Abstract
AIM: To investigate the effects of Helicobacter pylori (H pylori) eradication therapy for treatment of peptic ulcer on the incidence of gastric cancer.
METHODS: A multicenter prospective cohort study was conducted between November 2000 and December 2007 in Yamagata Prefecture, Japan. The study included patients with H pylori-positive peptic ulcer who decided themselves whether to receive H pylori eradication (eradication group) or conventional antacid therapy (non-eradication group). Incidence of gastric cancer in the two groups was determined based on the results of annual endoscopy and questionnaire surveys, as well as Yamagata Prefectural Cancer Registry data, and was compared between the two groups and by results of H pylori therapy.
RESULTS: A total of 4133 patients aged between 13 and 91 years (mean 52.9 years) were registered, and 56 cases of gastric cancer were identified over a mean follow-up of 5.6 years. The sex- and age-adjusted incidence ratio of gastric cancer in the eradication group, as compared with the non-eradication group, was 0.58 (95% CI: 0.28-1.19) and ratios by follow-up period (< 1 year, 1-3 years, > 3 years) were 1.16 (0.27-5.00), 0.50 (0.17-1.49), and 0.34 (0.09-1.28), respectively. Longer follow-up tended to be associated with better prevention of gastric cancer, although not to a significant extent. No significant difference in incidence of gastric cancer was observed between patients with successful eradication therapy (32/2451 patients, 1.31%) and those with treatment failure (11/639 patients, 1.72%). Among patients with duodenal ulcer, which is known to be more prevalent in younger individuals, the incidence of gastric cancer was significantly less in those with successful eradication therapy (2/845 patients, 0.24%) than in those with treatment failure (3/216 patients, 1.39%).
CONCLUSION: H pylori eradication therapy for peptic ulcer patients with a mean age of 52.9 years at registration did not significantly decrease the incidence of gastric cancer.
Keywords: Helicobacter pylori, Peptic ulcer, Gastric cancer, Eradication therapy, Cancer prevention
INTRODUCTION
Yamagata Prefecture is an area in which gastric cancer is particularly common. In Cancer Incidence in Five Continents, published by the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO), it is reported that the incidence of gastric cancer for men in Yamagata Prefecture, was the second highest in the world, at 91.6/100 000 (ASR world) in 1993-1997[1]. Yamagata Prefecture therefore has attempted aggressively to achieve secondary prevention of gastric cancer. In 1994, IARC/WHO concluded that Helicobacter pylori (H pylori) is a definite carcinogen in humans[2], and the results of a prospective cohort study[3] and animal studies[4-6] have demonstrated that H pylori causes gastric cancer. The possible role of H pylori eradication therapy in primary prevention of gastric cancer has thus attracted substantial interest in Yamagata Prefecture. In November 2000, when coverage of H pylori eradication for patients with H pylori-positive peptic ulcer by the National Health Insurance system in Japan began, the Yamagata H pylori Clinical Study Group was established to design a multicenter prospective cohort study to investigate whether H pylori eradication therapy for patients with peptic ulcer can decrease the incidence of gastric cancer.
Although animal studies have revealed that primary prevention of gastric cancer by H pylori eradication is more effective as the duration between H pylori infection and eradication is decreased[7], the effects in humans of this type of prevention have not been determined sufficiently. Non-randomized prospective studies[8,9] and retrospective studies[10,11] in Japan have suggested that H pylori eradication therapy prevents the development of gastric cancer, while a large-scale randomized controlled study in China did not support this conclusion. Although, a sub-population analysis of patients who did not have precancerous change at the time of eradication therapy has suggested gastric-cancer-preventive effects of H pylori eradication therapy[12]. No significant reduction in the incidence of gastric cancer by H pylori eradication therapy was observed in a meta-analysis[13]. In a multicenter, randomized controlled study in patients who underwent endoscopic resection of early gastric cancer and were thus at high risk of secondary gastric cancer, occurrence of secondary gastric cancer was prevented significantly by H pylori eradication therapy[14]. Evidence of prevention of gastric cancer by H pylori eradication therapy thus needs to be obtained.
This report describes the results of a multicenter, prospective cohort study that investigated whether H pylori eradication therapy in patients with peptic ulcer, living in an area where the incidence of gastric cancer is especially high, was effective in primary prevention of gastric cancer. The results of the present study, including endoscopy findings, were reconciled with those of the Yamagata Prefecture Cancer Registry to ensure accurate detection of gastric cancer.
MATERIALS AND METHODS
Study design
The present study was designed at Yamagata Prefectural Central Hospital, where the office of the Yamagata H pylori Clinical Study Group was located in May 2000. It included 82 participating institutions, 26 hospitals and 56 clinics, in Yamagata Prefecture. For ethical reasons, we selected performance of a non-randomized, multicenter, prospective cohort study in which patients decided themselves whether to receive H pylori eradication therapy (eradication group) or conventional antacid therapy (non-eradication group) for the treatment of H pylori-positive peptic ulcer. The sample size was calculated to detect a significant difference in incidence of gastric cancer in patients who received H pylori eradication therapy or conventional antacid treatment over a 7-year period (a 2-year registration period and a 5-year follow-up period), with a power of 90% and an alpha error of 5% on the basis of the following assumptions: the incidence of gastric cancer in patients who received conventional antacid therapy was 0.5%; H pylori eradication therapy decreased the incidence of gastric cancer by 50%-90%; the percentage of withdrawals was 20%; and patients were allocated to the non-eradication and eradication groups at a ratio of 1:5. We estimated that 560-2467 patients and 2797-12 333 patients were required for the non-eradication and eradication therapy groups, respectively. All tests and treatments performed were covered by the National Health Insurance (NHI) as determined by the Ministry of Health and Welfare of Japan (currently the Ministry of Health, Labor, and Welfare of Japan), and the study protocol was approved by the Ethics Committee of Yamagata Prefectural Central Hospital. All patients received a full explanation of the study using a standardized document, and provided written informed consent before registration in the study.
Patients with H pylori-positive peptic ulcer were considered eligible. Patients with a history of gastric cancer and those in whom endoscopy or biopsy at the time of registration revealed gastric cancer were excluded from the study. Patients were registered between November 2000 and December 2003, and followed up until the end of December 2007.
Diagnosis of H pylori-positive peptic ulcer and treatment
Prior to registration, all patients underwent upper gastrointestinal endoscopy and biopsy, if necessary, to diagnose peptic ulcer and exclude gastric cancer. During endoscopy, biopsy samples were collected from the greater curvature of the upper body and antrum of the stomach. The presence/absence of H pylori infection was evaluated by rapid urease test.
H pylori eradication therapy consisted of 30 mg lansoprazole or 20 mg omeprazole, plus 750 mg amoxicillin and 200 or 400 mg clarithromycin, all twice daily for 7 d. At least 1 mo after the completion of eradication therapy, patients underwent upper gastrointestinal endoscopy with the rapid urease test and urea breath test (with a cut-off value of 2.5‰; Ubit, Otsuka Pharmaceuticals, Tokyo, Japan). Successful H pylori eradication was defined as negative results on the rapid urease and urea breath tests. When the results of the two tests were inconsistent, retesting was performed.
Conventional antacid therapy consisted of antacids such as proton-pump inhibitors and histamine-H2 blockers.
Detection of gastric cancer
During the follow-up period up to December 2007, endoscopy was performed annually, in principle, to determine the presence/absence of gastric cancer. When follow-up endoscopy was performed, the investigators reported its results to the study office using a follow-up report form to provide information on the date of endoscopy, stage of ulcer, results of H pylori testing, and presence/absence of newly developed gastric cancer or other gastrointestinal diseases (such as reflux esophagitis, erosive gastritis/duodenitis, and esophageal adenocarcinoma).
To avoid overlooking gastric cancer due to the absence of annual endoscopy, a questionnaire survey was conducted and the data obtained in the present study were reconciled with those of the Yamagata Prefecture Cancer Registry. In October 2006, a questionnaire was mailed to all registered patients to determine the presence/absence of gastric cancer diagnosed after registration. The results of the questionnaire were compared with the data at registration to identify patients who might have developed gastric cancer after registration. Such cases were referred to the participating medical institutions to confirm the diagnosis of gastric cancer. In March 2008, record linkage between the cohort and Yamagata Prefectural Cancer Registry was conducted for identification of previous and new gastric cancer cases during the follow-up period up to the end of December 2007.
Statistical analysis
Person-years were calculated from the date of recruitment to the date of incidence of gastric cancer; end of follow-up in December 2007; date of change of residence to outside Yamagata Prefecture; death from causes other than gastric cancer; or the initiation of H pylori eradication therapy for patients in the antacid therapy group, whichever came first. For comparison between groups at baseline, Fisher’s exact test was used for sex and type of peptic ulcer, and Student’s t test or χ2 test for histological type, location and stage of cancer, and treatment, with a level of significance of P < 0.05 (two-tailed).
Poisson regression was used to estimate the relative risk of gastric cancer in relation to eradication therapy for H pylori. Analyses were adjusted routinely for sex and age (< 60, 60-70, > 70 years), and stratified for duration of follow-up (< 1, 1-3, > 3 years) and location of ulcer (gastric, gastroduodenal or duodenal). We also examined the effects of adjustment for other risk factors including location of ulcer, intake of salt, and smoking. These statistical analyses were performed using Intercooled Stata 8.0 for Windows software (StataCorp LP, College Station, TX, USA).
The accumulated incidence of gastric cancer in patients with successful and unsuccessful H pylori eradication was determined using data for patients for whom the results of H pylori eradication therapy were confirmed using the Kaplan-Meier method, and were tested for significance of difference between patients with and without successful eradication using the log-rank method. These analyses were performed using Dr. SPSS II for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
Baseline characteristics of study subjects
A total of 4203 patients were registered, and 70 patients who had a history of gastric cancer or had gastric cancer at the time of registration were excluded. Intention-to-treat (ITT) analysis was performed for the data from the remaining 4133 patients (2964 male and 1169 female), who were aged between 13 and 91 years (mean 52.9 years).
H pylori eradication therapy was administered to 3781 patients (91.5%; eradication group) and conventional antacid therapy to 352 patients (8.5%; non-eradication group). Table 1 summarizes the characteristics of the 4133 patients included in the present study. There were no significant differences between the eradication and non-eradication groups in baseline characteristics such as distribution of sex or location of ulcers, while mean age was lower in the eradication than in the non-eradication group (52.4 years vs 58.1 years). No results of H pylori eradication therapy were reported for 691 (18.3%) of the 3781 patients who received H pylori eradication therapy. The eradication rate evaluated in the ITT analysis and the per-protocol (PP) analysis were 64.8% (2451/3781) and 79.3% (2451/3090), respectively. There were no significant differences in any factors, including age, between patients with and without successful H pylori eradication (mean ages of patients with and without successful H pylori eradication were 52.5 ± 12.3 years and 52.1 ± 13.5, respectively; P = 0.51).
Table 1.
Baseline characteristics of 4133 patients
| Eradication group (n = 3781) | Non-eradication group (n = 352) | ||
| Male/Female | 2715/1066 | 249/103 | P = 0.671 |
| Male (%) | 71.80 | 70.70 | |
| Mean age | 52.4 ± 12.7 | 58.1 ± 12.6 | P < 0.0012 |
| Min-max | 13.2-85.9 | 22.2-91.9 | |
| Age (yr) | P < 0.0011 | ||
| < 60 | 2732 (72.3) | 186 (52.8) | |
| 60-70 | 701 (18.5) | 102 (29.0) | |
| > 70 | 348 (9.2) | 64 (18.2) | |
| Location of ulcer | P = 0.411 | ||
| GU | 2048 (54.2) | 195 (55.4) | |
| GDU | 418 (11.1) | 45 (12.8) | |
| DU | 1265 (33.5) | 110 (31.3) | |
| Unknown | 50 (1.3) | 2 (0.6) | |
| Salt consumption | P < 0.0011 | ||
| Restricted | 1377 (36.4) | 173 (49.1) | |
| No interest in salt consumption | 729 (19.3) | 29 (8.2) | |
| Not restricted | 1185 (31.3) | 105 (29.8) | |
| Unknown | 490 (13.0) | 45 (12.8) | |
| Smoking history | P < 0.0011 | ||
| Non-smokers | 1174 (31.0) | 120 (34.1) | |
| Past smokers | 565 (14.9) | 48 (13.6) | |
| Current smokers | 1931 (51.1) | 159 (45.2) | |
| Unknown | 111 (2.9) | 25 (7.1) | |
| Mean duration of follow-up (yr) | 5.6 ± 1.1 | 5.2 ± 1.8 | P < 0.0012 |
| Min-max | 0.09-7.96 | 0.11-8.41 |
χ2 test;
t test. Eradication group: patients who received Helicobacter pylori eradication therapy with or without successful eradication. Non-eradication group: Patients who received conventional antacid therapy. GU: Gastric ulcer; GDU: Gastroduodenal ulcer; DU: Duodenal ulcer.
Development of gastric cancer
During a total of 22 900 person-years of follow-up (mean follow-up period: 5.6 years), gastric cancer was found in 56 patients, including 47/3781 patients (1.24%, 0.21%/year) who received H pylori eradication therapy and 9/352 patients (2.56%, 0.50%/year) who received conventional antacid therapy. There were no differences in sex distribution; location of ulcer lesions; histological type, location or stage of cancer; or type of treatment for gastric cancer between patients with and without H pylori eradication therapy (Table 2). Poisson regression analysis of factors that affected the incidence of gastric cancer revealed that sex, age group, and location of ulcers were independent factors that affected the differences in incidence rate ratio (IRR) of gastric cancer between patients receiving and not receiving H pylori eradication therapy (Table 3). The IRR of gastric cancer adjusted for sex and age group was 0.58 (95% CI: 0.28-1.19). H pylori eradication therapy decreased the incidence of gastric cancer by about 40%, although this change was not statistically significant. IRR was by duration of follow-up 1.16 (0.27-5.00) for patients followed up for < 1 year, 0.50 (0.17-1.49) for 1-3 years, and 0.34 (0.09-1.28) for > 3 years. There were no significant differences in the incidence of gastric cancer between any subgroups of the eradication and non-eradication groups, although the difference in incidence between the groups tended to increase as the duration of follow-up was prolonged (Table 4). Gastric cancer was found in 6/1375 patients with duodenal ulcer (0.44%). All six patients were > 50 years of age at the time of registration. No cases of Barrett’s adenocarcinoma, for which the possibility of increase in occurrence after H pylori eradication therapy was a concern, were found in either of the two groups.
Table 2.
Distribution of cases of gastric cancer
| Sex | Male | Female | Total | |||
| Eradication group | 38 | 9 | 47 | P = 0.3281 | ||
| Non-eradication group | 9 | 0 | 9 | |||
| Type of peptic ulcer | GU/GDU | DU | ||||
| Eradication group | 43 | 4 | 47 | P = 0.2441 | ||
| Non-eradication group | 7 | 2 | 9 | |||
| Histological type of cancer | Intestinal | Diffuse | Unknown | |||
| Eradication group | 35 | 10 | 2 | 47 | P = 0.3042 | |
| Non-eradication group | 5 | 4 | 0 | 9 | ||
| Location of cancer | L | M | U | Unknown | ||
| Eradication group | 21 | 18 | 6 | 2 | 47 | P = 0.7592 |
| Non-eradication group | 3 | 5 | 1 | 0 | 9 | |
| Stage | Early | Advanced | Unknown | |||
| Eradication group | 34 | 11 | 2 | 47 | P = 0.1982 | |
| Non-eradication group | 9 | 0 | 0 | 9 | ||
| Treatment | Endoscopy (EMR/ESD) | Surgery | Chemotherapy | |||
| Eradication group | 12 | 31 | 2 | 47 | P = 0.7632 | |
| Non-eradication group | 2 | 7 | 0 | 9 |
Fisher’s direct test;
Student’s t test or χ2 test. ESD: Endoscopic submucosal dissection; L: Lower third of the stomach; M: Middle third of the stomach; U: Upper third of the stomach.
Table 3.
Results of poisson regression analysis
|
IRR |
||||||
| Univariate | 95% CI | P | Multivariate | 95% CI | P | |
| Eradication group | 0.45 | 0.22-0.92 | 0.03 | 0.61 | 0.29-1.27 | 0.18 |
| Non-eradication group | 1.00 | 1.00 | ||||
| Sex | ||||||
| Male | 1.00 | 0.05 | 1.00 | 0.03 | ||
| Female | 0.49 | 0.24-1.00 | 0.39 | 0.17-0.88 | ||
| Age (yr) | 1.09 | 1.05-1.12 | < 0.01 | |||
| < 60 | 1.00 | 1.00 | ||||
| 60-70 | 3.22 | 1.74-5.95 | < 0.01 | 2.59 | 1.37-4.91 | < 0.01 |
| > 70 | 5.18 | 2.69-10.0 | < 0.01 | 4.23 | 2.09-8.53 | < 0.01 |
| Location of ulcer | 0.69 | 0.40-1.19 | 0.18 | |||
| Stomach/Stomach + duodenum | 1.00 | 1.00 | ||||
| Duodenum | 0.28 | 0.13-0.63 | < 0.01 | 0.37 | 0.16-0.83 | 0.02 |
| Unknown | 1.16 | 0.16-8.43 | 0.88 | 1.69 | 0.23-12.48 | 0.61 |
| Salt consumption | 0.94 | 0.84-1.06 | 0.27 | |||
| Restricted | 1.00 | 1.00 | ||||
| No interest in salt consumption | 0.43 | 0.16-1.11 | 0.08 | 0.65 | 0.25-1.73 | 0.39 |
| Not restricted | 0.78 | 0.43-1.41 | 0.41 | 1.03 | 0.56-1.90 | 0.93 |
| Unknown | 0.58 | 0.24-1.39 | 0.22 | 0.58 | 0.22-1.60 | 0.29 |
| Smoking history | 1.04 | 0.89-1.22 | 0.63 | |||
| Non-smokers | 1.00 | 1.00 | ||||
| Past smokers | 1.56 | 0.74-3.29 | 0.25 | 0.97 | 0.43-2.20 | 0.95 |
| Current smokers | 0.94 | 0.50-1.76 | 0.85 | 0.78 | 0.38-1.61 | 0.50 |
| Unknown | 1.71 | 0.50-5.88 | 0.39 | 1.65 | 0.41-6.65 | 0.48 |
IRR: Incidence rate ratio.
Table 4.
IRRs of gastric cancer by follow-up period and location of ulcer
|
Eradication group |
Non-eradication group |
IRR |
IRR (sex- and age-adjusted) |
|||||||
| per 1000 person-years | n | Incidence | per 1000 person-years | n | Incidence | 95% CI | 95% CI | |||
| Overall | 21.2 | 47 | 2.22 | 1.82 | 9 | 4.93 | 0.45 | 0.22-0.92 | 0.58 | 0.28-1.19 |
| Follow-up period (yr) | ||||||||||
| < 1 | 3.77 | 17 | 4.51 | 0.34 | 2 | 5.81 | 0.78 | 0.18-3.33 | 1.16 | 0.27-5.00 |
| 1-3 | 7.48 | 20 | 2.67 | 0.64 | 4 | 6.27 | 0.43 | 0.15-1.25 | 0.50 | 0.17-1.49 |
| > 3 | 9.92 | 10 | 1.01 | 0.84 | 3 | 3.56 | 0.28 | 0.08-1.03 | 0.34 | 0.09-1.28 |
| Gastric/Gastric + duodenal ulcers | 13.8 | 41 | 2.97 | 1.21 | 7 | 5.76 | 0.52 | 0.23-1.15 | 0.62 | 0.27-1.39 |
| Duodenal ulcers | 7.12 | 5 | 0.70 | 0.60 | 2 | 3.35 | 0.21 | 0.04-1.08 | 0.31 | 0.06-1.61 |
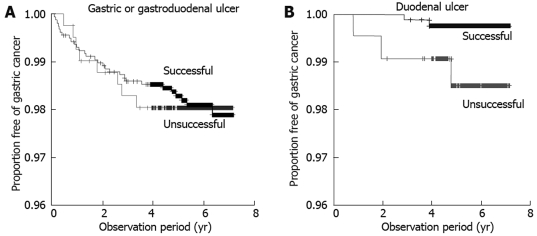
In a separate analysis, 3090 patients who received H pylori eradication therapy, with known results, were compared for incidence of gastric cancer according to presence/absence of successful eradication. Gastric cancer was detected in 43 of the 3090 patients, including 32/2451 patients (1.31%) and 11/639 patients (1.72%) with and without successful eradication, respectively. No significant difference in incidence of gastric cancer was observed between patients with and without successful eradication. Analysis by type of peptic ulcer at the time of registration revealed that the incidence of gastric cancer did not differ between patients with and without successful eradication in subgroups of patients with gastric or gastroduodenal ulcer, while successful eradication decreased the incidence of gastric cancer significantly in patients with duodenal ulcer (Figures 1 and 2).
Figure 1.
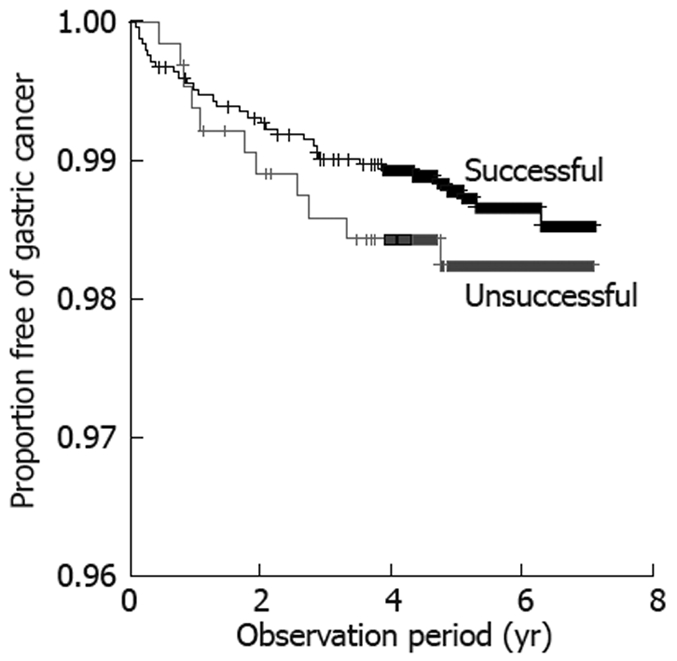
Proportion free of gastric cancer in the eradication group was compared according to results of eradication therapy, using Kaplan-Meier analysis. The incidence was 32/2451 (1.31%) in patients with successful eradication and 11/639 (1.72%) in patients with failure of eradication (log-rank test, P = 0.43).
Figure 2.
Proportion free of gastric cancer in patients with gastric or gastroduodenal ulcer (A) and duodenal ulcer (B) in the eradication group was compared according to results of eradication therapy, using Kaplan-Meier analysis. A: The incidence of gastric cancer was 29/1572 (1.85%) in patients with successful eradication and 8/413 (1.94%) in patients with failure of eradication (log-rank test, P = 0.92); B: The incidence of gastric cancer was 2/845 (0.24%) in patients with successful eradication and 3/216 (1.39%) in patients with failure of eradication (log-rank test, P = 0.03).
DISCUSSION
This was a prospective, multicenter, cohort study in patients with H pylori-positive peptic ulcer, which was designed to evaluate whether H pylori eradication therapy decreased the incidence of gastric cancer in Yamagata Prefecture, a region in which gastric cancer is especially common. Since continuous follow-up is often difficult in clinical observation studies, the data from the present study were reconciled with those of the Yamagata Prefecture Cancer Registry to avoid overlooking gastric cancer. This was a unique feature of the present study.
During the follow-up of 4133 patients with peptic ulcer (mean age: 52.9 years) for a mean of 5.6 years, the incidence of gastric cancer in patients who received H pylori eradication therapy was decreased by about 40% compared with that in patients who did not receive eradication therapy, although the difference between the groups was not statistically significant. Longer follow-up period tended to be associated with better prevention of gastric cancer, albeit not to a significant extent. Although there was no significant difference in incidence of gastric cancer according to the result of eradication therapy (success/failure) in those patients who received this treatment, successful eradication therapy did decrease significantly the incidence of gastric cancer in patients with duodenal ulcer.
There are four limitations to the interpretation of the results of the present study: (1) it was not a randomized controlled trial, and the number of patients not receiving eradication therapy was small; (2) the eradication rate was only 80%; (3) the follow-up period was not sufficiently long; and (4) the mean age of participants was high, at 53 years.
Factors (1) and (2) are limitations of the present study, in which randomization of patients was impossible for ethical reasons, and secondary H pylori eradication therapy was not covered by the NHI system. In addition to the four factors noted above, since the decrease in incidence of gastric cancer by H pylori eradication was smaller than expected, and the numbers of patients did not reach those targeted, especially in the non-eradication group, the study did not have the statistical power required to detect a significant difference in the incidence of gastric cancer between patients who received eradication therapy and conventional antacid therapy.
The finding that the efficacy of H pylori eradication in preventing gastric cancer tended to be better among patients with a longer follow-up period suggests that the length of follow-up in this study may have been insufficient. In a study of patients who underwent resection of gastric cancer, cancer in other locations was not detected upon preoperative evaluation[15]. Although all patients evaluated in the present study underwent endoscopy and biopsy prior to registration, if required, the possibility cannot be ruled out that some patients had undetectable gastric cancer before registration. Gastric cancer lesions detected during the early phase after eradication therapy may in many cases have been present before therapy. More accurate determination of the efficacy of eradication therapy in preventing gastric cancer will require that patients be followed up for a long period of time. Since follow-up endoscopy cannot be continued for many years, it is important that our data be reconciled with those of the Yamagata Prefecture Cancer Registry to continue follow-up of the participants.
As pointed out by Wong et al[12], the precancerous state may represent the point of no return at which development of gastric cancer can no longer be prevented by H pylori eradication. The participants in the present study were patients with peptic ulcer with a mean age of 53 years, and many patients with gastric ulcer also have atrophic gastritis or intestinal metaplasia. Among the registered patients, duodenal ulcer was more common in those < 50 years of age, while gastric ulcer was common in patients > 50 years of age. The risk of gastric cancer was higher in patients with gastric or gastroduodenal ulcer than in those with duodenal ulcer, and a significant decrease in the incidence of gastric cancer according to successful H pylori eradication was observed only in patients who underwent H pylori eradication for the treatment of duodenal ulcer. Since H pylori infection is usually established during childhood, it appears likely that antral gastritis and duodenal ulcer are common among young patients with a relatively short history of H pylori infection, and that eradication of H pylori may decrease the occurrence of gastric cancer. However, patients with a longer history of H pylori infection often have corpus gastritis, and eradication therapy does not prevent gastric cancer to a significant extent. The results of an experiment in Mongolian gerbils has shown that eradication of H pylori is more effective in preventing gastric cancer in animals with a shorter duration of H pylori infection[7]. These findings suggest the importance of the timing and target of H pylori eradication therapy in preventing gastric cancer.
In a randomized clinical study on the effects of eradication of H pylori after endoscopic mucosal resection of early gastric cancer, Fukase et al[14] have reported a significant decrease in the incidence of secondary gastric carcinoma during a 3-year follow-up period. The significant prevention of secondary gastric cancer by H pylori eradication was considered to be caused by the following: (1) the risk of development of gastric cancer in patients following endoscopic mucosal resection for early gastric cancer is about 10-fold higher than in patients with H pylori-positive peptic ulcer; (2) patients underwent accurate endoscopy several times before and after treatment for cancer; and (3) the number of cases of gastric cancer that were overlooked at the time of registration was therefore considered smaller than in other surveys.
Prior to initiation of the present study, there was concern regarding the possibility of a decrease in visits for endoscopy after symptomatic improvement, which could have resulted in an increase in advanced gastric cancer. Although the investigators fully explained to subjects the risk of development of gastric cancer after H pylori eradication therapy and the importance of follow-up endoscopy, > 15% of them did not undergo examination to confirm the results of H pylori eradication therapy. The percentage of patients who received follow-up endoscopy as specified was significantly lower among those who received H pylori eradication therapy compared with conventional antacid therapy. Although this may have biased the rate of detection of gastric cancer, we attempted to decrease bias by reconciling our data with those of the Yamagata Prefecture Cancer Registry. Of the 56 cases of gastric cancer detected in the present study, 11 were detected as advanced gastric cancer. Cases of advanced gastric cancer mainly consisted of diffuse-type cancer and those with a mixture of intestinal and diffuse cancer cells. Careful observation is thus needed for the development of gastric carcinoma, especially diffuse-type gastric cancer, which may progress rapidly and is often difficult to detect.
Although it has been reported that gastric cancer does not develop in patients with duodenal ulcer[3,8,9], gastric cancer developed in six patients with duodenal ulcer in the present study, including five aged ≥ 60 years and one 51-year-old patient. Since continuous infection with H pylori may result in the development of corpus gastritis in patients with antral gastritis associated with duodenal ulcer, middle-aged and elderly patients with duodenal ulcer often have gastritis in the corpus, and should thus be considered at high risk for development of gastric cancer, even after successful H pylori eradication therapy, especially in regions with a high incidence of gastric cancer.
In conclusion, H pylori eradication therapy for patients with peptic ulcer with a mean age of 52.9 years did not significantly decrease the incidence of gastric cancer during a mean follow-up period of 5.6 years. Although our results did not rule out a role for H pylori eradication therapy in preventing gastric cancer, the number of patients evaluated, duration of observation, and rate of eradication of H pylori were insufficient to obtain a significant difference in the incidence of gastric cancer between the groups with and without eradication therapy, and the mean age of patients was high. Further studies to clarify the efficacy of H pylori eradication in preventing gastric cancer will require eradication of H pylori as early as possible and careful and prolonged follow-up of patients.
COMMENTS
Background
Yamagata Prefecture in Japan has the second highest incidence of gastric cancer in the world. Helicobacter pylori (H pylori) plays an important role in the development of gastric cancer. It is thus of crucial importance to determine whether H pylori eradication therapy in this geographical area is effective in primary prevention of gastric cancer.
Research frontiers
Although it has been demonstrated that H pylori is a definite carcinogen in humans, previous studies that have examined the efficacy of eradication therapy in preventing gastric cancer have yielded inconsistent findings. In the present study, the authors found that eradication therapy in patients with peptic ulcer with a high mean age of 53 years did not significantly decrease the incidence of gastric cancer, at least over the mean 5.6-year follow-up period.
Innovations and breakthroughs
Single-center prospective studies and retrospective studies have reported significant prevention of gastric cancer by eradication therapy, while one randomized clinical trial has revealed no overall effects, but significant prevention of gastric cancer in patients without precancerous lesions. The present multicenter, prospective cohort study, conducted in an area where the incidence of gastric cancer is especially high, revealed no overall efficacy of H pylori eradication for preventing gastric cancer in patients with peptic ulcer. In contrast, it demonstrated that eradication was associated with a significant decrease of gastric cancer in patients with duodenal ulcer, which is known to be more prevalent in younger individuals.
Applications
Given the overall lack of efficacy of eradication therapy for peptic ulcer in preventing gastric cancer, the findings highlight the importance of longer and careful follow-up after eradication therapy. Furthermore, the significant efficacy of treatment observed in younger patients suggests the need to eradicate H pylori as early as possible.
Terminology
Conventional antacid therapy: a conventional method of treatment, covered by the National Health Insurance (NHI) as determined by the Ministry of Health, Labor, and Welfare of Japan, which consists of treatment with antacids including proton-pump inhibitors and histamine-H2 blockers given over 6-8 wk.
Peer review
The innovative content, as well as readability, reflects the advanced level of clinical research in gastroenterology both at home and abroad.
Acknowledgments
We acknowledge the cooperation with the study group of the 82 participating institutions and the registration of clinical data by 130 investigators.
Footnotes
Peer reviewers: Dusan M Jovanovic, Professor, Institute of Oncology, Institutski Put 4, Sremska Kamenica 21204, Serbia; Yoshio Yamaoka, MD, PhD, Associate Professor, Department of Medicine/Gastroenterology, Baylor College of Medicine and VA Medical Center (111D), 2002 Holcombe Blvd, Houston, Texas 77030, United States
S- Editor Tian L L- Editor Kerr C E- Editor Yin DH
References
- 1.Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer Incidence in Five Continents, Vol. VIII IARC Scientific Publications No.155. Lyon: IARC; 2002. [Google Scholar]
- 2.Infection with Helicobacter pylori. IARC monographs on the evaluation of carcinogenic risks to humans. Vol. 61. Schistosomes, liver flukes and Helicobacter pylori. Lyon: International Agency for Research on Cancer; 1994. pp. 177–241. [PMC free article] [PubMed] [Google Scholar]
- 3.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998;115:642–648. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- 5.Honda S, Fujioka T, Tokieda M, Satoh R, Nishizono A, Nasu M. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998;58:4255–4259. [PubMed] [Google Scholar]
- 6.Hirayama F, Takagi S, Iwao E, Yokoyama Y, Haga K, Hanada S. Development of poorly differentiated adenocarcinoma and carcinoid due to long-term Helicobacter pylori colonization in Mongolian gerbils. J Gastroenterol. 1999;34:450–454. doi: 10.1007/s005350050295. [DOI] [PubMed] [Google Scholar]
- 7.Nozaki K, Shimizu N, Ikehara Y, Inoue M, Tsukamoto T, Inada K, Tanaka H, Kumagai T, Kaminishi M, Tatematsu M. Effect of early eradication on Helicobacter pylori-related gastric carcinogenesis in Mongolian gerbils. Cancer Sci. 2003;94:235–239. doi: 10.1111/j.1349-7006.2003.tb01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Take S, Mizuno M, Ishiki K, Nagahara Y, Yoshida T, Yokota K, Oguma K, Okada H, Shiratori Y. The effect of eradicating helicobacter pylori on the development of gastric cancer in patients with peptic ulcer disease. Am J Gastroenterol. 2005;100:1037–1042. doi: 10.1111/j.1572-0241.2005.41384.x. [DOI] [PubMed] [Google Scholar]
- 9.Kamada T, Hata J, Sugiu K, Kusunoki H, Ito M, Tanaka S, Inoue K, Kawamura Y, Chayama K, Haruma K. Clinical features of gastric cancer discovered after successful eradication of Helicobacter pylori: results from a 9-year prospective follow-up study in Japan. Aliment Pharmacol Ther. 2005;21:1121–1126. doi: 10.1111/j.1365-2036.2005.02459.x. [DOI] [PubMed] [Google Scholar]
- 10.Takenaka R, Okada H, Kato J, Makidono C, Hori S, Kawahara Y, Miyoshi M, Yumoto E, Imagawa A, Toyokawa T, et al. Helicobacter pylori eradication reduced the incidence of gastric cancer, especially of the intestinal type. Aliment Pharmacol Ther. 2007;25:805–812. doi: 10.1111/j.1365-2036.2007.03268.x. [DOI] [PubMed] [Google Scholar]
- 11.Ogura K, Hirata Y, Yanai A, Shibata W, Ohmae T, Mitsuno Y, Maeda S, Watabe H, Yamaji Y, Okamoto M, et al. The effect of Helicobacter pylori eradication on reducing the incidence of gastric cancer. J Clin Gastroenterol. 2008;42:279–283. doi: 10.1097/01.mcg.0000248006.80699.7f. [DOI] [PubMed] [Google Scholar]
- 12.Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 13.Fuccio L, Zagari RM, Minardi ME, Bazzoli F. Systematic review: Helicobacter pylori eradication for the prevention of gastric cancer. Aliment Pharmacol Ther. 2007;25:133–141. doi: 10.1111/j.1365-2036.2006.03183.x. [DOI] [PubMed] [Google Scholar]
- 14.Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 15.Honmyo U, Misumi A, Murakami A, Haga Y, Akagi M. Clinicopathological analysis of synchronous multiple gastric carcinoma. Eur J Surg Oncol. 1989;15:316–321. [PubMed] [Google Scholar]