006;38:493–497. doi: 10.1055/s-2006-925398. [DOI] [PubMed] [Google Scholar]
- 12.Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Ichinose M, Omata M. Successful endoscopic en bloc resection of a large laterally spreading tumor in the rectosigmoid junction by endoscopic submucosal dissection. Gastrointest Endosc. 2006;63:178–183. doi: 10.1016/j.gie.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka S, Haruma K, Oka S, Takahashi R, Kunihiro M, Kitadai Y, Yoshihara M, Shimamoto F, Chayama K. Clinicopathologic features and endoscopic treatment of superficially spreading colorectal neoplasms larger than 20 mm. Gastrointest Endosc. 2001;54:62–66. doi: 10.1067/mge.2001.115729. [DOI] [PubMed] [Google Scholar]
- 14.Chiu PW. Endoscopic submucosal dissection-bigger piece, better outcome! Gastrointest Endosc. 2006;64:884–885. doi: 10.1016/j.gie.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 16.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 17.Stuart A, Ord JK. Kendall's Advanced Theory of Statistics. 6th ed. London: Edward Arnold; 1994. pp. 71–84. [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Deeks JJ. Systematic reviews of evaluations of diagnostic and screening tests. In: Egger M, Smith GD, Altman DG, editors. Systematic reviews in health care: meta-analysis in context. 2nd ed. London: BMJ Books; 2001. pp. 40–58. [Google Scholar]
- 20.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 21.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 23.Matsushita H, Yamano H, Imai Y, Nakazato M, Maeda S, Sato K, Fujita K, Yamanaka Y, Ono H. Strategy for residual/recurrent colorectal tumors. Early colorectal cancer. 2003;7:531–537. [Google Scholar]
- 24.Imai Y, Kudo S, Yamano H. A study of resectability of endoscopic mucosal resection (EMR) and endoscopic piecemeal mucosal resection (EPMR) for colorectal neoplasm. Early colorectal cancer. 1999;3:23–26. [Google Scholar]
- 25.Igarashi M, Katsumata T, Kobayashi K, Takahashi H, Yokoyama K. Study of surveillance colonoscopy and local recurrence after endoscopic treatment for the colorectal tumors. Stomach and Intestine. 1999;34:645–652. [Google Scholar]
- 26.Oka S, Tanaka S, Kaneko I, Kawamura T, Mohri R, Chayama K. Endoscopic mucosal resection for colorectal tumors. Rinsho shokaki naika. 2005;20:1759–1768. [Google Scholar]
- 27.Sano Y, Machida H, Fu KI, Ito H, Fujii T. Endoscopic mucosal resection and submucosal dissection method for large colorectal tumors. Dig Endosc. 2004;16:S93–S96. [Google Scholar]
- 28.Hotta K, Fujii T, Kozu T, Matsuda T, Kakugawa Y, Kobayashi N, Nakajima T, Hasuda K, Uraoka T, Kodani T, et al. Surveillance after endoscopic mucosal resection for colorectal tumors from the point of view of local recurrence: necessity of en-bloc resection. Shokaki naishikyo. 2003;15:965–970. [Google Scholar]
- 29.Matsuda T, Saito Y, Uraoka T, Ikehara H, Mashimo Y, Kikuchi T, Yokoi C, Takizawa K, Sakamoto T, Fukuzawa M, et al. Therapeutic strategy for laterally spreading tumors (LSTs) in the colorectum. Shokaki naishikyo. 2006;18:1151–1157. [Google Scholar]
- 30.Yasumoto S, Hirata I, Hamamoto N, Nishikawa T, Abe Y, Egashira Y. Endoscopic mucosal resection for laterally spreading tumors-technical procedure, results. Stomach and Intestine. 2005;40:1781–1789. [Google Scholar]
- 31.Terai T, Sakamoto N, Abe S, Beppu K, Namihisa A, Kurosawa A, Nagata T, Nagahara A, Okusa T, Hagiwara T, et al. Endoscopic treatment for laterally spreading tumors in the colon. Stomach and Intestine. 2003;38:1843–1846. [Google Scholar]
- 32.Nozaki R, Matsudaira M, Yamada K, Takano M. Clinical evaluation of therapeutic endoscopic methods for large colorectal tumors greater than 20mm, Focus on effectiveness and validity of scheduled piecemeal endoscopic mucosal resection. J colon exam. 2006;23:24–30. [Google Scholar]
- 33.Watari J, Saitoh Y, Ohta T, Honda M, Sasaki A, Fujiki T, Taruishi M, Ayabe T, Yokota K, Murakami M, et al. Endoscopic resection for nodule aggregating tumors of the colorectum. Rinsho shokaki naika. 1998;13:1269–1275. [Google Scholar]
- 34.Sugisaka H, Ikegami M, Kijima H, Fukata M, Furushima H, Sakabe S, Takagi I, Doi K, Nozawa H, Nishino H, et al. Pathological features of remnant or recurrent colonic lesions after endoscopic mucosal resection. Shokaki naishikyo. 2003;15:951–956. [Google Scholar]
- 35.Matsunaga A, Nomura M, Uchimi K, Kikuchi T, Noda Y, Senoo S, Ito K, Okubo K, Katakura Y, Fujita N. Evalustion of remnant or recurrent colonic lesions after endoscopic mucosal resection (EMR) and their additional treatment. Early colorectal cancer. 1999;3:27–33. [Google Scholar]
- 36.Nomura M, Fujita N, Matsunaga A, Uchimi K, Noda Y, Yuki T, Sano T, Ishida K, Senoo S, Ito K, et al. Scratch-stick-method for endoscopic mucosal resection of colorectal tumors. Gastroenterological Endoscopy. 2001;43:1821–1827. [Google Scholar]
- 37.Kobayashi H, Fuchigami T, Sakai Y, Oda H, Kikuchi Y, Nagamura S, Takemura S, Ishikawa N, Miyamoto R, Moriyama T, et al. A study of remnant or recurrent colorectal lesions (adenoma, mucosal carcinoma) after endoscopic resection. Stomach and Intestine. 1999;34:597–610. [Google Scholar]
- 38.Nakajima K, Miyazaki S, Aoki T, Okazaki Y, Sakama A, Inoue M, Kuboshima M, Horibe D, Kakuta S, Kitabayashi H, et al. Result of endoscopic resection and treatment strategy including operation for colorectal adenoma and early cancer of 20mm or more in diameter. Progress of Digestive Endoscopy. 2006;68:67–72. [Google Scholar]
- 39.Cho E, Mochizuki N, Tanaka K, Uno K, Tsukada K, Ueda M, Miyata M, Hasegawa K, Uenoyama Y, Kawahata H, et al. Local recurrence after endoscopic mucosal resection (EMR) in cases with colorectal large sessile mucosal tumors. Stomach and Intestine. 1999;34:619–628. [Google Scholar]
- 40.Saito Y, Fujii T, Kondo H, Mukai H, Yokota T, Kozu T, Saito D. Endoscopic treatment for laterally spreading tumors in the colon. Endoscopy. 2001;33:682–686. doi: 10.1055/s-2001-16213. [DOI] [PubMed] [Google Scholar]
- 41.Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002;55:390–396. doi: 10.1067/mge.2002.121881. [DOI] [PubMed] [Google Scholar]
- 42.Hurlstone DP, Sanders DS, Cross SS, Adam I, Shorthouse AJ, Brown S, Drew K, Lobo AJ. Colonoscopic resection of lateral spreading tumours: a prospective analysis of endoscopic mucosal resection. Gut. 2004;53:1334–1339. doi: 10.1136/gut.2003.036913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hurlstone DP, Sanders DS, Cross SS, George R, Shorthouse AJ, Brown S. A prospective analysis of extended endoscopic mucosal resection for large rectal villous adenomas: an alternative technique to transanal endoscopic microsurgery. Colorectal Dis. 2005;7:339–744. doi: 10.1111/j.1463-1318.2005.00813.x. [DOI] [PubMed] [Google Scholar]
- 44.Su MY, Hsu CM, Ho YP, Lien JM, Lin CJ, Chiu CT, Chen PC, Tung SY, Wu CS. Endoscopic mucosal resection for colonic non-polypoid neoplasms. Am J Gastroenterol. 2005;100:2174–2179. doi: 10.1111/j.1572-0241.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 45.Uraoka T, Fujii T, Saito Y, Sumiyoshi T, Emura F, Bhandari P, Matsuda T, Fu KI, Saito D. Effectiveness of glycerol as a submucosal injection for EMR. Gastrointest Endosc. 2005;61:736–740. doi: 10.1016/s0016-5107(05)00321-4. [DOI] [PubMed] [Google Scholar]
- 46.Kawamura YJ, Sugamata Y, Yoshino K, Abo Y, Nara S, Sumita T, Setoyama R, Kiribuchi Y, Kawano N. Endoscopic resection for submucosally invasive colorectal cancer: is it feasible? Surg Endosc. 1999;13:224–227. doi: 10.1007/s004649900949. [DOI] [PubMed] [Google Scholar]
- 47.Brooker JC, Saunders BP, Shah SG, Thapar CJ, Suzuki N, Williams CB. Treatment with argon plasma coagulation reduces recurrence after piecemeal resection of large sessile colonic polyps: a randomized trial and recommendations. Gastrointest Endosc. 2002;55:371–375. doi: 10.1067/mge.2002.121597. [DOI] [PubMed] [Google Scholar]
- 48.Stergiou N, Riphaus A, Lange P, Menke D, Köckerling F, Wehrmann T. Endoscopic snare resection of large colonic polyps: how far can we go? Int J Colorectal Dis. 2003;18:131–135. doi: 10.1007/s00384-002-0450-3. [DOI] [PubMed] [Google Scholar]