Abstract
Purpose
To validate lysyl oxidase (LOX), a hypoxia-related protein, as a marker for metastasis in an independent head and neck cancer (HNC) patient group enrolled onto a prospective trial.
Patients and Methods
We performed traditional immunohistochemical (IHC) staining and automated quantitative analysis (AQUA) for LOX expression in 66 HNC patients from one institution. We also performed AQUA staining for LOX in 306 of 1,113 patients treated on a phase III trial comparing four radiation fractionation schedules in locally advanced HNC (RTOG 90-03). Pretreatment characteristics and outcome were similar between patients with and without LOX assessment. We correlated AQUA LOX expression with time to metastasis (TTM), time to progression (TTP), and overall survival (OS).
Results
LOX expression from both staining methods predicted for TTM in the first 66 patients. Multivariate analysis, controlling for significant parameters including nodal stage and performance status, revealed tumor LOX expression, as a continuous variable, was an independent predictor for TTM (hazard ratio [HR], 1.21; 95% CI, 1.10 to 1.33; P = .0001), TTP (HR, 1.06; 95% CI, 1.02 to 1.10; P = .0069), and OS (HR, 1.04; 95% CI, 1.00 to 1.07; P = .0311) in RTOG 90-03 patients. This translates into a 259% increase in metastatic risk for a patient at the 75th percentile of LOX compared with one at the 25th percentile.
Conclusion
AQUA LOX expression was strongly associated with increased metastasis, progression, and death in RTOG 90-03 patients. This study validates that LOX is a marker for metastasis and survival in HNC.
INTRODUCTION
Head and neck cancer (HNC) is the fifth most common cancer worldwide.1 Despite improvements in treatment techniques, the 5-year survival rate has improved marginally.1 With the introduction of aggressive concurrent chemoradiotherapy (CRT) and more refined radiation (RT) delivery techniques, the pattern of failure for these tumors has shifted with a higher rate of distant metastasis observed in recent series.2 Therefore, it would be helpful to identify patients who are at higher risk for regional and distant relapse for treatment intensification.
Hypoxia, or a reduction of the tissue oxygen tension is a common phenomenon in solid tumors. Poorly oxygenated regions develop within tumors due to the inability of the existing tumor vasculature to meet the oxygen demands of rapid tumor expansion.3 Although hypoxia has traditionally been linked to treatment resistance and a higher risk of local failure, it has also been implicated in the development of distant metastasis.4,5 Gene expression studies have shown that many metastasis-mediated genes are induced by hypoxia.4,6 Recently, we have identified lysyl oxidase (LOX), an enzyme essential for the formation of the extracellular matrix, as a hypoxia and hypoxia inducible factor-1α (HIF-1α) regulated gene.7 We showed mechanistically that LOX is essential for hypoxia-mediated metastasis by affecting the invasive properties of hypoxic human carcinomas through the regulation of focal adhesion kinase (FAK) activity and cell to matrix adhesion. More importantly, increased LOX expression was associated with increased risk of tumor dissemination in two small cohorts of node-negative, estrogen receptor–positive breast cancer and locally advanced HNC patients.7 Therefore, we hypothesize that LOX is a major factor for hypoxia-induced metastasis for HNC.
The most common method of protein quantification is immunohistochemistry (IHC); however, it has several limitations including semi-quantitative nature, subjectivity of scoring, and difficulty of determining subcellular protein localization. Automated quantitative analysis (AQUA) of protein expression has been shown to provide reproducible analysis of target signal expression in fixed tissues on a quantitative scale while preserving spatial information such as subcellular localization. The use of this technology has been successfully applied to several proteins for biomarker identification in solid tumors, including HNC.8,9 In this study, we performed both IHC and AQUA staining in a small patient group from a single institution, compared the their signal intensities and determined their prognostic significance in these patients. We then applied AQUA on samples collected from an independent cohort of HNC treated on a phase III randomized trial of the Radiation Therapy Oncology Group (RTOG)10 to validate the role of LOX as a marker of metastasis in HNC. We confirmed that LOX is an independent predictor for metastasis, progression, and overall survival, and that hypoxia is a contributing factor to poor prognosis.
PATIENTS AND METHODS
Patient Characteristics
Comparison between traditional IHC and AQUA was performed on a group of HNC derived from patients treated at Stanford University. The patient characteristics have been published elsewhere.7,11 Since the initial tissue microarray (TMA) used for LOX IHC was exhausted, a new TMA was generated for Stanford patients, using different cores within the same tumor. This new TMA was used for AQUA LOX staining.
For validation of LOX as a marker for metastasis, we used tumors from patients enrolled on RTOG 90-03.10 Between September 30, 1991, and August 1, 1997, RTOG 90-03 accrued 1,113 patients, of which 1,068 were assessable for clinical outcome. The updated long-term outcomes used for this analysis were presented at the 2005 American Society for Radiation Oncology meeting.12 Of these 1,068 patients, paraffin blocks of pretreatment tumor and/or lymph node biopsies from 430 patients were available for TMA construction and for LOX analysis and 306 patients had interpretable LOX data for outcome correlation. The lack of interpretable LOX data on the remaining 124 patients was due to either core loss or insufficient tissue. The tumors deemed insufficient for analysis had less than 10% tumor area represented on the TMA.
AQUA Staining
Automated quantitative fluorescence (AQUA analysis) was performed using the HistoRx System (HistoRx, New Haven, CT). TMA slides were processed in Dako Target Retrieval Solution (Dako Cytomation, Carpinteria, CA) using a Biocare Medical Decloaking chamber (Biocare Medical, Concord, MA) for 8 minutes. Slides were blocked in Dako Serum-Free-Protein blocking solution. Areas of tumor were labeled using a mouse anticytokeratin (AE1/AE3) antibody (Dako Cytomation) and visualized using the goat antimouse Alexa 555 SFX kit from Invitrogen (Carlsbad, CA). LOX was stained using the rabbit anti-LOX antibody combined with a Rabbit EnVision Plus DAB Kit (Dako) as described above. The signal was amplified and visualized using a TSA-CY5 tyramide amplification kit (Perkin Elmer, Waltham, MA). Slides were mounted using Vectashield 4,6-diamidino-2-phenylindole (DAPI) –containing mounting media (Vector Laboratories, Burlingame, CA).
Statistical Methods
Three end points were evaluated for patients with interpretable LOX data: time to metastases (TTM), where failure was defined as regional progression in N0 patients only, or distant progression in all patients; time to disease progression (TTP), where failure was defined as local, regional, or distant progression; and overall survival (OS), where failure was defined as death due to any cause. For TTM, patients were censored at the date of local progression, regional progression in N+ patients, second primary tumor, or death due to any cause. For TTP, patients were censored at the date of second primary tumor or death due to any cause. All event times were measured from date of random assignment to RTOG 90-03 until the date of event occurrence or censoring. Cox proportional hazards models were used to estimate the impact of LOX on the three end points outlined above after adjustment for other patient and tumor characteristics.14 The factors included in the models (other than LOX) were determined by stepwise procedures (using α = .05 for both entry into the model and retention); various dichotomous groupings of assigned treatment, age, sex, Karnofsky performance status, primary site, T stage, and N stage were evaluated. After determining the base model for each end point, LOX, as a continuous variable, was added to the model to evaluate its prognostic value. Hazard ratios for LOX represent the change in risk for a 10-point increase in LOX. Cox models were also used to estimate the difference in prognosis for patients with and without LOX values (where patients with LOX values were coded as 1 and without coded as 0). For the Stanford patients, cancer-specific survival (CSS), where failure was defined as death due to the index HNC, was also evaluated; patients were censored at the date of death due to other causes.
RESULTS
Comparison Between IHC and AQUA Staining
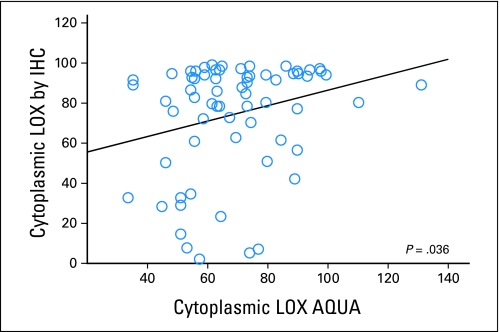
Sixty-six HNC patients on the Stanford TMA had interpretable LOX staining by both IHC and AQUA. Only cytoplasmic LOX signals were compared between the two systems as only cytoplasmic staining was identified by IHC. In general, cores with high IHC LOX staining also showed high AQUA signal and vice versa. There was a statistically significant, though small, correlation between the two staining and quantitation approaches (r = 0.26; P = .036; Fig 1). The low correlation was partially due to intrinsic differences in the quantitation approach used by each system, but most likely to due intratumoral heterogeneity of LOX expression since different cores within the tumor were used to generate the two separate TMAs for AQUA and IHC staining.
Fig 1.
Correlation of cytoplasmic lysyl oxidase (LOX) staining between traditional immunohistochemistry (IHC; as quantified by the Ariol system) and automated quantitative analysis (AQUA).
We also determined if AQUA LOX staining was predictive of treatment outcome in these patients, among whom we have previously established the role of LOX in predicting metastasis using traditional IHC staining.7 For most specimens evaluated, the AQUA approach consistently showed stronger nuclear than cytoplasmic staining. Quantitatively, the mean AQUA cytoplasmic LOX staining was 63.9 (standard deviation [SD], 14.9), whereas it was 103.5 for nuclear staining (SD, 22.2), and this difference was highly statistically significant (P < .0001). Therefore, we proceeded to focus on nuclear AQUA LOX staining for outcome analysis for this patient group and the RTOG 90-03 patients. Table 1 shows that nuclear LOX (nLOX) expression, as a continuous variable, was highly predictive of TTM, CSS, and OS in the Stanford patients. The predicting power of AQUA LOX staining was stronger than that for IHC (Table 1). For IHC data, negative and weak LOX staining were grouped together and compared with strong LOX staining as previously defined.7
Table 1.
LOX As Measured by AQUA and IHC Is a Predictor for Treatment Outcomes in Stanford Patients
| End Point | AQUA |
IHC |
||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P | |
| Time to metastasis | 1.03 | 1.01 to 1.06 | .004 | 0.47 | 0.22 to 0.99 | .049 |
| Cancer-specific survival | 1.02 | 1.01 to 1.04 | .003 | 0.40 | 0.18 to 0.87 | .02 |
| Overall survival | 1.02 | 1.00 to 1.03 | .01 | 0.53 | 0.28 to 1.01 | .05 |
NOTE. AQUA data were evaluated on a continuum with single unit increment. For IHC data, negative and weak LOX staining were grouped together and compared with strong LOX staining.
Abbreviations: LOX, lysyl oxidase; AQUA, automated quantitative analysis; IHC, immunohistochemistry.
Validation of LOX As a Prognostic Marker for Metastasis and Survival
Having established that AQUA performs similarly to IHC, we then used AQUA to validate LOX as a prognostic marker for metastasis and survival in RTOG 90-03 patients. Table 2 shows pretreatment characteristics and treatment assignment for the patients with and without available LOX AQUA staining. The patients with LOX assessable tumors were representative of the patients enrolled in RTOG 90-03. In addition, there were no clear differences in outcome for patients with and without LOX AQUA staining (TTM: hazard ratio 1.01; 95% CI, 0.71 to 1.43; TTP: hazard ratio, 0.94; 95% CI, 0.78 to 1.14; OS: hazard ratio, 1.00; 95% CI, 0.87 to 1.16). The AQUA LOX staining pattern for the RTOG 90-03 patients was similar to that seen in the Stanford patients. Overall, there was stronger nuclear (mean, 170.9; SD, 43.8) than cytoplasmic staining (mean, 130.0; SD, 45.3).
Table 2.
Pretreatment Characteristics and Assigned Treatment for RTOG 90-03 Patients
| Characteristic | Patients Without LOX Assay (n = 762) |
Patients With LOX Assay (n = 306) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Median age, years | 60 | 61 | ||
| Range | 31-88 | 30-90 | ||
| Sex | ||||
| Male | 609 | 79.9 | 242 | 79.1 |
| Female | 153 | 20.1 | 64 | 20.9 |
| KPS | ||||
| 60 | 34 | 4.5 | 14 | 4.6 |
| 70 | 79 | 10.4 | 42 | 13.7 |
| 80 | 173 | 22.7 | 70 | 22.9 |
| 90 | 355 | 46.6 | 139 | 45.4 |
| 100 | 121 | 15.9 | 41 | 13.4 |
| Primary site | ||||
| Oral cavity | 82 | 10.8 | 28 | 9.2 |
| Oropharynx | 475 | 62.3 | 171 | 55.9 |
| Hypopharynx | 101 | 13.3 | 39 | 12.7 |
| Larynx | 104 | 13.6 | 68 | 22.2 |
| T stage | ||||
| T1 | 51 | 6.7 | 11 | 3.6 |
| T2 | 215 | 28.2 | 72 | 23.5 |
| T3 | 270 | 35.4 | 135 | 44.1 |
| T4 | 225 | 29.5 | 88 | 28.8 |
| Tx | 1 | 0.1 | 0 | 0.0 |
| N stage | ||||
| N0 | 170 | 22.3 | 68 | 22.2 |
| N1 | 137 | 18.0 | 75 | 24.5 |
| N2a | 77 | 10.1 | 26 | 8.5 |
| N2b | 142 | 18.6 | 58 | 19.0 |
| N2c | 142 | 18.6 | 47 | 15.4 |
| N3 | 94 | 12.3 | 32 | 10.5 |
| Treatment assignment | ||||
| SFX | 196 | 25.7 | 70 | 22.9 |
| HFX | 174 | 22.8 | 87 | 28.4 |
| AFX-S | 190 | 24.9 | 84 | 27.5 |
| AFX-C | 202 | 26.5 | 65 | 21.2 |
Abbreviations: RTOG, Radiation Therapy Oncology Group; LOX, lysyl oxidase; KPS, Karnofsky performance status; SFX, standard fractionation; HFX, hyperfractionation; AFX-S, accelerated fractionation with split; AFX-C, accelerated fractionation with concomitant boost.
We evaluated the correlation between nuclear and total tumor LOX with other clinical and pathological variable. As presented in Appendix Table A1 (online only), Spearman's ρ correlation coefficient of LOX with all clinical variables is small.
Of the 306 patients with AQUA LOX staining, 45 had experienced failure with respect to the TTM end point, 147 had experienced failure for the TTP end point, and 250 had died at a median follow-up of 8.5 years for surviving patients. Appendix Table A2 (online only) presents the univariate analysis results for all parameters evaluated for the three end points. Table 3 presents the multivariate models for all 306 patients with nLOX included as a continuous variable for each of the three end points. The hazard ratio for LOX was presented for every 10-unit change in AQUA LOX intensity. nLOX has the greatest impact on TTM, followed by TTP, and finally OS. Since nLOX was evaluated in the models as a continuous variable, the hazard ratio of 1.15 for TTM means that for each 10-point increase in nLOX there is a 15% increase in risk of metastases. The effect of LOX is decreasing with time, as evidenced by the hazard ratio lower than 1 for the LOX by time interaction covariate. The effect in the first year is roughly twice that of the second year (hazard ratio, 1.16 v 1.08). There are not enough events to evaluate in the third year and later. No such interaction with time is evident for TTP or OS.
Table 3.
Results of Multivariate Analysis in Patients With AQUA LOX Staining Data (N = 306)
| End Point | No. of Events | Nuclear LOX |
Total Tumor LOX |
||||
|---|---|---|---|---|---|---|---|
| P | Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | ||
| Time to metastasis | |||||||
| N stage (1 if N2, 0 otherwise) | 45 | .0054 | 2.59 | 1.33 to 5.08 | .0028 | 2.83 | 1.43 to 5.60 |
| N stage (1 if N3, 0 otherwise) | .0002 | 5.59 | 2.29 to 13.61 | < .0001 | 6.02 | 2.45 to 14.81 | |
| Treatment (1 if HFX, 0 otherwise) | .0265 | 1.98 | 1.08 to 3.62 | .0113 | 2.21 | 1.20 to 4.07 | |
| LOX by time interaction | .0493 | 0.97 | 0.94 to 1.00 | .0316 | 0.96 | 0.92 to 1.00 | |
| LOX (continuous) | .0015 | 1.15 | 1.05 to 1.25 | .0001 | 1.21 | 1.10 to 1.33 | |
| Time to progression | 147 | ||||||
| KPS (1 if 60-80, 0 otherwise) | .0529 | 1.40 | 1.00 to 1.96 | .0276 | 1.46 | 1.04 to 2.05 | |
| T stage (1 if T4, 0 otherwise) | .0103 | 1.59 | 1.12 to 2.27 | .0058 | 1.64 | 1.15 to 2.33 | |
| N stage (1 if N3, 0 otherwise) | .0269 | 1.74 | 1.07 to 2.83 | .0265 | 1.74 | 1.07 to 2.83 | |
| LOX (continuous) | .0042 | 1.06 | 1.02 to 1.10 | .0069 | 1.06 | 1.02 to 1.10 | |
| Overall survival | |||||||
| KPS (1 if 60-80, 0 otherwise) | 250 | < .0001 | 1.82 | 1.41 to 2.35 | < .0001 | 1.87 | 1.44 to 2.42 |
| T stage (1 if T4, 0 otherwise) | .0529 | 1.32 | 1.00 to 1.75 | .0380 | 1.34 | 1.02 to 1.77 | |
| N stage (1 if N2, 0 otherwise) | .0064 | 1.45 | 1.11 to 1.88 | .0072 | 1.44 | 1.10 to 1.87 | |
| N stage (1 if N3, 0 otherwise) | .0033 | 1.89 | 1.24 to 2.88 | .0028 | 1.91 | 1.25 to 2.92 | |
| LOX (continuous) | .0265 | 1.03 | 1.00 to 1.06 | .0311 | 1.04 | 1.00 to 1.07 | |
NOTE. The hazard ratio for LOX was presented for every 10-unit change in LOX AQUA intensity for all three end points evaluated.
Abbreviations: AQUA, automated quantitative analysis; LOX, lysyl oxidase; KPS, Karnofsky performance status; HFX, hyperfractionated radiation therapy.
For ease of interpretation, we also estimated the increase in metastatic risk for a patient at the median and 75th percentile compared with a patient at the 25th percentile (Table 4). For TTM, there was a 54% increase in risk for a patient at the median nLOX value compared with the 25th percentile, and a 129% increase for a patient at the 75th percentile compared with the 25th percentile. These data are also shown graphically in Figure 2, which demonstrates the TTM for patients in the highest quartile of nLOX compared with those in the first three quartiles.
Table 4.
Hazard Ratios Comparing Median and 75th Percentile With 25th Percentile of Nuclear LOX and Total Tumor LOX for Each End Point
| End Point | Nuclear LOX |
Total Tumor LOX |
||||||
|---|---|---|---|---|---|---|---|---|
| Comparing Median With 25th Percentile |
Comparing 75th Percentile With 25th Percentile |
Comparing Median With 25th Percentile |
Comparing 75th Percentile With 25th Percentile |
|||||
| Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | |
| Time to metastasis | 1.54 | 1.18 to 2.02 | 2.29 | 1.37 to 3.81 | 1.58 | 1.25 to 2.00 | 3.59 | 1.87 to 6.89 |
| Time to progression | 1.19 | 1.06 to 1.35 | 1.40 | 1.11 to 1.77 | 1.15 | 1.04 to 1.27 | 1.47 | 1.11 to 1.94 |
| Overall survival | 1.11 | 1.01 to 1.22 | 1.22 | 1.02 to 1.46 | 1.09 | 1.01 to 1.17 | 1.26 | 1.02 to 1.56 |
Abbreviation: LOX, lysyl oxidase.
Fig 2.
Cumulative incidence of metastasis for patients in the highest nuclear lysyl oxidase (LOX) quartile compared with those in the lower three quartiles.
Although LOX protein expression and activity have been observed in the nucleus, it is predominantly a cytoplasmic and secreted protein. To address the possibility that nuclear LOX localization may be an AQUA detection bias, we performed AQUA staining of matched MDA-MB-231 xenografts with normal and knocked down LOX expression by short hairpin RNA (shRNA). Appendix Figures A1A and A1B (online only) show similar reduction of LOX signals in both cytoplasmic and nuclear compartments in the knocked-down tumor. Appendix Figure A1C (online only) shows significantly lower tumor mask LOX on AQUA quantitation of the knocked down tumor, which was confirmed on immunoblot (Appendix Fig A1D, online only).
We also quantified the tumor mask LOX level in 90-03 patients and determined its relationship to treatment outcomes. Tables 3 and 4 show the multivariate models for TTM, TTP, and OS with nLOX replaced by tumor LOX. Similar to nLOX, tumor LOX was an independent predictor for all three outcomes evaluated.
DISCUSSION
Cancer death due to metastases has become increasingly more prominent in HNC with improved locoregional control. Metastasis is a multistep process that can be influenced by the tumor microenvironment, of which hypoxia plays an important role.4,15 We have previously shown that LOX expression is elevated in hypoxic tumors and its levels were associated with hypoxia in patients with breast cancers or HNC.7 Furthermore, high LOX levels in the primary tumor correlated with a worse metastasis-free survival and OS in these patients. Herein, we have validated that LOX protein expression, as measured by AQUA in an independent laboratory, predicted for metastasis as defined above in a large cohort of homogeneously treated patients on a randomized cooperative group study. The results of this study strongly support the role of LOX as a biologic marker for metastasis in HNC.
One aspect of this study that differs from our previous single institutional report is the definition of TTM. Since the RTOG trial activated in 1991 enrolled patients with locally advanced disease for treatment with radiation alone, many died of locoregional failure before developing distant metastasis. The overall rate of distant metastasis as a first event, defined as failure below the clavicle before other events, was relatively low with a total of 147 events (14%) from 1,068 assessable patients over a period of 10 years and 44 events (14%) in 306 patients with LOX values. These numbers were too small to use by themselves to determine prognostic impact for any biologic marker. However, the process of developing nodal metastasis is biologically similar to that of distant dissemination, as it requires the tumor cells to acquire the ability to disrupt the interaction of surrounding cells, cross the basement membrane, migrate through the extracellular matrix, penetrate lymphatic channels, survive the host defense mechanism, and subsequently extravasate from the microlymphatics into foreign nodal tissue where it has to implant, proliferate, and establish new blood supplies.16 Hence, several metastatic effector molecules are involved in both regional and distant tumor spread.17–19 Hypoxia has been shown to promote both regional and distant metastasis in several solid tumors.20,21 Clinically, nodal involvement at diagnosis or nodal relapse is highly associated with subsequent distant metastasis in HNC.22,23 Therefore, taking these factors into consideration, we decided a priori to include regional relapses without local failure in N0 patients as an event in this study. This allows us to have more statistical power in the patients with available LOX staining. Similar definitions have been used to study metastasis in other HNC studies.24 In addition to TTM, LOX is also a strong predictor for TTP and OS in these patients.
Recently, it was shown that the human papillomavirus (HPV) is a strong prognostic factor in HNC.25 Patients with HPV had favorable outcome with any given treatment.25–27 Fifty-five point nine percent of the RTOG 90-03 patients with analyzable LOX had the primary tumor in the oropharynx. It is therefore conceivable that the prognostic effects seen with LOX may have interaction with the tumor HPV status. Since the incidence of HPV-related HNC only started to rise in the United States in the late 1990s28 and the RTOG 90-03 samples were collected before that, we expect that only a minority of the RTOG patients would be HPV positive. However, our study will need to be examined in context of the HPV status in these patients, and such a study is currently ongoing.
LOX is synthesized and secreted as a catalytically inactive 50 kDA proenzyme (proLOX), which is subsequently cleaved into a mature 32 kDA enzyme (LOX) and an 18 kDa LOX-propeptide (LOX-PP). Although the traditional function attributed to the LOX is initiation of the covalent crosslinking of extracellular collagens and elastin, other studies have shown that LOX can be found in the nucleus, where it may regulate gene expression as evidenced by interaction with histones, altered chromatin condensation, inactivation of NF-κB, and modulation of β-catenin and cyclin D1 expression.29–36 Recently, nuclear LOX activity, specifically deactivation of NF-κB, has been directly linked to the cleaved 18 kDa LOX-PP (rather than the active 34 kDa LOX enzyme), which has been suggested to support a tumor suppressor function.37 Outside the nucleus, LOX can induce chemotaxis in monocytes, fibroblasts, and smooth muscle cells.38–40 Our group has demonstrated not only that LOX is essential for hypoxia-mediated metastasis by affecting the invasive properties of hypoxic tumors through the regulation of FAK activity and cell to matrix adhesion, we also found that it is critical for premetastatic niche formation.41 Secreted LOX from hypoxic breast cancer cells accumulates at the premetastatic sites such as the lung, crosslinks collagen IV in the basement membrane, and is essential for recruitment of bone marrow derived cells, which in turn, creates a permissive environment for tumor cells to invade and survive.
The AQUA finding that LOX protein levels localized to or near the DAPI nuclear mask is the main predictor for outcomes in both patient groups is intriguing. Since our antibody does not recognize the 18 kDa LOX-PP, which is the main LOX-related peptide with nuclear function, the data suggest that either LOX has a strong nuclear expression in HNC, or more likely, the nuclear localization is a detection bias in AQUA with this LOX antibody. To address this issue, we quantify tumor mask LOX and correlated it with clinical outcomes. As shown earlier, tumor LOX also correlated with all evaluated end points, suggesting that the nuclear interpretation is an AQUA detection bias. We also stained xenograft tumors with LOX knocked down using shRNA. We showed that knocking down LOX resulted in a similarly change in both nuclear and cytoplasmic LOX on AQUA.
In summary, we have validated LOX as a marker for metastasis, and thereby the significance of hypoxia, in HNC patients treated on a large independent cooperative group study. Since RTOG 90-03 patients were treated with radiation alone and the addition of chemotherapy may affect the rate of metastasis, our future plan is to validate these findings in patients treated homogenously with concurrent chemoradiotherapy. In addition, studies are ongoing to determine the HPV status in these samples and to target LOX and its downstream pathways in order to develop novel therapies to prevent and treat metastasis in HNC and other solid tumors.
Supplementary Material
Acknowledgment
We thank Sushama Varma for Ariol LOX analysis and Alex Klimowicz for AQUA LOX analysis of the xenografts.
Appendix
Immunohistochemical Staining
The Stanford HNC TMA was constructed as previously described.13 Each sample was present on the TMA as duplicate cores. Immunoperoxidase stain for lysyl oxidase (LOX) was performed on 4 μmol/L-thick sections of the TMA. LOX polyclonal antibody was raised against a synthetic peptide of human LOX (EDTSCDYGYHRRFA, Open Biosystems) and has been shown to be specific for LOX by immunoblotting of cells with normal LOX expression and those with LOX downregulated by LOX-specific short hairpin RNA (shRNA) constructs.7 Peptide blocking studies also confirmed specificity of this antibody (data not shown). Finally, treatment of either cells or mice with this same antibody significantly blunted LOX enzymatic activity either in the condition media or the plasma of mice bearing LOX expressing orthotopic tumors.7 The following staining conditions were used for IHC: 1:20 dilution, 30 minutes incubation at room temperature. LOX staining was primarily cytoplasmic on visualization. Cytoplasmic staining was quantified using the Ariol automated scanning and quantification system (Applied Imaging/Genetix, San Jose, CA). In addition, traditional qualitative immunohistochemistry scoring by a pathologist, who was blinded to treatment outcomes, was also performed.7,11
Automated Image Acquisition and Analysis
Automated image acquisition and analysis using automated quantitative analysis (AQUA) has been described previously.9 Briefly, monochromatic, high-resolution (1,024 × 1,024 pixel; 0.5 μm) images were obtained of each histospot. We distinguished areas of tumor from stromal elements by creating a mask from the cytokeratin signal. The DAPI signal within this mask was then used to identify tumor nuclei. The LOX signal (AQUA score) was scored on a normalized scale of 1 to 255 expressed as pixel intensity divided by the target area (tumor mask or tumor nuclei). AQUA scores for duplicate tissue cores were averaged to obtain a mean AQUA score for each tumor. Since LOX has been found in both the cytoplasm and the nucleus, we also quantify tumor mask LOX, which is defined as the LOX signal in both the DAPI and the cytokeratin mask regions within the cancer cells.
Fig A1.
(A) Automated quantitative analysis (AQUA) staining of MDA-MB-231 tumor cells with wild-type and knocked-down lysyl oxidase (LOX) expression. 4,6-Diamidino-2-phenylindole (DAPI) signifies nuclear staining; MDA-Ctl, control tumor; MDA-shLOX24, knocked-down tumor. (B) Higher magnification showing a similar decrease in cytoplasmic and nuclear signals in MDA-shLOX24 tumor compared with control. (C) Box and whisker blot showing a significant reduction of tumor mask LOX signal on AQUA quantitation for the same tumors (P = .001). (D) Immunoblot of the cell lysates, confirming the knock-down effect of the short hairpin RNA construct.
Table A1.
Correlation Between LOX Expression and Other Clinical Variables That Are Available in the RTOG Database
| LOX | Assigned Treatment | Age | Sex | KPS | Primary Site | T Stage | N Stage |
|---|---|---|---|---|---|---|---|
| Nuclear | 0.01 | −0.04 | −0.08 | −0.02 | 0.03 | 0.11 | −0.07 |
| Total | −0.01 | −0.04 | −0.03 | 0.06 | −0.01 | 0.05 | −0.07 |
NOTE. As shown, Spearman's Rho correlation coefficient of LOX with all clinical variables is small.
Abbreviations: LOX, lysyl oxidase; RTOG, Radiation Therapy Oncology Group; KPS, Karnofsky performance status.
Table A2.
Results of the Univariate Analysis, Showing the P Values and Hazard Ratios for All Variables Evaluated for the Three Clinical End Points
| Variable by End Point | P | Hazard Ratio | 95% CI |
|---|---|---|---|
| Time to metastasis | |||
| RX (1 if HFX, 0 otherwise) | .0662 | 1.750 | 0.963 to 3.178 |
| Age (1 if > 65, 0 otherwise) | .2251 | 0.656 | 0.332 to 1.296 |
| Sex (1 if female, 0 otherwise) | .1307 | 0.488 | 0.192 to 1.237 |
| KPS (1 if 60-80, 0 otherwise) | .3319 | 1.350 | 0.736 to 2.476 |
| Site (1 if oropharynx, 0 otherwise) | .4217 | 1.277 | 0.703 to 2.319 |
| Differentiation (1 if high-grade, 0 otherwise) | .9694 | 0.988 | 0.531 to 1.838 |
| T stage (1 if T4, 0 otherwise) | .8602 | 0.936 | 0.449 to 1.950 |
| N stage (1 if N3, 0 otherwise) | .0059 | 2.956 | 1.367 to 6.393 |
| LOX (nuclear, continuous) | .0254 | 1.008 | 1.001 to 1.014 |
| LOX (tumor, continuous) | .0074 | 1.010 | 1.003 to 1.018 |
| Time to progression | |||
| RX (1 if HFX, 0 otherwise) | .2541 | 1.224 | 0.865 to 1.731 |
| Age (1 if > 65, 0 otherwise) | .1296 | 0.755 | 0.525 to 1.086 |
| Sex (1 if female, 0 otherwise) | .9921 | 1.002 | 0.671 to 1.497 |
| KPS (1 if 60-80, 0 otherwise) | .0074 | 1.569 | 1.128 to 2.180 |
| Site (1 if oropharynx, 0 otherwise) | .4685 | 1.128 | 0.814 to 1.565 |
| Differentiation (1 if high-grade, 0 otherwise) | .0224 | 0.650 | 0.449 to 0.941 |
| T stage (1 if T4, 0 otherwise) | .0003 | 1.882 | 1.335 to 2.654 |
| N stage (1 if N3, 0 otherwise) | .0095 | 1.899 | 1.170 to 3.083 |
| LOX (nuclear, continuous) | .0014 | 1.006 | 1.002 to 1.010 |
| LOX (tumor, continuous) | .0086 | 1.006 | 1.001 to 1.010 |
| Overall survival | |||
| RX (1 if HFX, 0 otherwise) | .2235 | 0.840 | 0.635 to 1.112 |
| Age (1 if > 65, 0 otherwise) | .2069 | 1.183 | 0.911 to 1.535 |
| Sex (1 if female, 0 otherwise) | .5561 | 0.912 | 0.670 to 1.240 |
| KPS (1 if 60-80, 0 otherwise) | < .0001 | 1.942 | 1.508 to 2.502 |
| Site (1 if oropharynx, 0 otherwise) | .6763 | 1.055 | 0.822 to 1.353 |
| Differentiation (1 if high-grade, 0 otherwise) | .0645 | 0.770 | 0.583 to 1.016 |
| T stage (1 if T4, 0 otherwise) | .0010 | 1.574 | 1.201 to 2.063 |
| N stage (1 if N3, 0 otherwise) | .0024 | 1.848 | 1.243 to 2.748 |
| LOX (nuclear, continuous) | .0088 | 1.004 | 1.001 to 1.007 |
| LOX (tumor, continuous) | .0525 | 1.003 | 1.000 to 1.006 |
Abbreviations: RX, treatment arm; HFX, hyperfractionated radiation therapy; KPS, Karnofsky performance status; LOX, lysyl oxidase.
Footnotes
Supported by an RTOG Translational grant (T.M., R.D., B.S.), National Cancer Institute RTOG Grant No. U10 CA21661 (J.H., T.F.P., K.K.A.), CCOP Grant No. U10 CA3742 (J.H., T.F.P.), Pennsylvania Department of Health Grant No. 4100026182 (J.H.), and Grants No. 1 R01 CA118582-01 (Q.T.L., C.S.K.), P01- CA67166 (Q.T.L., J.T.E., A.J.G.), and P01-CA06294 (K.K.A.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Quynh-Thu Le, Adam Dicker, Amato J. Giaccia, K. Kian Ang
Financial support: Adam Dicker
Administrative support: Brian Shin
Provision of study materials or patients: Andy Trotti, K. Kian Ang
Collection and assembly of data: Quynh-Thu Le, Anthony M. Magliocco, Christina S. Kong, Roman Diaz, Brian Shin, Hongbin Cao, Janine T. Erler
Data analysis and interpretation: Quynh-Thu Le, Jonathan Harris, Anthony M. Magliocco, Hongbin Cao, Janine T. Erler, Christine H. Chung, Thomas F. Pajak, Amato J. Giaccia, K. Kian Ang
Manuscript writing: Quynh-Thu Le, Jonathan Harris, Anthony M. Magliocco, Christina S. Kong, Roman Diaz, Brian Shin, Andy Trotti, Janine T. Erler, Christine H. Chung, Adam Dicker, Thomas F. Pajak, Amato J. Giaccia, K. Kian Ang
Final approval of manuscript: Quynh-Thu Le, Jonathan Harris, Anthony M. Magliocco, Christina S. Kong, Roman Diaz, Brian Shin, Hongbin Cao, Andy Trotti, Janine T. Erler, Christine H. Chung, Adam Dicker, Thomas F. Pajak, Amato J. Giaccia, K. Kian Ang
REFERENCES
- 1.Forastiere A, Koch W, Trotti A, et al. Head and neck cancer. N Engl J Med. 2001;345:1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 2.Argiris A, Haraf DJ, Kies MS, et al. Intensive concurrent chemoradiotherapy for head and neck cancer with 5-Fluorouracil- and hydroxyurea-based regimens: Reversing a pattern of failure. Oncologist. 2003;8:350–360. doi: 10.1634/theoncologist.8-4-350. [DOI] [PubMed] [Google Scholar]
- 3.Brown JM, Giaccia AJ. The unique physiology of solid tumors: Opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- 4.Le QT, Denko NC, Giaccia AJ. Hypoxic gene expression and metastasis. Cancer Metastasis Rev. 2004;23:293–310. doi: 10.1023/B:CANC.0000031768.89246.d7. [DOI] [PubMed] [Google Scholar]
- 5.Graeber TG, Osmanian C, Jacks T, et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 6.Denko NC, Fontana LA, Hudson KM, et al. Investigating hypoxic tumor physiology through gene expression patterns. Oncogene. 2003;22:5907–5914. doi: 10.1038/sj.onc.1206703. [DOI] [PubMed] [Google Scholar]
- 7.Erler JT, Bennewith KL, Nicolau M, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 8.Camp RL, Dolled-Filhart M, King BL, et al. Quantitative analysis of breast cancer tissue microarrays shows that both high and normal levels of HER2 expression are associated with poor outcome. Cancer Res. 2003;63:1445–1448. [PubMed] [Google Scholar]
- 9.Psyrri A, Yu Z, Weinberger PM, et al. Quantitative determination of nuclear and cytoplasmic epidermal growth factor receptor expression in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clin Cancer Res. 2005;11:5856–5862. doi: 10.1158/1078-0432.CCR-05-0420. [DOI] [PubMed] [Google Scholar]
- 10.Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: First report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 11.Le QT, Kong C, Lavori PW, et al. Expression and prognostic significance of a panel of tissue hypoxia markers in head-and-neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2007;69:167–175. doi: 10.1016/j.ijrobp.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 12.Trotti A, Fu KK, Pajak TF, et al. A comparison of hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinoma. Proc Am Soc Thera Rad Onc (ASTRO), Denver, CO. Int J Radiat Oncol Biol Phys. 2005;63(suppl 1):S70–S71. doi: 10.1016/s0360-3016(00)00663-5. abstr 116. [DOI] [PubMed] [Google Scholar]
- 13. Reference deleted.
- 14.Cox DR. Regression models and life tables. J Royal Stat Soc. 1972;34:187–229. [Google Scholar]
- 15.Lunt SJ, Chaudary N, Hill RP. The tumor microenvironment and metastatic disease. Clin Exp Metastasis. 2009;26:19–34. doi: 10.1007/s10585-008-9182-2. [DOI] [PubMed] [Google Scholar]
- 16.Cairns RA, Khokha R, Hill RP. Molecular mechanisms of tumor invasion and metastasis: An integrated view. Curr Mol Med. 2003;3:659–671. doi: 10.2174/1566524033479447. [DOI] [PubMed] [Google Scholar]
- 17.Rofstad EK, Rasmussen H, Galappathi K, et al. Hypoxia promotes lymph node metastasis in human melanoma xenografts by up-regulating the urokinase- type plasminogen activator receptor. Cancer Res. 2002;62:1847–1853. [PubMed] [Google Scholar]
- 18.Rofstad EK, Mathiesen B, Henriksen K, et al. The tumor bed effect: Increased metastatic dissemination from hypoxia-induced up-regulation of metastasis-promoting gene products. Cancer Res. 2005;65:2387–2396. doi: 10.1158/0008-5472.CAN-04-3039. [DOI] [PubMed] [Google Scholar]
- 19.Rofstad EK, Mathiesen B, Galappathi K. Increased metastatic dissemination in human melanoma xenografts after subcurative radiation treatment: Radiation-induced increase in fraction of hypoxic cells and hypoxia-induced up-regulation of urokinase-type plasminogen activator receptor. Cancer Res. 2004;64:13–18. doi: 10.1158/0008-5472.can-03-2658. [DOI] [PubMed] [Google Scholar]
- 20.Cairns RA, Hill RP. Acute hypoxia enhances spontaneous lymph node metastasis in an orthotopic murine model of human cervical carcinoma. Cancer Res. 2004;64:2054–2061. doi: 10.1158/0008-5472.can-03-3196. [DOI] [PubMed] [Google Scholar]
- 21.Rofstad EK, Galappathi K, Mathiesen B, et al. Fluctuating and diffusion-limited hypoxia in hypoxia-induced metastasis. Clin Cancer Res. 2007;13:1971–1978. doi: 10.1158/1078-0432.CCR-06-1967. [DOI] [PubMed] [Google Scholar]
- 22.Lim YC, Koo BS, Choi EC. Bilateral neck node metastasis: A predictor of isolated distant metastasis in patients with oral and oropharyngeal squamous cell carcinoma after primary curative surgery. Laryngoscope. 2007;117:1576–1580. doi: 10.1097/MLG.0b013e318093ee2b. [DOI] [PubMed] [Google Scholar]
- 23.Al-Othman MO, Morris CG, Hinerman RW, et al. Distant metastases after definitive radiotherapy for squamous cell carcinoma of the head and neck. Head Neck. 2003;25:629–633. doi: 10.1002/hed.10275. [DOI] [PubMed] [Google Scholar]
- 24.Brizel DM, Schroeder T, Scher RL, et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51:349–353. doi: 10.1016/s0360-3016(01)01630-3. [DOI] [PubMed] [Google Scholar]
- 25.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 26.Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24:5630–5636. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 27.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 28.Chaturvedi AK, Engels EA, Anderson WF, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 29.Li W, Nellaiappan K, Strassmaier T, et al. Localization and activity of lysyl oxidase within nuclei of fibrogenic cells. Proc Natl Acad Sci U S A. 1997;94:12817–12822. doi: 10.1073/pnas.94.24.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giampuzzi M, Botti G, Di Duca M, et al. Lysyl oxidase activates the transcription activity of human collagene III promoter: Possible involvement of Ku antigen. J Biol Chem. 2000;275:36341–36349. doi: 10.1074/jbc.M003362200. [DOI] [PubMed] [Google Scholar]
- 31.Nellaiappan K, Risitano A, Liu G, et al. Fully processed lysyl oxidase catalyst translocates from the extracellular space into nuclei of aortic smooth-muscle cells. J Cell Biochem. 2000;79:576–582. doi: 10.1002/1097-4644(20001215)79:4<576::aid-jcb60>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 32.Kagan HM, Williams MA, Calaman SD, et al. Histone H1 is a substrate for lysyl oxidase and contains endogenous sodium borotritide-reducible residues. Biochem Biophys Res Commun. 1983;115:186–192. doi: 10.1016/0006-291x(83)90987-7. [DOI] [PubMed] [Google Scholar]
- 33.Mello ML, Contente S, Vidal BC, et al. Modulation of ras transformation affecting chromatin supraorganization as assessed by image analysis. Exp Cell Res. 1995;220:374–382. doi: 10.1006/excr.1995.1328. [DOI] [PubMed] [Google Scholar]
- 34.Jeay S, Pianetti S, Kagan HM, et al. Lysyl oxidase inhibits ras-mediated transformation by preventing activation of NF-kappa B. Mol Cell Biol. 2003;23:2251–2263. doi: 10.1128/MCB.23.7.2251-2263.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giampuzzi M, Oleggini R, Albanese C, et al. Beta-catenin signaling and regulation of cyclin D1 promoter in NRK-49F cells transformed by down-regulation of the tumor suppressor lysyl oxidase. Biochim Biochim Biophys Acta. 2005;1745:370–381. doi: 10.1016/j.bbamcr.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Payne SL, Hendrix MJ, Kirschmann DA. Paradoxical roles for lysyl oxidases in cancer–a prospect. J Cell Biochem. 2007;101:1338–1354. doi: 10.1002/jcb.21371. [DOI] [PubMed] [Google Scholar]
- 37.Palamakumbura AH, Jeay S, Guo Y, et al. The propeptide domain of lysyl oxidase induces phenotypic reversion of ras-transformed cells. J Biol Chem. 2004;279:40593–40600. doi: 10.1074/jbc.M406639200. [DOI] [PubMed] [Google Scholar]
- 38.Lazarus HM, Cruikshank WW, Narasimhan N, et al. Induction of human monocyte motility by lysyl oxidase. Matrix Biol. 1995;14:727–731. doi: 10.1016/s0945-053x(05)80015-0. [DOI] [PubMed] [Google Scholar]
- 39.Nelson JM, Diegelmann RF, Cohen IK. Effect of beta-aminopropionitrile and ascorbate on fibroblast migration. Proc Soc Exp Biol Med. 1988;188:346–352. doi: 10.3181/00379727-188-42745. [DOI] [PubMed] [Google Scholar]
- 40.Li W, Liu G, Chou IN, et al. Hydrogen peroxide-mediated, lysyl oxidase-dependent chemotaxis of vascular smooth muscle cells. J Cell Biochem. 2000;78:550–557. [PubMed] [Google Scholar]
- 41.Erler JT, Bennewith KL, Cox TR, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.