Abstract
Purpose
Bicarbonate transport plays a role in aqueous humor (AH) secretion. Here, we examined bicarbonate transport mechanisms and carbonic anhydrase (CA) in porcine non-pigmented ciliary epithelium (NPE).
Methods
Cytoplasmic pH (pHi) was measured in cultured porcine NPE loaded with BCECF. Anion exchanger (AE), sodium bicarbonate cotransporter (NBC) and CA were examined by RT-PCR and immunolocalization. AH secretion was measured in the intact porcine eye using a fluorescein dilution technique.
Results
Anion exchanger AE2, CAII and CAIV were abundant in the NPE layer. In cultured NPE superfused with a CO2/HCO3− free HEPES buffer, exposure to a CO2/HCO3−-containing buffer caused a rapid acidification followed by a gradual pHi increase. Subsequent removal of CO2/HCO3− with HEPES buffer caused rapid alkalinization followed by gradual pHi decrease. The rate of gradual alkalinization after addition of HCO3−/CO2 was inhibited by sodium-free conditions, DIDS, CA inhibitors acetazolamide and methazolamide but not by Na-H exchange inhibitor dimethylamiloride or low chloride buffer. The phase of gradual acidification after removal of HCO3−/CO2 was inhibited by DIDS, acetazolamide, methazolamide and by low chloride buffer. DIDS reduced baseline pHi. In the intact eye, DIDS and acetazolamide reduced AH secretion by 25% and 44% respectively.
Conclusion
The results suggest the NPE uses a Na+-HCO3− cotransporter to import bicarbonate and a Cl−/HCO3− exchanger to export bicarbonate. CA influences the rate of bicarbonate transport. AE2, CAII and CAIV are enriched in the NPE layer of the ciliary body and their coordinated function may contribute to AH secretion by effecting bicarbonate transport into the eye.
Keywords: aqueous humor, AE2, bicarbonate, carbonic anhydrase, ciliary epithelium
INTRODUCTION
Aqueous humor (AH) is formed by active transport of solutes followed by osmotic flux of water across the double layer ciliary epithelium 1, 2. This process requires coordinated action of several different ion transport mechanisms and ion channels 3, 4. The ciliary epithelium bilayer consists of two types of cells joined by gap junctions in an apex to apex orientation 5. The inner layer of pigmented ciliary epithelium (PE) faces the stromal blood supply and the outer nonpigmented ciliary epithelium (NPE) faces the AH in the posterior chamber of the eye. Solute transport across the bilayer gives rise to osmotic water movement, resulting in AH formation. The ability of carbonic anhydrase (CA) inhibitors to reduce the rate of AH formation points to an important role for CA 6–9.
Mammalian CA is a family of enzymes consisting of at least 10 members, some localized in the cytoplasm and some membrane-associated 10, 11. All members catalyze the reversible inter-conversion of CO2 and HCO3− 12, 13. In human erythrocytes it has been shown that the speed of the reaction is increased 23,000 fold by CA 14. By increasing the availability of HCO3−, CA can have significant effect on bicarbonate and proton transporter mechanisms. Thus sodium-hydrogen exchanger NHE1 function is influenced by CA inhibitors 15, 16 as is anion exchanger (AE)-mediated bicarbonate and chloride transport 17–19. Here we show enrichment of AE2, CAII and CAIV in the porcine ciliary NPE layer. For consistency we also used the porcine eye to examine aqueous secretion and as a source of NPE cells for primary culture. In cultured porcine NPE, studies were conducted to determine effects of DIDS and acetazolamide on cytoplasmic pH responses to the HCO3− addition or removal. We also provide evidence for a reduced AH formation rate in arterially perfused intact eyes treated with DIDS and acetazolamide, inhibitors of bicarbonate transporters and CA respectively.
MATERIALS AND METHODS
Perfused eye preparation
Fresh pig eyes were obtained from a local abattoir and perfused based on a similar method as described earlier 20, 21. The eye was placed in a circulating warming jacket maintained at 37°C and covered with an insulated plastic cup. The ophthalmic artery was cannulated and the eye perfused with Krebs' solution at 37°C, that contained (mM) NaCl, 118; KCl, 4.0; MgSO4, 1.2; CaCl2, 2.0; NaHCO3, 25; KH2PO4, 1.2; glucose, 10; reduced glutathione, 1.0; ascorbate 0.05 and allopurinol, 1.8, at pH 7.4. The solution was bubbled with O2 containing 5% CO2. Allopurinol, a xanthine oxidase inhibitor, was added to the perfusate to suppress oxidative damage and reperfusion injury. Using a peristaltic pump (Watson Marlow, 505S) perfusion flow was increased stepwise to 1.5 ml.min−1.
The anterior chamber was cannulated with two 23G needles connected to silicon rubber tubing filled with 1.04 ml of artificial AH containing (in mM): NaCl, 110; KCl, 3; CaCl2, 1.4; MgCl2, 0.5; KH2PO4, 0.9; NaHCO3, 30; glucose, 6; ascorbic acid, 3 sodium and fluorescein, 0.0186 at pH 7.6 and equilibrated with 95% O2 - 5% CO2. The artificial AH formed a loop which circulated via a pump at 0.2 ml.min−1 from the anterior chamber to a spectrophotometer cuvette (Pharmacia Biotech, Spectronic 2000) then back to the anterior chamber. Absorbance was recorded every 5 min at 490 nm. The two needles were kept wide apart to optimize fluid mixing. A third 23G needle in the anterior chamber was connected to a water manometer to measure intraocular pressure.
The AH formation rate was estimated from the rate of fluorescein dilution (decrease in absorbance) that occurred due to continuous secretion of AH into the eye. After a settling in period, the plot of loge [absorbance] vs time (min) was a straight line whose slope is the rate constant, Kout (min−1) of aqueous flow. Test agents were added to the arterial perfusate (i.e., to the stromal side) at a fixed concentration (100µM for DIDS and 500µM for acetazolamide). Kout determined 30 min before the addition of DIDS or acetazolamide to the arterial perfusate was considered as the control value for comparison with Kout measured in the presence of the test compound.
Isolation and culture of porcine NPE
Porcine non-pigmented ciliary epithelium (NPE) was established in primary culture using a modification of our previous technique 22. Porcine eyes were dissected to obtain the entire ring of NPE remained attached to the vitreous, leaving the pigmented cell (PE) layer attached to the ciliary body. The NPE ring was separated from the vitreous using fine scissors then cut into 1–2 mm pieces. NPE from 5–7 eyes was pooled and transferred to a 90 mm petri dish containing 15 ml of 0.015% collagenase A and 500 U/ml of hyaluronidase (Sigma St. Louis, MO, USA) in a collagenase buffer containing (in mM): NaCl 66.7, KCl 13.4, HEPES 3.8, CaCl2 4.8, pH 7.4. The petri dish was placed for 5–7 min on a rotary shaker in a 37°C incubator then removed from the shaker and the collagenase and hyaluronidase neutralized by adding excess (7ml) of a 1:1 mixture of newborn calf serum (NCS) and fetal bovine serum (FBS). NPE cells were dispersed by gentle trituration using a round-tipped pasteur pipette and pelleted by centrifugation at 2,000 rpm (670g) for 10 min at 4°C. The pellet was dispersed and the cells incubated without changing the medium for 3–4 days in a small volume of Dulbecco's modified Eagle's medium (DMEM) (Sigma, St Louis, MO) containing 10% FBS and 100 IU/ml gentamycin at 37°C in 5% CO2/95% air. Thereafter the medium was them changed every alternate day. The cells grow to confluence in 7–10 days. At confluence the cells were trypsinized and seeded at a density of 2×104 - 5×104 cells.cm2 for subsequent passages. In the studies reported here, only the 4th passage cells prior to confuence were used.
Immunolocalization
Immunolocalization studies were conducted using fresh pig eyes. The cornea was removed then 4–6 mm of the anterior sclera was carefully peeled from the choroid all around the globe using a pair of curved scissors. The corneal remnant at the limbus was used as the handle to facilitate this dissection. The whole iris-ciliary body along with the lens and anterior vitreous was then removed from the posterior part of the globe. The tissue was placed in a petri dish (corneal face down) containing ice-cold Ringer solution comprising (mM) NaCl 113, KCl 4.6, NaHCO3 21.0, MgSO4 0.6, D-glucose 7.5, glutathione (reduced form) 1.0, Na2HPO4 1.0, HEPES 10.0, and CaCl2 1.4, pH adjusted to 7.4. and the lens removed by cutting the zonules. Vitreous was carefully trimmed away then the iris was dissected away to leave the ciliary body which was fixed in formalin and used to prepare 5–7µm paraffin sections. Cultured NPE cells also were used for immunolocalization. The cells, which were grown on specially designed chamber slides (Nalge Nunc, Lab-Tek II Chamber Slides), were washed with PBS containing 1.0mM MgCl2 and 0.1mM CaCl2 then fixed in acetone for 2 min at room temperature. To probe for the cytoplasmic protein CAII, the cells were permeabilized before fixation using 0.1%Triton X-100 in PBS.
Tissue sections or cultured NPE cells prior to confluence were incubated at room temperature for 90 min in 10% goat serum in PBS (blocking buffer). Primary antibodies directed against either AE2 (10µg/ml), CAII (10µg/ml) or CAIV (20µg/ml) were added for 24h at 4°C. Control specimens received only the blocking buffer. The specimens were washed with PBS and incubated 24h at 4°C with fluorescent secondary antibody (Alexa fluor 488 or 546 conjugated to either goat anti rabbit or anti mouse IgG, 1:200 dilution). The specimens were examined using a Zeiss microscope (Axiovert 200M) and photographed using a Hamamatsu digital camera (C4742-95).
Some samples were prepared for confocal microscopic study. In these cases, nuclear counterstaining with TO-PRO-3 iodide (Invitrogen, Carlsbad, CA) was used to visualize the nucleus and the images were captured using a Zeiss LSM 510 meta-NLO confocal microscope (Carl Zeiss Inc, Thornwood, NY). Fluorescence excitation was achieved using 488, 543 and 633nm laser excitation wavelengths for FITC, rhodamine and TO-PRO-3 iodide respectively.
RT-PCR
Design of oligonucleotide probes for porcine AE1, AE2, AE3 and kNBC1
There is currently no published sequence for the porcine ortholog of AE1. The bovine AE1 sequence (GenBank Acc# NM181036) was used to probe for a related sequence in the partially sequenced porcine genome (NCBI). This query resulted in several highly conserved hits located in proximity on chromosome 12 (genomic locales: 98698–98874 bp; 101907–102086 bp; 102522–102711 bp; 105983–106259 bp). Nucleotide sequences in these locales were 83–87% identical to the open reading frame of human AE1, but less than 80% (60–80%) identical to human AE2 and AE3, suggesting that the mined sequence is that of porcine AE1 (pAE1). The oligonucleotide probes used were sense: 5′-GTGACATCACAGACGCCTTGA-3′and antisense: 5′-CTCTGGTTTGCTGACGATCA-3′. The porcine ortholog of AE2 has been fully sequenced (GenBank Acc# AF120099), and the oligonucleotide probes used were sense: 5′-AGGAGATCTTCGCCTTCCTC-3′and antisense: 5′-AGCATCCAGGCATTTCATCT-3′. There is currently no published sequence for pAE3, and a sequence similar to hAE3 could not be found in the porcine genome. Nucleotide sequences conserved between the human, mouse, rabbit, rat and monkey AE3 cDNA sequences were used to design oligonucleotide probes for pAE3 (sense: 5′-AAGACCTTGGCTGTGAGCAG-3′and antisense: 5′-GCTGCTCCAAGAAAGGCAC-3′). The porcine ortholog of kNBC1 has been cloned (NM001030533), and the oligonucleotide probes used were sense: 5′-TCTTTTGCCTCTTTGCTGGT -3′and antisense: 5′-GCTTGAACTCACTTGGCACA -3′.
RNA isolation and RT-PCR
Total RNA from porcine renal cortex, cardiac muscle, native NPE and primary cultures of NPE at passage #4 was isolated using RNA-Bee (Tel-Test, Inc., Friendswood, TX). Porcine kidney was used as a positive control for AE1 and AE2, whereas porcine myocardial tissue served as a control for AE3. One microgram of total RNA was reverse transcribed using the QuantiTect Reverse Transcription kit according to the manufacturer’s protocol (Qiagen, Valencia, CA). This protocol includes a step to remove genomic DNA. Four microliters of cDNA was used in the PCR reaction. The PCR components were assembled followed by a single denaturing step for 2 min at 94C. This was followed by 35 cycles of: 94C for 30 sec, 55C for 30 sec and 72C for 2 min. A final elongation step of 7 min (72C) was included after the last cycle. PCR products were separated on 1% agarose gels and visualized with ethidium bromide. Amplified products were purified with the QIAquick gel extraction kit (Qiagen), and sequences were confirmed with an Applied Biosystems 3730xl DNA analyzer at the University of Arizona sequencing facility. The partially cloned pAE1 and pAE3 sequences were 87% and 92% identical to the hAE1 and hAE3 sequences, respectively. The cloned pAE2 sequence was identical to the previously published pAE2 sequence.
Measurement of cytoplasmic pH
NPE cells grown to pre-confluence on 35mm plastic dishes (Corning) were loaded for 10 min with the pH-sensitive dye BCECF-AM (5.0µM) as described earlier 23, placed in a temperature controlled perfusion micro-incubator (PDMI-2, Harvard Biosciences, Holliston, MA) on the stage of an upright epifluorescence microscope (Nikon Eclipse 80i, Japan) and superfused with a HEPES buffer containing (in mM): NaCl, 137; KCl, 4.5; D-glucose, 6.0; MgCl2, 1.0; CaCl2, 1.5 and HEPES, 10.0, adjusted with NaOH to pH 7.35. The flow rate was 3.0 ml.min−1. Cytoplasmic pH (pHi) was recorded using an Incyt imaging system (Intracellular Imaging Inc., Cincinnati OH) with an emission wavelength of 535nm and alternating excitation wavelengths of 488 nm and 460nm. pHi was calculated from the fluorescence intensity ratio I488/I460.
The cells were first superfused with the HEPES buffer for 5 min to obtain a stable pHi baseline then the superfusate was switched to bicarbonate/CO2 buffer containing (in mM): NaCl, 117; KCl, 4.5; NaHCO3, 20; D-glucose, 6.0; MgCl2, 1.0; CaCl2, 1.5 and HEPES, 10.0 adjusted to pH 7.35 and equilibrated by gassing with 5% CO2 and 95% air. In some experiments either a sodium-free or a low chloride buffer was used. The sodium-free bicarbonate /CO2 solution contained (in mM): choline chloride, 117; choline bicarbonate, 20; KCl, 4.5; D-glucose, 6.0; MgCl2, 1.0 and CaCl2, 1.5 and HEPES 10. The low chloride buffer contained (in mM): sodium gluconate, 117; potassium gluconate, 4.5; NaHCO3, 20; glucose, 6.0; MgCl2, 1.0 and CaCl2, 2.5; HEPES, 10. To prepare sodium-free/ bicarbonate-free or low chloride/bicarbonate-free buffers, the bicarbonate was omitted and replaced with an equimolar amount of either sodium gluconate or choline chloride, respectively.
Reagents
Mouse anti-human carbonic anhydrase IV monoclonal antibody was obtained from R & D Systems Inc. MN. Rabbit anti CAII polyclonal antibody was purchased from Santa Cruz Biotechnology, Inc, CA. Rabbit anti AE2 polyclonal antibody was obtained from Alpha Diagnostics International, TX. Secondary antibodies used to probe the bound primary antibodies were Alexa Fluor 488 goat anti-rabbit IgG and Alexa Fluor 546 goat anti-mouse IgG (Invitrogen Carlsbad, CA). Acetazolamide, methazolamide , 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS) and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich, St Louis, MO, USA. 2′,7′-bis(2-carboxyl)-5(6)-carboxyfluorescein-acetoxyethyl ester (BCECF) was purchased from Invitrogen. All other chemicals were purchased from Sigma-Aldrich. Stock solutions of test compounds were prepared in DMSO before addition to the cell superfusate or to the whole eye perfusate. Control solutions received DMSO only.
Statistical analysis
A two-sample ‘t’ test was used to analyze unpaired data and paired ‘t’ test was used to compare paired samples. One way analysis of variance (ANOVA) followed by Bonferroni’s post hoc multiple comparison tests was used to compare differences for more than two groups of data. A probability (P) value of <0.05 was considered significant.
RESULTS
The influence of DIDS and acetazolamide on aqueous humor formation
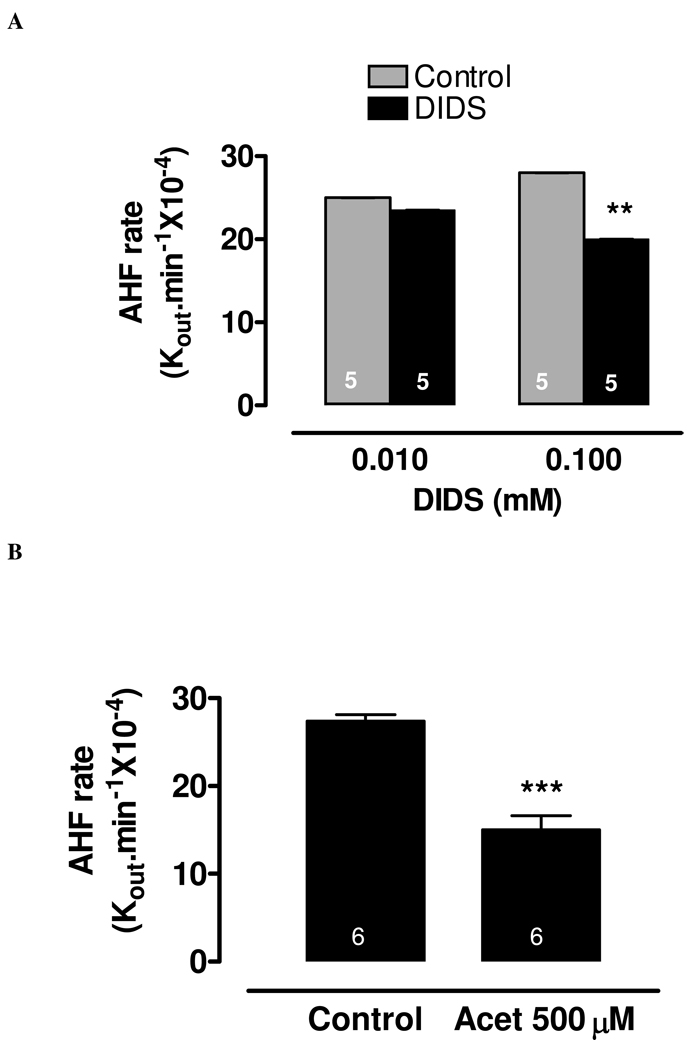
The rate of AH formation was measured in the intact, arterially perfused eye. Under control conditions the rate constant for AH formation (Kout.min−1×10−4) was 28.0±2.0 (n=5). To obtain the rate constant for AH formation under drug-treated condition, the anion transport inhibitor DIDS or the carbonic anhydrase inhibitor acetazolamide was added to the arterial perfusate. This represents stromal application. DIDS was added at a final concentration of either 10 or 100 µM and acetazolamide was added at a final concentration of 500µM. At a concentration of 100 µM, DIDS significantly reduced the rate constant for AH to 20.0±2.0 (n=5) (Fig 1A). The carbonic anhydrase inhibitor acetazolamide (500 µM) produced more pronounced reduction of the rate constant for AH formation to 15.0±2.0 (n=6) (Fig 1B).
Figure 1.
The effect of DIDS (10µM & 100µM) (A) and acetazolamide (500µM) (B) on AH formation rate measured in the porcine isolated perfused whole eye preparation. AH formation was measured by a fluorescein dilution technique. The results are expressed as a rate constant (Kout.min−1×10−4), and are shown as mean±SEM of 5 independent experiments for each condition. The rate measured during the first 30 min prior to drug addition was taken as the control value. After the addition of drug, a 20 min period was allowed to establish the drug effect, then the rate was measured over the subsequent 60 min. Significant differences from control were indicated by **P<0.01 (panel A) and ***P<0.001 (panel B).
Expression and localization of chloride-bicarbonate exchanger (AE2) and carbonic anhydrase in porcine NPE
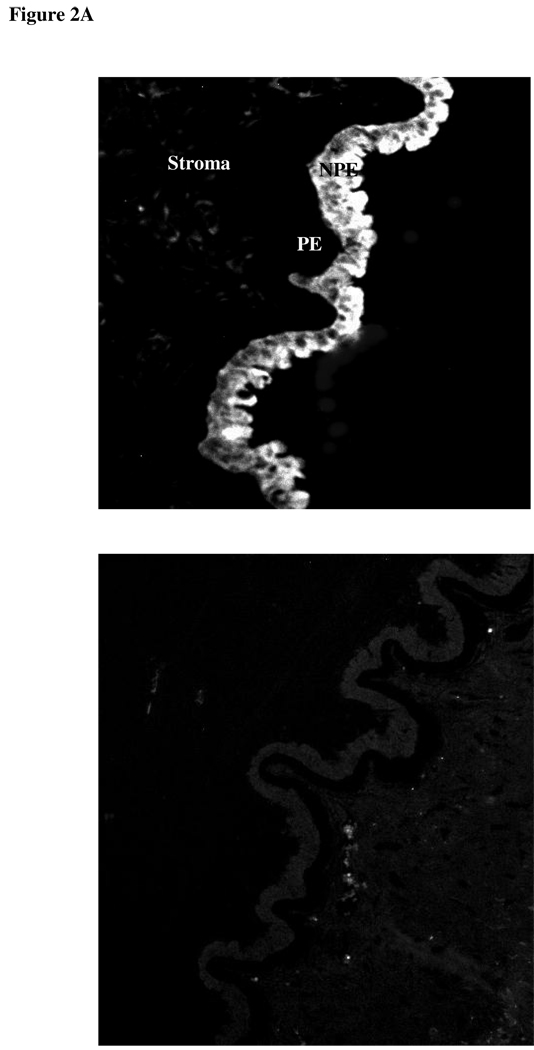
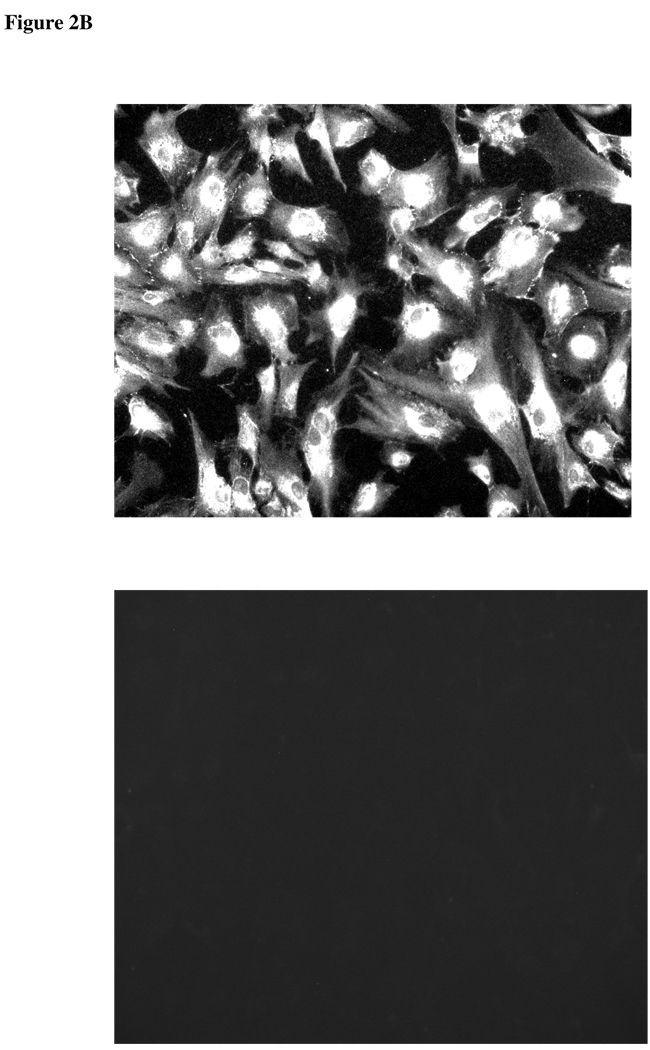
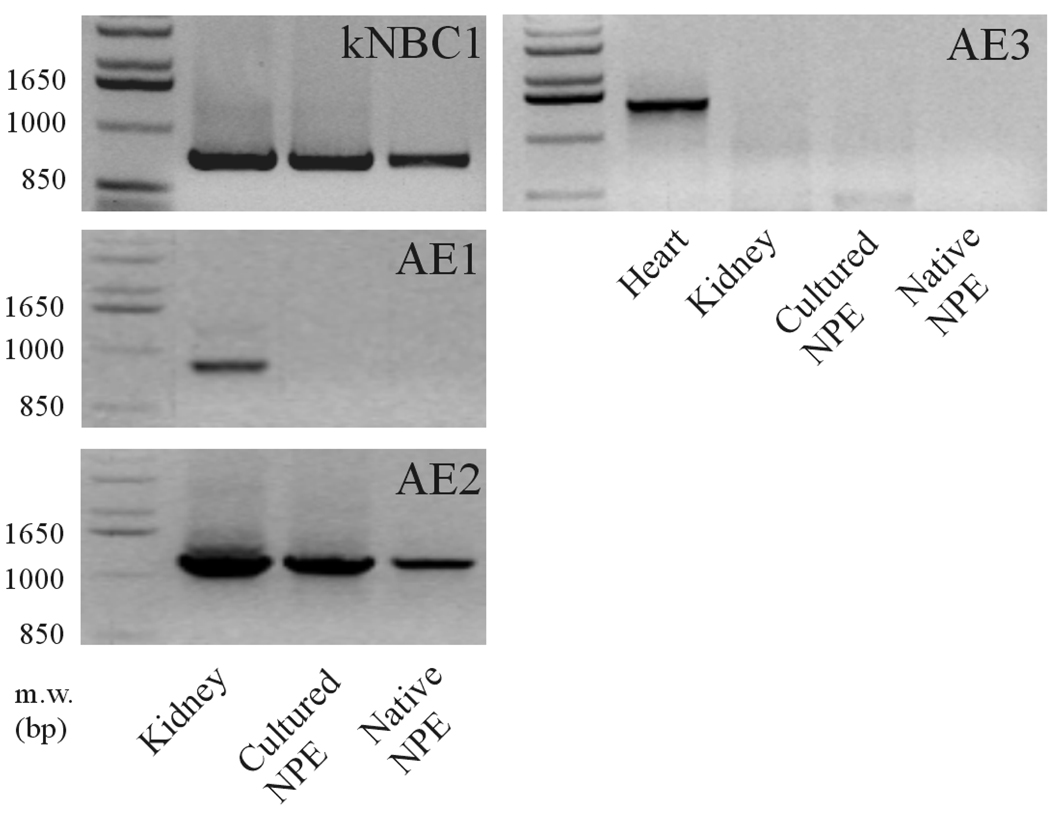
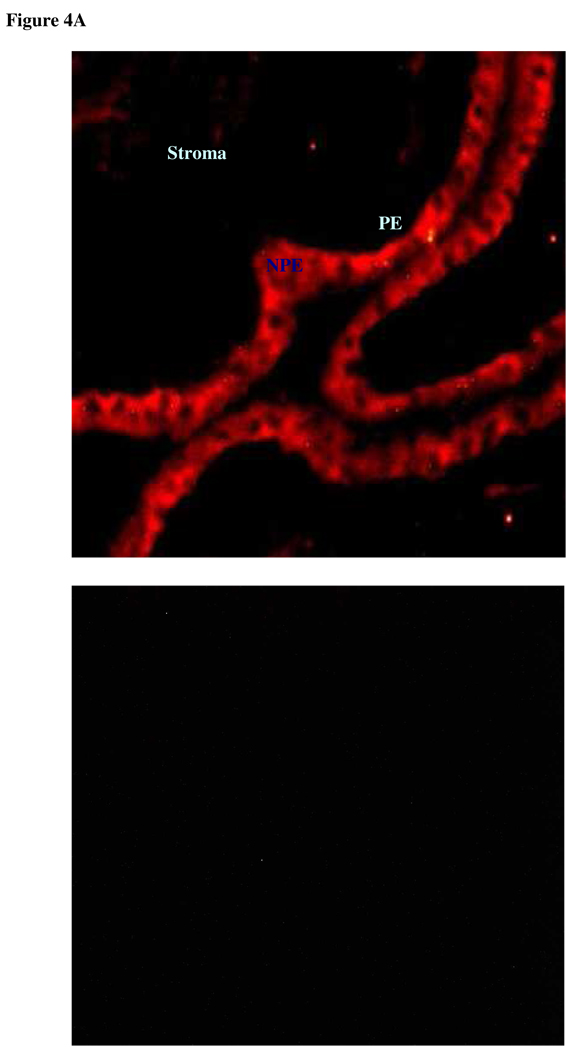
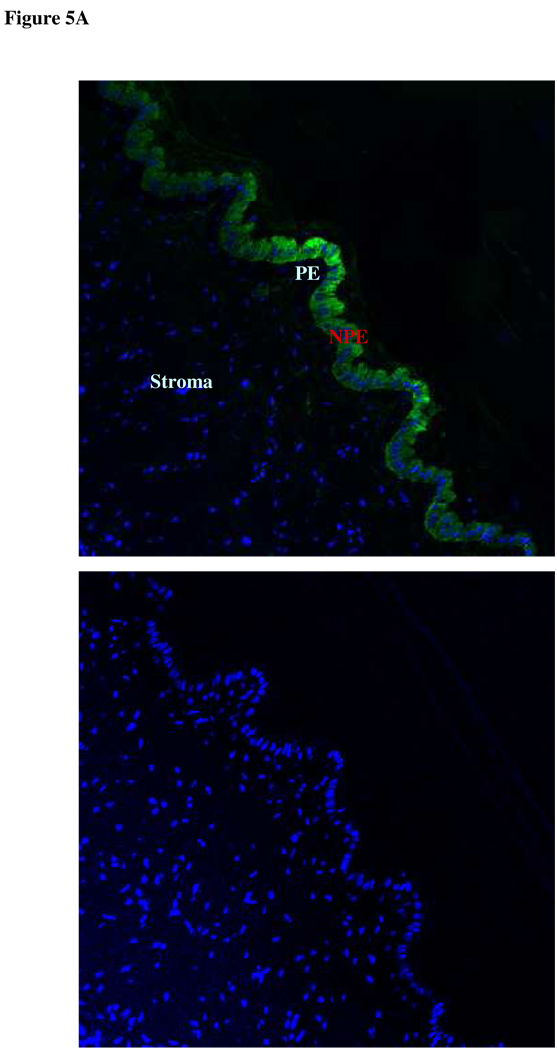
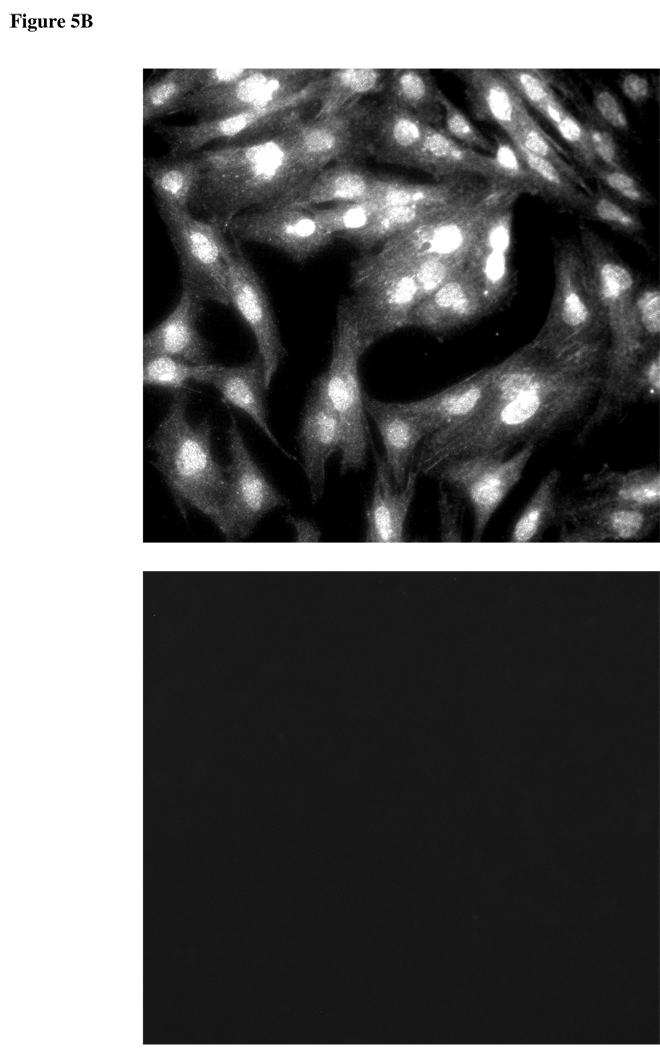
Since DIDS and acetazolamide reduce the rate of AH formation, experiments were conducted to examine chloride-bicarbonate exchanger and carbonic anhydrase expression in ciliary epithelium. Immunolocalization and RT-PCR studies were carried out to examine chloride-bicarbonate exchangers (AEs) in the ciliary epithelium bilayer and cultured NPE. AE2 was detected in the NPE cell layer where it was enriched at the basolateral membrane (Fig 2A). AE2 immunoreactivity was also present in cultured NPE (Fig 2B). AE1 and AE3 was not detected (result not shown). RT-PCR studies show presence of AE2 mRNA in both the native and cultured porcine NPE. AE1 or AE3 mRNA was not found (Fig 3). Carbonic anhydrase IV (CAIV) was localized to the surface of the NPE (Fig 4) but was not detectable in the PE layer. However, we acknowledge that the presence of heavy pigmentation in the PE cells might have masked the detection of fluorescence in these cells. In contrast, carbonic anhydrase II (CAII) distribution in the ciliary body was widespread as revealed by laser confocal microscopy (Fig 5A). CAII was abundant in cytoplasm of the NPE. CAIV and CAII immunoreactivity also was detected in the cultured NPE by laser confocal (Fig. 4B) and epifluorescence microscopy (Fig. 5B) respectively.
Figure 2.
Immunolocalization of Cl−/HCO3− exchanger AE2 in the porcine ciliary body where it appears on the NPE basolateral membrane (A). PE, pigmented epithelium; NPE, nonpigmented epithelium (Original Magnification 200x). AE2 also was detected in cultured NPE (B) (4th passage cells are shown). The negative controls, in which the primary antibody was replaced by PBS, show no staining. (Magnification 200x)
Figure 3.
RT-PCR for AE1, AE2, AE3 and kNBC1 in native and primary cultures of porcine non-pigmented epithelium (NPE). Porcine renal cortex (kidney) and porcine cardiac muscle (heart) served as positive controls for AE1, AE2, kNBC1 and AE3, respectively. Amplified products were separated on 1% agarose gels and visualized with ethidium bromide. The resulting amplified cDNA products were gel purified and their sequences were confirmed with DNA sequencing.
Figure 4.
Immunolocalization of carbonic anhydrase IV in the porcine ciliary body where it appears on the NPE membrane (A). PE, pigmented epithelium; NPE, nonpigmented epithelium (Original magnification 200x). Carbonic anhydrase IV also was detected in cultured NPE by laser confocal microscopy (B) (4th passage cells are shown). The negative controls, in which the primary antibody was replaced by PBS, show no staining (Original magnification 400x).
Figure 5.
Immunolocalization of carbonic anhydrase II in the porcine ciliary body by laser confocal microscopy, where it appears within the cytoplasm of the NPE (A). PE, pigmented epithelium; NPE, nonpigmented epithelium (Original magnification 200x). Carbonic anhydrase II also was detected in cultured NPE (B) (4th passage cells are shown). The negative controls, in which the primary antibody was replaced by PBS, show no staining (Original magnification 200x).
Cytoplasmic pH response of cultured NPE to HCO3−/CO2 addition
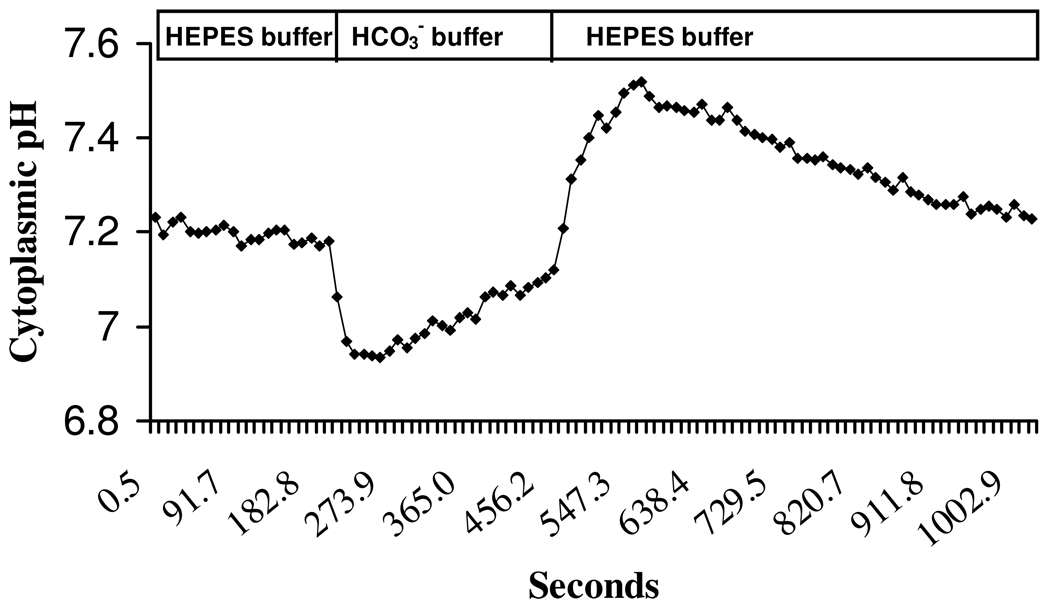
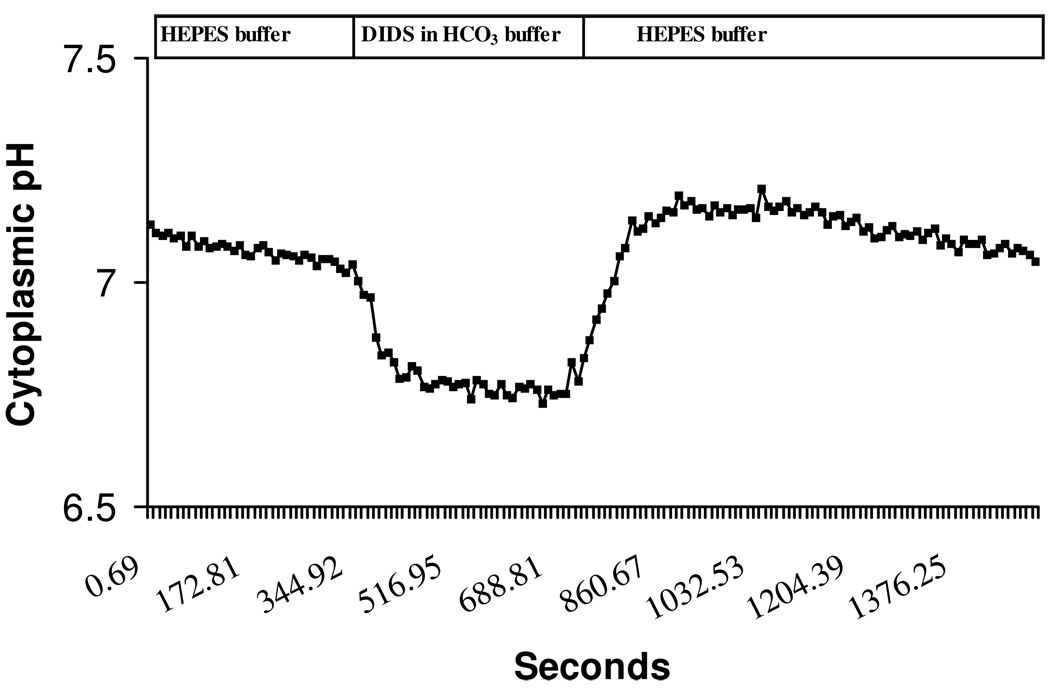
Figure 6 shows a typical cytoplasmic pH (pHi) response of cultured NPE when the bathing medium was switched for 5 min from bicarbonate-free HEPES-buffered solution to a HCO3−/CO2-buffered solution. Baseline pHi in HCO3−/CO2-free HEPES buffer was 7.25±0.13. When the superfusate was switched to HCO3−/CO2 buffer there was a rapid fall in pHi (0.4±0.02 units, n=10) followed by gradual recovery towards baseline. Subsequent replacement of HCO3−/CO2 medium with HEPES-buffered solution caused pHi to rise sharply (0.4±0.04 units, n=10) then gradually recover towards the baseline. The pHi response was examined under several different conditions: in sodium-free buffer, in low chloride buffer, in the presence of DMA, in the presence of DIDS and in the presence of acetazolamide or methazolamide.
Figure 6.
A typical cytoplasmic pH (pHi) response of BCECF-loaded porcine non-pigmented ciliary epithelial cells to the addition and subsequent removal of HCO3−/CO2 from the bathing solution. The cells first were superfused with bicarbonate-free HEPES buffered Krebs’ solution for 5 min to obtain a stable baseline. The superfusate was then switched to HCO3−/CO2 buffer. This caused a rapid fall in pHi, which gradually recovered towards baseline. Subsequent removal of HCO3−/CO2 and replacement with HEPES buffer caused a rapid pHi rise followed by a gradual recovery towards baseline.
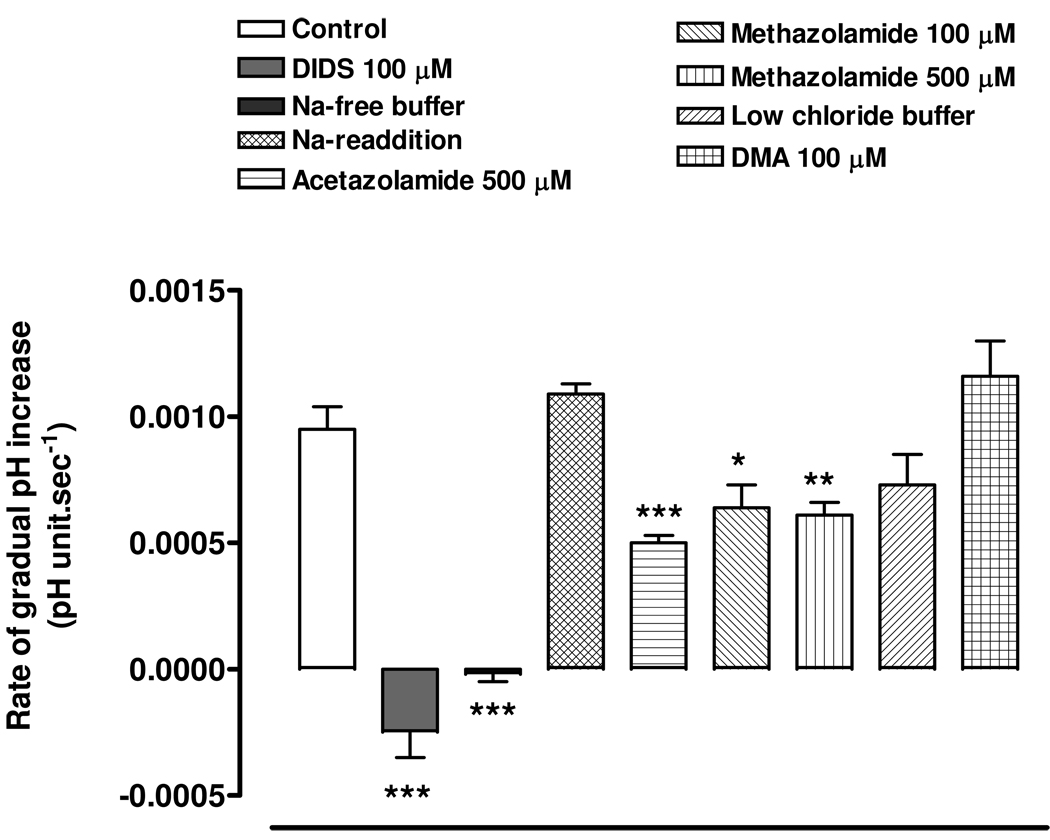
The addition of HCO3−/CO2 acidifies the cells due to rapid diffusion of CO2 into the cells, its carbonic anhydrase-mediated conversion to H2CO3 and subsequent dissociation of H2CO3 to HCO3− and H+. After this, we suggest the gradual alkalinization towards baseline occurs as the result of bicarbonate entry. DIDS at a concentration of 100 µM prevented the gradual alkalinization (Fig 7 & Fig 8). Sodium-free buffer also abolished the gradual alkalinization and on replacement of external sodium, the rate of pHi recovery returned to the normal (Fig 8). However, the rate of gradual alkalinization was not significantly different in low chloride buffer or in the presence of 100 µM DMA (Fig 8). The findings are consistent with the notion that the gradual increase of pHi is due to bicarbonate entry via a sodium-dependent anion transporter. Both acetazolamide (500 µM) and methazolamide (100 & 500 µM) significantly reduced the rate of gradual alkalinization (Fig 8).
Figure 7.
A typical cytoplasmic pH (pHi) response of BCECF-loaded NPE to the addition and subsequent removal of HCO3−/CO2 from the bathing solution in the presence of DIDS (100 µM). The cells first were superfused with bicarbonate-free HEPES buffered Krebs’ solution for 5 min to obtain a stable baseline, then the superfusate was then switched to HCO3−/CO2 buffer for 5 min.
Figure 8.
The effect of DIDS (100 µM), sodium-free buffer, low chloride buffer, CA inhibitors acetazolamide (500 µM) and methazolamide (100 and 500µM) and sodium-hydrogen exchange inhibitor DMA (100 µM) on the rate of gradual alkalinization towards baseline following the rapid pHi fall caused by the addition of HCO3−/CO2 to the bathing medium. The results are the mean±SEM of data from 5 or 10 independent experiments. A significant difference from control is indicated by *P<0.05, **P <0.01 or ***P <0.001.
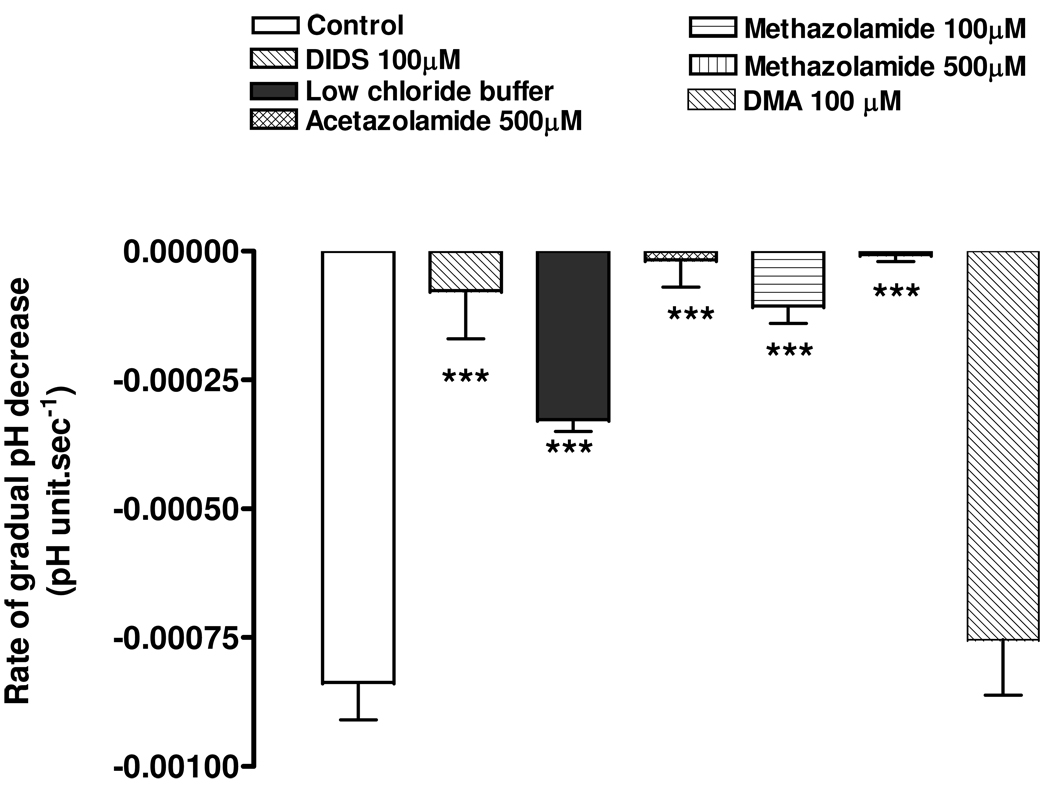
The removal of external HCO3−/CO2 causes pHi to increase sharply due to the rapid exit of CO2. After this, we suggest, the gradual acidification toward baseline was due to chloride-sensitive HCO3− efflux. The evidence is as follows. DIDS significantly inhibited the gradual acidification (Fig 9). The rate of gradual acidification also was reduced in low chloride buffer (Fig 9). The rate of gradual acidification measured in the presence of the sodium-hydrogen exchange inhibitor, DMA (100 µM), was not significantly different from the control rate (Fig 9). The findings are consistent with the gradual reduction of pHi due to bicarbonate exit via a sodium-independent anion exchanger. The carbonic anhydrase inhibitors acetazolamide (500 µM) and methazolamide (100 & 500 µM) both completely inhibited the gradual acidification (Fig 9).
Figure 9.
The effect of DIDS (100 µM), low chloride buffer, CA inhibitors acetazolamide (500 µM) and methazolamide (100 and 500µM) and sodium-hydrogen exchange inhibitor DMA (100 µM) on the rate of gradual acidification towards baseline following the rapid pHi rise caused by the removal of HCO3−/CO2 and replacement with HEPES buffer. The results are the mean±SEM of data from 6 or 10 independent experiments. A significant difference from control is indicated by ***P <0.0001.
Effect of DIDS and acetazolamide on baseline cytoplasmic pH
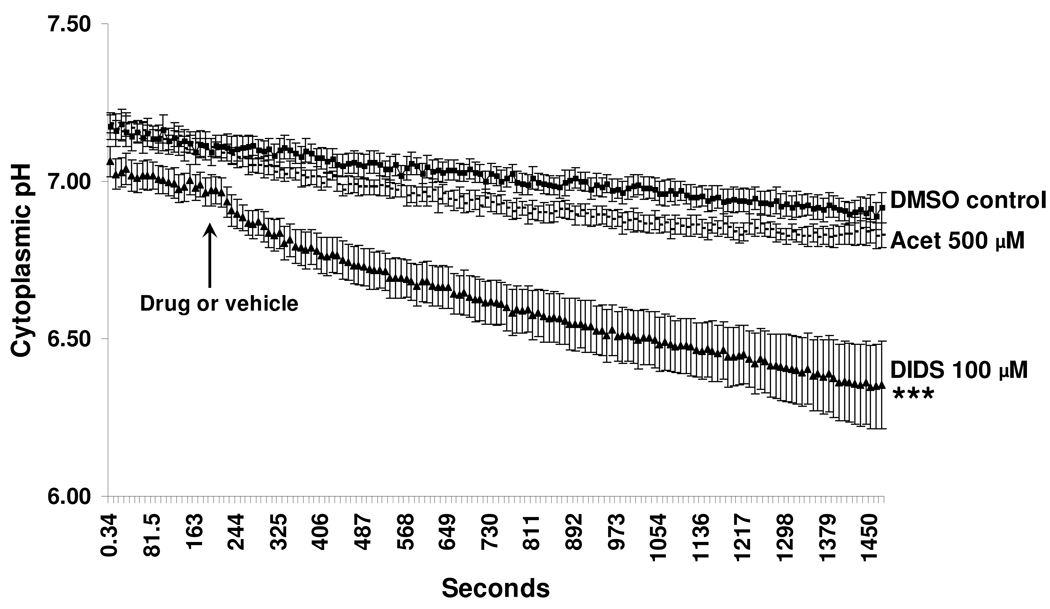
Since DIDS blocked and acetazolamide reduced the rate of cytoplasmic pH recovery in bicarbonate-containing buffer and since both the drugs reduced AH formation in isolated intact eye preparations, we studied the effect of these drugs on the baseline cytoplasmic pH of cultured NPE. DIDS (100µM) caused a significant progressive reduction of baseline cytoplasmic pH (Fig 10). After 20 min, the time point at which measurement of AH formation was started in the intact eye experiments, DIDS had lowered pH by ~0.6 pH units. In cells exposed to acetazolamide (500µM) pHi constantly was slightly lower than control pHi but at any one time point the difference was not significant (Fig 10). In control cells there is a slight and gradual drift in cytoplasmic pH, which may be due to dye bleaching.
Figure 10.
The effect of DIDS and acetazolamide on baseline cytoplasmic pH of porcine cultured NPE. BCECF-loaded cells were first superfused with bicarbonate-containing buffer for 3 min to establish baseline cytoplasmic pH. At this point DIDS 100 µ, acetazolamide 500 µM or vehicle DMSO 0.1% was introduced (arrow) and data collection was continued for a further 20 min. The results are shown as mean±SEM of 7–10 independent experiments. At the final time point, pHi in DIDS-treated cells showed a significant difference from control ***P <0.001.
DISCUSSION
Three lines of evidence point to expression of the AE2 chloride-bicarbonate exchanger in porcine NPE; RT-PCR detection of mRNA, protein immunolocalization, and the observation of chloride-sensitive pH responses. Consistent with a previous report from the human ciliary body 24, RT-PCR detected neither AE1 nor AE3. The ability of the ciliary epithelium bilayer to form aqueous humor is determined by the location of transport proteins and immunolocalization studies revealed expression of AE2 in the NPE layer. AE2 appeared most dense at the NPE basolateral surface, but it was not strictly limited to the cell border. The apparent cytoplasmic signal could stem from nonspecific antibody binding or the intracellular presence of AE2 trafficking vesicles. AE2 expression is consistent with earlier functional evidence for an electroneutral sodium-independent and DIDS-sensitive Cl−/HCO3− exchange mechanism in native rabbit NPE 25. Together with AE2, the porcine NPE layer also displayed abundant CAII, which appeared in the cytoplasm, and CAIV which was localized to the membrane. To our knowledge, this is the first report on localization of AE2 in native porcine ciliary epithelium but the presence of carbonic anhydrase has been demonstrated earlier in rabbit, monkey and human ciliary body using histochemical methods 26–28. In human NPE, carbonic anhydrase was reported at the basal and lateral membranes as well as in the cytoplasm 28. As well as CAII, other carbonic anhydrases reported in human ciliary body include membrane bound isoforms CAIX and CAXII 29. Western blot analysis and functional studies using a membrane impermeable carbonic anhydrase inhibitor pointed to the presence of the membrane bound CAIV in rabbit NPE 30. Matsui and coworkers suggested that CAIV could be linked to chloride/bicarbonate exchanger function in the NPE 9.
Using cultured porcine NPE we were able to examine anion transport indirectly by measuring pHi responses. Although there are likely to be significant differences between native and cultured cells, porcine NPE in primary culture was found to maintain expression of AE2, CAII and CAIV and NBC as judged by RT-PCR and immunolocalization. Earlier we have shown similar patterns of nitric oxide synthase (NOS 1, 2 and 3 isoforms), Na,K-ATPase (alpha 1, 2 and 3 isoforms) 22, and NHE (NHE1, 3 and 4 isoforms), aquaporin (AQP-1 and AQP-4) and connexins (Cx50 and Cx43) (unpublished observation, 2007) in cultured and native porcine NPE.
In cells superfused with a CO2/HCO3− free HEPES buffer, exposure to a CO2/HCO3−-containing buffer caused a rapid acidification followed by a gradual pHi increase. Subsequent replacement of CO2/HCO3− with HEPES buffer caused rapid alkalinization followed by gradual pHi decrease. A similar response to CO2/HCO3− addition and removal has been reported by Wolosin and coworkers in native rabbit NPE cells 25, 31 who suggested the initial rapid pHi changes likely resulted from rapid entry or exit of CO2. In the present study we focused on the subsequent slower pHi changes that are dependent, at least in part, on bicarbonate transport.
A gradual pHi increase back toward baseline was observed following the rapid acidification caused by addition of CO2/HCO3−. Alkalinizing systems reported in the ciliary epithelium include Na+-HCO3− cotransporter (NBC) 32, Na-H exchanger (NHE) 33 and vacuolar H-ATPase (V-ATPase) 34, 35. Here, the rate of alkalization was completely inhibited by Na-free solutions and DIDS, but not significantly altered in cells exposed to low chloride solution or to the NHE inhibitor DMA. The results are consistent with a Na+-HCO3− cotransporter that enables external bicarbonate to enter the cell. This notion fits with an earlier proposal that a Na+-HCO3− cotransporter and Cl−/HCO3− exchanger are the two principal determinants of NPE cytoplasmic pH 25. The lack of sensitivity to DMA suggests NHE-mediated proton export does not contribute to the observed alkalinization response. This apparently contrasts with our previous finding that porcine native and cultured NPE express abundant quantities of NHE1, NHE3 and NHE4 and that DMA significantly inhibits pHi recovery in this cell after acidification by a 20 mM ammonium chloride pulse (Shahidullah, et al. IOVS 2007;48:ARVO E-Abstract ). The explanation may lie in the fact that the smaller degree of acidification caused by exposure to CO2/HCO3− (~0.4 pH unit below baseline) compared to 20mM ammonium chloride pulse (>1 pH unit below baseline) is not sufficient to cause activation of NHE. It is well known that NHE activity is regulated primarily by pHi and increases markedly in response to intracellular acidosis 36. Such NHE activation is thought to occur through the interaction of H+ with an allosteric modifier site within the transport domain 37, 38. The ability of DIDS to completely inhibit the NPE alkalinization response following acidification by CO2/HCO3− addition argues against the contribution of NHE or H-ATPase to the pHi rise observed in the present studies.
A gradual acidification back towards baseline was observed after the rapid pHi rise on removal of extracellular CO2/HCO3− and return to HEPES buffer. The rate of gradual acidification was inhibited by both DIDS and low chloride solution. The results are consistent with a chloride-bicarbonate exchanger, such as AE2, that enables bicarbonate to exit the cell in exchange for external chloride entry. Removal of extracellular chloride results in the reversal of the exchanger due to favorable gradient for AE2-mediated chloride efflux in the presence of ample extracellular bicarbonate. The gradual acidification could be abolished by carbonic anhydrase inhibitors, either acetazolamide or methazolamide. The sodium-hydrogen exchange inhibitor DMA (100 µM) had no effect on the gradual acidification. The results are consistent with findings in other tissues where AE2-mediated bicarbonate export effects recovery from alkaline loading 39, 40. The sensitivity of the gradual acidification to acetazolamide or methazolamide can be explained if bicarbonate transport is rate-limited by availability of cytoplasmic HCO3− and can be inhibited by build up of extracellular HCO3−. On this basis, cytosolic CAII might provide the “push” for bicarbonate transport by making HCO3− available to the cytoplasmic face of AE2, and CAIV-catalyzed conversion of HCO3− to CO2 in the extracellular unstirred layer might provide the “pull” by diminishing the concentration of HCO3− at the extracellular AE2 face. Such a “push-pull” mechanism, resulting from CA activity on both sides of the NPE basolateral membrane, could accelerate AE2-mediated bicarbonate transport into the eye. The pH alkalinization response described above, which we propose is mediated by bicarbonate uptake via Na+-HCO3− cotransport, was only partially inhibited by carbonic anhydrase inhibitors acetazolamide or methazolamide. This may reflect the greater availablility of HCO3− in the extracellular solution compared to cytoplasm.
The fact that the gradual alkalinization after cytoplasmic acidification was abolished by DIDS and sodium-free solution but was insensitive to extracellular chloride removal indicates that the alkalinization is due to NBC-mediated bicarbonate entry into the cells. Since AE2 in the NPE cells localized mostly on the basolateral surface, and because AE2 enables bicarbonate to exit the cell in exchange for external chloride entry, it is tempting to speculate that the NBC might be localized on the apical membrane of the NPE cells. Apical localization of NBC has been reported in other secretory tissues, such as in human parotid and sublingual duct 41 and in rat pancreatic duct 42 epithelium.
The ability of acetazolamide and DIDS to abolish HCO3− export by cultured porcine NPE is interesting in light of the ability DIDS and acetazolamide to reduce AH secretion in the intact porcine eye. The findings are consistent with a contribution of bicarbonate transport to AH formation by the porcine eye. Acetazolamide reduced the rate of aqueous humor secretion by 40% and DIDS inhibited AH secretion by 25%. DIDS previously has been reported to reduce aqueous formation in the bovine eye 20. It is interesting to note that DIDS significantly reduced basal cytoplasmic pH in cultured NPE, lowering pHi by ~0.6 pH units after 20 min. In the perfused intact eye experiments, a 20 min interval was allowed to establish drug effect prior to measuring the effect of DIDS on AH formation rate. It should also be noted that DIDS is not a selective inhibitor of Cl−-HCO3− exchangers such as AE2. It also inhibits Na-HCO3− cotransport 24, 43, and chloride channels 44. The carbonic anhydrase inhibitor, acetazolamide had little effect on basal cytoplasmic pH in the cultured NPE, even though it had a robust effect in reducing AH formation rate in the perfused intact eye. One explanation for this apparent discrepancy might be that in vitro and with continuous CO2 bubbling of the bathing medium there might be sufficient hydration of CO2 in the absence of the catalysis by the carbonic anhydrase. Since CO2 is a membrane permeable, such noncatalytic hydration would be equally effective in the bathing medium as well as in the cytoplasm of cultured NPE. Perhaps this could help maintain the normal HCO3− transport and acid-base balance in the cultured cells. In the intact eye preparation or in vivo situation, aqueous humor CO2 equilibration might be less efficient and hence sufficient hydration of CO2 would be more dependent on the carbonic anhydrase. Consequently, inhibition of carbonic anhydrase would cause insufficient hydration of CO2 and hence insufficient production of HCO3− inside the cells for transport into the aqueous by basolateral AE2. On this basis, inhibition of AE2 by DIDS would inhibit transport of bicarbonate into the aqueous in exchange for Cl−. Inhibition of carbonic anhydrase would cause depletion of HCO3− required for transport by the AE2 and so have a similar effect; less bicarbonate transport to the aqueous and hence reduction in the rate of AH formation. Reduction of pHi, observed in response to DIDS in the present experiments, may also slow down the overall metabolic rate in the NPE, in a way that leads to inhibition of AH formation.
In contrast to the minimal effect of acetazolamide on pHi in porcine NPE in the present study, a 0.2 pH units reduction of pHi was detected in rabbit transformed NPE 30. This may be due to a difference in the contribution of bicarbonate transport to the formation of AH in different species. For example, it had been shown previously that bicarbonate depletion in the bathing medium of rabbit ciliary body preparation completely reversed the electrical polarity 45, whereas the same maneuver only inhibited the short circuit current (Isc) by approximately 30% in the ox 46 and ~90 in the pig 47. According to these results, the importance of HCO3− transport in the porcine ciliary body falls between that of rabbit and ox.
In summary, our results suggest that porcine NPE uses a Na+-HCO3− cotransporter to import bicarbonate and a Cl−/HCO3− exchanger to export bicarbonate. Carbonic anhydrase influences the rate of bicarbonate transport. The Cl−/HCO3− exchanger AE2 and carbonic anhydrases CAII and CAIV are enriched in the NPE layer of the ciliary body and their coordinated function may contribute to aqueous humor secretion by effecting bicarbonate transport into the eye.
Acknowledgments
Funding: NIH grant number EY006915
Contributor Information
Mohammad Shahidullah, Department of Physiology, University of Arizona, 1501 N Campbell Avenue, Tucson, AZ, 85724, USA..
To C-H, The Laboratory of Experimental Optometry, School of Optometry, The Hong Kong Polytechnic University, Hong Kong SAR, China.
Ryan M. Pelis, Department of Physiology, University of Arizona, 1501 N Campbell Avenue, Tucson, AZ, 85724, USA.
Nicholas A Delamere, Department of Physiology, University of Arizona, 1501 N Campbell Avenue, Tucson, AZ, 85724, USA.
REFERENCES
- 1.Burstein NL, Fischbarg J, Liebovitch L, Cole DF. Electrical potential, resistance, and fluid secretion across isolated ciliary body. Experimental Eye Research. 1984;39:771–779. doi: 10.1016/0014-4835(84)90076-9. [DOI] [PubMed] [Google Scholar]
- 2.Jacob TJ, Civan MM. Role of ion channels in aqueous humor formation. American Journal of Physiology. 1996;271:C703–C720. doi: 10.1152/ajpcell.1996.271.3.C703. [DOI] [PubMed] [Google Scholar]
- 3.Civan MM. The Eye's Aqueous Humor: From secretion to glaucoma. San Diego, London, Boston, New York, Sydney, Tokyo, Toronto: Academic Press; 1998. Transport components of net secretion of the aqueous humour and their integrated regulation; pp. 1–24. [Google Scholar]
- 4.To CH, Kong CW, Chan CY, Shahidullah M, Do CW. The mechanism of aqueous humour formation. Clinical & Experimental Optometry. 2002;85:335–349. [PubMed] [Google Scholar]
- 5.Raviola G, Raviola E. Intercellular junctions in the ciliary epithelium. Investigative Ophthalmology & Visual Science. 1978;17:958–981. [PubMed] [Google Scholar]
- 6.Brechue WF, Maren TH. A comparison between the effect of topical and systemic carbonic anhydrase inhibitors on aqueous humor secretion. Experimental Eye Research. 1993;57:67–78. doi: 10.1006/exer.1993.1100. [DOI] [PubMed] [Google Scholar]
- 7.Helbig H, Korbmacher C, Nawrath M, Erb C, Wiederholt M. Sodium bicarbonate cotransport in cultured pigmented ciliary epithelial cells. Current Eye Research. 1989;8:595–598. doi: 10.3109/02713688908995759. [DOI] [PubMed] [Google Scholar]
- 8.Maren TH. Carbonic anhydrase inhibition in ophthalmology: aqueous humor secretion and the development of sulfonamide inhibitors. EXS. 2000:425–435. doi: 10.1007/978-3-0348-8446-4_21. [DOI] [PubMed] [Google Scholar]
- 9.Matsui H, Murakami M, Wynns GC, et al. Membrane carbonic anhydrase (IV) and ciliary epithelium. Carbonic anhydrase activity is present in the basolateral membranes of the non-pigmented ciliary epithelium of rabbit eyes. Experimental Eye Research. 1996;62:409–417. doi: 10.1006/exer.1996.0046. [DOI] [PubMed] [Google Scholar]
- 10.Geers C, Gros G. Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiological Reviews. 2000;80:681–715. doi: 10.1152/physrev.2000.80.2.681. [DOI] [PubMed] [Google Scholar]
- 11.Kivela A, Parkkila S, Saarnio J, et al. Expression of a novel transmembrane carbonic anhydrase isozyme XII in normal human gut and colorectal tumors. American Journal of Pathology. 2000;156:577–584. doi: 10.1016/S0002-9440(10)64762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maren TH. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiological Reviews. 1967;47:595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- 13.Tashian RE. The carbonic anhydrases: widening perspectives on their evolution, expression and function. Bioessays. 1989;10:186–192. doi: 10.1002/bies.950100603. [DOI] [PubMed] [Google Scholar]
- 14.Wistrand PJ. The importance of carbonic anhydrase B and C for the unloading of CO2 by the human erythrocyte. Acta Physiologica Scandinavica. 1981;113:417–426. doi: 10.1111/j.1748-1716.1981.tb06918.x. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Alvarez B, Casey JR, Reithmeier RA, Fliegel L. Carbonic anhydrase II binds to and enhances activity of the Na+/H+ exchanger. J Biol Chem. 2002;277:36085–36091. doi: 10.1074/jbc.M111952200. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Liu Y, Alvarez BV, Casey JR, Fliegel L. A novel carbonic anhydrase II binding site regulates NHE1 activity. Biochemistry. 2006;45:2414–2424. doi: 10.1021/bi051132d. [DOI] [PubMed] [Google Scholar]
- 17.Morgan PE, Supuran CT, Casey JR. Carbonic anhydrase inhibitors that directly inhibit anion transport by the human Cl-/HCO3-exchanger, AE1. Molecular Membrane Biology. 2004;21:423–433. doi: 10.1080/09687860400014872. [DOI] [PubMed] [Google Scholar]
- 18.Sterling D, Alvarez BV, Casey JR. The extracellular component of a transport metabolon. Extracellular loop 4 of the human AE1 Cl-/HCO3-exchanger binds carbonic anhydrase IV. Journal of Biological Chemistry. 2002;277:25239–25246. doi: 10.1074/jbc.M202562200. [DOI] [PubMed] [Google Scholar]
- 19.Sterling D, Reithmeier RA, Casey JR. A transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. Journal of Biological Chemistry. 2001;276:47886–47894. doi: 10.1074/jbc.M105959200. [DOI] [PubMed] [Google Scholar]
- 20.Shahidullah M, Wilson WS, Yap M, To CH. Effects of ion transport and channel-blocking drugs on aqueous humor formation in isolated bovine eye. Investigative Ophthalmology & Visual Science. 2003;44:1185–1191. doi: 10.1167/iovs.02-0397. [DOI] [PubMed] [Google Scholar]
- 21.Shahidullah M, Yap MK, To CH. cGMP, sodiun nitroprusside and sodium azide reduce aqueous humour formation in the isolated arterially perfused pig eye. British Journal of Pharmacology. 2005;144:1–9. doi: 10.1038/sj.bjp.0706156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shahidullah M, Tamiya S, Delamere NA. Primary culture of porcine nonpigmented ciliary epithelium. Current Eye Research. 2007;32:511–522. doi: 10.1080/02713680701434899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandal A, Delamere NA, Shahidullah M. Ouabain-induced stimulation of sodium-hydrogen exchange in rat optic nerve astrocytes. Am J Physiol Cell Physiol. 2008;295 doi: 10.1152/ajpcell.90636.2007. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Counillon L, Touret N, Bidet M, et al. Na+/H+ and CI-/HCO3-antiporters of bovine pigmented ciliary epithelial cells. Pflugers Archiv -European Journal of Physiology. 2000;440:667–678. doi: 10.1007/s004240000302. [DOI] [PubMed] [Google Scholar]
- 25.Wolosin JM, Bonanno JA, Hanzel D, Machen TE. Bicarbonate transport mechanisms in rabbit ciliary body epithelium. Experimental Eye Research. 1991;52:397–407. doi: 10.1016/0014-4835(91)90035-d. [DOI] [PubMed] [Google Scholar]
- 26.Kishida K, Miwa Y, Iwata C. 2-Substituted 1, 3, 4-thiadiazole-5-sulfonamides as carbonic anhydrase inhibitors: their effects on the transepithelial potential difference of the isolated rabbit ciliary body and on the intraocular pressure of the living rabbit eye. Experimental Eye Research. 1986;43:981–995. doi: 10.1016/0014-4835(86)90076-x. [DOI] [PubMed] [Google Scholar]
- 27.Lutjen-Drecoll E, Lonnerholm G. Carbonic anhydrase distribution in the rabbit eye by light and electron microscopy. Investigative Ophthalmology & Visual Science. 1981;21:782–797. [PubMed] [Google Scholar]
- 28.Lutjen-Drecoll E, Lonnerholm G, Eichhorn M. Carbonic anhydrase distribution in the human and monkey eye by light and electron microscopy. Graefes Archive for Clinical & Experimental Ophthalmology. 1983;220:285–291. doi: 10.1007/BF00231357. [DOI] [PubMed] [Google Scholar]
- 29.Liao SY, Ivanov S, Ivanova A, et al. Expression of cell surface transmembrane carbonic anhydrase genes CA9 and CA12 in the human eye: overexpression of CA12 (CAXII) in glaucoma. Journal of Medical Genetics. 2003;40:257–261. doi: 10.1136/jmg.40.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Q, Delamere NA, Pierce W., Jr Membrane-associated carbonic anhydrase in cultured rabbit nonpigmented ciliary epithelium. Investigative Ophthalmology & Visual Science. 1997;38:2093–2102. [PubMed] [Google Scholar]
- 31.Wolosin JM, Chen M, Gordon RE, Stegman Z, Butler GA. Separation of the rabbit ciliary body epithelial layers in viable form: identification of differences in bicarbonate transport. Experimental Eye Research. 1993;56:401–409. doi: 10.1006/exer.1993.1054. [DOI] [PubMed] [Google Scholar]
- 32.Usui T, Hara M, Satoh H, et al. Molecular basis of ocular abnormalities associated with proximal renal tubular acidosis. Journal of Clinical Investigation. 2001;108:107–115. doi: 10.1172/JCI11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fidzinski P, Salvador-Silva M, Choritz L, Geibel J, Coca-Prados M. Inhibition of NHE-1 Na+/H+ exchanger by natriuretic peptides in ocular nonpigmented ciliary epithelium. American Journal of Physiology Cell Physiology. 2004;287:C655–C663. doi: 10.1152/ajpcell.00552.2003. [DOI] [PubMed] [Google Scholar]
- 34.Hou Y, Delamere NA. Studies on H(+)-ATPase in cultured rabbit nonpigmented ciliary epithelium. Journal of Membrane Biology. 2000;173:67–72. doi: 10.1007/s002320001008. [DOI] [PubMed] [Google Scholar]
- 35.Wax MB, Saito I, Tenkova T, et al. Vacuolar H+-ATPase in ocular ciliary epithelium. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:6752–6757. doi: 10.1073/pnas.94.13.6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leem CH, Lagadic-Gossmann D, Vaughan-Jones RD. Characterization of intracellular pH regulation in the guinea-pig ventricular myocyte. Journal of Physiology. 1999;517:159–180. doi: 10.1111/j.1469-7793.1999.0159z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wakabayashi S, Bertrand B, Shigekawa M, Fafournoux P, Pouyssegur J. Growth factor activation and "H(+)-sensing" of the Na+/H+ exchanger isoform 1 (NHE1). Evidence for an additional mechanism not requiring direct phosphorylation. Journal of Biological Chemistry. 1994;269:5583–5588. [PubMed] [Google Scholar]
- 38.Wakabayashi S, Ikeda T, Iwamoto T, Pouyssegur J, Shigekawa M. Calmodulin-binding autoinhibitory domain controls "pH-sensing" in the Na+/H+ exchanger NHE1 through sequence-specific interaction. Biochemistry. 1997;36:12854–12861. doi: 10.1021/bi9715472. [DOI] [PubMed] [Google Scholar]
- 39.Humphreys BD, Jiang L, Chernova MN, Alper SL. Functional characterization and regulation by pH of murine AE2 anion exchanger expressed in Xenopus oocytes. American Journal of Physiology. 1994;267:C1295–C1307. doi: 10.1152/ajpcell.1994.267.5.C1295. [DOI] [PubMed] [Google Scholar]
- 40.Jiang L, Stuart-Tilley A, Parkash J, Alper SL. pHi and serum regulate AE2-mediated Cl-/HCO3-exchange in CHOP cells of defined transient transfection status.[erratum appears in Am J Physiol 1995 Feb;268(2 Pt 1):section C following table of contents] American Journal of Physiology. 1994;267:C845–C856. doi: 10.1152/ajpcell.1994.267.3.C845. [DOI] [PubMed] [Google Scholar]
- 41.Roussa E. H+ and HCO3-Transporters in Human Salivary Ducts. An Immunohistochemical Study. The Histochemical Journal. 2001;33:337–344. doi: 10.1023/a:1012471023913. [DOI] [PubMed] [Google Scholar]
- 42.Thévenod F, Roussa E, Schmitt BM, Romero MF. Cloning and Immunolocalization of a Rat Pancreatic Na+ Bicarbonate Cotransporter. Biochemical and Biophysical Research Communications. 1999;264:291–298. doi: 10.1006/bbrc.1999.1484. [DOI] [PubMed] [Google Scholar]
- 43.Chu TC, Green K. Bicarbonate and DIDS effects on intracellular potential difference in rabbit ciliary epithelium. Current Eye Research. 1990;9:233–239. doi: 10.3109/02713689009044518. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell CH, Wang L, Jacob TJ. A large-conductance chloride channel in pigmented ciliary epithelial cells activated by GTPgammaS. Journal of Membrane Biology. 1997;158:167–175. doi: 10.1007/s002329900254. [DOI] [PubMed] [Google Scholar]
- 45.Krupin T, Reinach PS, Candia OA, Podos SM. Transepithelial electrical measurements on the isolated rabbit iris-ciliary body. Experimental Eye Research. 1984;38:115–123. doi: 10.1016/0014-4835(84)90096-4. [DOI] [PubMed] [Google Scholar]
- 46.Do CW, To CH. Chloride secretion by bovine ciliary epithelium: a model of aqueous humor formation. Investigative Ophthalmology & Visual Science. 2000;41:1853–1860. [PubMed] [Google Scholar]
- 47.Kong C-W, Li K-K, To C-H. Chloride secretion by porcine ciliary epithelium: New insight into species similarities and differences in aqueous humor formation. Investigative Ophthalmology & Visual Science. 2006;47:5428–5436. doi: 10.1167/iovs.06-0180. [DOI] [PubMed] [Google Scholar]