Abstract
Background and Purpose
This review focuses on the emerging principles of ICH management, emphasizing the natural history and treatment of intraventricular hemorrhage. The translational and clinical findings from recent randomized clinical trials are defined and discussed.
Summary of Review
Brain hemorrhage is the most severe of the major stroke subtypes. Extension of the hemorrhage into the ventricles (a 40% occurrence) can happen early or late in the sequence of events. Epidemiologic data demonstrate the amount of blood in the ventricles relates directly to the degree of injury and likelihood of survival. Secondary tissue injury processes related to intraventricular bleeding can be reversed by removal of clot in animals. Specific benefits of removal include limitation of inflammation, edema, and cell death as well as restoration of CSF flow, ICP homeostasis, improved consciousness, and shortening of ICU stay. Limited clinical knowledge exists about the benefits of IVH removal in humans, as organized attempts to remove blood have not been undertaken in large clinical trials on a generalized scale. New tools to evaluate the volume and location of IVH and to test the benefits/risks of removal have been employed in the clinical domain. Initial efforts are encouraging that increased survival and functional improvement can be achieved. Little controversy exists regarding the need to scientifically investigate treatment of this severity factor.
Conclusions
Animal models demonstrate clot removal can improve the acute and long term consequences of intraventricular extension from ICH by employing minimally invasive techniques coupled to rt-PA mediated clot lysis. The most recent human clinical trials show that severity of initial injury and the long term consequences of blood extending into the ventricles are clearly related to the amount of bleeding into the ventricular system. The failure of the last two pivotal brain hemorrhage RCTs may well relate to the consequences of intraventricular bleeding. Small proof of concept studies, meta-analyses, and preliminary pharmacokinetics studies support the idea of positive shifts in mortality and morbidity, if this one critical disease severity factor, IVH, is properly addressed. Understanding clinical methods for the removal of IVH is required, if survival and long term functional outcome of brain hemorrhage is to improve worldwide.
Keywords: IVH, ICH, thrombolysis, rt-PA, functional outcome
Introduction: Pragmatic treatment requirements
While mortality and functional outcomes for intracerebral hemorrhage (ICH) have not changed in the last 20 to 30 years,1-3 the results of recent animal4 and human investigations5, 6 radically alter the likelihood of changing poor outcome in this disease. It is now plausible to reverse the course of blood clot mediated injury of brain tissues and convert this reversal into gains in human function after a bleeding event. The clinical procedures needed to identify and treat this disease (computed tomography around the clock, extraventricular drainage use & image guidance) have proliferated worldwide at primary stroke centers, as has the knowledge of the specific disease processes in humans. Organized, multi-component intensive care, including acute blood pressure (BP) control and support for impaired cardio-respiratory functions, has improved and become widely available.7-9 Furthermore, several key treatment capabilities have been tested prospectively in clinical trials over the last decade. Specifically, practical experience with intracranial pressure (ICP) control, routine utilization of neurocritical care,7 experience with thrombolysis,10 knowledge of intracranial drug delivery,11 and image-based decision making11-13 are now widespread. Applied together as a coordinated treatment program, these tools offer the unique opportunity to reverse the effects of ICH. The scientific challenge that remains is to produce a definitive demonstration that the organized employment of a specific combination of treatments and treatment goals leads to a predictable set of good outcomes. This review will discuss the role of intraventricular extension of bleeding following spontaneous hypertensive brain hemorrhage as a critical, treatable event in the ICH disease process.
Cerebral Ventricular System
The cerebral ventricular system provides a low pressure pathway for the movement of cerebrospinal fluid. This system is frequently broken into when blood at near systolic pressures passes through a defect in the arterial wall, forming a spontaneous ICH as it dissects brain tissue.8 Brain hemorrhage can occur from defects in the vessel wall, such as aneurysms, arteriovenous malformations or small vessel microaneurysms, coagulation profile, or just increased blood pressure creating bleeding sites. Thus, multiple different diseases, including trauma, tumors, and BP elevation, are capable of producing collections of blood that may occlude or obstruct the intraventricular space.8 Bleeding at deep intracranial sites near the ventricles leads to early intraventricular rupture and compromise of the normal pressure regulation of the cranial vault, while bleeding sites further away from the ventricles may accumulate clotting blood before mechanical pressure and hemorrhage size produces a rupture into the ventricles. The actual rupture is often associated with a decreased consciousness that can be recognized clinically and is frequently associated with subsequent death.14, 15 Thus, the seriousness of rupture rapidly becomes evident.
Computed tomography (CT) and ICP monitoring rapidly moved intraventricular hemorrhage (IVH) out of the pathologic and epidemiologic domain and into the clinical domain permitting qualitative16 and quantitative measurement of intraventricular bleeding17, 18 and its effects on ICP.19 The utilization of serial brain imaging has gradually altered the diagnosis, management, and clinical response to the occurrence of intraventricular hemorrhage in the last three decades.10, 11, 20 The academically accepted approach of emergent ICP management and blood clot drainage until ventricular re-opening remains a pragmatic approach, the generalizability and value of which has not been rigorously tested in a large RCT.21, 22 However, this approach is supported by translational models.
Validating Therapy: reversibility of injury mechanisms in animals
Multiple animal models support the biologic rationale that IVH is one of the necessary disease factors to manage in order to make human recovery a reality for diseases like ICH. It appears that both mechanical and biochemical factors are responsible for brain tissue injury. In both canine and porcine IVH models, the greater the volume of blood clot injected into the ventricles, the greater the likelihood of animal death.23-25 Similarly, prolonged exposure of the ventricles to blood leads to altered consciousness and, at the tissue level, inflammation, fibrosis and hydrocephalus.26, 27 The removal of blood clot improves level of consciousness and prevents tissue inflammation in animal treatment simulations, even when there is substantial delay in initiating and accomplishing the removal. Histological assessments demonstrate that hydrocephalus and inflammation are prevented with thrombolytic-mediated removal of clot.27-29 Specific models have indicated reversal/prevention of ventricular enlargement,24, 27, 28 herniation and coma,25, 27 white cell infiltrations, 4, 26 peri-ventricular edema,27 and generalized edema.28, 30-32 Two important characteristics of these successful models are early control of ICP and significant removal of significant volumes of clot from tissue. The ability of clot removal to inhibit blood clot mediated progressive tissue injury and reverse prolonged neurological dysfunction provides the underlying biologic principle for the translation of the animal models of IVH to the human situation.
Epidemiologic observation demonstrates IVH as a severity factor in ICH
Observations from retrospective clinical series and prospective clinical trials have confirmed the importance of IVH as a clinical factor associated with poor outcomes including coma, mortality and long-term functional impairment. The presence of intraventricular blood has been strongly associated with impaired consciousness at presentation.14 Simple comparison between ICH subjects with and without IVH extension suggests that mortality is substantially increased if IVH is present (Table 1). Tuhrim was the first to consistently demonstrate a powerful relationship between the presence of IVH in a brain hemorrhage patient and the likelihood of death.33 This relationship was prospectively demonstrated in several subsequent studies15, 34 and noted to be continuously related to the complete range of incremental increases in the volume of intraventricular blood. 15 Multivariate regression analysis performed on other convenience samples almost always defines the presence of IVH as an independent risk factor for mortality and poor functional outcome.35-37 Randomized, controlled studies in the last decade have confirmed this point by demonstrating a similar relationship between poor outcomes and the extent of IVH. These post hoc evaluations of carefully collected clinical trial data from more than 2,000 subjects are consistent with animal model findings. These evaluations demonstrate that presence of IVH is associated with fewer than 20% good functional outcomes, as measured by the 90- to 180-day modified Rankin Scale.5, 38, 39 For example, a separate analysis of the CT images from the STICH trial demonstrated that, while 31% of the entire set of study subjects had good functional outcomes as defined by GOS (normal, good or moderate recovery), only 15% of the 377 STICH subjects with IVH experienced this same level of recovery. If radiographically-confirmed hydrocephalus occurs, then the frequency of a favorable recovery decreases to 11.5%.36 This same finding is repeated in the NovoSeven in ICH trial where 141 of 374 subjects had IVH at presentation and 169 subjects had IVH at 24 hours. Only 20% of those subjects having IVH at presentation experienced good outcomes (mRS 0-3) vs. 43% of those subjects with no IVH at presentation. When expansion of IVH occured in the first 24 hours, only 7% of subjects were noted to have good 90 day outcomes by these same mRS criteria. 35 Thus, in a recent multivariate analysis of the NovoSeven trial, the effect of IVH and IVH expansion (>2cm3) on functional outcome ranges from an odds ratio of 2.53 to 4.21; providing substantial support that IVH removal is an excellent therapeutic target. 35 Most importantly, no organized effort to remove IVH was undertaken in either trial.
Table 1.
Actual mortality calculated from selected peer-reviewed publications
| Publication | Type of Study | Number of Subjects | IVH% | Mortality w/IVH | Mortality w/o IVH |
|---|---|---|---|---|---|
| Steiner T, et al (2006) | Prospective Trial | n=374 | 45.00% | 59% | 14% |
| Bhattathiri P, et al (2006) | Prospective Trial | n=902 | 42% | 56.2% | 28.6% |
| Takahashi O, et al (2006) | Retrospective | n=347 | 45.53% | 29.11% | 12.7% |
| Marti-Fabregas, J, et al (2003) | Prospective Observational | n=48 | 54.17% | 46.15% | 13.64% |
| Cheung R, et al (2003) | Retrospective | n=142 | 40.4% | 45.6% | 5.9% |
| Hemphill J, etal (2001) | Retrospective | n=152 | 55.26% | 65.5% | 19.1% |
| Tuhrim S, et al (1999) | Prospective Observational | n=129 | 36.40% | 42.6% | 8.5% |
| Razzaq A, et al (1998) | Retrospective | n=146 | 19.1% | 56.25% | 19.67% |
| Quereshi A, et al (1995) | Retrospective | n=182 | 45.6% | 79.5% | 26.2% |
| Mase G, et al (1995) | Retrospective | n=138 | 28.26% | 65.8% | 13.13% |
| Franke C, et al (1992) | Prospective Observational | n=157 | 46.50% | 78.08% | 19.05% |
| Daverat P, et al (1991) | Retrospective | n=166 | 43.30% | 69% | 31% |
| Total and Population-Weighted Mean | n=2883 | 41.79% | 56.36 % | 20.30% |
IVH and poor outcomes
Early attempts to treat IVH focused on ICP elevation as the critical abnormality. This focus was reasonable, as severe ICP elevations are associated with herniation and ischemia—two common sequelae in IVH. However, detailed intensive care unit (ICU) assessments of the benefit of ICP management have not produced any biologic indication of improved consciousness, functional improvement, or improved mortality.10, 40-42 It is noted that ICP management reverses symptoms of herniation and leads to improved outcomes in a case series.43 Thus, European and North American guidelines define as clinically appropriate interventions a “gradual or graded” approach to the identification of mass effect and management to promote ICP control to “normal” levels.21, 22 However, utilization of ICP treatments in IVH or ICH remains low, most likely because of the absence of overall clinical benefit demonstrated in randomized trials. Recent trials of surgery (STICH) and recombinant factor VIIa (FVIIa, NovoSeven) therefore did not specify IVH removal or ICP treatment goals.
The Surgical Trial in ICH (STICH) tested the hypothesis that a policy of ICH removal would improve functional outcome. No attempt to stabilize clot enlargement was undertaken nor was there any provision in the protocol to remove the ventricular blood that occurred in 42% of subjects. A post hoc evaluation of the STICH trial segregated 221 subjects with lobar ICH and no IVH. The removal of ICH from these subjects who did not have IVH was more effective and produced a 12% absolute improvement in functional outcome compared to the medically treated subjects.44 This is a substantial improvement over all surgery-treated STICH subjects (i.e., those in whom 41% did have IVH); this group had only a 4% improvement in GOS outcome versus medically treated control subjects. STICH II is now actively investigating the hypothesis that removal of isolated lobar ICH (no IVH) is beneficial. Similarly, FVIIa has been evaluated for its ability to stop early clot expansion and produce improved functional outcome in ICH patients presenting in the first 4 hours after initial symptoms. There was no standard plan for removal of IVH despite a 49% rate of IVH occurrence in this trial. In fact, the frequency of ICP monitoring to manage IVH was 7%, suggesting limited attempts to treat ventricular expansion. The pivotal FAST trial used the same design and protocol as the prior successful NovoSeven in ICH trial, but demonstrated no effect of factor VIIa on functional outcome, in large part because of higher occurrence of IVH in the active arm of the study cohort.38, 45 The decision to avoid systematic attempts to remove IVH may have further masked the benefits of clot stabilization (a physiologic benefit that did occur). Taken together recent trials demonstrate a consistent picture of three major, independent severity factors, each strongly associated with poor outcome: ICH volume, early deterioration, and IVH size. Importantly, these well-organized, but unsuccessful trials chose to mitigate only one ICH severity factor (i.e., STICH—ICH size; NovoSeven—early deterioration/hematoma enlargement). The neutral findings in STICH and NovoSeven-FAST further support the likely independence of each factor's effect on outcome. These trial findings suggest the hypothesis that, for a treatment program to be sufficient to alter ICH outcome, a program must incorporate treatment of all three factors. In summary, recent clinical trial experiences suggest that an effective therapy for intracerebral hemorrhage must include 1) stability of the initial bleed, 2) removal of the IVH, and 3) minimization of the volume/mass effect of the ICH.45
Proof of treatment concepts in Humans
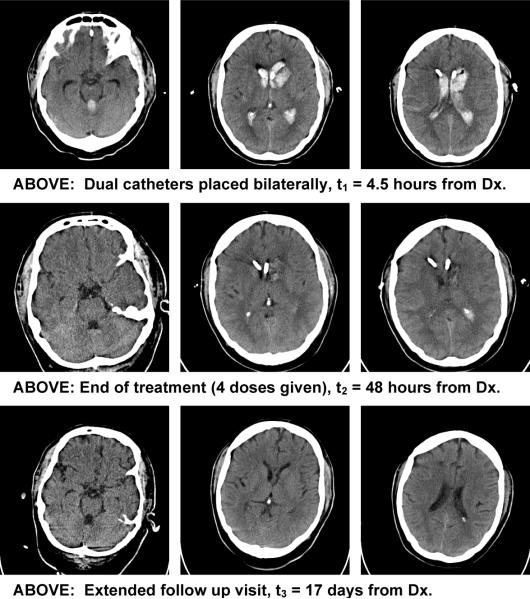
The CLEAR IVH trial tested removing IVH with catheter-delivered recombinant tissue plasminogen activator (rt-PA). This catheter-based treatment of IVH is consistent with the rationale that amelioration of intraventricular clot decreases the severity of the disease by decreasing mortality.17 Lower rates of mortality have been consistently observed when the treatment policy involves 1) the use of an intraventricular catheter to maintain ICP within the normal range and 2) efforts to remove clot by injecting low doses of thrombolytic.10, 11, 17, 20, 46 Treatment that solely maintains ICP is insufficient to alter mortality or morbidity10 and inconsistent with the critical therapeutic goal of blood clot removal after emergent control of ICP is achieved with the intraventricular catheter. 47 The initial results of the CLEAR IVH trial are consistent with the proposition that a protocol for removal of intraventricular clot from ICH subjects with small, stable parenchymal clots (i.e., ICH size < 30cm3) can produce close to 50% good functional outcomes as defined by mRS score at 180 days,48 despite the presence of, on average, 45 ml of intraventricular blood at the start of treatment (Fig. 1). Detailed analysis of the final results of this trial will be necessary to understand if a relationship exists between the amount of blood removed and the degree of benefit achieved in the treated group of subjects.Should such a relationship exist, it would be a link between therapeutic intent and therapeutic efficacy. Several expert groups have suggested that a trial testing the efficacy of IVH clot removal is an important goal for advancing our knowledge of ICH treatments. 10, 20, 49Plans for such a trial are now well described and await peer review.50
Figure 1.
Treatment of intraventricular hemorrhage in a patient requiring drug (rt-PA) instillation via two catheters
Refining removal therapy
Efforts to improve clot removal as a treatment are now underway. Animal models continue to improve our understanding of the sequence of inflammatory events that occur after hemorrhage and clot stabilization. Multiple biologic mechanisms could be tapped to minimize the negative effects of clot on tissue, particularly if substantial physical removal of blood clot volume can occur via a minimally traumatic mechanism prior to initiation of such a “biologic” clean-up. These biologic points of attack include blockade of thrombin-induced activation of inflammatory and cell death signals,51 scavenging of residual free iron,30 and enhanced phagocytosis of clot RBCs.52 Progress with enhanced, minimally-invasive surgical manipulations that lead to rapid atraumatic catheter-based clot removal appears possible.53 Each of these avenues can potentially improve the amount and qualities of clot removal from the ventricle. Post hoc analyses of best available data suggest a 10% to 15% absolute benefit might be achieved in selected populations of IVH and ICH subjects, if blood clot can be removed from the brain.48, 53 It is now time to test the viability of clot removal from the ventricle as the critically untested yet needed treatment in ICH.
Limitations of our knowledge
Despite the progress described, limitations of our knowledge regarding IVH are significant. We do not fully understand the role of coagulation events and vessel wall injury/repair in the sequence of bleeding events.13, 54 Improvements leading to a safe and more rapid termination of bleeding could further limit the injury to tissue.49 Similarly, identifying the critical steps from clotting to inflammation to cell death, while identified in animals, have only recently been initiated in human ICH.30, 31, 55, 56Additional knowledge regarding the mechanical49 and anatomic relationships57 that occur with spontaneous ICH is needed. Finally, our ability to prognosticate58, 59 while improving is still of limited value, as current diagnostic techniques provide anatomic rather than physiologic information. Additional methods that establish, with precise sensitivity, irreversible injury to vital brain tissues will be necessary, if prognostication can become sensitive enough to be employed routinely in clinical judgments regarding treatment.60, 61 Rigorous scientific investigation in clinical trials should bring additional information to clinical use within the next decade.
Acknowledgments
Acknowledgements and Funding: FDA orphan drugs program grant 5RO1- FD 001693, NINDS planning grant, 1R34- NS056638, MISITIE: NINDS, 1R01-NS 046309, Jeffrey and Harriet Legum professorship, Genentech, Inc., sponsored research agreement. The author acknowledges Shannon LeDroux and Eric Melnychuk for their editorial assistance and The CLEAR Investigators for their support and clinical efforts in collecting IVH trial materials.
Footnotes
Conflicts of Interest: The Johns Hopkins University use patent application # 10/509,694 (Dr. Hanley has disavowed interest in this patent)
References
- 1.Teernstra OP, Evers SM, Kessels AH. Meta analyses in treatment of spontaneous supratentorial intracerebral haematoma. Acta Neurochir (Wien) 2006;148:521–528. doi: 10.1007/s00701-005-0713-1. discussion 528. [DOI] [PubMed] [Google Scholar]
- 2.Dennis MS. Outcome after brain haemorrhage. Cerebrovasc Dis. 2003;16:9–13. doi: 10.1159/000069935. [DOI] [PubMed] [Google Scholar]
- 3.Lovelock CE, Molyneux AJ, Rothwell PM. Change in incidence and aetiology of intracerebral haemorrhage in oxfordshire, uk, between 1981 and 2006: A population-based study. Lancet Neurol. 2007;6:487–493. doi: 10.1016/S1474-4422(07)70107-2. [DOI] [PubMed] [Google Scholar]
- 4.Xi G, Keep FR, Hoff JT. Pathophysiology of brain edema formation. Neurosurg Clin N Am. 2002;13:371–383. doi: 10.1016/s1042-3680(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 5.Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, Karimi A, Shaw MD, Barer DH, Investigators S. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the international surgical trial in intracerebral haemorrhage (stich): A randomised trial. Lancet. 2005;365:387–397. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 6.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, Skolnick BE, Steiner T, Investigators RAFVIHT Recombinant activated factor vii for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777–785. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]
- 7.Diringer M, Edwards D. Admission to a neurologic/neurosurgical intensive care unit is associated with reduced mortality rate after intracerebral hemorrhage. Crit Care Med. 2001;29(3):635–640. doi: 10.1097/00003246-200103000-00031. [DOI] [PubMed] [Google Scholar]
- 8.Qureshi AI, Tuhrim S, Broderick J, Batjer HH, Hondo H, Hanley D. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 9.Qureshi AI. Antihypertensive treatment of acute cerebral hemorrhage (atach): Rationale and design. Neurocrit Care. 2007;6:56–66. doi: 10.1385/ncc:6:1:56. [DOI] [PubMed] [Google Scholar]
- 10.Nieuwkamp DJ, de Gans K, Rinkel GJ, Algra A. Treatment and outcome of severe intraventricular extension in patients with subarachnoid or intracerebral hemorrhage: A systematic review of the literature. J Neurol. 2000;247:117–121. doi: 10.1007/pl00007792. [DOI] [PubMed] [Google Scholar]
- 11.Naff NJ, Keyl PM, Tuhrim S, Bederson J, Bullock R, Mayer SA, Schmutzhard E, Hanley DF. Intraventricular thrombolysis speeds blood clot resolution: Results of a randomized, double-blinded controlled clinical trial. Neurosurgery. 2004;54:577–583. doi: 10.1227/01.neu.0000108422.10842.60. discussion 583-574. [DOI] [PubMed] [Google Scholar]
- 12.Brott T, Broderick J, Kothari R. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997:1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Broderick JP, Diringer MN, Hill MD, Brun NC, Mayer SA, Steiner T, Skolnick BE, Davis SM. Determinants of intracerebral hemorrhage growth: An exploratory analysis. Stroke. 2007;38:1072–1075. doi: 10.1161/01.STR.0000258078.35316.30. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi AI, Safdar K, Weil J. Predictors of early deterioration and mortality in black americans with spontaneous intracerebral hemorrhage. Stroke. 1995;26(10):1764–1767. doi: 10.1161/01.str.26.10.1764. [DOI] [PubMed] [Google Scholar]
- 15.Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med. 1999;27:617–621. doi: 10.1097/00003246-199903000-00045. [DOI] [PubMed] [Google Scholar]
- 16.Graeb DA, Robertson WD, Lapointe JS, Nugent RA, Harrison PB. Computed tomographic diagnosis of intraventricular hemorrhage. Etiology and prognosis. Radiology. 1982;143:91–96. doi: 10.1148/radiology.143.1.6977795. [DOI] [PubMed] [Google Scholar]
- 17.Naff NJ, Carhuapoma JR, Williams MA, Bhardwaj A, Ulatowski JA, Bederson J, Bullock R, Schmutzhard E, Pfausler B, Keyl PM, Tuhrim S, Hanley DF. Treatment of intraventricular hemorrhage with urokinase: Effect on 30-day survival. Stroke. 2000;31:841–847. doi: 10.1161/01.str.31.4.841. [DOI] [PubMed] [Google Scholar]
- 18.Zimmerman RD, Maldjian JA, Brun NC, Horvath B, Skolnick BE. Radiologic estimation of hematoma volume in intracerebral hemorrhage trial by ct scan. AJNR Am J Neuroradiol. 2006;27:666–670. [PMC free article] [PubMed] [Google Scholar]
- 19.Janny P, Papo I, Chazal J, Colnet G, Barretto LC. Intracranial hypertension and prognosis of spontaneous intracerebral haematomas: A correlative study of 60 patients. Acta Neurochir (Wien) 1982;61:181–186. doi: 10.1007/BF01740083. [DOI] [PubMed] [Google Scholar]
- 20.Lapointe M, Haines S. Fibrinolytic therapy for intraventricular hemorrhage in adults (cochrane review) Cochrane Database Syst Rev. 2002;3:CD003692. doi: 10.1002/14651858.CD003692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broderick J, Connolly S, Feldmann E, Hanley D, Kase CS, Krieger D, Mayberg MR, Morgenstern LB, Ogilvy CS, Vespa P, Zuccarello M. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: A guideline from the american heart association/american stroke association stroke council, high blood pressure research council, and the quality of care and outcomes in research interdisciplinary working group. Stroke. 2007;38:2001–2023. doi: 10.1161/STROKEAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 22.Steiner T, Kaste M, Forsting M, Mendelow D, Kwiecinski H, Szikora I, Juvela S, Marchel A, Chapot R, Cognard C, Unterberg A, Hacke W. Recommendations for the management of intracranial haemorrhage - part i: Spontaneous intracerebral haemorrhage. The european stroke initiative writing committee and the writing committee for the eusi executive committee. Cerebrovasc Dis. 2006;22:294–316. doi: 10.1159/000094831. [DOI] [PubMed] [Google Scholar]
- 23.Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 1: Canine intraventricular blood cast model. Neurosurgery. 1986;19:540–546. doi: 10.1227/00006123-198610000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Mayfrank L, Kissler J, Raoofi R. Ventricular dilatation in experimental intraventricular hemorrhage in pigs. Characterization of cerebrospinal fluid dynamics and the effects of fibrinolytic treatment. Stroke. 1997;28:141–148. doi: 10.1161/01.str.28.1.141. [DOI] [PubMed] [Google Scholar]
- 25.Qureshi AI, Wilson DA, Traystman RJ. Treatment of transtentorial herniation unresponsive to hyperventilation using hypertonic saline in dogs: Effect on cerebral blood flow and metabolism. J Neurosurg Anesthesiol. 2002;14:22–30. doi: 10.1097/00008506-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 2: In vivo safety study of intraventricular urokinase. Neurosurgery. 1986;19:547–552. doi: 10.1227/00006123-198610000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 3: Effects of intraventricular urokinase on clot lysis and posthemorrhagic hydrocephalus. Neurosurgery. 1986;19:553–572. doi: 10.1227/00006123-198610000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Mayfrank L, Kim Y, Kissler J, Delsing P, Gilsbach JM, Schroder JM, Weis J. Morphological changes following experimental intraventricular haemorrhage and intraventricular fibrinolytic treatment with recombinant tissue plasminogen activator. Acta Neuropathol (Berl) 2000;100:561–567. doi: 10.1007/s004010000219. [DOI] [PubMed] [Google Scholar]
- 29.Wagner KR, Xi G, Hua Y, Zuccarello M, de Courten-Myers GM, Broderick JP, Brott TG. Ultra-early clot aspiration after lysis with tissue plasminogen activator in a porcine model of intracerebral hemorrhage: Edema reduction and blood-brain barrier protection. J Neurosurg. 1999;90:491–498. doi: 10.3171/jns.1999.90.3.0491. [DOI] [PubMed] [Google Scholar]
- 30.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 31.Wagner KR, Xi G, Hau Y, Kleinholz M, de Courten-Myers GM, Myers RE. Early metabolic alterations in edematous perihematomal brain regions following experimental intracerebral hemorrhage. J Neurosurg. 1998;88:1058–1065. doi: 10.3171/jns.1998.88.6.1058. [DOI] [PubMed] [Google Scholar]
- 32.Narayan RK, Narayan TM, Katz DA, Kornblith P, Murano G. Lysis of intracranial hematomas with urokinase in a rabbit model. J Neurosurg. 1985;62:580–586. doi: 10.3171/jns.1985.62.4.0580. [DOI] [PubMed] [Google Scholar]
- 33.Tuhrim S, Dambrosia JM, Price TR, Mohr JP, Wolf PA, Heyman A, Kase CS. Prediction of intracerebral hemorrhage survival. Ann Neurol. 1988;24:258–263. doi: 10.1002/ana.410240213. [DOI] [PubMed] [Google Scholar]
- 34.Tuhrim S, Dambrosia JM, Price TR. Intracerebral hemorrhage: External validation and extension of a model for prediction of 30-day survival. Ann Neurol. 1991;29:658–663. doi: 10.1002/ana.410290614. [DOI] [PubMed] [Google Scholar]
- 35.Steiner T, Schneider D, Mayer S, Brun N, Begtrup K, Broderick J, Davis S, Diringer MN, Skolnick BE. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: Risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor vii. Neurosurgery. 2006;59:767–773. doi: 10.1227/01.NEU.0000232837.34992.32. discussion 773-764. [DOI] [PubMed] [Google Scholar]
- 36.Bhattathiri PS, Gregson B, Prasad KS, Mendelow AD. Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: Results from the stich trial. Acta Neurochir Suppl. 2006;96:65–68. doi: 10.1007/3-211-30714-1_16. [DOI] [PubMed] [Google Scholar]
- 37.Mayfrank L, Hutter BO, Kohorst Y, Kreitschmann-Andermahr I, Rohde V, Thron A, Gilsbach JM. Influence of intraventricular hemorrhage on outcome after rupture of intracranial aneurysm. Neurosurg Rev. 2001;24:185–191. doi: 10.1007/s101430100160. [DOI] [PubMed] [Google Scholar]
- 38.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, Skolnick BE, Steiner T. Efficacy and safety of recombinant activated factor vii for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–2137. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 39.Mendelow AD, Steiner T. Personal communication regarding stich and novoseven trials. 2007. [Google Scholar]
- 40.Adams RE, Diringer MN. Response to external ventricular drainage in spontaneous intracerebral hemorrhage with hydrocephalus. Neurology. 1998;50:519–523. doi: 10.1212/wnl.50.2.519. [DOI] [PubMed] [Google Scholar]
- 41.Diringer MN, Edwards DF, Zazulia AR. Hydrocephalus: A previously unrecognized predictor of poor outcome from supratentorial intracerebral hemorrhage. Stroke. 1998;29:1352–1357. doi: 10.1161/01.str.29.7.1352. [DOI] [PubMed] [Google Scholar]
- 42.Misra UK, Kalita J, Ranjan P, Mandal SK. Mannitol in intracerebral hemorrhage: A randomized controlled study. J Neurol Sci. 2005;234:41–45. doi: 10.1016/j.jns.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 43.Qureshi AI, Geocadin RG, Suarez Jl, Ulatowski JA. Long-term outcome after medical reversal of transtentorial herniation in patients with supratentorial mass lesions. Crit Care Med. 2000;28:1556–1564. doi: 10.1097/00003246-200005000-00049. [DOI] [PubMed] [Google Scholar]
- 44.Mendelow AD, Teasdale GM, Barer D, Fernandes HM, Murray GD, Gregson BA. Outcome assignment in the international surgical trial in intracerebral haemorrhage. Acta Neurochirurgica (Eur J Neurosurg) 2003;145:679–681. doi: 10.1007/s00701-003-0063-9. [DOI] [PubMed] [Google Scholar]
- 45.Tuhrim S. Intracerebral hemorrhage--improving outcome by reducing volume? N Engl J Med. 2008;358:2174–2176. doi: 10.1056/NEJMe0801856. [DOI] [PubMed] [Google Scholar]
- 46.Coplin WM, Vinas FC, Agris JM, Buciuc R, Michael DB, Diaz FG, Muizelaar JP. A cohort study of the safety and feasibility of intraventricular urokinase for nonaneurysmal spontaneous intraventricular hemorrhage. Stroke. 1998;29:1573–1579. doi: 10.1161/01.str.29.8.1573. [DOI] [PubMed] [Google Scholar]
- 47.Ziai WC TM, Naff NJ, Williams MA, Bullock R, Marmarou A, Tuhrim S, Pfausler B, Hanley D. Frequency of sustained intracranial pressure elevation during treatment of severe intracranial hemorrhage. Cerebrovascular Disease. 2008 doi: 10.1159/000209241. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanley D. On behalf of the clear investigators -- recent results from the clear-ivh trial. European Stroke Conference, http://www.eurostroke.org/PDF/esc_nice_press_release_08.pdf. 2008;25(suppl 2):1–192. http://www.eurostroke.org/PDF/esc_nice_press_release_08.pdf Cerebrovasc Dis 2008. [Google Scholar]
- 49.Priorities for clinical research in intracerebral hemorrhage: Report from a national institute of neurological disorders and stroke workshop. Stroke. 2005;36:e23–41. doi: 10.1161/01.STR.0000155685.77775.4c. [DOI] [PubMed] [Google Scholar]
- 50.CLEAR Phase III trial website. Http://clearivh.Com/default.Aspx.
- 51.Xi G, Wagner KR, Keep FR. Role of blood clot formation on early edema development after experimental intracerebral hemorrhage. Stroke. 1998;29:2580–2586. doi: 10.1161/01.str.29.12.2580. [DOI] [PubMed] [Google Scholar]
- 52.Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, Grotta JC, Aronowski J. Hematoma resolution as a target for intracerebral hemorrhage treatment: Role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol. 2007;61:352–362. doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]
- 53.Thiex R, Rohde V, Rohde I, Mayfrank L, Zeki Z, Thron A, Gilsbach JM, Uhl E. Framebased and frameless stereotactic hematoma puncture and subsequent fibrinolytic therapy for the treatment of spontaneous intracerebral hemorrhage. J Neurol. 2004;251:1443–1450. doi: 10.1007/s00415-004-0554-5. [DOI] [PubMed] [Google Scholar]
- 54.Mayer SA. Ultra-early hemostatic therapy for intracranial henorrhage. Stroke. 2003;34:224–229. doi: 10.1161/01.str.0000046458.67968.e4. [DOI] [PubMed] [Google Scholar]
- 55.Silva Y, Leira R, Tejada J, Lainez JM, Castillo J, Davalos A. Molecular signatures of vascular injury are associated with early growth of intracerebral hemorrhage. Stroke. 2005;36:86–91. doi: 10.1161/01.STR.0000149615.51204.0b. [DOI] [PubMed] [Google Scholar]
- 56.Hua Y, Keep RF, Hoff JT, Xi G. Brain injury after intracerebral hemorrhage: The role of thrombin and iron. Stroke. 2007;38:759–762. doi: 10.1161/01.STR.0000247868.97078.10. [DOI] [PubMed] [Google Scholar]
- 57.Broderick J, Brott T, Tomsick T, Leach A. Lobar hemorrhage in the elderly. The undiminishing importance of hypertension. Stroke. 1993;24:49–51. doi: 10.1161/01.str.24.1.49. [DOI] [PubMed] [Google Scholar]
- 58.Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Validation and comparison of models predicting survival following intracerebral hemorrhage. Crit Care Med. 1995;23:950–954. doi: 10.1097/00003246-199505000-00026. [DOI] [PubMed] [Google Scholar]
- 59.Hemphill JC, 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ich score: A simple. Reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 60.Ariesen MJ, Algra A, van der Worp HB, Rinkel GJ. Applicability and relevance of models that predict short term outcome after intracerebral haemorrhage. J Neurol Neurosurg Psychiatry. 2005;76:839–844. doi: 10.1136/jnnp.2004.048223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Godoy DA, Pinero G, Di Napoli M. Predicting mortality in spontaneous intracerebral hemorrhage: Can modification to original score improve the prediction? Stroke. 2006;37:1038–1044. doi: 10.1161/01.STR.0000206441.79646.49. [DOI] [PubMed] [Google Scholar]