Abstract
The synergistic combination of hydrodynamics-based gene delivery and ultrasound was investigated to achieve improved gene transfer to the kidney. Plasmids encoding firefly luciferase and Erythropoietin (EPO) gene were delivered into the left kidney of rats by single or combinative application of renal vein hydrodynamic injection and ultrasound treatment with or without the addition of ultrasound contrast agents (UCA). Ultrasound exposure was found to enhance the efficiency of hydrodynamics-based gene delivery for both luciferase and EPO expression. An ultrasound exposure intensity of 2 W/cm2 at 10% duty cycle for 15 min., produced a maximal gene expression 4.5 times higher than hydrodynamic delivery alone. Duration, location, and tissue-specificity of gene expression were not changed by ultrasound exposure. Application of UCA reduced the intensity and exposure duration of ultrasound treatment needed for optimal expression. Appropriate application of ultrasound and UCA did not alter histological structure or impair physiological function of the treated kidney.
Introduction
Gene therapy holds significant promise for the treatment of a variety of renal diseases [1]. To produce a therapeutic effect in the kidney without systemic adverse effects to other tissues, an efficient and targeted gene delivery method to the kidney is needed. Non-viral vectors based on the delivery of plasmid DNA (pDNA) have been developed as relatively simple gene therapy solutions that are both safe and versatile. Direct syringe injection of naked pDNA into mouse skeletal muscle was observed to produce significant transgene expression [2]. Subsequently, the use of direct pDNA injection has been applied to many other organs and biological models [3, 4].
Hydrodynamic delivery [5, 6] has emerged as a promising modification of direct injection that significantly enhances efficiency, achieving successful gene expression in a variety of organs and tissues [7]. In this method, hydrodynamic forces are generated by a rapid, pressurized injection of a large volume of DNA solution into the blood vessel, permeabilizing the capillary endothelium and generating “pores” in the plasma membrane of the surrounding parenchyma cells. These pores, in turn, facilitate the entry of DNA into the cytoplasm [7, 8]. Previous studies have demonstrated the feasibility of hydrodynamic injection-targeted gene delivery to the liver, kidney, skeletal muscle, and solid tumors [7]. In particular, hydrodynamic injections have been shown to be effective at overcoming tissue barriers, producing large localized concentrations of genetic material in target organs.
Despite this, it has been observed that, following hydroporation, much of the plasmid is bound to the outer surface of the plasma membrane for more than 1 hour, indicating insufficient permeabilization from hydrodynamic pressure [5, 9]. It has also been shown that massage of the delivery site can improve transgene expression from hydroporation [10], suggesting that additional mechanical forces capable of enhancing the entry of DNA molecules into the cytoplasm may further enhance the efficiency of hydrodynamic-induced gene delivery.
Ultrasound has emerged as another promising method for gene delivery because of its noninvasiveness, safety, and versatility in focusing acoustic energy on a specific region of organs [11]. It is hypothesized that acoustic cavitation plays a primary role in ultrasound-induced membrane permeability, or sonoporation [12]; consequently, ultrasound contrast agents (UCA) have often been employed in ultrasound-mediated gene delivery as a means of boosting cavitational activities. However, for effective ultrasound-mediated gene delivery, plasmids need to be localized at the site of exposure because ultrasound is limited in transporting genetic material across various tissue barriers without causing significant collateral damage.
The respective advantages of ultrasound and hydrodynamic injection are complementary, suggesting that their combination may yield a synergistically improved physical method for gene delivery. In this study, the feasibility of this combinative delivery for enhanced transgene expression in rat kidney was explored. The union of the two methods should provide a high concentration of plasmid at the target site from hydrodynamic injection, while increasing membrane permeability from ultrasound-induced cavitation effects. This combination has the potential to enhance gene expression while maintaining safe, site-specific delivery.
Materials and Methods
Animals
Eight-week-old male Wistar rats were purchased from Charles River Laboratories (Wilmington, MA) and maintained at the Duke University Vivarium. All animals were treated in accordance with NIH guidelines for animal experiments, and the protocols were approved by Duke University IACUC.
DNA plasmid preparation
Luciferase reporter plasmid (4.2 kb), gWIZ-Luc, was obtained from Gene Therapy Systems (San Diego, CA). Plasmid encoding hEPO gene (pCD2/EPO) was provided by Prof. Airu Zhou of the Dept. of Biochemistry and Molecular Biology at Peking University Medical Center. For plasmid preparation, the plasmids were transformed into DH-5α and selected on kanamycin containing agar plate. The positive clone was then amplified in Luria-Bertani (LB) medium. The final products of the plasmids in bacteria were extracted and purified using Qiagen Endofree Giga kit (Qiagen, Chatsworth, CA, USA). The purity and concentration of the isolated plasmids were confirmed by agarose gel electrophoresis and spectrophotometry.
Hydrodynamic injection of the plasmid DNA
Rats were anesthetized with 35-45 mg/kg Nembutal (Hospira, Lake Forest, IL) by intraperitoneal injection. A midline incision was performed to expose the left kidney and isolate the left renal vein. Immediately before the injection, the renal vein was clamped with a Diethrich bulldog clamp without occlusion of the adrenal and spermatic vein. Using a 26-gauge I.V. catheter (Kendall, Tyco Healthcare, Mansfield, MA), 0.5 ml of 100 μg/ml DNA solution was injected rapidly into the vein within 5 seconds, and the blood flow was re-established immediately after the injection. The site of injection was pressured for 15-30 s to achieve homeostasis. For experiments involving UCA, a mixed solution of pDNA and Optison (GE Healthcare, Princeton, NJ, plasmid DNA solution:Optison = 3:1) was injected into the renal vein within 5 seconds of the hydrodynamic injection.
Ultrasound treatment
For ultrasound treatment, a hand-held portable ultrasound probe (D = 10 mm) was placed on top of the kidney coupled by a thin layer of ultrasound gel. Kidneys were treated with the Sonotron 2000 ultrasound system (Rich-Mar Corp, Inola, OK). After ultrasound treatment, the skin incision was closed, and the animal was allowed to recover from anesthesia.
Bioluminescent evaluation
Bioluminescent imaging (BLI) was performed 1, 3, and 7 days after the hydrodynamic injection and/or ultrasound treatment. After anesthesia and intraperitoneal administration of 100 μl of D-luciferin at 15 mg/mL through the renal vein, BLI of the kidney and other organs was performed with the IVIS Imaging System (Xenogen, Alameda, CA,). Regions of interest from displayed images were designated around the injection origin and quantified as total photon counts or photons per second using the Xenogen system software. Background bioluminescence was measured to be in the range of 1∼2×105 photons/s.
EPO ELISA
EPO protein level in the animal was evaluated by a human EPO ELISA kit (StemCell Technologies Inc, Vancouver, Canada) according to the manufacturer's instructions. The presence of human EPO in serum was analyzed with a sensitivity of 0.6 mIU/mL and a detection range of 2.5∼200 mIU/mL.
Ex vivo luciferase and luciferase activity assay
Rats were euthanizied via an overdose injection of Nembutal. 15 minutes prior to euthanasia, 150 mg/kg D-luciferin (15 mg/ml) was injected intraperitoneally for analysis of luciferase activity. Immediately after necropsy, brain, heart, lung, liver, kidney, intestine, spleen, bladder, and testicle were placed individually into 35 mm dishes with sufficient D-luciferin (300ug/ml) to cover the tissues. Tissues were then imaged with the IVIS Imaging System, as described previously.
For luciferase report assay, tissues from various organs were harvested, homogenized and sonicated using a tissue disrupter (Sonic Dismembrator 60; Fisher Scientific Co., Pittsburgh, PA) and BCA Protein Assay Kit (Pierce, Rockford, IL). Luciferase activity was determined using the Luciferase Assay System (Promega, Madison, WI). Luciferase activities were normalized to relative light units per milligram of total protein in the homogenates.
Renal function evaluation
To eliminate the potential influence on renal function from the compensatory capacity of uninjected kidneys uninjected kidneys were removed immediately after the renal vein injection of DNA and ultrasound exposure. Uni-nephrectomized rats without DNA injection were used as controls. Blood urea nitrogen (BUN) and serum creatinine (Cr) levels, both endogenous markers for kidney function [13], were measured 1, 3, and 7 days after the treatment.
Immunohistochemistry staining and histology
To detect the location of luciferase transgene, immunohistochemistry was performed following published protocols [14].
For histological analysis, kidneys were harvested 1 day, 7 days, and 4 weeks after the hydrodynamic injection and/or sonication. They were fixed in 10% buffered formaldehyde, embedded in paraffin, and processed for routine light microscopy. To detect possible tissue injury produced by the gene transfer procedure, 5-μm sections with periodic acid-Schiff (PAS) were stained and examined.
Statistical analysis
For all described studies, the sample size for each experimental group consisted of 5 rats. Renal function evaluations were performed on 3 rats per experimental group. Data is presented as the mean values ± standard deviation of the mean. All data were analyzed using the SPSS 15.0 statistical program (SAS, Cary, NC), and the statistical significance was evaluated with the Anova and t-tests, with p values less than 0.05 considered to be statistically significant.
Results
Effect of ultrasound exposure on hydrodynamic gene transfer
Based on the measured luciferase activity, ultrasound exposure at 1 W/cm2 and 2 W/cm2 intensity levels increased the resultant gene expression produced by hydrodynamic injection. Specifically, enhancements in gene expression were produced at 2 W/cm2 with 3 min. and 15 min. exposure times, leading to 3.4-fold and 4.5 fold increases in expression yield, respectively (Fig. 1A). In comparison, ultrasound exposure at 1 W/cm2 intensity for the same treatment durations only increased the expression yield by factors of 1.2 and 3.1, respectively. Interestingly, at these intensity levels, a 30 min. treatment time caused a weaker increase in luciferase expression (< 1.8-fold), presumably due to increased tissue injury under long exposure durations.
Fig. 1.
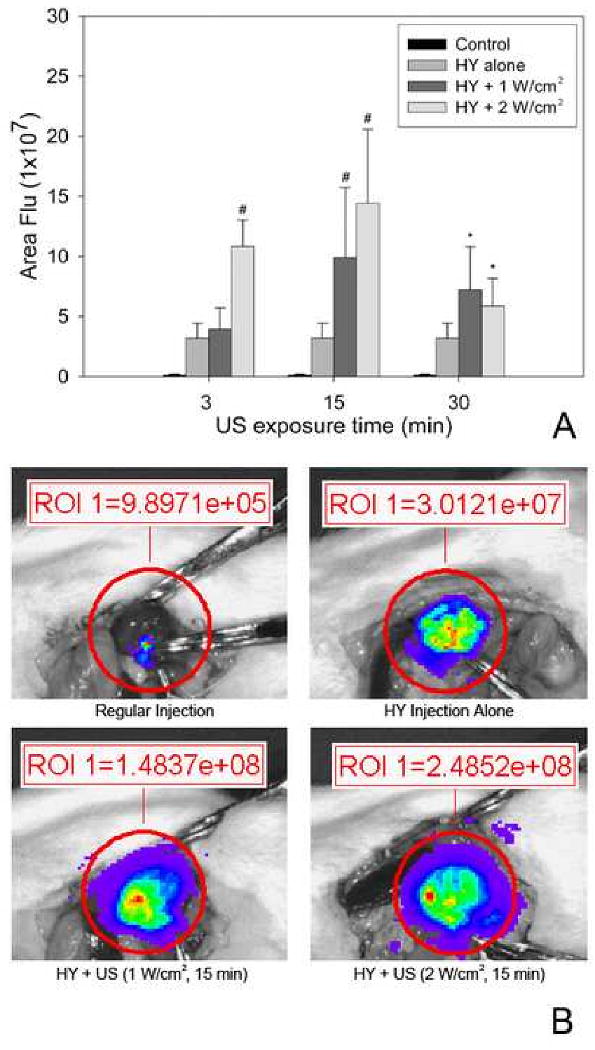
(A) Hydrodynamic-induced transgene expression was enhanced by ultrasound exposure at 1 W/cm2 and 2 W/cm2 intensity levels. Peak expression occurred for 15 minute ultrasound exposures. (B) Representative photographs from BLI. Compared with hydrodynamic alone, *: p<0.05, # p<0.01 (n = 5).
Ultrasound does not change the profiles of hydrodynamic injection-mediated gene delivery
The time-course of gene expression was evaluated using the ultrasound exposure condition (2 W/cm2 for 15 min) that led to maximally enhanced hydrodynamic-based gene expression. Similar to hydrodynamic injection alone, the highest gene expression for the combinative setting was found on day 1 after delivery, followed by a rapid decline. Gene expression on day 3 and day 7 dropped to approximately 1/10 and 1/100 of that on day 1, respectively (Figure 2A). Moreover, ultrasound exposure did not alter the distribution of transgene expression produced by hydrodynamic injection. As shown in Figure 2B, gene expression produced by the combinative delivery was observed primarily near PTC. Furthermore, the combination of ultrasound and hydrodynamic injection led to tissue-specific expression of exogenous reporter gene with significant luciferase activity observed only in the left kidney (Fig. 3).
Fig. 2.
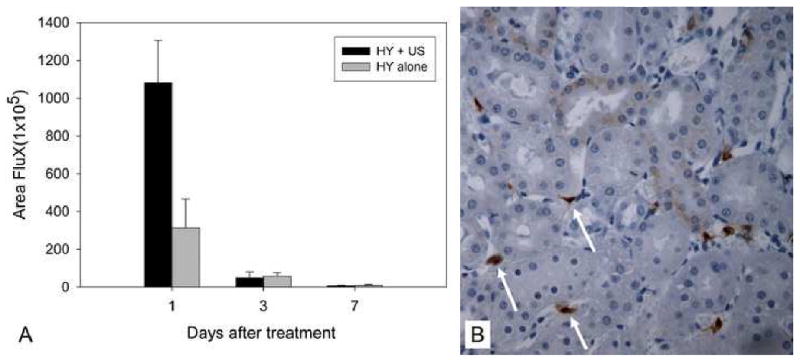
(A) Time course of transgene expression mediated by hydrodynamic injection alone and combinative delivery (n = 5). (B) Representative photograph of gene expression from combinative treatment. Expression occurred predominantly at interstitial fibroblasts near peritubular capillaries (PTC) (400 × magnification). HY: hydrodynamic injection; US: ultrasound.
Fig. 3.
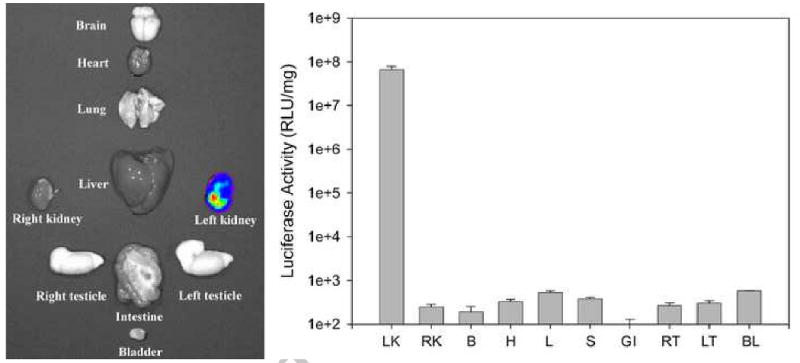
Introduction of ultrasound maintained tissue-specific expression, as determined by ex vivo BLI (left panel) and luciferase activity assay (right panel). LK: left kidney, RK: right kidney, B: brain, H: heart, L: liver, S: spleen, GI: intestine, RT: right testicle, LT: left testicle, BL: bladder.
The effect of UCA
The addition of Optison was found to augment the efficiency of gene delivery at lower doses of ultrasound exposure (Fig. 4A). The maximal gene expression was achieved at 1 W/cm2 intensity for 3 min exposure, which is comparable to that at 2 W/cm2 intensity for 15 min without Optison (Fig. 1A). At both intensity levels, gene expression dramatically declined in 15 and 30 min exposure groups. Also, the highest expression in the 2 W/cm2 group was significantly lower than its counterpart in the 1 W/cm2 group (p<0.05). The combination of UCA and hydrodynamic injection of plasmid DNA without ultrasound exposure did not affect the efficiency of gene expression. Also, UCA did not enhance gene expression mediated by “regular injection” of plasmid DNA (p>0.05, data not shown).
Fig. 4.
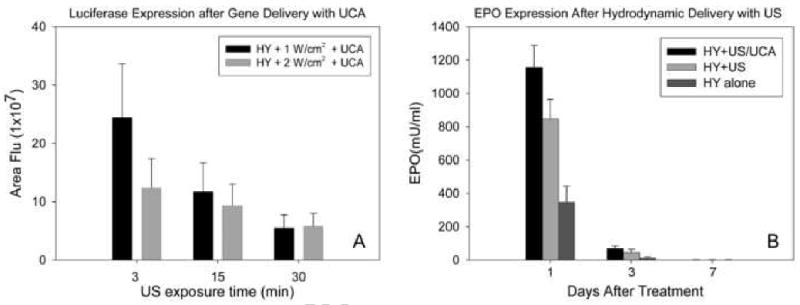
(A) Effect of UCA on combined ultrasound and hydrodyamic gene delivery. UCA (Optison, in a 25% solution with pDNA) lowered the energy and shortened the duration of ultrasound exposure necessary to achieve the highest gene expression (n = 5). (B) Efficacy of combinative delivery of EPO gene to rat kidney with and without UCA (25% solution with pDNA). Ultrasound was delivered for 3 minutes at an intensity of 1 W/cm2. For the combinative delivery alone, the ultrasound exposure setting was at 2 W/cm2 for a 15 minute duration. HY: hydrodynamic injection; US: ultrasound; UCA: ultrasound contrast agent, n = 5.
EPO gene expression assessment
EPO plasmid was employed to verify if exogenous gene transfected by hydrodynamic injection alone or in combination with ultrasound treatment could exert biological function as a result of EPO protein production. Ultrasound exposure settings that produced maximum gene expression of luciferase were selected. Compared with hydrodynamic injection alone, initial EPO expression on day 1 was increased by 2.3-fold with the addition of ultrasound and UCA (3 min. at 1 W/cm2). The use of ultrasound alone (15 min. at 2 W/cm2) yielded a 1.4-fold increase in EPO expression. Similar to the time-course of luciferase reporter gene transfer (Fig 2A), serum EPO reached a peak level at day 1, then decreased sharply with undetectable levels at day 7 (Fig. 4B).
Combinative treatment dose not deteriorate renal function
Serum creatinine and BUN levels were somewhat elevated at day 1 following treatment in the combination group; however, the differences were not of statistical significance when compared to the hydrodynamic injection alone or the uni-nephrectomized control groups at three time points (p>0.05, Table 1). Furthermore, despite these elevated levels, both the creatinine and BUN data were in the normal control range, indicating undeteriorated kidney function (Table 1). In comparing kidney sections from rats treated by combinative treatments with those from rats in the control group, no apparent pathological changes were found in the cortex, medulla or papilla of the kidneys at day 1, day 7, or day 28 (data not shown).
Table 1. Functional assessment of the treated kidneys.
| Normal range | Day | Control | HY | HY+US | HY + US/UCA |
|---|---|---|---|---|---|
| Creatinine (mg/dl) | 1 | 0.54 ± 0.10 | 0.61 ± 0.05 | 0.46 ± 0.09 | 0.69 ± 0.11 |
| 0.4-0.7 | 3 | 0.51 ± 0.05 | 0.55 ± 0.05. | 0.50 ± 0.06 | 0.56 ± 0.08 |
| 7 | 0.56 ± 0.06 | 0.57 ± 0.08 | 0.54 ± 0.04 | 0.57 ± 0.02 | |
| BUN (mg/dl) | 1 | 27.0 ± 7.2 | 30.0 ± 1.0 | 29.7 ± 3.5 | 31.7 ± 5.0 |
| 9-34 | 3 | 19.7 ± 5.1 | 17.7 ± 7.0 | 26.7 ± 2.5 | 22.7 ± 2.5 |
| 7 | 23.0 ± 1.7 | 24.0 ± 1.0 | 26.3 ± 1.5 | 26.0 ± 1.7 |
HY: Hydrodynamic injection, US: ultrasound, UCA: ultrasound contrast agent, n = 3
Discussion
For effective gene delivery, plasmid must first traverse three biological barriers before entering into target cells: the spatial barrier between the site of entry and the target organ, the structural barrier to the target cells, and finally, the cell membrane [8]. While both hydrodynamic injection and ultrasound are safe, simple, and relatively effective physical methods of gene delivery, each has its relative advantages and limitations with respect to overcoming these biological barriers. Hydrodynamic injection does not require sophisticated equipment, and, in comparison with electroporation and ballistic delivery (e.g., gene gun), it is less invasive for gene delivery to deeper tissues while still maintaining high levels of expression [15]. This method is highly effective for transporting large quantities of pDNA to target organs such as kidney, liver, and skeletal muscle; however, studies have shown large amounts of plasmid bound to the outer surface of the plasma membrane after transient permeabilization has ceased [9, 16]. Other reports demonstrating improved in vivo gene expression from hydrodynamic injection after physical massage also suggest that a large portion of injected pDNA remains in interstitial spaces without entering target cells [10]. Conversely, ultrasound-mediated gene delivery lacks an effective means of promoting localization of pDNA; thus, unlike hydrodynamic injection, ultrasound is limited in overcoming the tissue and structural barriers. The advantage of ultrasound, though, is that sonoporation is effective at inducing transient permeability of the cell membrane, facilitating transport into the cytoplasm [12]. In addition, ultrasound can be administered using a variety of transducer configurations and exposure conditions, enabling highly specific, non-invasive access to most internal organs.
In seeking a safe, convenient system of gene delivery that can yield efficient levels of gene expression, the union of ultrasound and hydrodynamic injection is an ideal fit that combines each mechanism's relative strengths while addressing their respective shortcomings, synergistically augmenting transport of pDNA. Specifically, hydrodynamic delivery of pDNA provides increased localized concentrations in the interstitial spaces at the target site, while ultrasound exposure provides additional disruption of the plasma membrane for improved uptake. In this manner, combinative delivery can effectively overcome the three major biological barriers.
In this study, the feasibility of combining hydrodynamic injection of plasmid DNA with localized ultrasound exposure to augment gene delivery in rat kidney has been demonstrated. This combinative means of gene delivery produced increased levels of both luciferase and EPO expression when compared with hydrodynamic injection alone, particularly during the first 24 hours after treatment. Functional and histological examinations of treated kidney also showed no apparent nephrotoxicity from the combinative treatment under the conditions used in this study. The augmentation of ultrasound-induced membrane permeability through the addition of ultrasound contrast agents further increased gene expression levels for shorter duration exposures. Furthermore, neither physical method poses any negative effects to the other system, providing site- and tissue-specific gene delivery to the kidney, with resultant luciferase expression isolated in the targeted interstitial fibroblasts near the PTC.
For combinative delivery, the intensity setting was chosen based on previous observations that longer duration exposures (∼30min.) at 2 W/cm2 led to improved transport of pDNA into the nuclei of targeted cells [17], which is believed to be the rate-limiting step in ultrasound-mediated gene therapy. Thus, the addition of ultrasound has the potential to further enhance gene expression through facilitation of pDNA transport into the nucleus. However, while the described results show an overall improvement in gene delivery, the highest enhancement occurred only for 3 and 15 min. exposure durations. The lower levels of gene expression from the 30 min. exposure may be caused by a potentially increased cellular damage from prolonged cavitation activity [18]. This speculation was supported by post-operative observation, with hematuria detected in some animals, and endpoint histology (not shown), which showed minor degrees of cellular fibrosis in groups receiving ultrasound treatment for 30 min. or ultrasound combined with Optison for 15 min.
The peak of luciferase expression was found to be at 24 h following the renal vein hydrodynamic injection, declining rapidly thereafter to an undetectable level at day 7 (Fig. 2). This is in agreement with a previous report in which an inferior vena cava injection of pDNA was applied [19]. The rapid decline of luciferase expression is mainly attributable to the SV-40 promoter employed in this study. Because the promoter cannot be integrated into the cell nuclei, only transient transfection can be achieved, regardless of the duration of permeability provided by both hydrodynamic injection and ultrasound exposure. However, others have demonstrated longer duration EPO expression using CAG promoter [13].
In ultrasound-mediated gene delivery, UCA have been employed as a means of introducing cavitation nuclei for increased cell membrane permeability [20]. Here, the use of UCA was found to increase the expression of luciferase and EPO for lower ultrasound exposure settings. This is consistent with previous studies in ultrasound-mediated gene delivery, where UCA typically lower the cavitation threshold [21]. The fact that Optison alone did not increase gene expression from hydrodynamic injection without ultrasound (data not shown) indicates that microbubbles may only affect sonoporation. However, the short lifetime of UCA at the site of ultrasound exposure and the increased cavitation activity at high ultrasound intensity likely contribute to the dramatic reduction in luciferase and EPO expression for longer treatment times. While increased cavitation activity improves membrane permeability, it has also been shown to reduce cell viability [21]. Future work is needed to develop techniques to produce safe, sustainable levels of cavitation in vivo that will greatly enhance the efficacy of this combinative approach.
Delivery of genetic material through the combination of ultrasound and hydrodynamic injection has significant clinical potential. Efficient gene transfer to porcine liver has been achieved by hydrodynamic injection [22], and similar large animal models have demonstrated the feasibility of ultrasound gene delivery. Clinically available ultrasound devices can be employed in conjunction with image-guided catheterization techniques to inject DNA. In particular, this technique is suitable for ex vivo naked DNA delivery during renal transplantation procedures. Other investigations have recently demonstrated improved control of hydrodynamic delivery to avoid injection-related tissue damage or low gene delivery efficiency due to insufficient volume or injection speed [23]. Future work is needed to develop techniques for producing sustainable cavitation activity in vivo and to determine the optimal ultrasound exposure conditions to prolong the delivery window and maximize transgene expression with minimal tissue and functional alterations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Imai E, Takabatake Y, Mizui M, Isaka Y. Gene therapy in renal diseases. Kidney international. 2004;65(5):1551–1555. doi: 10.1111/j.1523-1755.2004.05409.x. [DOI] [PubMed] [Google Scholar]
- 2.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science (New York, NY. 1990;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 3.Hickman MA, Malone RW, Lehmann-Bruinsma K, Sih TR, Knoell D, Szoka FC, Walzem R, Carlson DM, Powell JS. Gene expression following direct injection of DNA into liver. Human gene therapy. 1994;5(12):1477–1483. doi: 10.1089/hum.1994.5.12-1477. [DOI] [PubMed] [Google Scholar]
- 4.Lin H, Parmacek MS, Morle G, Bolling S, Leiden JM. Expression of recombinant genes in myocardium in vivo after direct injection of DNA. Circulation. 1990;82(6):2217–2221. doi: 10.1161/01.cir.82.6.2217. [DOI] [PubMed] [Google Scholar]
- 5.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6(7):1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 6.Zhang G, Budker V, Wolff JA. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Human gene therapy. 1999;10(10):1735–1737. doi: 10.1089/10430349950017734. [DOI] [PubMed] [Google Scholar]
- 7.Suda T, Liu D. Hydrodynamic Gene Delivery: Its Principles and Applications. Mol Ther. 2007 doi: 10.1038/sj.mt.6300314. [DOI] [PubMed] [Google Scholar]
- 8.Zhang G, Gao X, Song YK, Vollmer R, Stolz DB, Gasiorowski JZ, Dean DA, Liu D. Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther. 2004;11(8):675–682. doi: 10.1038/sj.gt.3302210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lecocq M, Andrianaivo F, Warnier MT, Wattiaux-De Coninck S, Wattiaux R, Jadot M. Uptake by mouse liver and intracellular fate of plasmid DNA after a rapid tail vein injection of a small or a large volume. J Gene Med. 2003;5(2):142–156. doi: 10.1002/jgm.328. [DOI] [PubMed] [Google Scholar]
- 10.Liu F, Huang L. Noninvasive gene delivery to the liver by mechanical massage. Hepatology (Baltimore, Md. 2002;35(6):1314–1319. doi: 10.1053/jhep.2002.33467. [DOI] [PubMed] [Google Scholar]
- 11.Newman CM, Bettinger T. Gene therapy progress and prospects: ultrasound for gene transfer. Gene Ther. 2007;14(6):465–475. doi: 10.1038/sj.gt.3302925. [DOI] [PubMed] [Google Scholar]
- 12.Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nature reviews. 2005;4(3):255–260. doi: 10.1038/nrd1662. [DOI] [PubMed] [Google Scholar]
- 13.Maruyama H, Higuchi N, Nishikawa Y, Hirahara H, Iino N, Kameda S, Kawachi H, Yaoita E, Gejyo F, Miyazaki J. Kidney-targeted naked DNA transfer by retrograde renal vein injection in rats. Human gene therapy. 2002;13(3):455–468. doi: 10.1089/10430340252792585. [DOI] [PubMed] [Google Scholar]
- 14.Lavon I, Goldberg I, Amit S, Landsman L, Jung S, Tsuberi BZ, Barshack I, Kopolovic J, Galun E, Bujard H, Ben-Neriah Y. High susceptibility to bacterial infection, but no liver dysfunction, in mice compromised for hepatocyte NF-kappaB activation. Nature medicine. 2000;6(5):573–577. doi: 10.1038/75057. [DOI] [PubMed] [Google Scholar]
- 15.Nishikawa M, Huang L. Nonviral vectors in the new millennium: delivery barriers in gene transfer. Human gene therapy. 2001;12(8):861–870. doi: 10.1089/104303401750195836. [DOI] [PubMed] [Google Scholar]
- 16.Herweijer H, Wolff JA. Gene therapy progress and prospects: hydrodynamic gene delivery. Gene Ther. 2007;14(2):99–107. doi: 10.1038/sj.gt.3302891. [DOI] [PubMed] [Google Scholar]
- 17.Duvshani-Eshet M, Baruch L, Kesselman E, Shimoni E, Machluf M. Therapeutic ultrasound-mediated DNA to cell and nucleus: bioeffects revealed by confocal and atomic force microscopy. Gene Ther. 2006;13(2):163–172. doi: 10.1038/sj.gt.3302642. [DOI] [PubMed] [Google Scholar]
- 18.Zarnitsyn VG, Prausnitz MR. Physical parameters influencing optimization of ultrasound-mediated DNA transfection. Ultrasound in medicine & biology. 2004;30(4):527–538. doi: 10.1016/j.ultrasmedbio.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Wu X, Gao H, Pasupathy S, Tan PH, Ooi LL, Hui KM. Systemic administration of naked DNA with targeting specificity to mammalian kidneys. Gene Ther. 2005;12(6):477–486. doi: 10.1038/sj.gt.3302433. [DOI] [PubMed] [Google Scholar]
- 20.Lawrie A, Brisken AF, Francis SE, Cumberland DC, Crossman DC, Newman CM. Microbubble-enhanced ultrasound for vascular gene delivery. Gene Ther. 2000;7(23):2023–2027. doi: 10.1038/sj.gt.3301339. [DOI] [PubMed] [Google Scholar]
- 21.Pua EC, Zhong P. Ultrasound-mediated drug delivery. IEEE Eng Med Biol Mag. 2009;28(1):64–75. doi: 10.1109/MEMB.2008.931017. [DOI] [PubMed] [Google Scholar]
- 22.Yoshino H, Hashizume K, Kobayashi E. Naked plasmid DNA transfer to the porcine liver using rapid injection with large volume. Gene Ther. 2006;13(24):1696–1702. doi: 10.1038/sj.gt.3302833. [DOI] [PubMed] [Google Scholar]
- 23.Suda T, Suda K, Liu D. Computer-assisted hydrodynamic gene delivery. Mol Ther. 2008;16(6):1098–1104. doi: 10.1038/mt.2008.66. [DOI] [PubMed] [Google Scholar]


