Introduction
“This is not the end. It’s not even the beginning of the end. But it is, perhaps, the end of the beginning.”
Sir Winston Churchill
Immune reconstitution after hematopoietic stem cell transplantation (HSCT) has been well studied in mouse and man and the relationship between delayed immune reconstitution and post-transplant morbidity and mortality from infections and relapse has been well documented (1). Whereas erythroid, myeloid and platelet reconstitution occurs in most cases in the first weeks after HSCT and is primarily determined by engraftment of donor HSCs, B cell and especially T cell reconstitution takes much longer and recipients of an allogeneic HSCT can have an impaired T and B cell function even years after their HSCT (2). In recent years a number of strategies to enhance post-transplant immune reconstitution have been developed and successfully tested in preclinical models. These studies in experimental models mark “the end of the beginning” and in this chapter we will discuss the first attempts to introduce these strategies in clinical trials with HSCT recipients.
Notch-mediated Regulation of Hematopoiesis
Irwin D. Bernstein, Colleen Delaney, Mari Dallas, Shelly Heimfeld and Barbara Varnum-Finney. Fred Hutchinson Cancer Research Center, Seattle, Washington.
The factors governing hematopoietic stem/progenitor cells are not well understood, but are thought to result from an interplay between soluble factors such as cytokines, and intercellular interactions, including those with marrow stroma. These latter interactions involve a number of evolutionary conserved pathways known to govern cell fate decisions in a wide variety of developing systems, such as the Notch pathway. Studies of hematopoiesis have revealed a role for Notch signalling in regulating early lymphoid development, specifically in regulating T vs. B cell-fate decisions (3, 4). Although the physiologic role of Notch signalling in regulating hematopoietic stem cell fate decisions in vivo has been controversial, in vitro studies have shown that enforced expression of the constitutively active intracellular domain of Notch1 in murine hematopoietic stem cells (HSCs) induces inhibition of myeloid differentiation, promotion of early T cell differentiation and enhanced generation of marrow repopulating cells (5). Furthermore, activation of endogenous Notch receptors using an immobilized, engineered form of the Notch ligand Delta,1 (Delta1ext-IgG) in non-mutant murine stem cells also profoundly affected differentiation in a cytokine context-dependent manner, including the promotion of early T cell differentiation and generation of a multi-log increase in the number of precursors with short-term lymphoid and myeloid repopulating ability (6). These findings suggest a potential role for Notch signaling in regulating stem cell growth and differentiation, and further suggest that compensatory effects, in vivo such as those mediated by cytokines may obscure such effects.
In this review, we will further address: 1) the role of Notch signaling in hematopoietic stem/progenitor cell self-renewal and differentiation in vivo, including during non-homeostatic states; 2) mechanisms whereby Notch signaling can mediate different cellular fates. These studies will address how quantitative and qualitative differences in ligand type and density lead to differential activation of specific Notch receptors, in turn differentially activating target genes and self-renewal vs. differentiation pathways. We will also address 3) the potential for exploiting the profound effects of Notch signaling ex vivo to generate hematopoietic stem/progenitor cells to improve clinical stem cell transplantation. In these studies of human cord blood precursor cells, culture with Delta1ext-IgG led to an approximate 15-fold increase in the SCID repopoulating cell frequency and resulted in more rapid engraftment compared to non-cultured cells. Based on these preclinical studies demonstrating enhanced early engraftment of Delta-cultured cells, we have developed clinically relevant methods for expanding cord blood repopulating cells using Notch ligand (7, 8). The potential efficacy of these expanded progenitors is currently being tested in a Phase I clinical trial, in which patients receive cord blood progenitors that were cultured with Delta1ext-IgG and a second, non-cultured cord blood unit. Promising results from initial patients suggesting the ability to overcome the delayed engraftment often encountered in patients undergoing cord blood transplantation will be discussed.
Enhanced post-transplant T cell reconstitution with Interleukin-7, Keratinocyte Growth Factor and Lymphoid Precursors
Gabrielle L. Goldberg, Johannes L. Zakrzewski, and Marcel R.M. van den Brink. Memorial Sloan-Kettering Cancer Center, New York, New York.
Interleukin-7
IL-7 is produced by enterocytes and keratinocytes as well as thymic and bone marrow stromal cells. It is a 25kD glycoprotein that binds IL-7 receptor (IL-7R) which comprises the common cytokine receptor γ-chain (γc) and the IL-7R α-chain (also known as CD127). IL-7R is expressed by a variety of hematopoietic cells including: common lymphoid precursors, developing B cells, triple negative and single positive thymocytes, thymic dendritic cells, both CD4+ and CD8+ peripheral T cells, γδ T cells and monocytes. It is also expressed on nonhematopoietic cells including keratinocytes and intestinal epithelial cells (reviewed in (9)).
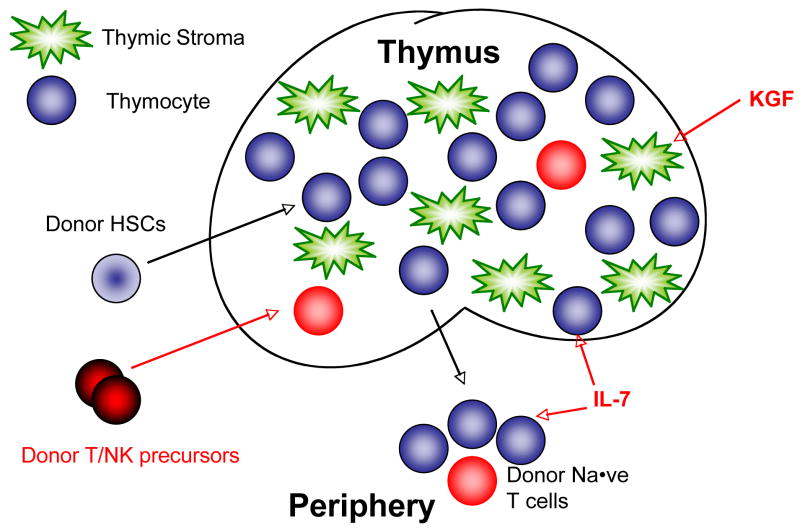
Both T and B cell development in mice require IL-7 but IL-7 is not essential for normal B cell development in humans. A defect in IL7Rα leads to severe combined immunodeficiency syndrome (SCID) and a complete lack of T cells (10). In mouse models of bone marrow transplantation, IL-7 augments T cell reconstitution in syngeneic and allogeneic recipients. This is due to improved thymopoiesis and increased homeostatic proliferation of mature T cells as well as decreased apoptosis of peripheral T-cells (Figure 1). Following IL-7 administration, T cell function is enhanced but T cell repertoire remained unaffected (11). Several studies have demonstrated that graft-versus-host-disease (GVHD) is not exacerbated by IL-7 administration. However, at high doses and with prolonged administration IL-7 may exacerbate GVHD when given in a T cell replete setting (12, 13). Finally, graft-vs-tumor (GVT) remains intact following IL-7 adminstration and allogeneic BMT (11).
Figure 1.
IL-7, KGF and Donor T/NK precursors enhance post-transplant T cell reconstitution
Phase 1 clinical trials have been performed with CYT 99 007 (Cytheris, Inc.), a recombinant non-glycosylated form of human IL-7. Patients have been treated, in dose-escalation trials, with subcutaneous CYT 99 007 in a dose range varying from 3 to 60 mcg/kg/dose (R. Buffet, personal communication). Toxicity effects were minimal and immunological efficacy was confirmed in these studies. Following repeated doses of CYT 99 007, naïve and memory CD4+ and CD8+ T cells were elevated, as were recent thymic emigrants (RTEs) (14). As defined by Ki67 and BCL-2, proliferation and survival of peripheral T cells were also enhanced with IL-7 administration. T cell expansion appeared to be more profound in those patients that had lower baseline T cell counts. This finding is consistent with the role of IL-7 in homeostatic expansion. Further studies with a less immunogenic and appropriately glycosylated form of IL-7 (CYT107, Cytheris) are underway.
Keratinocyte Growth Factor
Keratinocyte Growth Factor (KGF), also know as fibroblast growth factor 7 (FGF7), is primarily produced by cells of mesenchymal origin and is an epithelial mitogen (summarized in (15)). In the thymus, fibroblasts are the major source of KGF (16). Thymocyte subsets also produce KGF, and their production increases as the thymocytes mature (17). FGFR2IIIb is the only known receptor for KGF and is expressed primarily by epithelial cells (15). In the thymus, FGFR2IIIb is expressed on epithelial cells but not on cells of hematopoietic origin (18). KGF is approved by the FDA for the prophylaxis of oral mucositis in patients receiving high-dose therapy, including myeloablative conditioning associated with HSCT.
KGF administration prior to cyclophosphamide, irradiation or dexamethasone increases thymic cellularity in young and old mice and peripheral T cell numbers in aged mice (19). Using KGF −/− mice as donors or hosts in transplant experiments reveals that the KGF required for post-irradiation thymic recovery is produced by host non-haematopoietic cells. Thymopoiesis and peripheral T cell reconstitution are enhanced following KGF pretreatment of recipients prior to syngeneic and allogeneic HSCT (19, 20) (Figure 1). Furthermore, KGF administration decreases GVHD morbidity and mortality in allogeneic recipients (18). Recent primate studies revealed that KGF improved thymic-dependent T cell reconstitution in rhesus monkeys following transplantation (21). Thymic architecture was restored, thymic output increased and naïve T cell numbers elevated in the KGF-treated group. The enhanced T cell reconstitution translated to an increase in peripheral T cell function (21).
The above preclinical studies suggest that KGF administration enhances T cell reconstitution. Clinical studies are currently underway in allogeneic HSCT patients to assess whether KGF administration will enhance T cell recovery in patients receiving an allograft consisting of CD34+ hematopoietic stem cells.
Lymphoid precursors
As mentioned above, Notch signaling is involved in a variety of fetal and adult cellular differentiation processes and lineage decisions required for the development of a multi-cellular organism (22). Activation of the Notch1 pathway promotes T lineage commitment of hematopoietic progenitors while blocking B cell development and has been implicated in several critical steps during T cell differentiation including commitment to αβ versus γδ T cell lineage, Th1 versus Th2 cell differentiation, and development of regulatory T cells. Culture systems utilizing Notch1 signaling can be used for the in vitro development of T lineage cells at various differentiation stages. These systems include Delta1ex-IgGas described above and the OP9-DL1 system, which uses a mouse bone marrow stromal cell line transduced to express the Notch1 ligand Delta-like 1 (DL1) to coculture hematopoietic stem cells in the presence of IL-7 and FLT3-ligand (23). This system can be modified for the generation of large numbers of lymphoid progenitors committed to the T lineage for adoptive immunotherapy. We recently demonstrated that co-transplanted allogeneic OP9-DL1 derived early T progenitors can mature in an immunosuppressed host, mediating immunity including anti-tumor activity without causing GVHD (24), and can even be transferred in the absence of allogeneic stem cells to any immunosuppressed individual irrespective of MHC disparities for adoptive ‘off-the-shelf’ immunotherapy (unpublished data by van den Brink and coworkers). Such cells also protect the thymus from atrophy which otherwise hinders future lymphopoiesis, and can be genetically modified in vitro for targeted immunotherapy. Notch-based culture systems show promise for clinically applicable therapeutic use. Human cord blood and human adult BM derived CD34+ progenitor cells have been cultured in these systems to generate human T cells (20).
Manipulation of the thymus and bone marrow to improve engraftment and restoration of immune competence in autologous and allogeneic HSCT
Richard Boyd, Jayne Sutherland, Tracy Heng, Gabby Goldberg, Daniel Gray, Anne Fletcher, Natalie Seach and Ann Chidgey. Monash University, Clayton, Victoria, Australia.
Paradoxical to its importance in developing immunity and preventing disease, the thymus also undergoes profound age-related degeneration as a consequence of the natural aging process, beginning early in life but becoming more prominent post-puberty. This subsequently results in over 90% loss of thymus output from ~30 years of age and is the primary cause of increased levels of diseases including cancer and severe infections in the aged. Ironically, with aging there is also a steep increase in immune dysfunction such as autoimmune diseases (eg multiple sclerosis, lupus, rheumatoid arthritis, scleroderma). The consequences of thymic atrophy become even more paramount following immune insult such as HIV and the standard cancer treatments of chemotherapy and radiation therapy. While children can recover effective immunity by 6 months after treatment, adults rarely attain new naïve T cells and even this minor level can require several years. In addition it is clear that the bone marrow also declines in function with age. The collective impact of a highly compromised thymus and bone marrow is manifest as high susceptibility to infection of adults undergoing standard of care cancer treatments particularly myeloablative chemotherapy and HSCT. To be able to reverse in particular the thymus aging process, and also that of the bone marrow, thus represents one of the great challenges of modern medicine; it would have a major impact in the treatment of some of the most important diseases in the clinic.
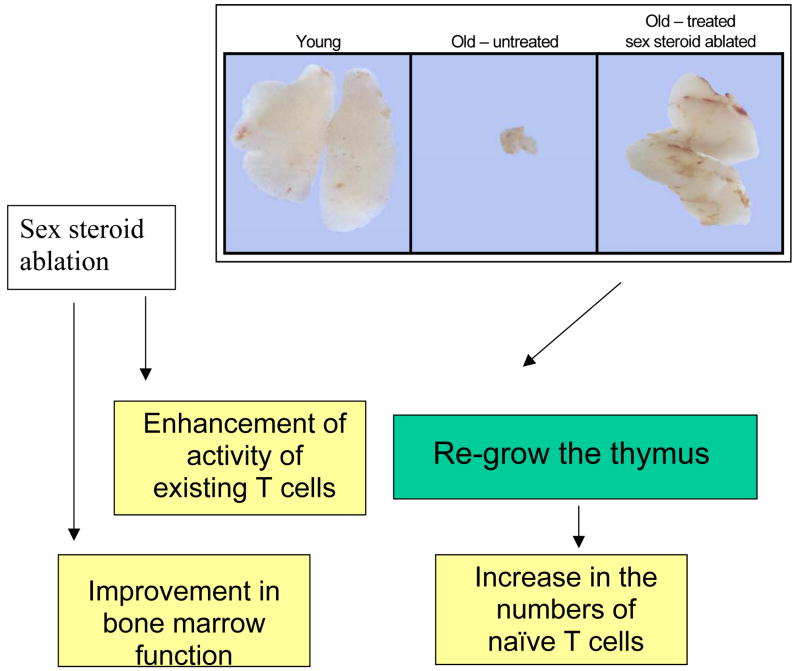
Our research over the past 3 decades has focussed on how the thymus develops, functions and degenerates. Recognizing that steroids are the natural enemy of the immune system, we are now able to reverse the natural thymus aging process (Figure 2). This allows the bodies own endogenous recuperative mechanisms to be reactivated. Accordingly, we have shown that inhibition of sex steroids results in a profound rejuvenation of thymus function with extensive replenishment of the T cell pool. This can be achieved in males and females by surgical or biochemical (LHRH/GnRH) means. The latter hormone treatment is fully reversible and is currently used as standard of care in sex hormone exacerbated conditions such as prostate and breast cancer. The intrathymic thymic events are first manifest as an increase in the TN populations including enhance proliferation of the early thymic progenitor (ETP) subset by day 5-post castration (25). Subsequently all thymocyte subpopulations increase numerically, including the mature SPs in the medulla, but in a homeostatically controlled manner with no major proportional changes within the subsets. Thymic rejuvenation is not restricted to the T cell compartment. We have shown by gene chip analysis that thymic stromal cells undergo marked alterations in expression profiles within 24 hours. Thymic epithelial cells (TEC) begin to upregulate MHC class II (which is decreased with aging) by 5 days. The TEC proliferate by day 7 -10, marginally lagging behind the T cells. There is also a proportional increase in medullary thymic epithelial cells (mTEC) again reversing changes consequential to aging (26). The cellular basis to the TEC recovery is unclear but collective evidence suggest cells expressing low levels of MHC class II and the cortical (cTEC) and mTEC markers Ly51/6C3 and UEA-1 respectively, maybe of precursor potential. The increased thymopoiesis is reflected in renewed export of naïve T cells to the periphery, reversing the decline in CD4:CD8 ratio with age and restoring full immune competence of the aged animal to viral and resistance to transplantable tumours, to levels equivalent to the young (27).
Figure 2.
Sex steroids are key to the degradation of the adult immune system and the immune system can be rejuvenated after re-growth of the thymus
A fundamental feature of the thymic recovery was the input of bone marrow derived progenitor cells. Sex steroid ablation increased the levels of bone marrow HSC and CLP and reversed the age related bias towards myelopoiesis, and improved the status of bone marrow stromal cells forming the endosteal and vascular niches. One important consequence of the improved bone marrow and thymus was the enhanced recovery of the immune system following ablation by chemotherapy and radiotherapy. It also greatly enhanced the recovery from myeloablative conditioning and the efficacy of autologous and allogeneic HSCT (28, 29). The clinical applicability of LHRH therapy to address immune deficiency was shown in prostate cancer patients who had strong evidence of renewed thymic output 4 months after onset of LHRH agonist therapy.
Collectively the preclinical data and that from the prostate cancer patients formed the basis to the protocol for a pilot phase II clinical trial on patients with haematological malignancies (acute leukaemia, chronic leukemia, lymphoma, multiple myeloma) or non-malignancies myelodysplasia (1 patient) and aplastic anemia (4 patients). Eighty patients in total (40 control, 40 LHRH treated, non-randomised), male and female, aged 17– 69 were included in the trial. LHRH was given over 4 months beginning approximately 3 weeks prior to transplant, timed to induce castration levels of sex steroids at the time of transplant. Patients were monitored for 12 months. LHRH increased HSC engraftment (day 9 for allogeneic and day 10 for autologous) in both transplant groups but the most significant finding was the restoration of naïve CD4+ T cell production from ~6 months in the allogeneic group and 9–12 months in the autologous group. This involved a full spectrum of T cell spectratypes. The increase in naïve T cells was demonstrated phenotypically and confirmed by TREC analysis.
This provides a fundamentally new approach to overcoming the immunodeficiency caused by thymic atrophy in adults requiring HSCT and has immediate applications to the development of new therapies aimed at improving vaccines to infections and cancer, AIDS, autoimmune diseases and transplantation tolerance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peggs KS. Immune reconstitution following stem cell transplantation. Leukemia & lymphoma. 2004;45:1093–1101. doi: 10.1080/10428190310001641260. [DOI] [PubMed] [Google Scholar]
- 2.Storek J, Gooley T, Witherspoon RP, Sullivan KM, Storb R. Infectious morbidity in long-term survivors of allogeneic marrow transplantation is associated with low CD4 T cell counts. Am J Hematol. 1997;54:131–138. doi: 10.1002/(sici)1096-8652(199702)54:2<131::aid-ajh6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 3.Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S, Pear WS, Bernstein ID. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nature medicine. 2000;6:1278–1281. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- 4.Dallas MH, Varnum-Finney B, Delaney C, Kato K, Bernstein ID. Density of the Notch ligand Delta1 determines generation of B and T cell precursors from hematopoietic stem cells. The Journal of experimental medicine. 2005;201:1361–1366. doi: 10.1084/jem.20042450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohishi K, Varnum-Finney B, Bernstein ID. Delta-1 enhances marrow and thymus repopulating ability of human CD34(+)CD38(−) cord blood cells. J Clin Invest. 2002;110:1165–1174. doi: 10.1172/JCI16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varnum-Finney B, Brashem-Stein C, Bernstein ID. Combined effects of Notch signaling and cytokines induce a multiple log increase in precursors with lymphoid and myeloid reconstituting ability. Blood. 2003;101:1784–1789. doi: 10.1182/blood-2002-06-1862. [DOI] [PubMed] [Google Scholar]
- 7.Delaney C, Varnum-Finney B, Aoyama K, Brashem-Stein C, Bernstein ID. Dose-dependent effects of the Notch ligand Delta1 on ex vivo differentiation and in vivo marrow repopulating ability of cord blood cells. Blood. 2005;106:2693–2699. doi: 10.1182/blood-2005-03-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dallas MH, Varnum-Finney B, Martin PJ, Bernstein ID. Enhanced T-cell reconstitution by hematopoietic progenitors expanded ex vivo using the Notch ligand Delta1. Blood. 2007;109:3579–3587. doi: 10.1182/blood-2006-08-039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alpdogan O, van den Brink MR. IL-7 and IL-15: therapeutic cytokines for immunodeficiency. Trends Immunol. 2005;26:56–64. doi: 10.1016/j.it.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 11.Alpdogan O, Schmaltz C, Muriglan SJ, Kappel BJ, Perales MA, Rotolo JA, Halm JA, Rich BE, van den Brink MR. Administration of interleukin-7 after allogeneic bone marrow transplantation improves immune reconstitution without aggravating graft-versus-host disease. Blood. 2001;98:2256–2265. doi: 10.1182/blood.v98.7.2256. [DOI] [PubMed] [Google Scholar]
- 12.Gendelman M, Hecht T, Logan B, Vodanovic-Jankovic S, Komorowski R, Drobyski WR. Host conditioning is a primary determinant in modulating the effect of IL-7 on murine graft-versus-host disease. J Immunol. 2004;172:3328–3336. doi: 10.4049/jimmunol.172.5.3328. [DOI] [PubMed] [Google Scholar]
- 13.Sinha ML, Fry TJ, Fowler DH, Miller G, Mackall CL. Interleukin 7 worsens graft-versus-host disease. Blood. 2002;100:2642–2649. doi: 10.1182/blood-2002-04-1082. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg SA, Sportes C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, Stetler-Stevenson M, Morton KE, Mavroukakis SA, Morre M, Buffet R, Mackall CL, Gress RE. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006;29:313–319. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finch PW, Rubin JS. Keratinocyte growth factor/fibroblast growth factor 7, a homeostatic factor with therapeutic potential for epithelial protection and repair. Adv Cancer Res. 2004;91:69–136. doi: 10.1016/S0065-230X(04)91003-2. [DOI] [PubMed] [Google Scholar]
- 16.Gray DH, Tull D, Ueno T, Seach N, Classon BJ, Chidgey A, McConville MJ, Boyd RL. A unique thymic fibroblast population revealed by the monoclonal antibody MTS-15. J Immunol. 2007;178:4956–4965. doi: 10.4049/jimmunol.178.8.4956. [DOI] [PubMed] [Google Scholar]
- 17.Erickson M, Morkowski S, Lehar S, Gillard G, Beers C, Dooley J, Rubin JS, Rudensky A, Farr AG. Regulation of thymic epithelium by keratinocyte growth factor. Blood. 2002;100:3269–3278. doi: 10.1182/blood-2002-04-1036. [DOI] [PubMed] [Google Scholar]
- 18.Rossi S, Blazar BR, Farrell CL, Danilenko DM, Lacey DL, Weinberg KI, Krenger W, Hollander GA. Keratinocyte growth factor preserves normal thymopoiesis and thymic microenvironment during experimental graft-versus-host disease. Blood. 2002;100:682–691. doi: 10.1182/blood.v100.2.682. [DOI] [PubMed] [Google Scholar]
- 19.Alpdogan O, V, Hubbard M, Smith OM, Patel N, Lu S, Goldberg GL, Gray DH, Feinman J, Kochman AA, Eng JM, Suh D, Muriglan SJ, Boyd RL, van den Brink MR. Keratinocyte growth factor (KGF) is required for postnatal thymic regeneration. Blood. 2006;107:2453–2460. doi: 10.1182/blood-2005-07-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Min D, Taylor PA, Panoskaltsis-Mortari A, Chung B, Danilenko DM, Farrell C, Lacey DL, Blazar BR, Weinberg KI. Protection from thymic epithelial cell injury by keratinocyte growth factor: a new approach to improve thymic and peripheral T-cell reconstitution after bone marrow transplantation. Blood. 2002;99:4592–4600. doi: 10.1182/blood.v99.12.4592. [DOI] [PubMed] [Google Scholar]
- 21.Seggewiss R, Lore K, Guenaga FJ, Pittaluga S, Mattapallil J, Chow CK, Koup RA, Camphausen K, Nason MC, Meier-Schellersheim M, Donahue RE, Blazar BR, Dunbar CE, Douek DC. Keratinocyte growth factor augments immune reconstitution after autologous hematopoietic progenitor cell transplantation in rhesus macaques. Blood. 2007;110:441–449. doi: 10.1182/blood-2006-12-065623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radtke F, Wilson A, Mancini SJ, MacDonald HR. Notch regulation of lymphocyte development and function. Nat Immunol. 2004;5:247–253. doi: 10.1038/ni1045. [DOI] [PubMed] [Google Scholar]
- 23.Schmitt TM, Z-PJ Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 24.Zakrzewski JL, Kochman AA, Lu SX, Terwey TH, Kim TD, Hubbard VM, Muriglan SJ, Suh D, Smith OM, Grubin J, Patel N, Chow A, Cabrera-Perez J, Radhakrishnan R, Diab A, Perales MA, Rizzuto G, Menet E, Pamer EG, Heller G, Zuniga-Pflucker JC, Alpdogan O, van den Brink MR. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med. 2006;12:1039–1047. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 25.Heng TS, Goldberg GL, Gray DH, Sutherland JS, Chidgey AP, Boyd RL. Effects of castration on thymocyte development in two different models of thymic involution. J Immunol. 2005;175:2982–2993. doi: 10.4049/jimmunol.175.5.2982. [DOI] [PubMed] [Google Scholar]
- 26.Gray DH, Seach N, Ueno T, Milton MK, Liston A, Lew AM, Goodnow CC, Boyd RL. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood. 2006;108:3777–3785. doi: 10.1182/blood-2006-02-004531. [DOI] [PubMed] [Google Scholar]
- 27.Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, Blazar BR, Millar JL, Malin MA, Chidgey AP, Boyd RL. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175:2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg GL, Sutherland JS, Hammet MV, Milton MK, Heng TS, Chidgey AP, Boyd RL. Sex steroid ablation enhances lymphoid recovery following autologous hematopoietic stem cell transplantation. Transplantation. 2005;80:1604–1613. doi: 10.1097/01.tp.0000183962.64777.da. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg GL, Alpdogan O, Muriglan SJ, Hammett MV, Milton MK, Eng JM, Hubbard VM, Kochman A, Willis LM, Greenberg AS, Tjoe KH, Sutherland JS, Chidgey A, van den Brink MR, Boyd RL. Enhanced Immune Reconstitution by Sex Steroid Ablation following Allogeneic Hemopoietic Stem Cell Transplantation. J Immunol. 2007;178:7473–7484. doi: 10.4049/jimmunol.178.11.7473. [DOI] [PubMed] [Google Scholar]