The higher oestrogen concentrations in women have been suggested as the reason for their slower development of atherosclerosis compared with men. Oestrogen receptors have been located on macrophages, smooth muscle cells, and endothelial cells in women and men, but it is not known whether the protective effect at the level of the arterial wall is mediated by these receptors. It has been reported that premenopausal women have fewer α oestrogen receptors in atherosclerotic than in normal coronary arteries.1 The gene for human α oestrogen receptor contains a polymorphism in the regulatory (upstream) region of the gene: this polymorphism consists of a dinucleotide (thymine and adenine) repeat, the length of which has been associated with bone mineral density, suggesting an effect on oestrogen receptor transcription.2 This prompted us to study whether this polymorphism is associated with coronary artery disease in men.
Subjects, methods, and results
The associations of the polymorphism with atherosclerosis and myocardial infarction were studied in the Helsinki sudden death study, a prospective series of necropsies of white Finnish men who died suddenly.3 Atherosclerotic changes in the coronary arteries were measured by computer assisted planimetry, and coronary narrowings were determined from plastic casts.3 The presence of myocardial infarction was confirmed by macroscopic and histological examination of the myocardium. We selected the 119 cases (with mean age 53.4 (SD 8) years) for our analyses according to phenotype—52 men with severe coronary atherosclerosis (mean coronary stenosis 65.9% (10.8%)) and 67 men with only slightly narrowed coronary arteries (mean 22.1% (13.7%)). The causes of deaths were coronary heart disease in 59, violent death or accident in 38, and other diseases in 22. DNA was extracted from blood by a standard method, amplified by polymerase chain reaction, and analysed by capillary gel electrophoresis as described previously.4
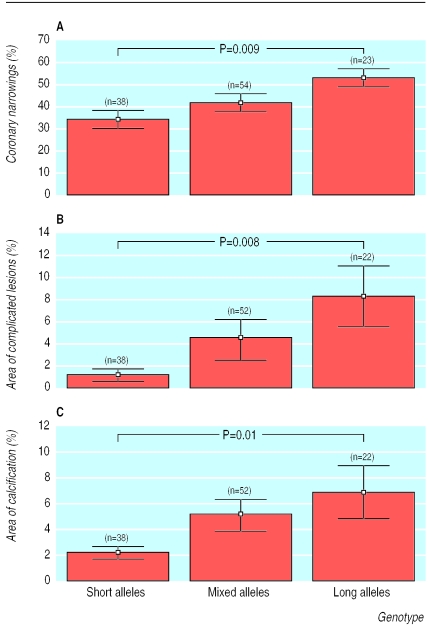
Because of the large number of dinucleotide repeats,4 we used the median number of the repeat (n=19) to categorise the study population into three groups: those with short allele genotypes (both alleles of <19 repeats), those with long allele genotypes (both alleles of ⩾19 repeats), and those with mixed genotypes (one short and one long allele). In analysis of covariance, with age and body mass index as covariates, men with long allele genotypes had a significantly greater number of severely narrowed coronary arteries (P=0.009), larger areas of complicated lesions (P=0.008), and more calcification of the coronary arteries (P=0.01) than men with short alleles (see figure). Atherosclerotic changes in the mixed genotype group were intermediate and not significantly different from those in the short or long allele genotype group.
A stepwise logistic regression analysis with age and body mass index as covariates showed that the group with long allele genotypes had a higher risk of myocardial infarction compared with the group with short allele genotypes (odds ratio 4.4 (95% confidence interval 1.21 to 15.70); P=0.025) and seemed to be more predisposed to coronary thrombosis (odds ratio 11.4 (1.2 to 108.8); P=0.04). When all confounding factors (age, body mass index, smoking, alcohol consumption, diabetes, hypertension) were forced in statistical models, the tendencies of the results were the same, but the small number of cases with all data (n=42) weakened the statistical significance.
Comment
Our preliminary results suggest that the length of the dinucleotide repeat in the regulatory region of the α oestrogen receptor gene is associated with severity of coronary artery disease in men. This may partly explain differences between individuals in the development of coronary artery disease. Of the three other polymorphisms in this gene, the most extensively studied, intronic PvuII polymorphism, showed no association with coronary artery stenosis as measured by angiography.5 Although the biochemical evidence is presently lacking, we speculate that carriers of the long repeat variants have lower expression of the oestrogen receptor gene and benefit less from the cardiovascular protective effect of oestrogen receptors.
Figure.
Association of length of oestrogen receptor dinucleotide repeat with coronary narrowing (A), amount of complicated lesions in coronary plaques (B), and area of calcification in coronary arteries (C). Graphs show means (SE), and P values are from analysis of covariance with Scheffe's post hoc test, adjusted for body mass index and age
Acknowledgments
We thank Outi Lumme and Mervi Niittylahti for their skilful technical assistance, Seppo Tyynelä for planimetric measurements, and Markus Perola for DNA isolation.
Footnotes
Funding: This work was funded by the Medical Research Fund of Tampere University Hospital, the Yrjö Jahnsson Foundation, the Finnish Foundation of Alcohol Research, and the Tampere Regional Fund of the Finnish Cultural Foundation.
Competing interests: None declared.
References
- 1.Losordo DW, Kearney M, Kim EA, Jekanowski J, Isner JM. Variable expression of the estrogen receptor in normal and atherosclerotic coronary arteries of premenopausal women. Circulation. 1994;8:1501–1510. doi: 10.1161/01.cir.89.4.1501. [DOI] [PubMed] [Google Scholar]
- 2.Sano M, Inoue S, Hosoi T, Ouchi Y, Emi M, Shiraki M, et al. Association of estrogen receptor dinucleotide repeat polymorphism with osteoporosis. Biochem Biophys Res Commun. 1995;217:378–383. doi: 10.1006/bbrc.1995.2787. [DOI] [PubMed] [Google Scholar]
- 3.Mikkelsson J, Perola M, Laippala P, Savolainen V, Pajarinen J, Lalu K, et al. Glycoprotein IIIa PlA polymorphism associates with progression of coronary artery disease and with myocardial infarction in an autopsy series of middle-aged men who died suddenly. Arterioscler Thromb Vasc Biol. 1999;19:2573–2578. doi: 10.1161/01.atv.19.10.2573. [DOI] [PubMed] [Google Scholar]
- 4.Kunnas TA, Holmberg-Marttila D, Karhunen PJ. Analysis of estrogen receptor dinucleotide polymorphism by capillary gel electrophoresis with a population genetic study in 180 Finns. Hum Hered. 1999;49:142–145. doi: 10.1159/000022862. [DOI] [PubMed] [Google Scholar]
- 5.Matsubara Y, Murata M, Kawano K, Koichi Z, Zama T, Aoki N. Genotype distribution of estrogen receptor polymorphism in men and postmenopausal women from healthy and coronary populations and its relation to serum lipid levels. Arterioscler Thromb Vasc Biol. 1997;17:3006–3012. doi: 10.1161/01.atv.17.11.3006. [DOI] [PubMed] [Google Scholar]