Abstract
Background
Encapsulation of cells has the potential to eliminate the need for immunosuppression for cellular transplantation. Recently, the TheraCyte® device was shown to provide long-term immunoprotection of murine islets in the NOD/SCID mouse model of diabetes. In this report, translational studies were undertaken using skin fibroblasts from an unrelated rhesus monkey donor that were transduced with an HIV-1-derived lentiviral vector expressing firefly luciferase permitting the use of bioluminescence imaging (BLI) to monitor cell survival over time and in a noninvasive manner.
Methods
Encapsulated cells were transplanted subcutaneously (N=2) or cells were injected without encapsulation (N=1) and outcomes compared. BLI was performed to monitor cell survival.
Results
The BLI signal from the encapsulated cells remained robust post-insertion, and in one animal persisted for up to 1 year. In contrast, the control animal that received unencapsulated cells exhibited a complete loss of cell signal within 14 days.
Conclusions
These data demonstrate that TheraCyte® encapsulation of allogeneic cells provides robust immune protection in transplanted rhesus monkeys.
Keywords: Encapsulation, Transplantation, Optical Imaging, Rhesus Monkeys
INTRODUCTION
Conditioning regimens, chronic immune suppression, and graft-versus-host disease present significant challenges for cellular transplantation (1, 2). To avoid the need to modulate the immune system, cell encapsulation devices have been considered (3, 4). Many encapsulation studies, to date, have employed microencapsulation technology, in which small numbers of cells are placed within alginates or other hydrogels. Larger encapsulation devices, such as the TheraCyte® macroencapsulation system, offer advantages which includes the encapsulation of a relatively large number of cells (e.g., ≥ 1×106), and the convenience that the device can be removed for analysis (5–8). Prior challenges with early prototypes such as fibrosis and a barrier to diffusion across the membrane have been overcome with current technology using a bilayered membrane. In studies with these devices cell survival has typically been monitored by secreted proteins or the immunohistochemical analysis of explanted devices.
Experimental studies focused on encapsulation have involved rodents and in some cases larger species (5–8). While rodent models are important they present some limitations because of a less complex immune system when compared to humans. In contrast, there are important similarities between human and nonhuman primate MHC genes and development of the T-cell repertoire, providing important preclinical and translational opportunities for study (9). Advances in in vivo imaging also present a means to monitor graft survival in real time, and in a noninvasive manner (10). This study describes the transplantation of encapsulated allogeneic rhesus monkey fibroblasts from an unrelated donor, and monitoring of graft survival by in vivo bioluminescent imaging (BLI) over time. The findings from this study represent an important step toward clinical translation of encapsulation technology.
MATERIALS AND METHODS
All animal procedures conformed to the requirements of the Animal Welfare Act and protocols were approved prior to implementation by the Institutional Animal Care and Use Committee at the University of California, Davis. A total of three young rhesus monkeys (Macaca mulatta) were included in these studies. Rhesus monkey skin fibroblasts from an unrelated donor were previously cryopreserved using a controlled rate cryopreservation protocol were thawed, expanded, and prepared for transduction with a lentiviral vector as previously described (10, 11). Briefly, fibroblasts were plated at 5×103 cells/cm2 then incubated at 37°C overnight, and transduced with an HIV-1-derived lentiviral vector (1×106 infectious particles/ml) expressing firefly luciferase under the control of the MND promoter in medium containing 4 mg/ml polybrene. After cells reached approximately 80% confluence, cells were trypsinized and replated in culture medium at 5×103 cells/cm2.
Transduced fibroblasts were plated at 3.9×102 – 1.0×105 cells/well with 100 mg/ml D-luciferin added to the medium and imaged using a Xenogen IVIS®200 Series imaging system (Caliper Life Sciences, Alameda, CA) with Living Image Software for post-imaging analysis (10) and to confirm luciferase expression. Cells were expanded and at approximately 80% confluence washed with PBS, incubated with 0.25% trypsin-EDTA for 5 min at 37°C, and culture medium added to inactivate trypsin activity. Fibroblasts were collected, counted, and washed three times in PBS using established techniques. Sterile TheraCyte® encapsulation devices (20 µl ported; Irvine, CA) were prepared according to the manufacturer’s instructions. Approximately 1×107 transduced fibroblasts were gently injected into each TheraCyte device through the port under sterile conditions then sealed with medical adhesive (Dow Corning, MI). Each device was washed in sterile PBS and transported in sterile PBS in preparation for insertion into rhesus monkeys (~2 years of age; N=2). The device was inserted subcutaneous (SC) between the scapulae in telazol-sedated animals (5–8 mg/kg) after sterile preparation of the site and blunt dissection of a pocket of sufficient size to insert the device with the port end in a cranial orientation. Fibroblasts (1×107 transduced cells) without encapsulation were injected SC at the same anatomical location in a third monkey. Immediately after insertion of the device (N=2) or direct cell injection (N=1) each animal was injected intravenously with 100 mg/ml D-luciferin via a peripheral vessel for in vivo imaging. Luciferin administration and imaging was then performed at 1 week, every 2 weeks up to 3 months, then monthly thereafter in telazol-sedated animals. Complete blood counts and clinical chemistry panels were assessed prior to insertion and at each imaging session (all parameters were within normal limits).
RESULTS
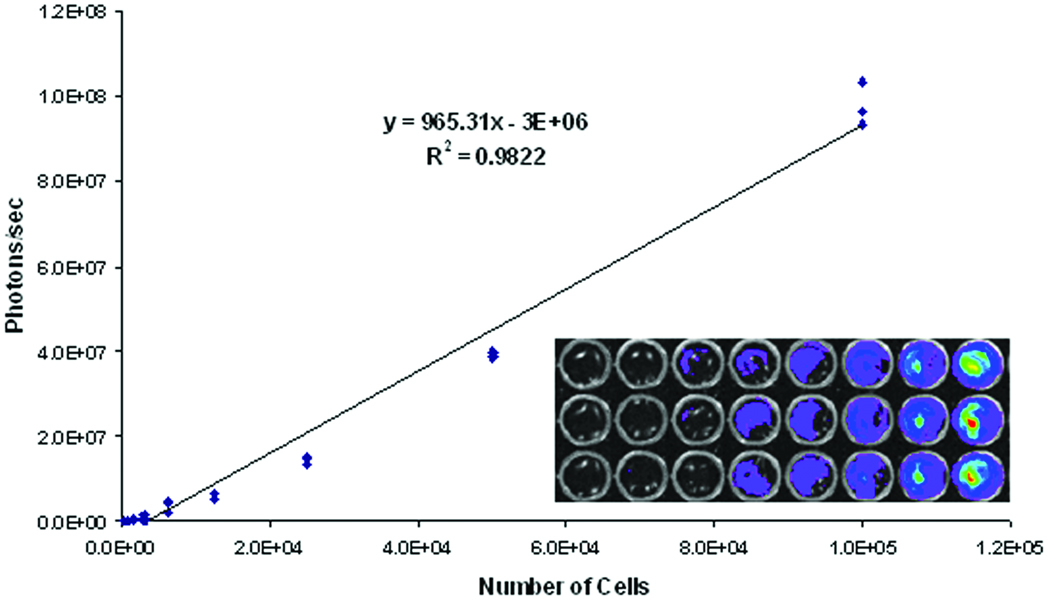
BLI and quantitative analysis of fibroblasts showed that an average of 459.5 ± 57.8 photons/sec/cm2 (p/s) were emitted from each cell by the firefly luciferase-luciferin reaction when the cells were initially assessed in vitro. A standard curve was generated by plating transduced fibroblasts and imaging for photon emissions. A strong correlation was shown between the number of cells plated and photons detected (Fig. 1). As few as 300 cells (5×104 p/s) per well were detected. The number of photons emitted from encapsulated fibroblasts was also assessed after placing the device in a sterile culture dish with sterile PBS and adding luciferin (Fig. 2, left panel). The number of photons observed under these conditions was 1.5×1010 p/s, which was equivalent to 1.5×107 cells.
Figure 1. Expression of luciferase by monkey fibroblasts.
Allogeneic rhesus monkey fibroblasts were transduced with a lentiviral vector expressing firefly luciferase under the control of the MND promoter. Luciferase-expressing fibroblasts were plated in a 96-well plate containing 100 mg/ml of D-luciferin and imaged for emission of photons. A strong correlation is shown between the number of cells plated and the number of photons detected.
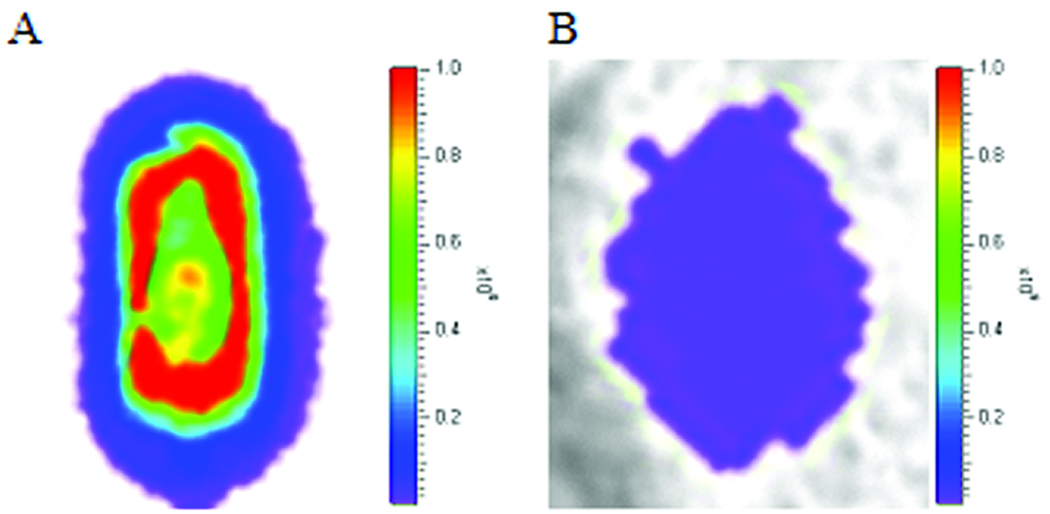
Figure 2. In vitro and in vivo BLI.
Transduced fibroblasts were encapsulated in the TheraCyte® device and imaged before (A) and after (B) SC placement. A 50-fold decline of luciferase signal was observed after insertion into the animal and as a result of attenuation.
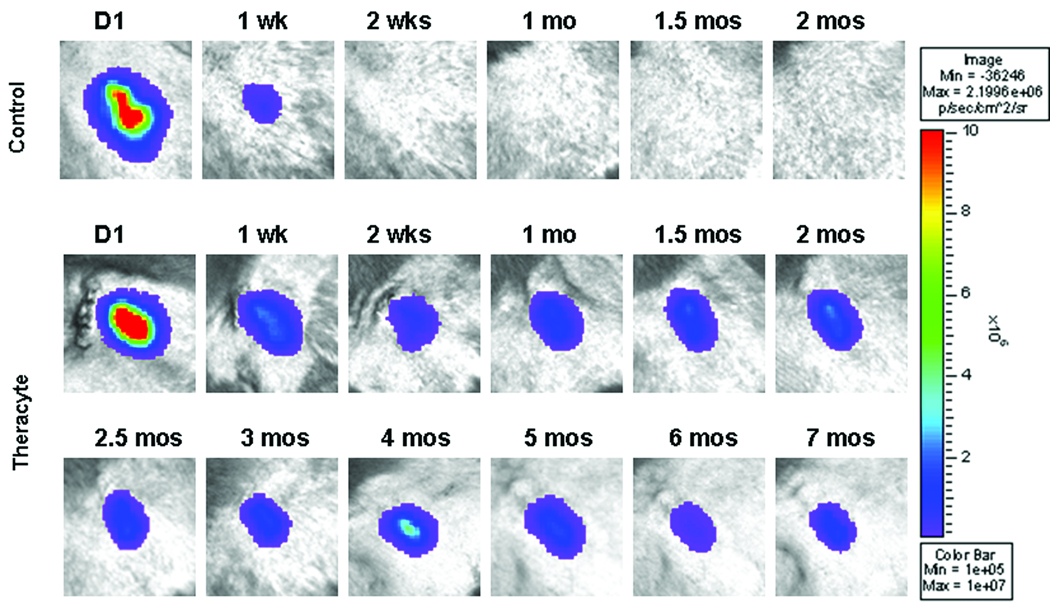
Compared to the in vitro imaging of the encapsulated cells, an approximate 50-fold decrease in the number of photons (2.8×108 p/s) was detected immediately following SC placement (Fig. 2, right panel), resulting from attenuation of light through skin (~3 mm) (10). Since cells expressing luciferase were confined within the TheraCyte® device, this outcome accurately reflects the quantity of light scattered. During the first week post-transplant, an 81.6 ± 4.8% decline in the number of photons emitted from the site was observed (Fig. 3). The level of photon emissions remained stable up to 7 months post-insertion, then the signal declined gradually to background levels by 12 months (data not shown). The Theracyte® device was removed at approximately 12 months post-insertion and immediately imaged and showed 5×106 p/s (data not shown). The additional animal transplanted with encapsulated fibroblasts also showed robust cell survival and transgene expression post-transplant up to the time points evaluated (2 months) (data not shown). There was no evidence of fibrosis at the insertion site in either of these animals. In the control animal, the number of photons declined approximately 97% at one week after insertion, with no detectable signal at 2 weeks (see Fig. 3) or thereafter.
Figure 3. In vivo BLI.
Upper panels show a control animal that received cells injected SC without encapsulation. Bottom panel shows one monkey implanted SC with the TheraCyte® device containing allogeneic skin fibroblasts expressing luciferase and imaged immediately after insertion then at 1 week, 2 weeks, and monthly up to 12 months. After an initial decline in the level of photon emission stable levels of photons/sec (p/s) were observed for 7 months.
DISCUSSION
The long-term survival of encapsulated rhesus monkey skin fibroblasts provides evidence to support the potential to develop such techniques for cell transplantation protocols, and to use in vivo imaging to monitor cell survival. While the ability of the device to provide xenograft protection has been controversial (6–8, 12), the semi-permeable membrane of the TheraCyte® device was shown in this study to be very effective in protecting encapsulated cells from host immune responses. Lee et al. (13) also recently demonstrated that the TheraCyte® device protects murine β-cells from both allo- and auto-immune rejection. Further, in studies in NOD/SCID mice, encapsulated human β-cell progenitors survived, differentiated, and functioned to ameliorate diabetes.
In the study described here, BLI of macroencapsulated fibroblasts expressing luciferase was performed one week post-transplantation and revealed a decline in the number of photons. This may represent a downregulation of luciferase expression or cell loss (13). It is important to note that transduced fibroblasts used in this study were at the log phase of growth at the time of encapsulation and when the photon emission measurements were collected. Upon insertion, cells can undergo contact inhibition and may downregulate a number of genes including luciferase. Zou et al. (14) reported a similar loss of reporter gene activity by contact inhibition in NIH3T3 cells.
These preliminary studies have demonstrated that TheraCyte® encapsulation can support the survival of allogeneic nonhuman primate fibroblasts, protecting the cells from rejection. These findings have important implications for the use of such technology for clinical-based therapies such as those proposed for diabetes and other similar congenital or acquired diseases, and might also be useful for studies focused on cell loss in other allogeneic cell therapy settings (e.g., stem and progenitor cells, T cells, NK cells). This study also supports the usefulness of in vivo imaging and preclinical studies for monitoring the viability of encapsulated cells over time.
ACKNOWLEDGEMENTS
The authors thank Christine Mall for expert technical assistance. This study was supported by the National Institutes of Health (NIH) Center of Excellence in Translational Human Stem Cell Research (grant #HL069748) (A.F.T), the California National Primate Research Center (CNPRC) base operating grant (grant #RR00169) (A.F.T.), and the Juvenile Diabetes Research Foundation (P.I.A.).
ABBREVIATIONS
- MND
Myeloproliferative sarcoma virus enhancer, negative control region deleted
REFERENCES
- 1.Lekakis L, De Padua Silva L, de Lima M. Novel preparative regimens in hematopoietic stem cell transplantation. Curr Pharm Des. 2008;14:1923. doi: 10.2174/138161208785061409. [DOI] [PubMed] [Google Scholar]
- 2.Soiffer RJ. Immune modulation and chronic graft-versus-host disease. Bone Marrow Transplant. 2008;42:S66. doi: 10.1038/bmt.2008.119. [DOI] [PubMed] [Google Scholar]
- 3.Wilson JT, Chaikof EL. Challenges and emerging technologies in the immunoisolation of cells and tissues. Adv Drug Deliv Rev. 2008;60:124. doi: 10.1016/j.addr.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson JT, Cul W, Sun X-L, Tucker-Burden C, Weber CJ, Chaikof EL. In vivo biocompatibility and stability of a substrate-supported polymerizable membrane-mimetic film. Biomaterials. 2007;28:609. doi: 10.1016/j.biomaterials.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Binette TM, Seeberger KL, Lyon JG, Rajotte RV, Korbutt GS. Porcine endogenous retroviral nucleic acid in peripheral tissues is associated with migration of porcine cells post islet transplant. Am J Transplant. 2004;4:1051. doi: 10.1111/j.1600-6143.2004.00460.x. [DOI] [PubMed] [Google Scholar]
- 6.Garkavenko O, Emerich DF, Muzina M, et al. Xenotransplantation of neonatal porcine liver cells. Transplant Proc. 2005;27:477. doi: 10.1016/j.transproceed.2004.12.183. [DOI] [PubMed] [Google Scholar]
- 7.Elliott RB, Escobar L, Calafiore R, Basta G, Garkavenko O, Vasconcellos A, Bambra C. Transplantation of micro- and macroencapsulated piglet islets into mice and monkeys. Transplant Proc. 2005;37:466. doi: 10.1016/j.transproceed.2004.12.198. [DOI] [PubMed] [Google Scholar]
- 8.Sörenby AK, Kumagai-Braesch M, Sharma A, et al. Preimplantation of an immunoprotective device can lower the curative dose of islets to that of free islet transplantation-Studies in a rodent model. Transplantation. 2008;86:364. doi: 10.1097/TP.0b013e31817efc78. [DOI] [PubMed] [Google Scholar]
- 9.Bontrop RE. Non-human primates: Essential partners in biomedical research. Immunol Rev. 2001;183:5. doi: 10.1034/j.1600-065x.2001.1830101.x. [DOI] [PubMed] [Google Scholar]
- 10.Tarantal AF, Lee CC, Jimenez DF, Cherry SR. Fetal gene transfer using lentiviral vectors: in vivo detection of gene expression by microPET and optical imaging in fetal and infant monkeys. Hum Gene Ther. 2006;17:1254. doi: 10.1089/hum.2006.17.1254. [DOI] [PubMed] [Google Scholar]
- 11.Lee CI, Kohn DB, Ekert JE, Tarantal AF. Morphological analysis and lentiviral transduction of fetal monkey bone marrow-derived mesenchymal stem cells. Mol Ther. 2004;9:112. doi: 10.1016/j.ymthe.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Brauker J, Martinson LA, Young SK, Johnson RC. Local inflammatory response around diffusion chambers containing xenografts. Nonspecific destruction of tissues and decreased local vascularization. Transplantation. 1996;61:1671. doi: 10.1097/00007890-199606270-00002. [DOI] [PubMed] [Google Scholar]
- 13.Lee S-H, Hao E, Savinov AY, Geron I, Strongin AY, Itkin-Ansari P. Human β-cell precursors mature into functional insulin-producing cells in an immunoisolation device: implications for diabetes cell therapies. Transplantation. doi: 10.1097/TP.0b013e31819c86ea. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou Y, Mirbaha F, Stastny P. Contact inhibition causes strong downregulation of expression of MICA in human fibroblasts and decreased NK cell killing. Hum Immunol. 2006;67:183. doi: 10.1016/j.humimm.2006.02.018. [DOI] [PubMed] [Google Scholar]