Abstract
Purpose
National Cancer Institute of Canada Clinical Trials Group JBR.10 demonstrated that adjuvant vinorelbine and cisplatin after resection of stage IB-II non–small-cell lung cancer (NSCLC) improved relapse-free and overall survival. However, many patients either are not referred for chemotherapy or decline treatment. To aid in treatment decision making, quality-adjusted survival estimates of the JBR.10 trial were derived using a quality-adjusted time without symptoms or toxicity (Q-TWiST) analysis.
Methods
Survival curves for treatment (N = 242) and observation groups (N = 240) were partitioned into three health states: time with ≥ grade 2 (early or late) chemotherapy-related toxicity (TOX), time in relapse (REL), and time without toxicity or relapse (TWiST). Q-TWiST = uTOX × TOX + uTWiST × TWIST + uREL × REL, where weights uTOX, uTWIST, and uREL range from 0 to 1. Threshold utility analysis was performed to test the sensitivity of the results to changes in the weights. Weights were derived in an exploratory fashion using different methods. Methods included use of arbitrary values, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30) quality-of-life data prospectively collected in JBR.10 (global assessment questions and symptom-based questions), and lastly weights European Quality of Life–Five Dimensions questionnaire collected from early-stage NSCLC (nontrial) patients after resection with discounting for toxicity and relapse. The α level was .05.
Results
Threshold utility analysis revealed that adjuvant chemotherapy was preferred for all possible weight values for relapse and toxicity (uREL, uTOX), although the result was not always statistically significant. The adjuvant chemotherapy group had better Q-TWiST in the range of 5 to 6 additional months, which was statistically significant using all methods.
Conclusion
Adjuvant chemotherapy in early-stage NSCLC improves quality-adjusted survival despite chemotherapy toxicity.
INTRODUCTION
Lung cancer remains a leading cause of cancer-related mortality.1 Until recently, early stage non–small-cell lung cancer (NSCLC) was managed with complete resection followed by observation alone, as randomized trials comparing chemotherapy to observation had failed to demonstrate significant benefit from adjuvant chemotherapy.2 Possible reasons include underpowered trials and poor compliance with postoperative chemotherapy regimens,3 as well as the use of less active chemotherapeutic agents.
One of the first trials to show a significant survival benefit was the National Cancer Institute of Canada Clinical Trial Group (NCIC CTG) JBR.10 study that was conducted in collaboration with United States (US) National Cancer Institute Intergroup partners (Southwest Oncology Group, Eastern Cooperative Oncology Group, and Cancer and Leukemia Group B). This trial showed an absolute improvement in survival of 15% at 5 years in patients with stage IB and stage II NSCLC who were treated postoperatively with vinorelbine and cisplatin chemotherapy.4 The European Adjuvant Navelbine International Trialist Association (ANITA) trial also showed an 8% improvement in 5-year survival with adjuvant vinorelbine and cisplatin in resected stage I to III NSCLC.5 Based on these trials, the American Society of Clinical Oncology (ASCO) guidelines recommend adjuvant cisplatin-based chemotherapy for patients with completely resected stages IIA, IIB, and IIIA NSCLC.6 Despite this, our recent review suggests that only two thirds of patients with completely resected NSCLC are referred for adjuvant chemotherapy,7 and less than half go on to receive chemotherapy, the most common reason being patient refusal. Patients who decline adjuvant chemotherapy are more likely to focus on the potential for severe toxicity and quality-of-life impairment associated with chemotherapy treatment, compared to those who accept adjuvant chemotherapy.8 This suggests that a proportion of patients are willing to trade-off some degree of potential survival gain for perceived better quality of life and avoidance of toxicity. It also underscores the difficulty of treatment decision making, even where the data clearly support the benefit of adjuvant therapy. An improved understanding of treatment impact on quality of life has the potential to enhance informed consent for adjuvant chemotherapy in lung cancer.
Quality-Adjusted Time Without Symptoms and Toxicity (Q-TWiST) is a measure of quality-adjusted survival that was initially developed to portray the impact of adjuvant therapy for breast cancer, where there is often a trade-off between toxicity of treatment and delayed recurrence of disease.9,10 Overall survival is partitioned into clinical health states that differ in quality of life, and the duration of each state is adjusted by a weight between 0 and 1 that corresponds to its perceived value. Then, Q-TWiST is obtained by summing up the adjusted health state durations.
We conducted a Q-TWiST analysis using the NCIC JBR.10 database to evaluate the effectiveness of adjuvant chemotherapy by considering simultaneously both quality and quantity of life during and after adjuvant vinorelbine and cisplatin chemotherapy. An assessment of the quality of life of patients in the JBR.10 was reported elsewhere.11
PATIENTS AND METHODS
Persons age 18 years or older with completely resected T2N0, T1N1, or T2N1 NSCLC with acceptable baseline characteristics and an Eastern Cooperative Oncology Group performance status of 0 or 1 were eligible to participate in the NCIC CTG JBR.10 trial.4 Patients were enrolled in the JBR.10 from July 1994 to April 2001. A total of 482 patients were randomly assigned (240 for observation and 242 for chemotherapy), of which 173 patients from the observation group and 186 from the chemotherapy group completed quality-of-life questionnaires. From these patients, 166 questionnaires were used to calculate the weight for toxicity, and 56 questionnaires were used to calculate the weight for relapse.
Health states are defined as follows: TOX, time in which the patient experiences ≥ grade 2 chemotherapy-related toxicity (Appendix V BR.10 Protocol, NCIC CTG Expanded Common Toxicity Criteria, online only); TWiST, time without relapse and without chemotherapy-related toxicity; and REL, time in relapse.
The intended chemotherapy duration was 16 weeks (cisplatin was given on days 1 and 8 every 4 weeks for four cycles, and vinorelbine was given weekly for 16 weeks). TOX was calculated as the duration starting from the date of onset of the toxicity to the date of resolution of the toxicity, as assessed during the trial. The issue of multiple toxicities was handled as follows. If any two toxicities did not overlap in time, then the individual toxicity durations were summed. If toxicities overlapped, then the duration was calculated from the start of the first toxicity until resolution of the last toxicity. Intermittent episodes of the same toxicity were treated as multiple toxicities that did not overlap in time. In calculating TOX, laboratory toxicities were excluded, as these rarely impact patient quality of life.
To estimate the mean duration of TWiST and REL, partitioned survival plots were constructed using the Kaplan- Meier product limit method to graph the transitional survival curves TOX, disease-free survival (DFS; from the day of random assignment until the date of disease recurrence or death), and overall survival (OS; from the day of random assignment to date of death). TWiST is represented by the area between Kaplan-Meier curves for DFS and TOX. The mean duration of TWiST is estimated by the difference between the mean DFS and the mean duration in TOX (TWiST = DFS – TOX). This estimate was restricted to the first 6 years of study follow-up and referred to as the restricted mean, as the median follow-up time was 5.3 years. REL is represented by the area between Kaplan-Meier curves for OS and DFS (REL = OS – DFS). The restricted mean duration of REL is estimated by the difference between mean OS and mean duration of DFS. Q-TWiST was calculated as a linear combination of the restricted mean duration of each clinical health state and its corresponding weight (eg, uTOX, uTWiST, uREL):
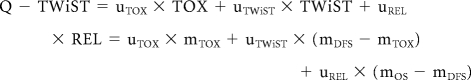 |
where mTOX, mDFS, mOS are the estimates of the restricted mean durations of TOX, DFS, and OS, respectively.
The treatment effect was estimated by subtracting the mean Q-TWiST for the chemotherapy group from the mean Q-TWiST for the observation group:
with a SE (se) of:
We tested the null hypothesis that the mean quality-adjusted survival is equal in the two treatment groups using the Z statistic:
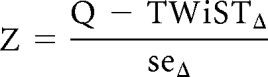 |
that follows a standard normal distribution under the null hypothesis.
The CIs were estimated using the bootstrap method. The bootstrap was done by repeatedly sampling (stratifying by stage), with replacement, from the N individuals in the trial, to obtain a new sample of size N. The restricted means for the new sample were found from the area under the Kaplan-Meier plot. This process was repeated 1,000 times. Based on the distribution of the means obtained by the bootstrap, we use the (α/2) × 1,000th value for the lower CI limit and the (1−α/2) ×1,000th value for the higher CI limit. The α level used was .05.
As Q-TWiST analyses contain some degree of uncertainty regarding the weights of the health states, a threshold utility analysis was performed to test the sensitivity of the results and conclusions to changes in the weights. Q-TWiST was calculated as uTOX and uREL are varied from 0 to 1. Contour lines were added to the threshold utility analysis to indicate the number of months of Q-TWiST gained for different values of uTOX and uREL.
Patient quality-of-life data (but not utilities or weights) were collected prospectively in the JBR.10 trial. Therefore, weights were derived in an exploratory fashion using different predefined methods to complement the threshold utility analysis. Method 1 used arbitrary values for uTOX, uREL, and uTWIST as 0.75, 0.5, and 1.0, respectively. Methods 2 and 3 derived weights based on the EORTC QLQ-C30 quality-of-life data from the JBR.10 trial. The QLQ-C30 is a self-administered, cancer-specific questionnaire that has multidimensional scales consisting of five functional domains (physical, role, emotional, cognitive, and social); three symptom domains (fatigue, nausea/vomiting, and pain); six single items for symptoms (dyspnea, sleep, appetite, constipation, diarrhea, and financial impact) and two global quality-of-life questions (overall health and overall quality of life in the past week).12 The functional and symptom domain answer scale ranges from 1 (not at all) to 4 (very much), and the global quality-of-life domain scale ranges from 1 (very poor) to 7 (excellent).
Method 2 used the two global quality-of-life assessment items (scale range, 1 to 7) and defined the weight as u = [(mean quality-of-life score − 1)/6], where the mean quality-of-life score = [(score1 + score2 + score3 +…+ scorei+…+ scoren)/n], scorei is the average score for the two global quality-of-life domain questions of the ith patient in TOX (or REL) state, and n is the number of patients in TOX (or REL) state. Method 3 used the symptom- related QLQ-C30 items (scale range, 1 to 4), and defined the weight as u = 1 – [(average quality-of-life score − 1)/3] (as the scale is out of 4, with higher scores reflecting worse symptoms), where the average quality-of-life score = [(score1 + score2 + score3 +… scorei +… + scoren)/n], scorei, is the average score for all the symptom and functional questions in the QLQ-C30 of the ith patient in TOX (or REL) state, and n is the number of patients in TOX (or REL) state.
Method 4 used prospectively collected European Quality of Life–Five Dimensions questionnarie (EQ5D) scores from 158 nontrial early-stage NSCLC patients postresection surveyed at the Princess Margaret Hospital in 2007, with discounting of 10% for uTOX and 20% for uREL. Of the 158 patients, 34 were postoperative and had not received any adjuvant chemotherapy, nine were receiving adjuvant chemotherapy, 27 were postadjuvant chemotherapy and disease free, and 88 had relapsed disease (including patients on no treatment, palliative chemotherapy, and erlotinib). The EQ5D is a simple, generic, health-related quality-of-life instrument that consists of five dimensions/questions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, and a visual analog scale for self-assessment of current general health.13 For this study, EQ5D tariffs were converted into weights using health state preference values derived in a study of a nationally representative sample of the United States population.14
Lastly, the gain function, which represents the mean number of months of Q-TWiST gained for the vinorelbine and cisplatin group compared with the observation group as a function of years from random assignment, was calculated for all four methods.
The protocol was approved by the institutional review boards at all participating institutions, and all patients provided written informed consent to participate. The research ethics board of the Princess Margaret Hospital approved the collection of data from nontrial patients in this analysis.
RESULTS
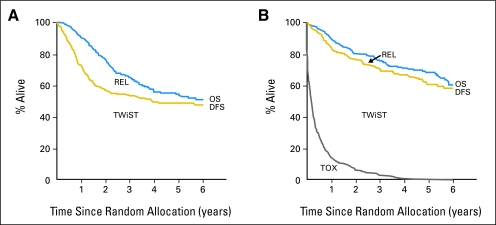
Figure 1 shows the partitioned survival curves for the observation and vinorelbine and cisplatin groups. Note that the observation group has a lower OS and does not have a TOX curve (as no chemotherapy was administered).
Fig 1.
Partitioned survival curves for (A) the observation group and (B) the vinorelbine and cisplatin group. REL, relapse; TOX, toxicity; TWiST, time without symptoms or toxicity; OS, overall survival; DFS, disease-free survival.
Weights assigned to each health state based on the four methods of deriving weights are presented in Table 1. Method 2, based on the global questions of the QLQ-C30, had the lowest weights, while method 3, derived from the symptom-related questions of the QLQ-C30, had the highest weights. Weights for REL were lower than those for TOX for all methods.
Table 1.
Arbitrary and Calculated Weights
| Method | TWIST |
Toxicity |
Relapse |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Observation |
Vinorelbine and Cisplatin |
Observation |
Vinorelbine and Cisplatin |
Observation |
Vinorelbine and Cisplatin |
|||||||
| Weight | SD | Weight | SD | Weight | SD | Weight | SD | Weight | SD | Weight | SD | |
| 1. Arbitrary values | 1 | 1 | — | — | 0.75 | 0.50 | 0.50 | |||||
| 2. Global questions QLQ-C30 | 1 | 1 | — | — | 0.57 | 0.21 | 0.50 | 0.25 | 0.50 | 0.25 | ||
| 3. Symptom-related questions QLQ-C30 | 1 | 1 | — | — | 0.86 | 0.09 | 0.83 | 0.10 | 0.83 | 0.10 | ||
| 4. EQ5D index score* | 0.75 | 0.75 | — | — | 0.68 | 0.60 | 0.60 | |||||
Abbreviations: TWIST, time without symptoms or toxicity; SD, standard deviation; QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30; EQ5D, European Quality of Life–Five Dimensions questionnaire.
Restricted means for the different health end points and Q-TWiST are presented in Table 2. After 6 years of follow-up, the difference in mean OS and DFS between the two arms was 6.8 months and 9.7 months, respectively, both in favor of the chemotherapy group. However, patients treated with chemotherapy spent 6.3 months (mean) in TOX, after 16 weeks of chemotherapy.
Table 2.
Restricted Mean Duration of Health States and Q-TWiST Values
| End Point | Duration (months) |
|||
|---|---|---|---|---|
| Treatment Group |
Difference | 95% CI | ||
| Vinorelbine and Cisplatin | Observation | |||
| Overall survival | 55.0 | 48.1 | 6.8 | 2.6 to 13.5 |
| Disease-free survival | 50.1 | 40.5 | 9.7 | 2.9 to 22.1 |
| Toxicity | 6.3 | 0.0 | 6.3 | 5.1 to 7.5 |
| TWiST | 43.8 | 40.5 | 3.4 | −3.2 to 15.8 |
| Relapse | 4.8 | 7.7 | −2.8 | −12.4 to 3.0 |
| Q-TWiST weights | ||||
| Arbitrary | 51.0 | 44.3 | 6.7 | 1.8 to 15.9 |
| From the global questions of QLQ-C30 | 49.8 | 44.3 | 5.5 | 0.7 to 14.7 |
| From symptom-related questions of QLQ-C30 | 53.3 | 46.8 | 6.4 | 2.1 to 13.7 |
| From EQ5D index score | 40.1 | 34.9 | 5.1 | 1.8 to 10.7 |
Abbreviations: Q-TWIST, quality-adjusted time without symptoms or toxicity; TWIST, time without symptoms or toxicity; QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30; EQ5D, European Quality of Life–Five Dimensions questionnaire.
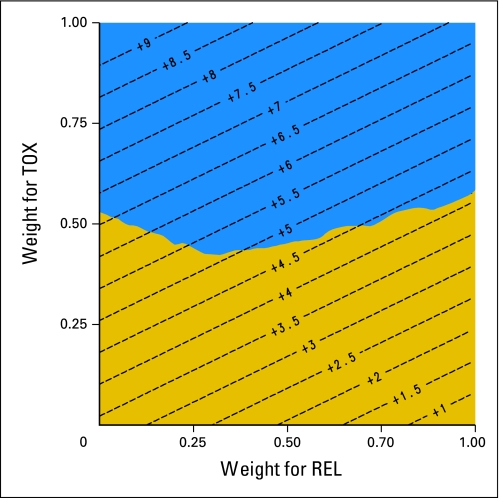
A threshold utility analysis is presented in Figure 2. The adjuvant chemotherapy arm was preferred to the observation arm for all possible weights, and was statistically significant for the majority of utox and urel pairs. The Q-TWiST estimates, regardless of the method used for calculating weights, were higher for the chemotherapy group than the observation group, consistently in the range of 5 to 6 months. The difference in Q-TWiST time favored adjuvant chemotherapy for all four methods: method 1 (arbitrary weights; P = .007), method 2 (QLQ-C30 global questions; P = .024), method 3 (QLQ-C30 symptom-related questions; P = .004), and method 4 (EQ5D Index Score; P = .003).
Fig 2.
Threshold utility analysis with a 95% CI and contour lines. The diagonal contour lines indicate the units of months gained in quality-adjusted time without symptoms or toxicity (Q-TWiST) for different weights of toxicity (TOX) and relapse (REL). The weight for TWiST is defined as uTWIST = 1. The gold area represents weights for which the improvement in Q-TWiST for vinorelbine and cisplatin is not statistically significant. The blue area represents weights for which the improvement in Q-TWiST for vinorelbine and cisplatin is statistically significant.
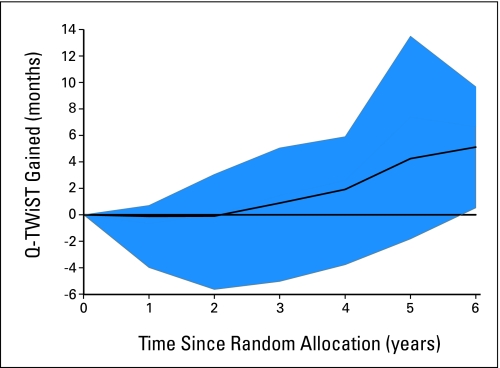
The Q-TWiST gain function for method 4 is presented in Appendix Figure A1 (online only), as all four functions were similar. For the first 2 to 3 years of follow-up, the benefit was in favor of the observation group or neutral because of the early toxic effects of chemotherapy. After 2 to 3 years, the benefits of chemotherapy increased until year 5, at which point they either plateaued or decreased depending on the weight valuation method used.
DISCUSSION
The primary analysis of the JBR.10 trial showed a significant OS benefit for adjuvant chemotherapy with a difference in median OS of 21 months (94 v 73 months). Mean survival times in each arm were 55.0 and 48.0 months for the adjuvant chemotherapy and observation arm, respectively, and using a restricted mean at 6 years of follow-up, the survival benefit was 6.8 months. However, the impact of adjuvant chemotherapy on quality of life for patients during and after treatment is more challenging to convey to patients, and to incorporate into treatment decision making. This may explain why some patients refuse chemotherapy or are not even referred for adjuvant chemotherapy.
Quality-adjusted mean survival favored the chemotherapy group by a range of 5 to 6 months, out of the total mean survival benefit of 7 months. This magnitude of benefit was consistent across the different methods used to derive weights, and is well demonstrated by the contour lines in Figure 2. It clearly establishes that the majority of survival benefit derived from adjuvant chemotherapy is time with good quality.
The valuation of weights is the most challenging aspect of defining quality-adjusted survival, as most clinical trials do not collect utility values or weights prospectively. Reasons for this include time constraints and costs, and lack of interest from sponsors and even investigators. The threshold utility analysis overcomes the inherent uncertainty associated with derivation of quality-adjusted weights. For all possible values of uTOX and uREL, adjuvant chemotherapy yielded better quality-adjusted survival than observation, although the vast majority but not all results were statistically significant. Higher quality-adjusted weight for toxicity (uTOX) is more likely to lead to statistically significant results, representing greater willingness to tolerate chemotherapy toxicity, and its potential impact on patient function.
The threshold utility analysis can also be used as a communication tool for oncologists and patients. It provides a concise visual representation, showing how differences in a patient's tolerance for, and hence valuation for, different health states will predict changes in their quality-adjusted survival. As an example, as long as the patient's valuation for TOX is at least 0.5, regardless of the patient's valuation for REL, the gain in Q-TWiST will be statistically significant. In other words, the oncologist could explain that as long as living with chemotherapy-related adverse effects, such as fatigue, nausea, and vomiting seen in the JBR.10 trial, is worth half as much as being healthy, then chemotherapy will pay off.
The four methods of deriving weights complement the threshold utility analysis by providing reasonable valuations that patients may have for the different health states. Of the four methods used to determine quality-adjusted survival, one was arbitrary, but methods 2 and 3 harnessed some aspect of the prospectively collected quality-of-life data from the NCIC JBR.10 trial. We felt that incorporating some component of the patient's real-time quality of life throughout the different stages of lung would better inform the valuation of the weight. The global questions of the QLQ-C30 used for method 2 yielded much lower utilities than the symptom-related questions. This may be due either to the additive effects of each symptom on the overall quality of life, or to certain dimensions of health that are not represented by the symptom-related questions of the QLQ-C30.
Methods 2 and 3 in this study, using linearly summed quality-of- life aggregates, are not validated methods of utility derivation, such as time trade-off or standard gamble exercises, and may underestimate true utility scores. Method 4 was used to produce weights that were closer to true utilities from a group of lung cancer patients similar to those in JBR.10. The mean weights from the EQ5D were approximately 0.75 for all groups. These values, especially for patients with relapsed disease, were surprisingly high and may reflect a higher performance status group of patients who were willing to complete the questionnaires. Because of the similar weights among groups, we used discounting as a reasonable but arbitrary alternative to calculate uTOX and uREL.
The use of the gain function may help explain the time frame of the toxicities, risks, and benefits associated with adjuvant chemotherapy. Patients who opt for chemotherapy will experience at least some toxicity of chemotherapy, and therefore for the first 2 or 3 years, they may have slightly lower quality-adjusted survival. Thus patients are paying up front in exchange for longer quality-adjusted and OS in future.
Adjuvant chemotherapy can be a difficult concept to explain to patients, as some patients will not benefit, but all will have at least some treatment-related toxicity. At the present time, despite advances in our understanding of both clinical and molecular markers of prognosis as well as the identification of some molecular markers that may be predictive of response to chemotherapy,15,16 it remains impossible to know a priori which specific individuals will benefit from therapy. It is important, therefore, that oncologists empower patients to make the most informed decisions possible. Our study shows that over the long-term, vinorelbine and cisplatin adjuvant chemotherapy not only improves OS, but also improves quality-adjusted OS. This finding should help mitigate concerns, expressed by lung cancer patients considering adjuvant chemotherapy, about the adverse impact of treatment on their quality of life. It may also serve to better inform patients in the treatment decision-making process.
Appendix
The Appendix is included in the full-text version of this article, available online at www.jco.org. It is not included in the PDF version (via Adobe® Reader®).
Fig A1.
Quality-adjusted time without symptoms or toxicity (QTWiST) gain function with method 4: European Quality of Life–Five Dimensions Index Score. The solid line gives the average months of Q-TWIST gained for the vinorelbine and cisplatin group compared with the observation group as a function of years from random assignment. The blue region shows the ranges for the Q-TWiST gain function as the weight coefficients vary between 0 and 1.
Footnotes
The JBR.10 trial was supported by the Canadian Cancer Society, the National Cancer Institute of Canada, the National Cancer Institute, the US Intergroup members (Southwest Oncology Group, Eastern Cooperative Oncology Group, and Cancer and Leukemia Group B), and GlaxoSmithKline. R.W.J. was supported by the Princess Margaret Hospital/University Health Network Medical Oncology and the University of Toronto and received an American Society of Clinical Oncology Merit Award and Novartis Oncology Young Canadian Investigator Award.
Presented in part at the 44th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2008; and the Annual Meeting of the Canadian Association of Medical Oncology, Toronto, Canada, May 1, 2008.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Frances A. Shepherd, Pierre Fabre Research Funding: Lesley Seymour, GlaxoSmithKline Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Raymond W. Jang, Andrea Bezjak, Natasha Leighl
Collection and assembly of data: Raymond W. Jang, Aurélie Le Maître, Keyue Ding, Natasha Leighl
Data analysis and interpretation: Raymond W. Jang, Aurélie Le Maître, Keyue Ding, Andrea Bezjak, Natasha Leighl
Manuscript writing: Raymond W. Jang, Aurélie Le Maître, Keyue Ding, Andrea Bezjak, Frances Shepherd, Lesley Seymour, Natasha Leighl
Final approval of manuscript: Raymond W. Jang, Aurélie Le Maître, Keyue Ding, Tim Winton, Andrea Bezjak, Lesley Seymour, Frances A. Shepherd, Natasha B. Leighl
REFERENCES
- 1.Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: A meta-analysis using updated data on individual patients from 52 randomized clinical trials. BMJ. 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 3.Alam N, Shepherd FA, Winton T, et al. Compliance with post-operative adjuvant chemotherapy in non-small cell lung cancer: An analysis of National Cancer Institute of Canada and intergroup trial JBR. 10 and a review of the literature. Lung Cancer. 2005;47:385–394. doi: 10.1016/j.lungcan.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 5.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): A randomised controlled trial. Lancet Oncol. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 6.Pisters K, Evans WK, Azzoli CG, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA respectable non-small cell lung cancer. J Clin Oncol. 2007;25:5506–5518. doi: 10.1200/JCO.2007.14.1226. [DOI] [PubMed] [Google Scholar]
- 7.Kassam F, Shepherd FA, Johnston M, et al. Referral patterns for adjuvant chemotherapy in patients with completely resected non-small cell lung cancer. J Thorac Oncol. 2007;2:39–43. doi: 10.1097/JTO.0b013e31802baff6. [DOI] [PubMed] [Google Scholar]
- 8.Howe KL, Leighl NB. Factors contributing to adjuvant therapy decisions. J Thorac Oncol. 2008;3:195–196. doi: 10.1097/JTO.0b013e318160f37d. [DOI] [PubMed] [Google Scholar]
- 9.Gelber RD, Goldhirsch A, Cole BF. Evaluation of effectiveness: Q-TWiST: The International Breast Cancer Study Group. Cancer Treat Rev. 1993;19(suppl A):73–84. doi: 10.1016/0305-7372(93)90060-5. [DOI] [PubMed] [Google Scholar]
- 10.Glasziou PP, Simes RJ, Gelber RD. Quality adjusted survival analysis. Stat Med. 1990;9:1259–1276. doi: 10.1002/sim.4780091106. [DOI] [PubMed] [Google Scholar]
- 11.Bezjak A, Lee CW, Ding K, et al. Quality-of-life outcomes for adjuvant chemotherapy in early-stage non–small-cell lung cancer: Results from a randomized trial, JBR. 10. J Clin Oncol. 2008;26:5052–5059. doi: 10.1200/JCO.2007.12.6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organisation for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 13.EuroQol Group. EuroQol: A new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 14.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: Development and testing of the D2 valuation model. Med Care. 2005;43:203–220. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Azzoli CG, Park BJ, Pao W, et al. Molecularly tailored adjuvant chemotherapy for resected non-small cell lung cancer: A time for excitement and equipoise. J Thorac Oncol. 2008;3:84–93. doi: 10.1097/JTO.0b013e31815efe24. [DOI] [PubMed] [Google Scholar]
- 16.Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]