Abstract
BACKGROUND
NADPH oxidases (Nox) regulate vascular physiology and contribute to the pathogenesis of vascular disease. In vascular smooth muscle cells (VSMCs), the interactions of individual Nox homologues with regulatory proteins are poorly defined.
OBJECTIVE
The objective of this study was to identify novel NADPH oxidase regulatory proteins.
METHODS and RESULTS
Using a yeast 2 hybrid screen, we identified a novel binding partner, Poldip2, and demonstrate that it associates with p22phox, Nox1 and Nox4 and co-localizes with p22phox at sites of Nox4 localization. Poldip2 increases Nox4 enzymatic activity by 3-fold and positively regulates basal reactive oxygen species (ROS) production in VSMCs (O2•−: 86.3±15.6% increase; H2O2: 40.7±4.5% increase). Overexpression of Poldip2 activates Rho (180.2±24.8% increase), strengthens focal adhesions and increases stress fiber formation. These phenotypic changes are blocked by dominant negative Rho. In contrast, depletion of either Poldip2 or Nox4 results in a loss of these structures, which is rescued by adding back active Rho. Cell migration, which requires dynamic cytoskeletal remodeling, is impaired by either excess (70.1±14.7% decrease) or insufficient Poldip2 (63.5±5.9% decrease).
CONCLUSION
These results suggest that Poldip2 associates with p22phox to activate Nox4, leading to regulation of focal adhesion turnover and VSMC migration, thus linking ROS production and cytoskeletal remodeling. Poldip2 may be a novel therapeutic target for vascular pathologies with a significant VSMC migratory component, such as restenosis and atherosclerosis.
Keywords: Nox4, Poldip2, vascular smooth muscle cells, reactive oxygen species, cytoskeleton
Introduction
Reactive oxygen species (ROS), such as superoxide (O2•−) and hydrogen peroxide (H2O2), are implicated in the development of multiple cardiovascular disease pathologies, including hypertension, atherosclerosis and restenosis.1 Physiologically, ROS mediate many cellular functions such as proliferation, gene expression, migration, differentiation, and cytoskeletal remodeling.2 One major source of ROS is the NADPH oxidase (Nox) family of enzymes.
The catalytic moieties of NADPH oxidases are homologues of the flavin- and NADPH-binding protein gp91phox (Nox2) termed Nox1, Nox3, Nox4, Nox5, Duox1 and Duox2. Most cell types express multiple Nox enzymes that are differentially regulated and have distinct sub-cellular localizations, suggesting that these oxidases serve unique roles. For example, Nox1 and Nox4 are the predominant homologues in rodent vascular smooth muscle cells (VSMCs) from large vessels. While Nox1 is primarily found in caveolae, Nox4 is found in the nucleus, in focal adhesions and along stress fibers.3 Nox1 mediates VSMC growth and migration, while Nox4 is involved in differentiation.4
Nox enzymes also differ in their mode of regulation. The Nox2-based oxidase consists of five subunits. Together, the membrane proteins Nox2 and p22phox comprise the cytochrome b558 membrane complex, which is localized in sub-membranous vesicles and the plasma membrane. Catalytic activity is initiated by translocation to the membrane of cytosolic subunits p47phox, p67phox, and the small molecular weight G-protein Rac.4 Nox1 and Nox3 are similarly regulated by the p47phox and p67phox homologues Nox organizer 1 (NoxO1) and Nox activator 1 (NoxA1), respectively, as well as with Rac.4 However, none of the presently known cytosolic regulatory subunits are required for Nox4 activation.5
The mechanism by which Nox4 activity is regulated remains unclear. Some studies suggest that the principal mechanism of Nox4 regulation may be induction at the mRNA level, rather than assembly of an enzyme complex or post-translational protein modifications.6 However, while it is known that Nox4 requires p22phox, there has been no systematic search for proteins that bind to the Nox4/p22phox complex. In this study, we show that Poldip2, polymerase (DNA-directed) delta interacting protein 2,7, 8 is a novel Nox4/p22phox interacting protein and is a potent positive regulator of Nox4 activity in VSMCs. The Nox4/p22phox/Poldip2 complex has profound effects on Rho-dependent cytoskeletal reorganization and cellular migration. These results thus provide mechanistic insight into the unique regulation of Nox4 and the fundamental physiological and pathophysiological cellular functions that Poldip2/Nox4 modulates.
Materials and Methods
An expanded Materials and Methods section is available in the online data supplement at http://circres.ahajournals.org.
Cell Culture
Rat aortic VSMCs and human aortic smooth muscle cells (HASMCs) (passages 6–12) were grown in Dulbecco’s Modified Eagle’s Media (DMEM). HEK293 cells were cultured in DMEM with 10% Fetal Bovine Serum. Rat VSMCs were used for all VSMC experiments, except where indicated.
Antibodies
Poldip2 Goat antibody was custom made by GenScript Corporation (Piscataway, NJ) against the peptide sequence NPAGHGSKEVKGKTC.
Yeast Two-Hybrid Assay
We utilized the Matchmaker LexA yeast two-hybrid system (Clontech) and a VSMC cDNA library constructed in pB42AD. The cytosolic tail of rat p22phox (nt 360–579) served as bait. Positive colonies were amplified in minimal medium (-His/-Trp/-Ura) and false positives were eliminated by mating with YM4271 yeast bearing the pLexA containing the BD domain alone.
siRNA
VSMCs were plated at 40–50% confluence on collagen-coated substrate for 4–6 h, washed with OPTI-MEM, and incubated with siRNA + Oligofectamine complexes for 48 h. Two stealth siRNAs (Invitrogen) against Poldip2 (siPoldip2; #1-5’GCCCACAUAUAUCUCAGAGAUCUCA3’, #2-5’UGAGAUCUCUGAGAUAUAUGUGGGC3’) or a stealth control siRNA (siControl) were used at 15 nmol/L. Nox4 siRNA (siNox4; 25 nmol/L) was used as described previously,9 with the Allstars Negative Control (Qiagen). Cells were incubated in OPTIMEM for 2–4 days before use.
Constructs
HA-tagged Nox1 (Nox1-HA) and V5-tagged p22phox (V5-p22phox) were prepared as described previously.10 Myc-tagged rat Poldip2 was prepared using the coding region of Poldip2 obtained by PCR.
Adenoviruses
The AdEasy System was used to prepare adenoviruses with either no insert (AdGFP), hemagglutinin (HA)-tagged Nox1 (AdNox1HA), antisense Nox1 (AdNox1AS),11 antisense Nox4 (AdNox4AS),9 and N-terminal myc-tagged Poldip2 (AdPoldip2). The LacZ control (AdLacZ), constitutively active RhoA (AdRhoAGV), and dominant negative RhoA adenoviruses (AdRhoTN) were also used. VSMCs were transduced as described previously.9
GST-Pulldown
VSMCs were transfected with AdGFP or AdPoldip2 and labeled with 35S-methionine (20 µCi; 3 h). Lysates were incubated with GST-fusion proteins (GST-vector or GST-p22phox) prepared using the TNT T7 Quick coupled transcription/translation system (Promega). Binding partners were detected by autoradiography.
Immunoblotting and Immunoprecipitation
VSMCs were lysed in standard lysis buffer3, 10 or in Hunter’s buffer. Whole cell lysates were utilized for Western blot and immunoprecipitation.9, 10 Band intensity was quantified by densitometry using ImageJ software.
RNA Isolation and Quantitative RT-PCR
Total RNA was extracted using the RNeasy kit (Qiagen). Superscipt II (Invitrogen) and random primers were used for RT. Poldip2 (primer sequences: GTATGAGACGGGACAGCTATTTCTCCA and CTGACATAGTCCAAGCCTGGGATG), nox1, nox4, p22phox, 18S rRNA, were measured by amplification of VSMC cDNA using the LightCycler real-time thermocycler and SYBR green dye and normalized to 18S rRNA.9
Immunocytochemistry and Confocal Microscopy
VSMCs were plated serum deprived for 48 h, transiently transfected with siRNA for 72 h, or grown to 50–60% confluence before treatment with adenovirus. Immunocytochemistry was carried out as described,3 and images were acquired with a Zeiss LSM 510 META Laser Scanning Confocal Microscope.
Amplex Red Assay
H2O2 was measured using the oxidation of Amplex Red (100 µmol/L) in the presence of horseradish peroxidase.12 H2O2 production was normalized to cellular protein, measured by the Bradford Assay (Bio-Rad).
Lucigenin-enhanced Chemiluminescense
NADPH oxidase activity in membrane fractions was assessed using 5 µmol/L Lucigenin in the presence of 100 µmol/L NADPH and normalized to protein concentration, as described previously.13
Detection of Superoxide
Intracellular O2•− production was evaluated by measuring the conversion of DHE to 2-hydroxyethidium using HPLC.9 Results are expressed as PEG-SOD-inhibitable signal.
Immunohistochemistry
Tissues from rats were harvested and prepared for immunohistochemistry as described previously.9
Rho Activity Assay
Rho activity was measured by 2 independent methods: Rho pulldown assay and Rho G-LISA.
Migration Assay
Migration was measured using Boyden Chamber assays as described previously.14
Statistical Analysis
Results are expressed as mean ± S.E.M. from at least three independent experiments. Statistical significance was assessed using analysis of variance (ANOVA), followed by Bonferroni’s Multiple Comparison post-hoc test. A value of p<0.05 was significant.
Results
Identification of Poldip2 as a novel p22phox-interacting partner
Using the proline-rich region of the C-terminal tail of p22phox as bait, a likely binding site for regulatory molecules,4 we performed a yeast two hybrid screen on a cDNA library from rat VSMCs (cells with high Nox4 expression). After stringent elimination of false positives, we isolated the cDNA of a protein that interacted with p22phox and obtained its full-length sequence using RT-PCR, and identified the clone as Poldip2 (GenBank accession #FJ515740). Poldip2 bears no homology to the classical Nox cytosolic regulatory subunits p47phox or p67phox or their homologues NoxO1 and NoxA1. It consists of 368 amino acids and has a predicted molecular weight of ~42 kDa, with a potential signal peptide cleavage site after the first N-terminal 48 residues, which would result in a protein of ~37 kDa. Additional predicted features include an ApaG domain, a PX domain, N-Myristoylation sites, phospho-Serine/Threonine sites, and Tyrosine sulfation sites.
Poldip2 association with Nox subunits
To verify that Poldip2 associates with p22phox, we initially used a GST-pulldown assay. Indeed, 35S-Poldip2 pulls down with GST-p22phox (Fig. 1a), confirming the association of these two proteins. To further validate the association of Poldip2 and p22phox, we co-transfected HEK293 cells with V5-tagged p22phox (V5-p22phox) and either vector control or Myc-tagged Poldip2 (Myc-Poldip2). Myc-Poldip2 co-immunoprecipitates with V5-p22phox (Fig. 1b). To confirm an association between endogenous proteins, we generated and characterized an antibody against Poldip2 (Online Figure I,a–b), which recognizes both the 42 kDa and 37 kDa forms of Poldip2. Endogenous Poldip2 co-immunoprecipitates with p22phox in VSMCs (Fig. 1c), as expected from our observations with tagged proteins.
Figure 1. Poldip2 associates with Nox1 and Nox4 in a p22phox-dependent manner.
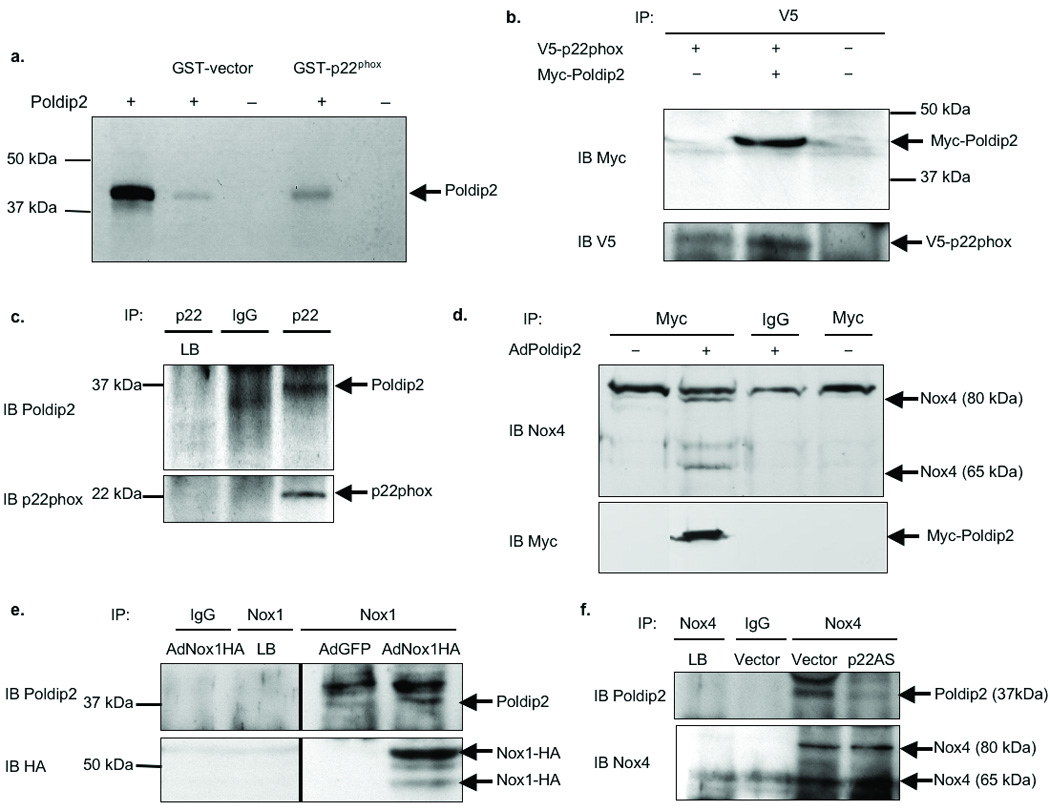
a. VSMCs were transfected with vector control (−) or Poldip2 (+), labeled with 35S-Methionine, and used in a GST-pulldown assay. Binding partners were detected by autoradiography. Positive control: in vitro translated Poldip2 (lane 1). b. HEK 293 cells were co-transfected with V5-tagged p22phox (V5-p22phox) and either empty vector (−) or vector expressing Myc-tagged Poldip2 (+; Myc-Poldip2). Cells were immunoprecipitated (IP) with V5 antibody and immunoblotted (IB) with a Myc (upper) or V5 (lower) antibody. c. Human VSMC lysates were immunoprecipitated with rabbit IgGs (IgG) or p22phox antibody and immunoblotted with a Poldip2 (upper) or p22phox (lower) antibody. Lysis buffer (LB) is a negative control in all IP experiments. Poldip2 is detected as a 37 kDa band; IgGs interfere with resolution of the 42 kDa band. d. VSMCs were transduced with no adenovirus (No Ad), 125 µL/dish of control adenovirus (AdGFP) or Myc-tagged Poldip2 adenovirus (AdPoldip2). Lysates were immunoprecipitated with a Myc antibody and immunoblotted with a Nox4 (upper) or Myc (lower) antibody. e. VSMCs transduced with AdGFP or HA-tagged Nox1 (Nox1HA) adenovirus were immunoprecipitated with goat IgGs or a Nox1 antibody and immunoblotted with a Poldip2 (upper) or HA (lower) antibody. f. VSMCs stably transfected with empty vector (Vector) or antisense p22phox (p22AS) vector were immunoprecipitated with rabbit IgGs or a Nox4 antibody and immunoblotted with a Poldip2 (upper) or Nox4 (lower) antibody.
Previously published data show that both Nox1 and Nox4 co-localize with and co-immunoprecipitate with p22phox in rat VSMCs, but that other Nox homologues are not expressed.3, 15 Therefore, we examined a potential association of Poldip2 with these proteins. VSMCs were transduced with adenovirus to overexpress Myc-Poldip2 (AdPoldip2) (Online Figure I,c) and co-immunoprecipitation revealed an association of Nox4 and Myc-Poldip2 (Fig. 1d). To ascertain if the association with Nox4 is dependent on p22phox, we utilized a rat VSMC line stably transfected with antisense p22phox (p22AS) in which p22phox expression is ablated16 (Online Figure I,d). Poldip2 co-immunoprecipitates with Nox4 in vector-transfected cells, but not in cells lacking p22phox (Fig. 1f), suggesting that Poldip2 requires p22phox to associate with Nox4. As shown in Fig. 1e, Poldip2 also co-immunoprecipitates with HA-tagged Nox1 in VSMCs.
Poldip2 stimulates ROS production via Nox4
To determine if Poldip2 regulates oxidase function in VSMCs, we measured NADPH oxidase activity in membrane fractions of VSMCs transduced with AdPoldip2. Overexpressing Poldip2 alone caused a significant increase in basal oxidase activity in a dose-dependent manner when compared to VSMCs transduced with control adenovirus (AdGFP) (Fig. 2a). This increase in activity was not caused by an increase in nox1 mRNA (AdGFP: 2.4±1.1 ×105 vs. AdPoldip2: 1.6±0.7 ×105 copies/µL cDNA, p=NS), nox4 mRNA (AdGFP: 1.3±0.4 ×107 vs. AdPoldip2: 1.0±0.5 ×107 copies/µL cDNA, p=NS), or p22phox expression (AdGFP: 4.3±0.2 ×106 vs. AdPoldip2: 3.0±1.1 ×106 copies/µL cDNA, p=NS), nor by an increase in Nox4 or p22phox protein levels (Online Figure I,f). Because Poldip2 structure does not predict intrinsic oxidase activity, we tested whether this increase in NADPH-dependent O2 •− production results from activation of either of the VSMC Nox catalytic subunits. Reduction of Nox4 by expression of antisense Nox4 (AdNox4AS) (Online Figure I,e) prior to the overexpression of Poldip2 abolishes the Poldip2-mediated increases in NADPH oxidase activity (Fig. 2b), suggesting that Nox4 mediates the effects of Poldip2. This dependency on Nox4 is reflected in levels of O2•− and H2O2 in intact cells transduced with both constructs (Fig. 2c–d). Of interest, Poldip2 was unable to significantly increase H2O2 production in VSMCs lacking p22phox expression (Online Figure III). In contrast, when Nox1 is depleted using antisense Nox1 (AdNox1AS), the increase in H2O2 production caused by Poldip2 overexpression is potentiated, rather than inhibited (Fig. 2e). However, Poldip2 overexpression significantly increases ROS production by ~2.5 fold (p<0.05) in both Nox1 wild-type (Nox1 y/+; 2.65±0.2 fold increase) and Nox1 knockout (Nox1 y/−; 2.56±0.2 fold increase) cells, as measured by ESR, suggesting that Nox1 is not directly involved in Poldip2-mediated ROS production. The potentiation of ROS production after Nox1 depletion may occur because the absence of Nox1 may increase the amount of p22phox available to stabilize Nox4, or may release a pool of Poldip2 that is then free to interact with Nox4. Taken together, these data strongly indicate that Poldip2 positively regulates Nox4, but not Nox1, activity, leading to subsequent increases in ROS production.
Figure 2. Poldip2 stimulates ROS production via Nox4.
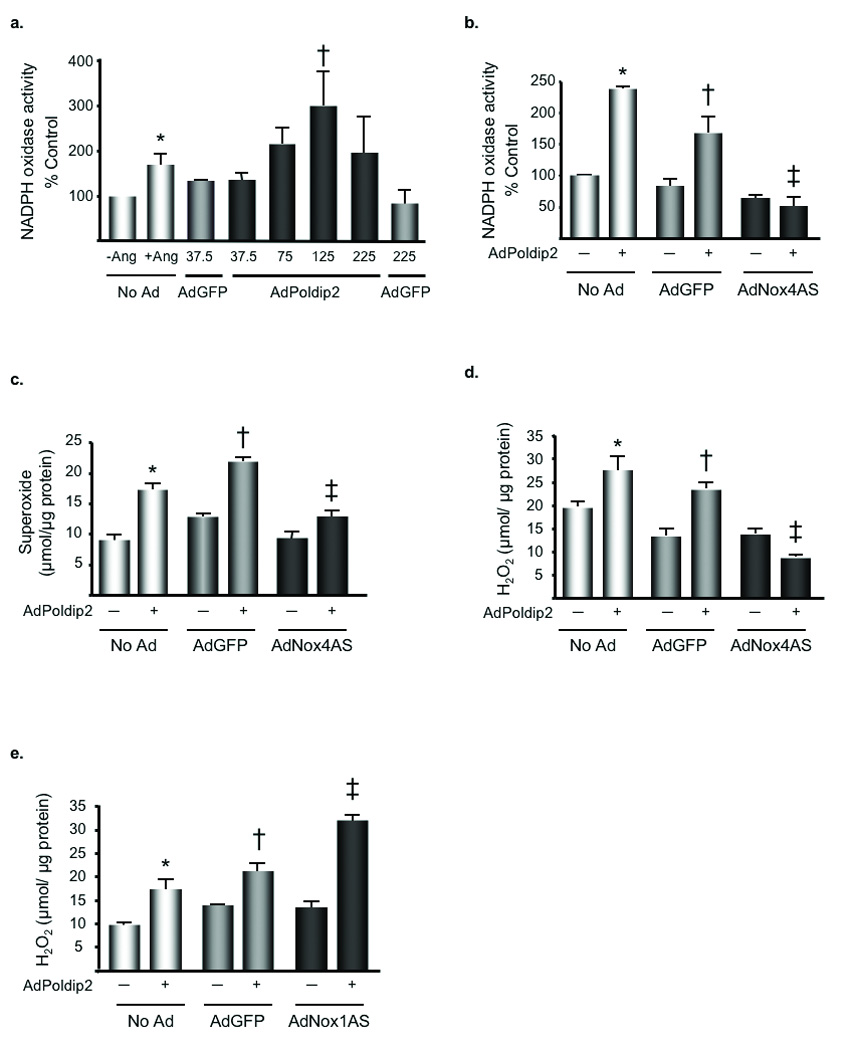
a. VSMCs were transduced with no adenovirus (No Ad), 37.5 or 225 µL/dish of AdGFP or 37.5 µL/dish to 225 µL/dish of AdPoldip2. Lucigenin enhanced chemiluminescence was used to measure NADPH oxidase activity in membrane fractions. Bars are mean ± S.E.M. of 3–4 independent experiments, †p<0.05 vs. AdGFP. Positive control: VSMCs stimulated with 100 nmol/L Ang II for 4 h. *p<0.05 vs. No Ad - Ang. b. VSMCs were transduced with AdGFP or AdNox4AS for 48 h prior to transduction with AdGFP (−) or AdPoldip2 (+) for 72 h. Lucigenin enhanced chemiluminescence was used to measure NADPH oxidase activity. Bars are mean ± S.E.M. of four independent experiments, *p<0.001 vs. No Ad- AdPoldip2; †p<0.01 vs. AdGFP-AdPoldip2; ‡p<0.001 vs. AdGFP+AdPoldip2. c. VSMCs were transduced with the same adenoviral combinations as panel b prior to using DHE-HPLC to measure O2•− production (bars are mean ± S.E.M. of 4 independent experiments, *p<0.001 vs. No Ad-AdPoldip2; †p<0.001 vs. AdGFP-AdPoldip2; ‡p<0.001 vs. AdGFP+AdPoldip2). d. VSMCs transduced with the same adenoviral combinations as in panel b were used for the Amplex red assay to measure H2O2 production (bars are mean ± S.E.M. of 4 independent experiments, *p<0.05 vs. No Ad- AdPoldip2; †p<0.01 vs. AdGFP-AdPoldip2; ‡ p<0.001 vs. AdGFP+ AdPoldip2). e. VSMCs were transduced with AdGFP or adenovirus expressing AdNox1AS for 48 h before transducing cells with AdGFP (−) or AdPoldip2 (+) for 72 h. The Amplex Red Assay was used to measure H2O2. Bars are mean ± S.E.M. of 4 independent experiments, *p<0.05 vs. No Ad-AdPoldip2; †p<0.05 vs. AdGFP-AdPoldip2; ‡p<0.001 vs. AdGFP+AdPoldip2).
Poldip2 co-localizes with Nox4 and p22phox
Because Poldip2 functionally regulates Nox4 activity, we utilized immunocytochemistry to determine if Poldip2 co-localizes with Nox4 and p22phox in VSMCs. As shown in Fig. 3, Poldip2 and p22phox co-localize in focal adhesions (Fig. 3a, upper), along stress fibers (Fig. 3a, middle), and in the nucleus of VSMCs, which are the patterns of p22phox distribution reported previously.3 Similar results were obtained using a Myc-tagged Poldip2 construct (Fig. 3a, lower). In VSMCs, Nox4 has been detected in comparable locations.3, 9 Indeed, co-localization experiments showed a clear overlap between Myc-Poldip2 and Nox4 staining in all three major subcellular sites (Fig. 3b). Together these data suggest that Poldip2 associates with p22phox and Nox4 in specific subcellular compartments.
Figure 3. Poldip2 co-localizes with Nox4 and p22phox.
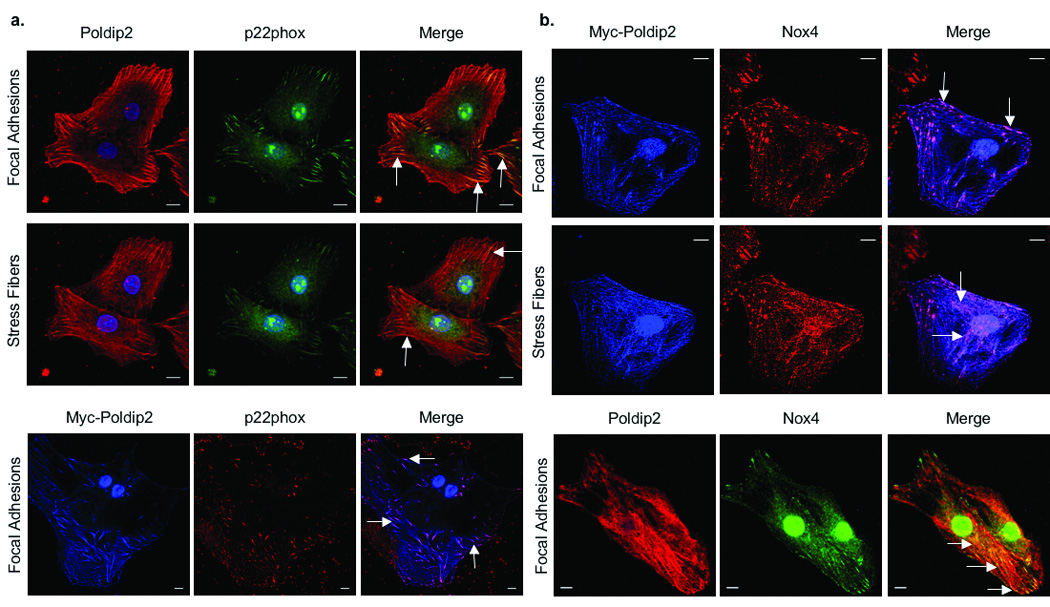
a, upper. Confocal images of VSMCs double labeled with anti-Poldip2 (red) and anti-p22phox (green) antibodies. Nuclei are labeled with DAPI (blue). Images acquired at the focal adhesion (upper panel) and stress fiber (lower panel) planes are depicted. Arrows indicate areas of co-localization (yellow) in the merge. a, lower. VSMCs were transduced with AdPoldip2 and double labeled with anti-Myc (pseudo-colored blue) and anti-p22phox (red) antibodies. Arrows indicate areas of co-localization (purple) in the merge. b, upper. VSMCs were transduced with AdPoldip2 and double labeled with anti-Myc (pseudocolored blue) and anti-Nox4 (red) antibodies. Arrows indicate areas of co-localization (purple) in the merge at the focal adhesion (upper panel) and stress fiber (lower panel) planes. b, lower. VSMCs were double labeled with anti-Poldip2 (red) and anti-Nox4 (green) antibodies. Arrows indicate areas of co-localization (yellow) in the merge. Scale bars, 10 µm.
The association between Nox4 and Poldip2 prompted us to examine the expression of Poldip2 in tissues rich in Nox4, such as aorta, lung and kidney.4 Analysis of tissue distribution using immunohistochemistry, Western blot and qRT-PCR indicates that Poldip2 is highly expressed in all three tissues (Fig. 4). In contrast, Poldip2 is barely detectable in spleen and thymus, which are rich in Nox2.
Figure 4. Tissue distribution of Poldip2.
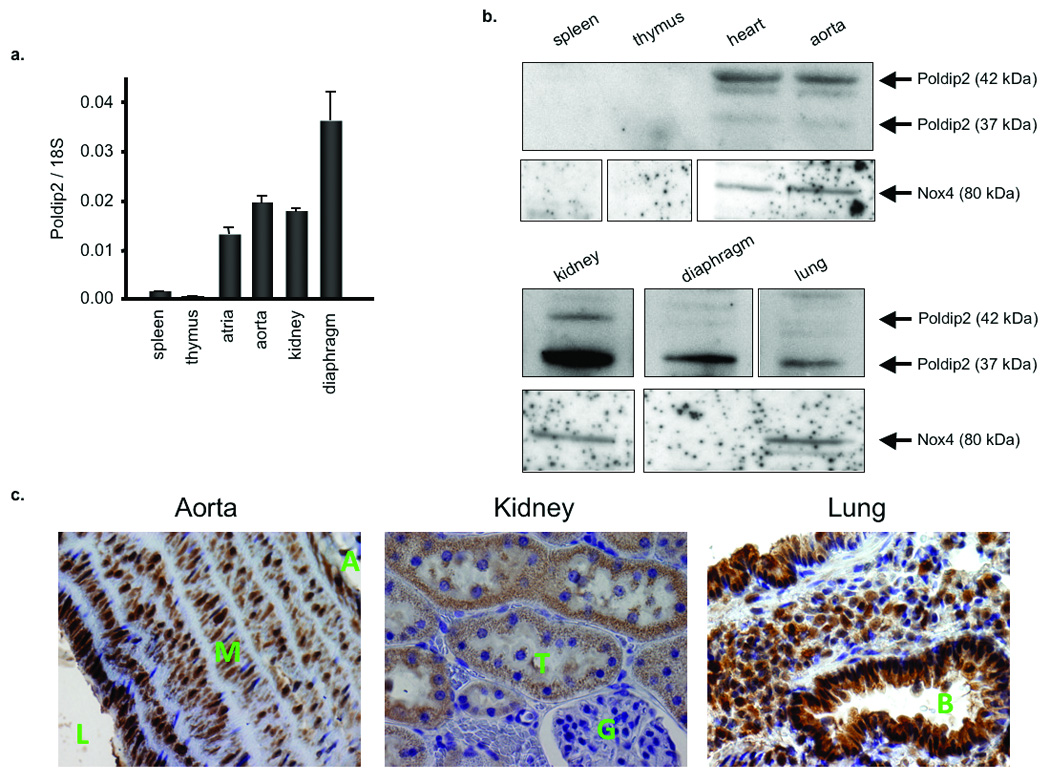
a. Quantitative real-time PCR of poldip2 expression in tissues from Sprague Dawley rats; mRNA is normalized to 18S. Bars are mean ± S.E.M. of triplicates from a representative experiment, repeated twice. b. Western blot of Poldip2 protein expression in rat tissue lysates. The 42 kDa Poldip2, containing the predicted signal peptide, and the 37 kDa Poldip2, after peptide cleavage, are shown. c. Immunohistochemical distribution of Poldip2 and Nox4 in rat aorta (L-lumen, M-media, A-adventitia), kidney (G-glomerulus, T-tubule), and lung (B-bronchiole) tissues using an anti-Poldip2 antibody. Poldip2 signal appears brown. Nuclei are counterstained with hematoxylin (blue/purple).
siPoldip2 decreases ROS production by Nox4 and alters VSMC phenotype
Overexpression of Poldip2 establishes its ability to regulate Nox4, but does not indicate whether Poldip2 is required for Nox4 activation. We have previously shown that basal ROS production in VSMCs is due to Nox4 activity;9 therefore, we tested the effect of Poldip2 knockdown on basal O2•− and H2O2 production in VSMCs transfected with siRNA to deplete Poldip2 (siPoldip2). As shown in Fig. 5a,b, siPoldip2 significantly decreases Poldip2 mRNA and protein levels, as well as O2•− and H2O2 production (Fig. 5c,d), compared to control siRNA (siControl). Interestingly, we observed distinct changes in VSMC morphology after siPoldip2 treatment; cells became elongated, spindly, and seemed to have fewer points of contact with the dish, reminiscent of the phenotype observed in siNox4 treated cells (Fig. 5f).
Figure 5. siPoldip2 alters VSMC phenotype and decreases basal ROS production by Nox4.
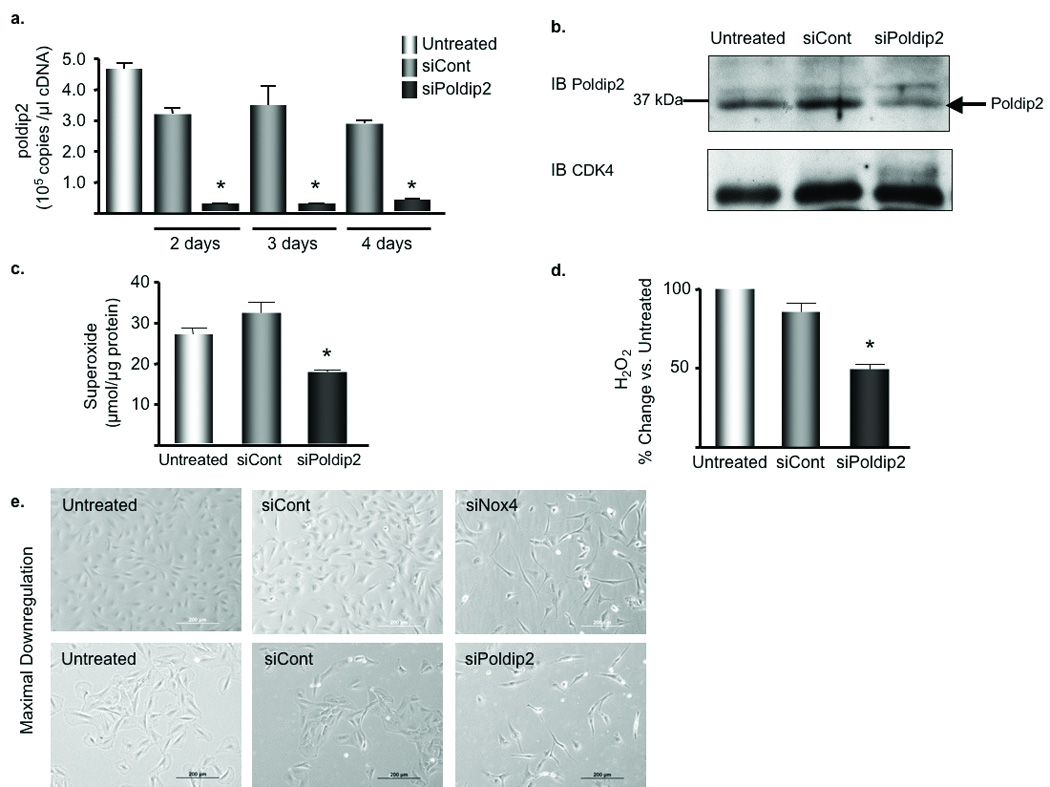
a. Quantitative real-time PCR of poldip2 expression in VSMCs transfected with control siRNA (siCont) or siRNA against Poldip2 (siPoldip2) for the indicated times. Bars are mean ± S.E.M. of three independent experiments, *p<0.001 vs. siCont. b. Western blot analysis of Poldip2 expression in protein extracts from VSMCs transfected for 4 days. CDK4 (lower blot) serves as a loading control. c. DHE-HPLC was used to assess intracellular O2•− production in VSMCs were treated as described in b. Bars are mean ± S.E.M. of four independent experiments, *p<0.01 vs. siCont. d. Amplex Red Assay was used to assess H2O2 production. Bars are means ± S.E.M. of 4 independent experiments, *p<0.001 vs. siCont. e. VSMCs were transiently transfected for 4 (upper) or 5 (lower) days and visualized by phase contrast microscopy; scale bars are 200 µm.
Poldip2 regulates proper Nox4 and p22phox localization, focal adhesion integrity and stress fiber formation
The localization of Poldip2, Nox4 and p22phox in focal adhesions and the apparent loss of these structures in siPoldip2-treated cells raise the possibility that Nox4 and p22phox localization is impaired when Poldip2 is depleted. Both Nox4 and p22phox protein levels seem slightly reduced in siPoldip2 treated VSMCs; however, the most striking effect is the loss of Nox4 and p22phox localization to focal adhesions (Fig. 6a). The profound cytoskeletal phenotype observed in cells treated with siPoldip2 or siNox4 suggests that this enzyme complex may regulate focal adhesion integrity and/or stress fiber formation. As shown in Fig. 6a,b siPoldip2 or siNox4 treatment results in wavy, disorganized stress fibers, as detected by phalloidin staining, and complete loss of focal adhesion-like structures, as detected by staining for the resident focal adhesion proteins vinculin and paxillin. In contrast, overexpressing Poldip2 results in an increase in both focal adhesion and stress fiber formation (Fig. 6c). Thus, the Poldip2/Nox4 complex appears to be a potent regulator of these important cytoskeletal structures.
Figure 6. Poldip2 regulates proper Nox4 and p22phox localization, focal adhesion integrity and stress fiber formation.
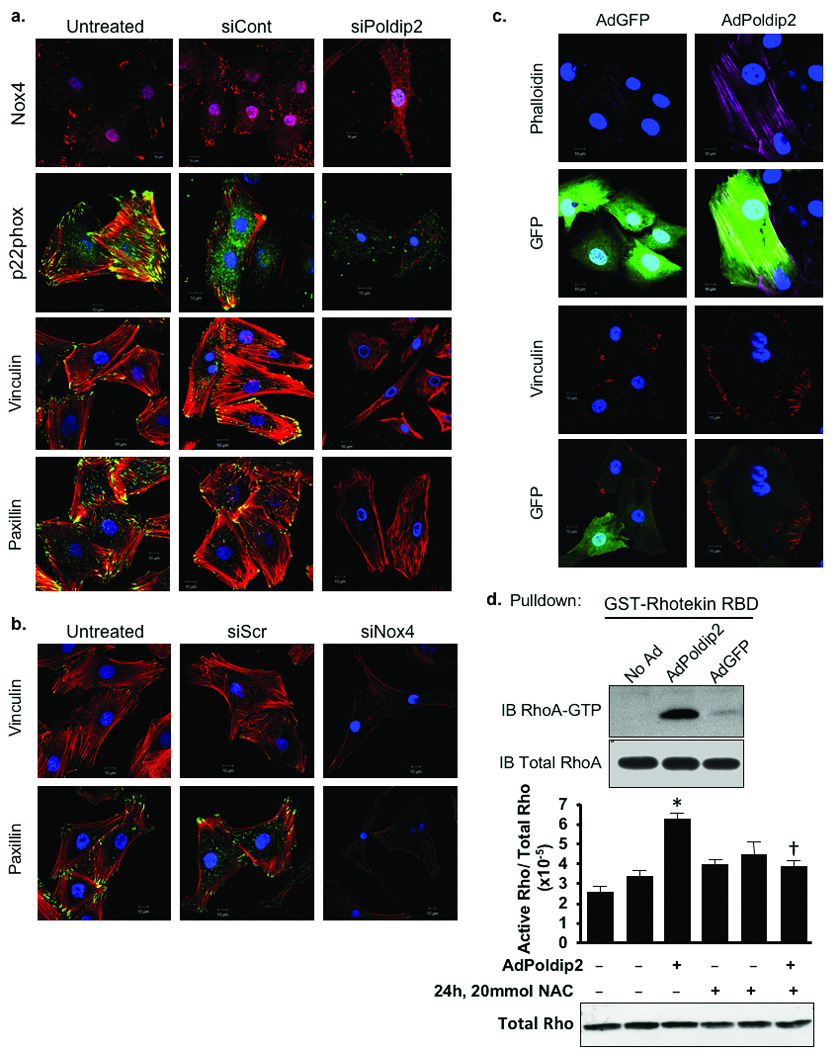
a. Confocal images of VSMCs either untreated or transiently transfected with 15 nmol/L of either siCont or siPoldip2. Nox4. single labeled with anti-Nox4 (red) antibody, p22phox. double labeled with anti-p22phox (green) and phalloidin (red) to stain stress fibers, Vinculin. double labeled with anti-vinculin (green) and phalloidin (red), Paxillin. double labeling with anti-paxillin (green) and phalloidin (red). Yellow appears where stress fibers (red) insert into focal adhesions (green). Nuclei are labeled with DAPI (blue). Images in a acquired at the focal adhesion plane. b. Confocal images of VSMCs either untreated or transiently transfected with 25 nmol/L of Allstars negative control siRNA (siCont) or siNox4. Vinculin. double labeled with anti-vinculin (green) and phalloidin (red). Paxillin. double labeled with anti-paxillin (green) and phalloidin (red). images in b acquired at the focal adhesion plane. c. Confocal images of VSMCs transduced with AdGFP or AdPoldip2 (detected as green in GFP panels) before labeling with phalloidin (purple) or with vinculin (red). Scale bars in a, b, and c are 10 µm. d. VMSCs that were non-transduced (No Ad) or transduced for 72 h with AdPoldip2 or AdGFP and that were either untreated or treated with 20 mmol/L N-acetyl cysteine (NAC) for 24 h prior to a Rho activity assay to measure active, GTP-bound RhoA. Western blots of GTP-bound RhoA (upper) and total RhoA (lower) are from a representative experiment. The bar graph quantifies three independent Rho activity assays. Bars are mean ± S.E.M., *p<0.01 vs. AdGFP-NAC; †p<0.05 vs. AdPoldip2-NAC.
Nox4 and Poldip2 stabilize focal adhesions and promote stress fiber formation through RhoA activation
Because both focal adhesion turnover and stress fiber formation are mediated through RhoA activation,17 we hypothesized that Poldip2/Nox4 may exert their effects by activating RhoA. Previous work has shown that RhoA is activated by ROS, suggesting that RhoA is a potential target of this complex.18 Overexpression of Poldip2 results in a substantial increase in RhoA activation (180.2%±24.8 increase vs. AdGFP, p<0.05), and this increase is significantly blocked by treatment with the potent antioxidant N-acetyl cysteine (Fig. 6d).
To evaluate the role of RhoA in mediating the effects of Poldip2/Nox4, we transduced siPoldip2-treated VSMCs with constitutively active RhoA (AdRhoGV) to determine if we could rescue the loss of focal adhesions and stress fibers. Indeed, as shown in Fig. 7a, RhoGV countered the phenotypic loss of stress fibers and focal adhesions caused by Poldip2 depletion. Additionally, dominant negative RhoA (AdRhoTN) blocked the phenotypic increase in stress fiber and focal adhesion formation in VSMCs overexpressing Poldip2 (Fig. 7b). Taken together, these data strongly indicate that Poldip2 functionally regulates focal adhesion and stress fiber formation through a RhoA-dependent pathway.
Figure 7. Nox4 and Poldip2 regulate focal adhesion and stress fiber maintenance through RhoA activation.
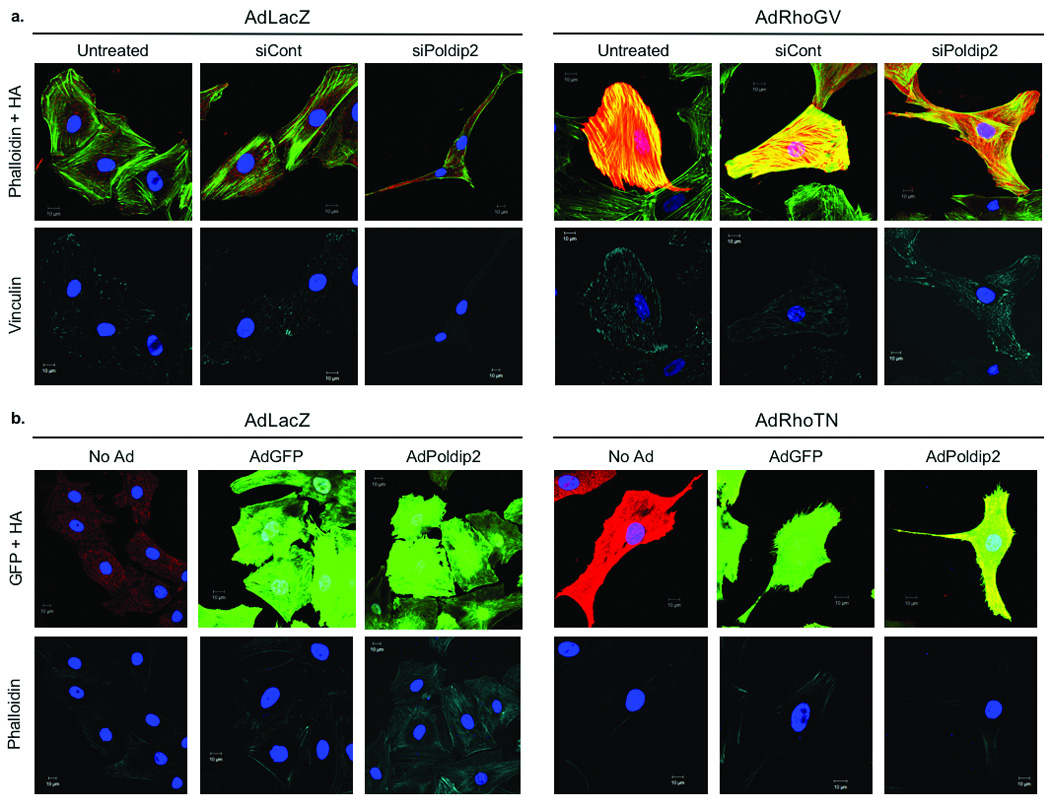
a. Confocal images VSMCs either untreated or transiently transfected with 15 nmol/L of either siCont or siPoldip2 prior to transduction with either control adenovirus (AdLacZ) or an HA-tagged constitutively active Rho (AdRhoGV). Cells were triple labeled with anti-HA antibody (HA; red) to detect cells transduced with AdRhoGV, Phalloidin (green), to detect stress fibers, and anti-vinculin (pseudo-colored cyan) to detect focal adhesions. Images acquired at the stress fiber (upper) and focal adhesion (lower) planes. b. VSMCs non-transduced (No Ad) or transduced for 24 h with AdGFP or AdPoldip2 prior to transduction with either AdLacZ or an HA-tagged dominant negative Rho (AdRhoTN). Cells transduced with AdGFP or AdPoldip2 appear green from GFP. Cells were double labeled with anti-HA antibody (HA; red) to detect cells transduced with AdRhoTN, and Phalloidin (pseudo-colored cyan) to detect stress fibers. Nuclei are labeled with DAPI (blue). Images acquired at the stress fiber plane. Scale bars in a and b are 10 µm.
Nox4 and Poldip2 regulate VSMC migration
VSMCs participate in the development of vascular lesions through their ability to proliferate and migrate.19 Migration, in particular, is dependent upon dynamic focal adhesion turnover and cytoskeletal reorganization. Because either upregulation or downregulation of Poldip2/Nox4 negatively impacts focal adhesion turnover (Fig. 6a,b), we hypothesized that either overexpression or knockdown of these proteins would impair cell migration. As shown in Fig. 8a, overexpression of Poldip2, which strengthens focal adhesions, blocks migration in response to PDGF (10 nmol/L, 4 hours), perhaps because focal adhesions cannot release from the trailing edge of the cell to allow forward movement. Knockdown of Poldip2 or Nox4, which induces a loss of focal adhesions, also inhibits migration (Fig. 8b,c), in this case presumably because new focal adhesions cannot form or mature at the leading edge of the cell.
Figure 8. Poldip2 regulates VSMC migration.
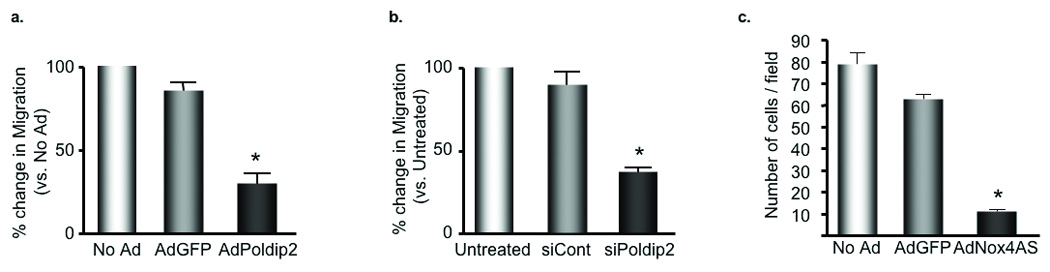
The Boyden Chamber Assay was used to measure cell migration in response to 10 nmol/L platelet-derived growth factor (PDGF) for 4 h. a. VSMCs were transduced with no adenovirus (No Ad), AdGFP or AdPoldip2 prior to the Boyden Chamber Assay, *p<0.01 vs. AdGFP. b. VSMCs were transfected with no siRNA (Untreated), siCont or siPoldip2 prior to the Boyden Chamber Assay, *p<0.01 vs. siCont. c. VSMCs were transduced with no adenovirus (No Ad), AdGFP or AdNox4AS prior to the Boyden Chamber Assay, *p<0.01 vs. AdGFP.
Discussion
In this study, we identify a novel role for Poldip2 as a unique positive regulator of Nox4 via its association with p22phox. Poldip2, together with Nox4, has profound effects on Rho-dependent cytoskeletal remodeling. Very little is known about the physiological role of Poldip2, except as a regulator of cell division.20 On the other hand, Nox4 is functionally linked to senescence, proinflammatory responses, oxygen sensing, migration,21 differentiation,9 proliferation and apoptosis.4 The fact that Nox4 regulates such diverse physiological and pathophysiological responses suggests that it regulates a fundamental cellular process. Our data showing a functional association of Nox4 with Poldip2, and subsequent modulation of cytoskeletal integrity, provide a possible mechanism to explain the known functions of both proteins.
The association of Poldip2 and p22phox/Nox4 was first detected in our yeast two-hybrid screen designed to search for new p22phox-interacting proteins. Further investigation using multiple techniques (Fig. 1a–c; Fig. 2a) confirm this interaction and show a clear functional association between Nox4 and Poldip2. Both Myc-tagged Poldip2 and endogenous Poldip2 associate with Nox4 and p22phox (Fig. 1d,f). In VSMCs, Nox4 and Poldip2 co-localize specifically in focal adhesions, stress fibers, and the nucleus (Fig. 2b). Nox4 and Poldip2 also co-localize in similar cell types and cell structures in tissues from mouse aorta, kidney, and lung (Fig. 4c). Interestingly, knockdown of either Poldip2 or Nox4 using siRNA causes the same cytoskeletal and phenotypic changes in VSMCs (Fig. 5f; Fig. 6a). The ability of Nox4 antisense to block Poldip2-induced increases in NADPH oxidase activity and ROS production (Fig. 2a–d) indicates that Poldip2/Nox4-mediated ROS generation is responsible for these changes. However, as shown in Fig. 1e, Poldip2 can also associate with HA-tagged Nox1, suggesting that Poldip2 may complex with the other Nox enzyme expressed in VSMCs, and raising the possibility that an alteration in Nox1 activity could explain the phenotype. This seems unlikely because antisense Nox1 does not inhibit Poldip2-stimulated ROS production (Fig. 2e) and Nox1 is not found in focal adhesions or the nucleus.3 Furthermore, overexpression or knockdown of Poldip2 in Nox1y/-VSMCs has the same effect on ROS production and cytoskeletal remodeling as it does in wild-type VSMCs, suggesting that Nox1 is not involved in the cytoskeletal effects of Poldip2. Nonetheless, we cannot rule out the possibility that Poldip2 may also influence Nox1-specific signaling.
There appear to be multiple levels of regulation of Nox4 by Poldip2. First, Poldip2 increases Nox4-dependent ROS production (Fig. 2), suggesting Poldip2 may function similarly to other NADPH oxidase regulatory proteins such as p47phox, p67phox, NoxO1 or NoxA1. Second, Poldip2 appears to be involved in targeting Nox4 to specific subcellular compartments in VSMCs. Nox4 and p22phox have been detected in stress fibers, the nucleus and focal adhesions.9 In the absence of Poldip2, Nox4 and p22phox no longer localize in focal adhesions, but are still present in the nucleus (Fig. 6 and unpublished observations). Therefore, binding to Poldip2 may ensure proper Nox4 and p22phox localization to cytoskeletal structures. In cells with low or absent Poldip2 expression, Nox4 may thus have different subcellular distributions. This is supported by the observation that Nox4 is detected in the endoplasmic reticulum in endothelial cells (ECs).22, 23 A third possibility is that Poldip2 mediates processing of Nox4 and p22phox in the endoplasmic reticulum and Golgi. For other Nox homologues, such as Nox2 and Nox3, heme incorporation and interaction with p22phox during transit through the endoplasmic reticulum are required for complex stabilization and full enzyme activity.24, 25 Moreover, Duox1 and 2 require coexpression of a maturation factor (DuoxA1 or DuoxA2) for proper processing.26 These proteins mediate the endoplasmic reticulum-to-Golgi transition of the Duox proteins, and participate in their maturation and translocation to the plasma membrane. Because Nox4 is highly expressed in the endoplasmic reticulum in some cells, this potential function requires further investigation.
As noted earlier, the fact that Nox4 regulates such diverse physiological and pathophysiological responses suggests that it modulates a fundamental cellular process that may influence multiple functions such as proliferation, apoptosis, senescence, and migration. The association between Poldip2 and Nox4 predicts that Poldip2 should mediate these functions as well. Our data clearly implicate the cytoskeleton as a target of Poldip2. siPoldip2 causes cells to become elongated and spindly, with few detectable focal adhesions and wavy stress fibers (Fig. 5f, Fig.6a). In contrast, overexpression of Poldip2 increases the thickness of stress fibers and causes focal adhesions to elongate and mature (Fig. 6c). It is likely that Nox4 mediates these effects, since others have suggested that ROS are potent regulators of cytoskeletal remodeling. Moldovan et al.27 showed that actin polymerization in migrating ECs is blocked by the flavin containing oxidase inhibitor diphenylene iodonium. Similar results were found for LDL-induced reorganization of the actin cytoskeleton.28 Wu et al.29 identified oxidative modifications of focal adhesion proteins induced by overexpression of a NADPH oxidase component, which raises the interesting possibility that ROS produced in focal adhesions may regulate their integrity by specifically modifying resident proteins.
A major target of Nox4/Poldip2 appears to be RhoA. Rho is activated in Poldip2 overexpressing cells, and dominant negative Rho inhibits cytoskeletal changes induced by Poldip2 overexpression (Fig. 6d, Fig.7b). Moreover, expression of constitutively active Rho reverses the loss of stress fibers and focal adhesions induced by knockdown of Poldip2 (Fig. 7a). RhoA is one of the most important factors regulating focal adhesion turnover. Basal Rho activity is required to maintain cell substrate adhesion,30 the maturation of focal adhesions from focal complexes is mediated by Rho-dependent actin-myosin contraction of stress fibers,17 and focal adhesion dissolution requires inhibition of Rho.31 Rho also regulates microtubule stabilization,32 suggesting an additional mechanism to explain the siPoldip2 phenotype. This suggests that other cytoskeleton-mediated events such as cell division, proliferation, and differentiation-associated stress fiber formation, may also be mediated by the Poldip2/Nox4 axis. Moreover, it explains why both Nox1 and Nox4 are required for PDGF-induced VSMC migration. Nox1 is involved in signaling events at the cell membrane, regulating cofilinmediated actin remodeling,33 while Nox4/Poldip2 likely regulates focal adhesion turnover in the trailing edge of the cell.
In this regard, it is of interest to examine what little is presently known about the functions of Poldip2. Poldip2 (also known as PDIP38 and Mitogenin I) was originally identified as a PCNA-and DNA polymerase δ-interacting protein, implicating a possible function in the regulation of gene expression, DNA duplication, or DNA repair.7 In the present context, this is of particular import because we also see Nox4 in the nucleus, but the role of Nox4 in these processes is unknown. While Poldip2 is reportedly associated with the mitochondria, these studies were performed in HeLa cells transfected with GFP-tagged Poldip2, which may confer different subcellular localizations compared to endogenous Poldip2.8, 34 In fact, a more recent study reported localization of Poldip2 in the nucleus, cytoplasm and plasma membrane in epithelial and endothelial cells.35 Interestingly, these authors found an interaction of Poldip2 with carcinoembryonic antigen-related cell adhesion molecule-1 (CD66a) at the plasma membrane, which induces shuttling of Poldip2 to the nucleus, a process that requires cytoskeletal integrity. Additional reports link Poldip2 to mitotic spindle organization and chromosome segregation, which both require proper microtubule and cytoskeletal dynamics.20 Taken in conjunction with the observations reported here, these data substantiate a role for Poldip2 as a novel modulator of cytoskeletal coordination, a function important in VSMC migration and endothelial barrier function.36
In light of these observations, we propose that the ability of Poldip2 to regulate Nox4 enzymatic activity and to modulate the cytoskeleton via RhoA may underlie many of the described functions of Poldip2. While Poldip2 likely has additional binding partners, its association with Nox4 seems to be a major determinant of cell phenotype. Conversely, previous knowledge of cell processes affected by Poldip2 suggests new potential functions for Nox4 in the regulation of DNA repair. The identification of Poldip2 as a Nox4 regulatory protein provides an important new mechanism for regulation of basal ROS production. Previous studies have focused on transcriptional control of Nox4 as the principal mechanism of regulation,6 but our data implicate Poldip2 as an additional critical regulator not only of Nox4 activity, but also of its subcellular localization. It is thus conceivable that the interaction of these two proteins to coordinate ROS generation and cytoskeletal organization represents a novel mechanism that explains their shared and individual functions.
Supplementary Material
Acknowledgements
We thank Dr. David Lambeth and Dr. Mark Quinn for kindly providing the Nox4 and p22phox antibodies. We thank Dr. Karl-Heinz Krause for providing the nox1 knockout animals. We also thank Dr. Aviv Hassid for providing the LacZ, Rho-TN, and Rho-GV adenoviruses.
Sources of Funding
This work was supported by NIH grants HL38206 and HL05863 (KKG) and a pre-doctoral grant to Alicia N. Lyle from the American Heart Association.
Non-Standard Abbreviations and Acronyms
- Ad
Adenovirus
- AS
Anti-sense
- ECs
Endothelial Cells
- ESR
Electron Spin Resonance
- GFP
Green Fluorescent Protein
- GST
Glutathione S-transferase
- H2O2
Hydrogen Peroxide
- ICC
Immunocytochemistry
- IP
Immunoprecipitation
- Nox
NADPH oxidase
- NoxA1
Nox activator 1
- NoxO1
Nox organizer 1
- O2•−
Superoxide
- PDGF
Platelet Derived Growth Factor
- ROS
Reactive Oxygen Species
- VSMCs
Vascular Smooth Muscle Cells
Footnotes
Subject Codes: [137] Cell biology/structural biology, [138] Cell signalling/signal transduction, [91] Oxidant stress
Disclosures
None.
References
- 1.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 4.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 5.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L, Rodriguez-Belmonte EM, Mazloum N, Xie B, Lee MY. Identification of a novel protein, PDIP38, that interacts with the p50 subunit of DNA polymerase delta and proliferating cell nuclear antigen. J Biol Chem. 2003;278:10041–10047. doi: 10.1074/jbc.M208694200. [DOI] [PubMed] [Google Scholar]
- 8.Xie B, Li H, Wang Q, Xie S, Rahmeh A, Dai W, Lee MY. Further characterization of human DNA polymerase delta interacting protein 38. J Biol Chem. 2005;280:22375–22384. doi: 10.1074/jbc.M414597200. [DOI] [PubMed] [Google Scholar]
- 9.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanna IR, Hilenski LL, Dikalova A, Taniyama Y, Dikalov S, Lyle A, Quinn MT, Lassegue B, Griendling KK. Functional association of nox1 with p22phox in vascular smooth muscle cells. Free Radic Biol Med. 2004;37:1542–1549. doi: 10.1016/j.freeradbiomed.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 12.Weber DS, Rocic P, Mellis AM, Laude K, Lyle AN, Harrison DG, Griendling KK. Angiotensin II-induced hypertrophy is potentiated in mice overexpressing p22phox in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2005;288:H37–H42. doi: 10.1152/ajpheart.00638.2004. [DOI] [PubMed] [Google Scholar]
- 13.Sorescu D, Somers MJ, Lassegue B, Grant S, Harrison DG, Griendling KK. Electron spin resonance characterization of the NAD(P)H oxidase in vascular smooth muscle cells. Free Radic Biol Med. 2001;30:603–612. doi: 10.1016/s0891-5849(00)00507-4. [DOI] [PubMed] [Google Scholar]
- 14.Weber DS, Taniyama Y, Rocic P, Seshiah PN, Dechert MA, Gerthoffer WT, Griendling KK. Phosphoinositide-dependent kinase 1 and p21-activated protein kinase mediate reactive oxygen species-dependent regulation of platelet-derived growth factor-induced smooth muscle cell migration. Circ Res. 2004;94:1219–1226. doi: 10.1161/01.RES.0000126848.54740.4A. [DOI] [PubMed] [Google Scholar]
- 15.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 16.Ushio-Fukai M, Zafari AM, Fukui T, Ishizaka N, Griendling KK. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem. 1996;271:23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- 17.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montezano AC, Callera GE, Yogi A, He Y, Tostes RC, He G, Schiffrin EL, Touyz RM. Aldosterone and angiotensin II synergistically stimulate migration in vascular smooth muscle cells through c-Src-regulated redox-sensitive RhoA pathways. Arterioscler Thromb Vasc Biol. 2008;28:1511–1518. doi: 10.1161/ATVBAHA.108.168021. [DOI] [PubMed] [Google Scholar]
- 19.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812–819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klaile E, Kukalev A, Obrink B, Muller MM. PDIP38 is a novel mitotic spindle-associated protein that affects spindle organization and chromosome segregation. Cell Cycle. 2008;7:3180–3186. doi: 10.4161/cc.7.20.6813. [DOI] [PubMed] [Google Scholar]
- 21.Haurani MJ, Cifuentes ME, Shepard AD, Pagano PJ. Nox4 oxidase overexpression specifically decreases endogenous Nox4 mRNA and inhibits angiotensin II-induced adventitial myofibroblast migration. Hypertension. 2008;52:143–149. doi: 10.1161/HYPERTENSIONAHA.107.101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal. 2005;7:308–317. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 23.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeLeo FR, Burritt JB, Yu L, Jesaitis AJ, Dinauer MC, Nauseef WM. Processing and maturation of flavocytochrome b558 include incorporation of heme as a prerequisite for heterodimer assembly. J Biol Chem. 2000;275:13986–13993. doi: 10.1074/jbc.275.18.13986. [DOI] [PubMed] [Google Scholar]
- 25.Nakano Y, Banfi B, Jesaitis AJ, Dinauer MC, Allen LA, Nauseef WM. Critical roles for p22phox in the structural maturation and subcellular targeting of Nox3. Biochem J. 2007;403:97–108. doi: 10.1042/BJ20060819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grasberger H, Refetoff S. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J Biol Chem. 2006;281:18269–18272. doi: 10.1074/jbc.C600095200. [DOI] [PubMed] [Google Scholar]
- 27.Moldovan L, Moldovan NI, Sohn RH, Parikh SA, Goldschmidt-Clermont PJ. Redox changes of cultured endothelial cells and actin dynamics. Circ Res. 2000;86:549–557. doi: 10.1161/01.res.86.5.549. [DOI] [PubMed] [Google Scholar]
- 28.Holland JA, Goss RA, O'Donnell RW, Chang MM, Johnson DK, Ziegler LM. Low-density lipoprotein induced actin cytoskeleton reorganization in endothelial cells: mechanisms of action. Endothelium. 2001;8:117–135. doi: 10.3109/10623320109165321. [DOI] [PubMed] [Google Scholar]
- 29.Wu MH. Endothelial focal adhesions and barrier function. J Physiol. 2005;569:359–366. doi: 10.1113/jphysiol.2005.096537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwanicki MP, Vomastek T, Tilghman RW, Martin KH, Banerjee J, Wedegaertner PB, Parsons JT. FAK, PDZ-RhoGEF and ROCKII cooperate to regulate adhesion movement and trailing-edge retraction in fibroblasts. J Cell Sci. 2008;121:895–905. doi: 10.1242/jcs.020941. [DOI] [PubMed] [Google Scholar]
- 32.Bartolini F, Moseley JB, Schmoranzer J, Cassimeris L, Goode BL, Gundersen GG. The formin mDia2 stabilizes microtubules independently of its actin nucleation activity. J Cell Biol. 2008;181:523–536. doi: 10.1083/jcb.200709029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee MY, San Martin A, Mehta PK, Dikalova AE, Garrido AM, Datla SR, Lyons E, Krause KH, Banfi B, Lambeth JD, Lassegue B, Griendling KK. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler Thromb Vasc Biol. 2009;29:480–487. doi: 10.1161/ATVBAHA.108.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arakaki N, Nishihama T, Kohda A, Owaki H, Kuramoto Y, Abe R, Kita T, Suenaga M, Himeda T, Kuwajima M, Shibata H, Higuti T. Regulation of mitochondrial morphology and cell survival by Mitogenin I and mitochondrial single-stranded DNA binding protein. Biochim Biophys Acta. 2006;1760:1364–1372. doi: 10.1016/j.bbagen.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Klaile E, Muller MM, Kannicht C, Otto W, Singer BB, Reutter W, Obrink B, Lucka L. The cell adhesion receptor carcinoembryonic antigen-related cell adhesion molecule 1 regulates nucleocytoplasmic trafficking of DNA polymerase delta-interacting protein 38. J Biol Chem. 2007;282:26629–26640. doi: 10.1074/jbc.M701807200. [DOI] [PubMed] [Google Scholar]
- 36.Bogatcheva NV, Verin AD. The role of cytoskeleton in the regulation of vascular endothelial barrier function. Microvasc Res. 2008;76:202–207. doi: 10.1016/j.mvr.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


