Abstract
Progressive familial intrahepatic cholestasis (PFIC) type 2 is caused by mutations in ABCB11, which encodes bile salt export pump (BSEP). We report a Thai female infant who presented with progressive cholestatic jaundice since 1 mo of age, with normal serum γ-glutamyltransferase. Immunohistochemical staining of the liver did not demonstrate BSEP along the canaliculi, while multidrug resistance protein 3 was expressed adequately. Novel mutations in ABCB11, a four-nucleotide deletion in exon 3, c.90_93delGAAA, and a single-nucleotide insertion in exon 5, c.249_250insT, were identified, with confirmation in her parents. These mutations were predicted to lead to synthesis of truncated forms of BSEP. Immunostaining and mutation analysis thus established the diagnosis of PFIC type 2.
Keywords: ABCB11, Bile salt export pump, Progressive familial intrahepatic cholestasis
INTRODUCTION
Progressive familial intrahepatic cholestasis (PFIC) refers to a heterogeneous group of autosomal recessive liver disorders of childhood in which cholestasis of hepatocellular origin often presents in the neonatal period or first year of life, and leads to death from liver failure at ages ranging from infancy to adolescence[1,2]. Three types of PFIC have been found; each is related to mutations in hepatocellular transport system genes involved in bile formation[1-3]. PFIC type 1 (Byler disease) is caused by mutations in ATP8B1 (chromosome 18q21-22), which encodes familial intrahepatic cholestasis 1 (FIC1). PFIC type 2 is caused by mutations in ABCB11 (chromosome 2q24), which encodes bile salt export pump (BSEP). Mutations in ABCB4 (chromosome 7q21), which encodes multidrug resistance protein 3 (MDR3), which is responsible for biliary secretion of phospholipids, cause PFIC type 3. In PFIC types 1 and 2, low or normal serum γ-glutamyltransferase (GGT) levels are found, whereas GGT levels are high in PFIC type 3[1,2].
The diagnosis of PFIC can be difficult, especially where genetic testing is not readily available, as in Thailand. We report here a Thai infant diagnosed with PFIC type 2.
CASE REPORT
A 2-mo-old female infant presented with a 1-mo history of icteric sclerae associated with pale yellowish stools. She was a normal full-term infant (birth weight 2700 g). Hypothyroidism had been diagnosed at age 1 mo [free T4 0.94 ng/dL, thyroid stimulating hormone (TSH) 20.5 mIU/L], and thyroxin therapy begun. Growth and development were otherwise normal. No family history of liver disease was elicited. She had mildly icteric sclerae and hepatomegaly without splenomegaly or ascites. All other physical-examination findings were normal.
Conjugated-bilirubin and transaminase values were elevated but albumin, cholesterol, and GGT values were within expected ranges (Table 1). Prolonged coagulogram values were corrected by intravenous vitamin K administration. No IgM-class antibodies against cytomegalovirus were found, and VDRL testing was non-reactive. Plasma amino acid analysis found only mildly elevated methionine levels, interpreted as a nonspecific consequence of liver disease. Urine-reducing substances were absent. Other laboratory investigation results were normal, including free T4 and TSH, complete blood count, electrolytes, glucose, urea nitrogen, creatinine, ammonia and alpha-fetoprotein levels. Ultrasonography revealed a normal liver and bile duct. DISIDA scanning showed good hepatic function with demonstrable intraduodenal tracer at 3 h. Microscopy of a liver-biopsy specimen found changes interpreted as neonatal hepatitis. Ursodeoxycholic acid (UDCA) and fat-soluble vitamins were given.
Table 1.
Evolution of clinical biochemistry test results with body weight and height
| Age (mo) | ALP (U/L)1 | AST (U/L) (15-37) | ALT (U/L) (30-65) | GGT (U/L)2 | Alb (g/L) (34-50) | TB (mg/dL) (0-1.5) | DB (mg/dL) (0-0.5) | Chol (mg/dL) (114-203) | BW (kg) | Ht (cm) |
| 2 | 417 | 354 | 264 | 47 | 37.4 | 5.7 | 3.9 | 155 | 4.95 | 56.0 |
| 6 | 298 | 375 | 344 | 37 | 49.8 | 7.7 | 6.4 | 229 | 7.20 | 64.0 |
| 12 | 267 | 472 | 354 | 66 | 33.8 | 5.3 | 4.5 | 191 | 8.20 | 68.0 |
| 18 | 332 | 311 | 237 | 59 | 38.1 | 6.2 | 5.2 | 151 | 10.4 | 71.4 |
| 24 | 269 | 239 | 171 | 59 | 44.1 | 6.1 | 4.5 | 121 | 11.4 | 78.0 |
| 30 | 373 | 340 | 240 | 60 | 41.2 | 7.9 | 6.8 | 102 | 11.5 | 80.0 |
ALP: Alkaline phosphatase; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; Alb: Albumin; TB: Total bilirubin; DB: Direct bilirubin; Chol: Cholesterol; BW: Body weight; Ht: Height. Units and expected ranges for test-result values are given in parentheses.
Normal values for female children < 1 year, 185-555; 1-2 years, 185-520; 3 years, 185-425 U/L[17];
Normal values for children 1-2 mo, 12-123; 2-4 mo, 8-90; 4 mo-10 years, 5-32 U/L[18].
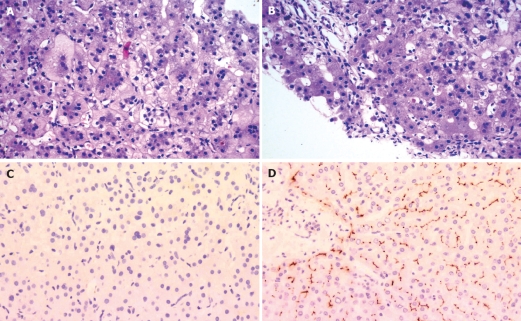
Cholestasis persisted (Table 1), with development of severe pruritus and hepatosplenomegaly. On repeat liver biopsy aged 10 mo, hepatocellular swelling with multinucleation was found, as was canalicular and hepatocellular cholestasis (Figure 1A). Mild portal lymphocytic infiltration and fibrosis were also observed (Figure 1B). BSEP was not detected along the canaliculi on immunostaining (Figure 1C and D), while the homologous transport protein MDR3 was expressed adequately, which demonstrated that tissue fixation was adequate and permitted the inference that lack of BSEP expression was BSEP-specific. As these results were compatible with PFIC type 2, ABCB11, which encodes BSEP, was sequenced after parental consent was obtained.
Figure 1.
Liver, second biopsy (10 mo). Hepatocellular swelling and multinucleation, with intralobular cholestasis (A), accompanying mild portal-tract lymphocytic infiltration and fibrosis (B). On immunostaining, bile salt export pump (BSEP) expression was not detected (C); canaliculi in control liver stained normally for BSEP (D). [Hematoxylin/eosin, A and B; rabbit anti-BSEP polyclonal antibody (generous gift of Dr B Stieger)/hematoxylin, C and D; original magnification, all images, × 200].
Treatment with UDCA and fat-soluble vitamins was continued. At the time of writing, the patient is 30 mo old, with persistent jaundice and growth delay [Table 1; body weight 11.5 kg (P25) and height 80 cm (< P3)]. Serum alpha-fetoprotein concentrations are normal and sonographic monitoring has found no focal changes. She awaits liver transplantation.
Mutation analysis
ABCB11 was analyzed by direct sequencing of PCR products obtained from genomic DNA extracted from peripheral blood leukocytes. All exons, together with the adjacent parts of the intronic sequences, were amplified by PCR with intronic oligonucleotide primers as reported previously[4]. The amplicons were gel-purified, extracted with QIAquick spin columns (Qiagen, Hilden, Germany), and used as templates for the sequencing reaction with Big Dye Terminator kit v3.1 (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol. The products were analyzed on a Genetic Analyzer 3130 (Applied Biosystems).
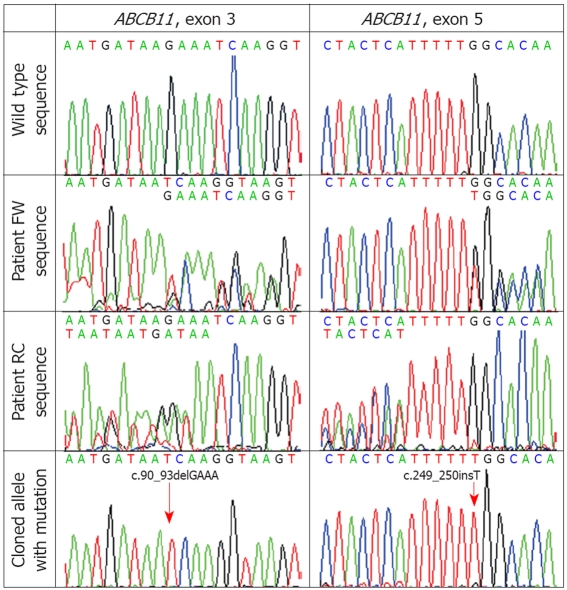
Two suspected sequence disturbances were predicted from the dual sequence readings at 20 bp from the potential mutation start site in both directions. The presence of both mutations was verified by molecular cloning into a plasmid vector pCR4.1-TOPO (Invitrogen, Carlsbad, CA), and sequencing the cloned wild-type and the mutated allele separately. In addition, the presence of suspected mutations was also examined by direct sequencing of appropriate amplicons obtained from the proband’s parents. Two different heterozygous ABCB11 mutations were found in the patient: a four-nucleotide deletion in exon 3 (protein coding exon 2), c.90_93delGAAA, inherited from the patient’s mother, and a single-nucleotide insertion in exon 5 (protein coding exon 4), c.249_250insT, inherited from the patient’s father (Figure 2). Both mutations are predicted to cause reading frame shift and premature termination of DNA translation, respectively p.Lys30AsnfsX31 and p.Gly84TrpfsX9, and therefore are considered to be pathogenic.
Figure 2.
Mutations c.90_93delGAAA and c.249_250insT detected in exon 3 and 5 of ABCB11. FW: Forward reading (coding strand sequence); RC: Reversed and complemented reading (reversed and complemented sequence of the complementary strand).
DISCUSSION
To the best of our knowledge, this is the first case report of PFIC type 2 in Thailand. The patient had a typical clinical presentation of PFIC type 2 with cholestasis of onset in early infancy, with development of hepatomegaly and severe pruritus[1,2]. In PFIC type 2 serum bile acid concentrations are high, biliary bile-salt concentrations are very low, and GGT and cholesterol values are normal[2,5]. While serum bile-acid and biliary bile-salt concentrations could not be determined, as such studies are unavailable in Thailand, her severe pruritus indicated hypercholanemia. This feature and our patient’s GGT and cholesterol values were compatible with PFIC type 2. Although hypothyroidism in this patient might have caused cholestasis by delayed emptying of the biliary tract, and by changes in bile composition and excretion rate[6], normalization of free T4 and TSH levels after thyroxin therapy argued against this possibility. Since hypoglycemia was not present and initial growth was unremarkable, hypopituitarism was not pursued.
While PFIC type 1 has initial clinical and laboratory findings similar to those of PFIC type 2, histological features may differ. In PFIC type 1, bland canalicular cholestasis with variable fibrosis is found. In PFIC type 2, variable features include canalicular cholestasis and a neonatal hepatitis pattern, with hepatocellular swelling and giant cell transformation[1,2]. Lobular and portal inflammation and fibrosis are more pronounced in PFIC type 2 than type 1[2]. Immunostaining is a useful diagnostic tool for PFIC type 2 since most patients with ABCB11 mutations and hepatobiliary disease of onset in infancy have no canalicular BSEP expression[7]. At initial liver biopsy (2 mo) changes of neonatal hepatitis were found. At follow-up biopsy, these changes persisted, and BSEP was not detected along the canaliculi. The clinicopathological picture, felt to be compatible with PFIC type 2, thus prompted ABCB11 analysis.
BSEP is an ATP-dependent transporter located on the canalicular membrane of hepatocytes[8,9]. It is a major exporter of primary bile salts from hepatocyte cytoplasm into the bile-canaliculus lumen, and works against an extreme concentration gradient[2,9]. Mutations in ABCB11 that lead to failure of BSEP expression, or to expression of functionally defective BSEP, in turn lead to accumulation of bile salts inside hepatocytes, with ongoing severe hepatocellular damage and diminished bile flow.
To date, more than 100 mutations in ABCB11 have been identified[7,10-14]; however, genotype-phenotype correlations are not wholly clear. Severe phenotypes are often associated with mutations that lead to premature protein truncation or failure of protein production. Insertion, deletion, nonsense, and splicing mutations result in damaging effects, and patients who have clinical PFIC associated with such mutations exhibit little or no BSEP expression in hepatocyte canaliculi[2,7]. Missense mutations are also common. These can affect protein processing and trafficking or disrupt functional domains and protein structure[2,7,15]. Detectable BSEP expression does not exclude functional BSEP deficiency[2,7].
E297G and D482G are the two most common mutations in persons of European descent, and account for approximately 58% of BSEP mutations in European studies[7]. In Asian patients, few reports of mutations in PFIC type 2 exist[12-14]. Goto et al[14] have reported four mutations in ABCB11, predicted to yield V330X, R487H, R575X and E636G, in two Japanese PFIC patients. Chen et al[13] have reported seven BSEP mutations (M183V, V284L, R303K, R487H, W493X, G1004D and 1145delC) in four PFIC patients of Chinese descent; none of these mutations has been described in Caucasian patients. To the best of our knowledge, the mutations identified in our patient are novel. These mutations are predicted to lead to synthesis of truncated forms of BSEP.
Patients with PFIC type 2 are at risk for hepatobiliary malignancy[7,16]. Hepatocellular carcinoma or cholangiocarcinoma developed in 19 of 128 patients (15%)[7] and those who had two protein-truncating mutations were at particular risk[7]. Close surveillance of BSEP-deficient patients who retain their native liver is therefore essential.
In conclusion, we report a Thai infant with clinical features of normal-GGT PFIC. Her liver did not express immunohistochemically demonstrable BSEP. Novel mutations in ABCB11 were identified in the infant, with confirmation in her parents. These mutations were predicted to lead to synthesis of truncated forms of BSEP. Immunostaining and mutation analysis thus established the diagnosis of PFIC type 2.
Footnotes
Supported by “IKEM MZO 00023001”
Peer reviewers: John Y Chiang, MD, PhD, Professor, Department of Biochemistry and Molecular Pathology, Northeastern Ohio Univ. College of Medicine, 4209 State Route 44, PO Box 95, Rootstown, OH 44272, United States; Pietro Invernizzi, MD, PhD, Division of Internal Medicine and Hepatobiliary Immunopathology Unit, IRCCS Istituto Clinico Humanitas, via A. Manzoni 113, 20089 Rozzano, Milan, Italy
S- Editor Tian L L- Editor Kerr C E- Editor Yin DH
References
- 1.Alissa FT, Jaffe R, Shneider BL. Update on progressive familial intrahepatic cholestasis. J Pediatr Gastroenterol Nutr. 2008;46:241–252. doi: 10.1097/MPG.0b013e3181596060. [DOI] [PubMed] [Google Scholar]
- 2.Davit-Spraul A, Gonzales E, Baussan C, Jacquemin E. Progressive familial intrahepatic cholestasis. Orphanet J Rare Dis. 2009;4:1. doi: 10.1186/1750-1172-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris MJ, Le Couteur DG, Arias IM. Progressive familial intrahepatic cholestasis: genetic disorders of biliary transporters. J Gastroenterol Hepatol. 2005;20:807–817. doi: 10.1111/j.1440-1746.2005.03743.x. [DOI] [PubMed] [Google Scholar]
- 4.Kotalova R, Cebecauerova D, Knisely AS, Hrebicek M, Jirsa M. Progressive familial intrahepatic cholestasis-manifestation and diagnosis in infancy. Ces Slov Pediatr. 2006;61:200–206. [Google Scholar]
- 5.Whitington PF, Freese DK, Alonso EM, Schwarzenberg SJ, Sharp HL. Clinical and biochemical findings in progressive familial intrahepatic cholestasis. J Pediatr Gastroenterol Nutr. 1994;18:134–141. doi: 10.1097/00005176-199402000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Laukkarinen J, Sand J, Saaristo R, Salmi J, Turjanmaa V, Vehkalahti P, Nordback I. Is bile flow reduced in patients with hypothyroidism? Surgery. 2003;133:288–293. doi: 10.1067/msy.2003.77. [DOI] [PubMed] [Google Scholar]
- 7.Strautnieks SS, Byrne JA, Pawlikowska L, Cebecauerová D, Rayner A, Dutton L, Meier Y, Antoniou A, Stieger B, Arnell H, et al. Severe bile salt export pump deficiency: 82 different ABCB11 mutations in 109 families. Gastroenterology. 2008;134:1203–1214. doi: 10.1053/j.gastro.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 8.Suchy FJ, Ananthanarayanan M. Bile salt excretory pump: biology and pathobiology. J Pediatr Gastroenterol Nutr. 2006;43 Suppl 1:S10–S16. doi: 10.1097/01.mpg.0000226385.71859.5f. [DOI] [PubMed] [Google Scholar]
- 9.Stieger B, Meier Y, Meier PJ. The bile salt export pump. Pflugers Arch. 2007;453:611–620. doi: 10.1007/s00424-006-0152-8. [DOI] [PubMed] [Google Scholar]
- 10.Pauli-Magnus C, Stieger B, Meier Y, Kullak-Ublick GA, Meier PJ. Enterohepatic transport of bile salts and genetics of cholestasis. J Hepatol. 2005;43:342–357. doi: 10.1016/j.jhep.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Noe J, Kullak-Ublick GA, Jochum W, Stieger B, Kerb R, Haberl M, Müllhaupt B, Meier PJ, Pauli-Magnus C. Impaired expression and function of the bile salt export pump due to three novel ABCB11 mutations in intrahepatic cholestasis. J Hepatol. 2005;43:536–543. doi: 10.1016/j.jhep.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Chen HL, Chang PS, Hsu HC, Ni YH, Hsu HY, Lee JH, Jeng YM, Shau WY, Chang MH. FIC1 and BSEP defects in Taiwanese patients with chronic intrahepatic cholestasis with low gamma-glutamyltranspeptidase levels. J Pediatr. 2002;140:119–124. doi: 10.1067/mpd.2002.119993. [DOI] [PubMed] [Google Scholar]
- 13.Chen HL, Liu YJ, Su YN, Wang NY, Wu SH, Ni YH, Hsu HY, Wu TC, Chang MH. Diagnosis of BSEP/ABCB11 mutations in Asian patients with cholestasis using denaturing high performance liquid chromatography. J Pediatr. 2008;153:825–832. doi: 10.1016/j.jpeds.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 14.Goto K, Sugiyama K, Sugiura T, Ando T, Mizutani F, Terabe K, Ban K, Togari H. Bile salt export pump gene mutations in two Japanese patients with progressive familial intrahepatic cholestasis. J Pediatr Gastroenterol Nutr. 2003;36:647–650. doi: 10.1097/00005176-200305000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi H, Takada T, Suzuki H, Akita H, Sugiyama Y. Two common PFIC2 mutations are associated with the impaired membrane trafficking of BSEP/ABCB11. Hepatology. 2005;41:916–924. doi: 10.1002/hep.20627. [DOI] [PubMed] [Google Scholar]
- 16.Knisely AS, Strautnieks SS, Meier Y, Stieger B, Byrne JA, Portmann BC, Bull LN, Pawlikowska L, Bilezikçi B, Ozçay F, et al. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology. 2006;44:478–486. doi: 10.1002/hep.21287. [DOI] [PubMed] [Google Scholar]
- 17.Knisely AS . St. Louis PJ. Biochemical studies: Liver and intestine. In: Walker WA, Durie PR, Hamilton JR, Walker-Smith JA, Watkins JB, et al., editors. Pediatric gastrointestinal disease, pathophysiology, diagnosis, management. 1st ed. Ontario: BC Decker Inc; 1991. pp. 1364–1374. [Google Scholar]
- 18.Nicholson JF, Pesce MA. Reference ranges for laboratory tests and procedures. In: Kliegman RM, Behrman RF, Jenson HB, Stanton BF, et al., editors. Nelson textbook of Pediatrics. 18th ed. Philadelphia: Saunders Elsevier; 2007. pp. 2943–2954. [Google Scholar]