Abstract
Purpose
Although, for patients with cancer, comorbidity can affect the timing of cancer detection, treatment, and prognosis, there is little information relating to the question of whether the choice of comorbidity index affects the results of studies. Therefore, to compare the association of comorbidity with mortality after surgery for colon cancer, this study evaluated the Adult Comorbidity Evaluation-27 (ACE-27), the National Institute on Aging (NIA) and National Cancer Institute (NCI) Comorbidity Index, and the Charlson Comorbidity Index (CCI).
Patients and Methods
The study population consisted of colon cancer patients (N = 496) who underwent surgery at the University of Alabama at Birmingham Hospital from 1981 to 2002. Hazard ratios (HRs) with 95% CIs were obtained using the method of Cox proportional hazards for the three comorbidity indices in predicting overall and colon cancer–specific mortality. The point estimates obtained for comorbidity and other risk factors across the three models were compared.
Results
For each index, the highest comorbidity burden was significantly associated with poorer overall survival (ACE-27: HR = 1.63; 95% CI, 1.24 to 2.15; NIA/NCI: HR = 1.83; 95% CI, 1.29 to 2.61; CCI: HR = 1.46; 95% CI, 1.14 to 1.88) as well as colon cancer–specific survival. For the other risk factors, there was little variation in the point estimates across the three models.
Conclusion
The results obtained from these three indices were strikingly similar. For patients with severe comorbidity, all three indices were statistically significant in predicting shorter survival after surgery for colon cancer.
INTRODUCTION
For patients, comorbidity can affect cancer detection, treatment, and prognosis.1 Comorbidity assessment is a primary determinant for establishing eligibility for patients enrolling onto clinical trials, and in general practice, patient age and overall health, which are factors of comorbidity, are considered by physicians in development of individualized therapies.2 A high comorbidity burden for patients decreases their likelihood of receiving chemotherapy when it is otherwise indicated.3 The use of comorbidity indices to guide treatment decisions is not common practice. In general, clinical trials exclude patients with chronic comorbid conditions. By using a comorbidity index for each patient, physicians would have relevant information for deciding on the best treatment. Although several comorbidity indices have been used in cancer research, few studies have evaluated their predictive capacity to determine whether the conclusions reached differ according to the index used.
Assessment of comorbidity is important for the recruitment of patients onto clinical trials; older patients with comorbidity are often not considered.4 At the time of diagnosis of colon cancer, most patients are ≥ 65 years of age and have comorbid conditions.5,6 The logic for excluding these patients is to eliminate the confounding influence of comorbidity in the evaluation of treatment efficacy. By excluding older patients with comorbidities, the conclusions drawn from clinical trials may not be applicable to most cancer patients.7 Another reason for not enrolling older patients with comorbidities onto trials is the potential toxicity of the treatment regimen.7 The assessment of comorbidity among patients enrolled onto clinical trials would identify those patients at higher risk of toxicity. In addition, investigators could compare the efficacy of a treatment regimen across different levels of comorbidity. Comorbidity assessment in clinical trials would allow more accurate prognoses for patients and would prevent patients with comorbidities from being denied potentially beneficial treatments.
In cancer research, assessment of comorbidity is essential because it may be a confounder for other risk factors.8 Failing to account for comorbidity in statistical analyses may result in erroneous conclusions regarding the parameters of interest (eg, biomarker development studies). Because comorbidity is associated with mortality, good research practice dictates that it be assessed in studies of cancer outcomes.9
Because the comparability of results for different measures of comorbidity is unexplored, the current study compared the results obtained for three comorbidity indices in predicting mortality after surgery for colon cancer. These were the Adult Comorbidity Evaluation-27 (ACE-27), the National Institute on Aging (NIA) and National Cancer Institute (NCI) Comorbidity Index, and the Charlson Comorbidity Index (CCI).6,10–12 The aims were to emphasize the importance of comorbidity assessment, to assess the prognostic capacity of the three indices in predicting death as a result of any cause and colon cancer–specific death, and to compare the point estimates obtained for other risk factors according to comorbidity index. We will determine whether the tools used to assess comorbidity burden provide equivalent risk assessment for patients with intercurrent medical problems.
PATIENTS AND METHODS
Patient and Inclusion Criteria
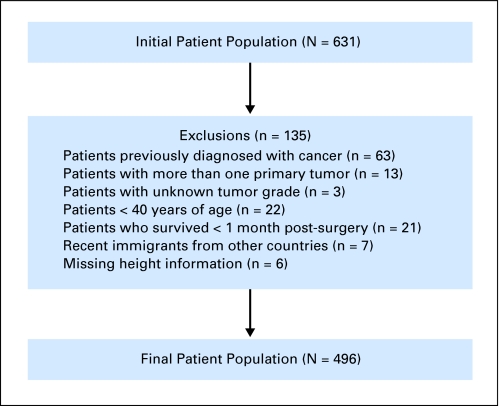
This study was approved by the institutional review board at the University of Alabama at Birmingham (UAB). The study population consisted of patients who underwent surgery for sporadic (nonhereditary) adenocarcinoma of the colon at UAB Hospital from 1981 to 2002. The termination date for accrual of follow-up information was June 1, 2008. The initial patient population consisted of 631 participants. To eliminate potential sources of selection or information bias, the exclusion criteria (Fig 1) were applied. Because the primary aim was to assess the effect of comorbidity on survival and because the impact of comorbidity increases with age, patients younger than 40 years of age (n = 22) were removed from the study. By excluding these patients, the probability of the tumor arising in individuals with a family or genetic history of colorectal cancer was minimized. This resulted in a final study population of 496 patients.
Fig 1.
Study population and exclusions.
Tumor-specific characteristics were obtained from pathology reports and adjudicated by two of the authors (C.C. and U.M.). Tumors were classified using the TNM system and staged according to the American Joint Committee on Cancer system as stages I, II, III, or IV.13 Tumor grade was recorded as well, moderately, or poorly differentiated or as unknown (no tumors were undifferentiated). The tumor grade was ascertained by a pathologist (C.C.). Well-differentiated and moderately differentiated tumors were low grade, and poorly differentiated tumors were high grade.14
Demographic, clinical, and patient data regarding age at time of surgery, sex, race (self-identified), surgery date, insurance status, comorbidity, height and weight, smoking status, receipt of chemotherapy after surgery, and perioperative variables were obtained from medical records. From height and weight data, body mass index (BMI; weight in kilograms divided by height in meters squared) was calculated, and participants were categorized as underweight (BMI < 18.5 kg/m2), normal weight (BMI = 18.5 to 24.9 kg/m2), overweight (BMI = 25.0 to 29.9 kg/m2), or obese (BMI ≥ 30.0 kg/m2), according to recommended guidelines.15 Whether or not the patient experienced weight loss before surgery, presence of bowel obstruction, and receipt of blood during surgery were also documented.
Comorbidity information was abstracted from the medical record up to the date of surgery by the primary author (R.B.H.). These data were obtained from physician notes, anesthesia notes, nursing notes, and discharge summaries. On the basis of their comorbid burden, patients were categorized using the following three indices: the ACE-27 index, the NIA/NCI comorbidity index, and the CCI. Because having a previous cancer was an exclusion criterion for this study, information pertaining to cancer was not used in calculation of the comorbidity burden. For the ACE-27 index, each patient was given an overall grade of none, mild, moderate, or severe comorbidity, as detailed by Piccirillo et al.16 In obtaining a total comorbidity count for the NIA/NCI index, patients were placed into one of the following four groups corresponding to the total number of comorbid conditions: zero to one, two to three, four to five, or ≥ six comorbidities. This categorization was based on the distribution within the study population. The list of comorbid conditions for the NIA/NCI index was slightly modified by excluding anemia. Smoking information was also excluded for the NIA/NCI index because this information was recorded as a separate variable. Finally, using the CCI, patients were categorized based on the sum of weighted comorbidities into one of the following three groups: score of 0, 1, or ≥ 2, as previously reported.17
Follow-up data on each patient were obtained from the UAB tumor registry. This information was updated by the registry every 6 months by contacting each patient or a family member by telephone or mail. If a patient had died since the last follow-up contact, the date and cause (colon cancer-specific or other) of death were recorded. This information was validated by examination of the state death registry. Patients who were alive at the termination date were right-censored at the last contact date. If a patient was recorded as alive and ≥ 3 years had elapsed since the last contact, the patient was designated as lost to follow-up.
Statistical Analysis
Survival time was calculated from the date of surgery until death, the termination date, or the last date of contact for patients who were still alive. The events of interest were death from any cause and colon cancer–specific death. All reported P values are two-sided, and statistical significance is defined as P < .05.
The Cox proportional hazards method was used to obtain hazard ratios (HRs) with 95% CIs for the bivariate association of risk factors and other covariates with all-cause mortality. For categorical variables (surgery date, smoking status, comorbidity, tumor stage, and BMI), the overall association of the variable with mortality was obtained using the likelihood ratio test. Cox multivariable models were constructed for each of the comorbidity indices. For variable selection, those associated with all-cause mortality at P < .20 were considered as potential confounders and were included in the initial multivariable model for each index. To obtain the final multivariable model, the least significant variable was removed in a stepwise manner for each of the three models. If a covariate was significant in any of the three models, it was retained in all models. This process ensured that the final model for all three indices contained the same set of variables and thus allowed comparison of ratio measures across the three models. After obtaining the final model for each of the indices, the proportional hazards assumption was evaluated and met for each comorbidity index by testing the interaction with time. Once the final model was obtained for each index in predicting overall mortality, the same models were reanalyzed with cancer-specific death as the event of interest.
RESULTS
Study Population
The characteristics of the study population and the bivariate association of each characteristic with survival are listed in Table 1. Most patients were deceased at the end of the follow-up period (n = 333, 67.1%), and most died as a result of colon cancer (n = 219, 65.8%). The median follow-up time for study participants was 58.2 months. Most participants had no or mild comorbidity, although differences were apparent based on the categorization for each index. Most patients had localized disease (stage I or II: n = 260, 52.5%), and histologically, most tumors were low grade (n = 398, 80.2%).
Table 1.
Characteristics of the Study Population and Bivariate Associations With All-Cause Mortality
| Characteristic | Study Participants (N = 496) |
Association With All-Cause Mortality |
P | ||
|---|---|---|---|---|---|
| No. | % | Unadjusted Hazard Ratio | 95% CI | ||
| Status | |||||
| Alive | 163 | 32.9 | |||
| Dead | 333 | 67.1 | |||
| Cause of death (n = 333) | |||||
| Colon cancer | 219 | 65.8 | |||
| Other | 114 | 34.2 | |||
| Median follow-up time, months | 58.2 | ||||
| Age, years | 1.25* | 1.14 to 1.37 | |||
| Mean | 66.9 | ||||
| Standard deviation | 12.2 | ||||
| Sex | |||||
| Male | 225 | 45.4 | Ref | ||
| Female | 271 | 54.6 | 1.08 | 0.87 to 1.34 | |
| Race | |||||
| White | 303 | 61.2 | Ref | ||
| African American | 193 | 38.9 | 1.23 | 0.99 to 1.53 | |
| Time of surgery | .08 | ||||
| 1981-1986 | 76 | 15.3 | Ref | ||
| 1987-1991 | 96 | 19.4 | 0.67 | 0.48 to 0.94 | |
| 1992-1996 | 133 | 26.8 | 0.69 | 0.50 to 0.95 | |
| 1997-2002 | 191 | 38.5 | 0.75 | 0.55 to 1.02 | |
| Private insurance | |||||
| Yes | 334 | 67.6 | Ref | ||
| No | 160 | 32.4 | 1.26 | 1.01 to 1.58 | |
| Smoking status | .77 | ||||
| No | 297 | 59.9 | Ref | ||
| Former | 117 | 23.6 | 0.91 | 0.69 to 1.19 | |
| Current | 82 | 16.5 | 0.98 | 0.73 to 1.32 | |
| ACE-27 comorbidity | < .001 | ||||
| None | 98 | 19.8 | Ref | ||
| Mild | 184 | 37.1 | 0.81 | 0.60 to 1.11 | |
| Moderate | 128 | 25.8 | 1.20 | 0.87 to 1.66 | |
| Severe | 86 | 17.3 | 1.56 | 1.11 to 2.20 | |
| CCI score | .003 | ||||
| 0 | 252 | 50.8 | Ref | ||
| 1 | 133 | 26.8 | 1.01 | 0.78 to 1.32 | |
| ≥ 2 | 111 | 22.4 | 1.58 | 1.21 to 2.05 | |
| NIA/NCI comorbidities | .02 | ||||
| 0-1 | 150 | 30.2 | Ref | ||
| 2-3 | 189 | 38.1 | 0.85 | 0.65 to 1.11 | |
| 4-5 | 113 | 22.8 | 1.00 | 0.74 to 1.35 | |
| ≥ 6 | 44 | 8.9 | 1.56 | 1.06 to 2.29 | |
| Tumor stage | < .001 | ||||
| I | 91 | 18.4 | Ref | ||
| II | 169 | 34.1 | 1.43 | 1.00 to 2.05 | |
| II | 137 | 27.6 | 2.48 | 1.72 to 3.58 | |
| IV | 99 | 20.0 | 9.37 | 6.40 to 13.71 | |
| Tumor grade | |||||
| Low | 398 | 80.2 | Ref | ||
| High | 98 | 19.8 | 1.70 | 1.32 to 2.20 | |
| Chemotherapy | |||||
| No | 362 | 73.0 | Ref | ||
| Yes | 134 | 27.0 | 1.74 | 1.38 to 2.20 | |
| BMI | < .01 | ||||
| Underweight | 24 | 4.8 | 1.81 | 1.15 to 2.83 | |
| Normal | 199 | 40.1 | Ref | ||
| Overweight | 168 | 33.9 | 0.86 | 0.67 to 1.11 | |
| Obese | 105 | 21.2 | 0.77 | 0.57 to 1.03 | |
| Recent weight loss | |||||
| No | 336 | 67.7 | Ref | ||
| Yes | 160 | 32.3 | 1.52 | 1.22 to 1.90 | |
| Obstruction | |||||
| No | 349 | 70.4 | Ref | ||
| Yes | 147 | 29.6 | 1.98 | 1.58 to 2.48 | |
| Received blood | |||||
| No | 471 | 95.0 | Ref | ||
| Yes | 25 | 5.0 | 1.96 | 1.26 to 3.06 | |
Abbreviations: Ref, referent; ACE-27, Adult Comorbidity Evaluation-27; CCI, Charlson Comorbidity Index; NIA/NCI, National Institute on Aging and National Cancer Institute; BMI, body mass index.
For each 10-year increase in age.
Bivariate Associations With Overall Mortality
For each of the characteristics listed in Table 1, unadjusted HRs were obtained using the Cox proportional hazards method. The same conclusions were drawn for the three comorbidity indices. For the three indices, the HRs for the most severe category of comorbidity were similar. For the ACE-27 index, the severe category of comorbidity was associated with a 56% increased risk of death (HR = 1.56; 95% CI, 1.11 to 2.20). The highest level of comorbidity in the NIA/NCI index conferred a 56% increased risk (HR = 1.56; 95% CI, 1.06 to 2.29), and those with CCI scores of ≥ 2 had a 58% increased risk of death (HR = 1.58; 95% CI, 1.21 to 2.05).
When considered in the context of the entire study population, it seems that receipt of chemotherapy is hazardous (HR = 1.74; 95% CI, 1.38 to 2.20). However, this is a result of confounding by indication because those patients for whom chemotherapy is indicated (stage III patients) have more advanced tumors. Therefore, receipt of chemotherapy is a marker of more advanced disease and is not, in itself, hazardous.
Multivariable Models
Overall mortality.
Models for Cox proportional hazards were constructed separately for the three comorbidity indices. On the basis of the results obtained from the unadjusted association of each risk factor with survival, categories were combined when the HRs were similar. Therefore, strata for comorbidity, tumor stage, and BMI were combined. For each index, results of the models for multivariable Cox regression are listed in Table 2. As was the case for the unadjusted measures, only the highest level of comorbidity in each model was associated with an impact on mortality. The highest level (severe) of the ACE-27 index increased the risk of death by more than 60% (HR = 1.63; 95% CI, 1.24 to 2.15). A stronger association was found when comparing the highest level (≥ six comorbidities) of the NIA/NCI index (HR = 1.83; 95% CI, 1.29 to 2.61), although the CI was wider, reflecting the small number of participants in this category (n = 44). There was an increased risk among those with scores of ≥ 2 by the CCI, although the magnitude of association was smaller compared with the other two indices (HR = 1.46; 95% CI, 1.14 to 1.88).
Table 2.
Association of Comorbidity Indices With Mortality
| Variable | ACE-27 |
NIA/NCI Index |
CCI |
|||
|---|---|---|---|---|---|---|
| Adjusted* HR | 95% CI | Adjusted* HR | 95% CI | Adjusted* HR | 95% CI | |
| Comorbidity level | ||||||
| Not severe,† < 6,‡ < 2§ | Ref | Ref | Ref | |||
| Severe,† ≥ 6,‡ or ≥ 2§ | 1.63 | 1.24 to 2.15 | 1.83 | 1.29 to 2.61 | 1.46 | 1.14 to 1.88 |
| Race | ||||||
| White | Ref | Ref | Ref | |||
| African American | 1.34 | 1.06 to 1.68 | 1.34 | 1.07 to 1.69 | 1.36 | 1.08 to 1.71 |
| Stage | ||||||
| I-II | Ref | Ref | Ref | |||
| III | 1.95 | 1.50 to 2.54 | 2.06 | 1.57 to 2.69 | 1.95 | 1.49 to 2.54 |
| IV | 8.96 | 6.60 to 12.18 | 8.66 | 6.39 to 11.73 | 8.54 | 6.30 to 11.57 |
| Grade | ||||||
| Low | Ref | Ref | Ref | |||
| High | 1.55 | 1.19 to 2.03 | 1.66 | 1.27 to 2.16 | 1.59 | 1.22 to 2.08 |
| BMI | ||||||
| Underweight | 1.54 | 0.96 to 2.45 | 1.56 | 0.98 to 2.49 | 1.54 | 0.97 to 2.46 |
| Normal | Ref | Ref | Ref | |||
| Overweight/obese | 0.77 | 0.61 to 0.97 | 0.80 | 0.63 to 1.00 | 0.77 | 0.61 to 0.97 |
| Bowel obstruction | 1.51 | 1.19 to 1.91 | 1.54 | 1.21 to 1.94 | 1.52 | 1.20 to 1.93 |
Abbreviations: ACE-27, Adult Comorbidity Evaluation-27; NIA/NCI, National Institute on Aging and National Cancer Institute; CCI, Charlson Comorbidity Index; HR, hazard ratio; Ref, referent; BMI, body mass index.
Adjusted for the variables listed as well as age.
According to ACE-27.
According to NIA/NCI index.
According to CCI.
Nearly identical results were obtained when comparing the range of HRs (HRrange) for the other risk factors across each model. In all models, the association with African American race was equivalent (HRrange, 1.34 to 1.36) and statistically significant. The results obtained for tumor stage were also within a narrow range (stage III: HRrange, 1.95 to 2.06; stage IV: HRrange, 8.54 to 8.96), as were the results for tumor grade (HRrange, 1.55 to 1.66). The results for BMI were virtually identical for underweight (HRrange, 1.54 to 1.56) and overweight/obese (HRrange, 0.77 to 0.80), as were the point estimates for the presence of bowel obstruction (HRrange, 1.51 to 1.54). In the multivariable models, surgery date, insurance status, receipt of chemotherapy, weight loss before surgery, and receipt of blood during surgery did not remain statistically significant.
Colon cancer–specific mortality.
The results obtained for each of the comorbidity indices with cancer-specific mortality as the end point are listed in Table 3. Because most of the deaths that occurred in this population of patients were a result of cancer, it is not surprising that results similar to those for overall mortality were obtained when colon cancer–specific death was the outcome.
Table 3.
Association of Comorbidity Indices With Colon Cancer–Specific Mortality
| Variable | ACE-27 |
NIA/NCI Index |
CCI |
|||
|---|---|---|---|---|---|---|
| Adjusted* HR | 95% CI | Adjusted* HR | 95% CI | Adjusted* HR | 95% CI | |
| Comorbidity level | ||||||
| Not severe,† < 6,‡ < 3§ | Ref | Ref | Ref | |||
| Severe,† ≥ 6,‡ or ≥ 3§ | 1.61 | 1.14 to 2.27 | 2.05 | 1.33 to 3.15 | 2.02 | 1.29 to 3.16 |
| Race | ||||||
| White | Ref | Ref | Ref | |||
| African American | 1.27 | 0.96 to 1.68 | 1.30 | 0.98 to 1.73 | 1.29 | 0.97 to 1.70 |
| Stage | ||||||
| I-II | Ref | Ref | Ref | |||
| III | 3.19 | 2.24 to 4.54 | 3.41 | 2.38 to 4.89 | 3.20 | 2.24 to 4.57 |
| IV | 13.15 | 9.07 to 19.05 | 12.82 | 8.88 to 18.52 | 12.77 | 8.85 to 18.45 |
| Grade | ||||||
| Low | Ref | Ref | Ref | |||
| High | 1.63 | 1.19 to 2.24 | 1.78 | 1.30 to 2.43 | 1.77 | 1.30 to 2.42 |
| BMI | ||||||
| Underweight | 1.45 | 0.79 to 2.66 | 1.38 | 0.75 to 2.54 | 1.50 | 0.82 to 2.74 |
| Normal | Ref | Ref | Ref | |||
| Overweight/obese | 0.79 | 0.59 to 1.05 | 0.83 | 0.62 to 1.10 | 0.80 | 0.61 to 1.07 |
| Bowel obstruction | 1.64 | 1.24 to 2.16 | 1.62 | 1.22 to 2.15 | 1.70 | 1.28 to 2.24 |
Abbreviations: ACE-27, Adult Comorbidity Evaluation-27; NIA/NCI, National Institute on Aging and National Cancer Institute; CCI, Charlson Comorbidity Index; HR, hazard ratio; Ref, referent; BMI, body mass index.
Adjusted for the variables listed as well as age.
According to ACE-27.
According to NIA/NCI index.
According to CCI.
DISCUSSION
Three comorbidity indices were used to assess differences in the comorbidity assessment tools in a population of colon cancer patients immediately before surgery. In survival models with overall and cancer-specific death as the outcomes of interest, the point estimates for each comorbidity index and other risk factors were compared. All three indices were statistically significant for the association with all-cause and colon cancer–specific mortality. In addition, the point estimates obtained for the other risk factors in each of the multivariable models were essentially equivalent for overall and cancer-specific mortality. In conclusion, the assessment of comorbidity burden was similar in all three indices. On the basis of these results, selection of any of the three comorbidity indices is justifiable because the conclusions reached were the same for all three.
For colon cancer patients, a higher comorbidity burden is associated with decreased survival.3,6,16,18–21 To the authors' knowledge, no previous study has compared the results obtained by use of different comorbidity indices in populations of these patients, although other cancer patient populations have been used. A study by Soares et al22 compared the ACE-27 and the CCI in predicting 6-month mortality for critically ill cancer patients. The ACE-27 index was superior in identifying a significant association between comorbidity and patient survival. Similar to results for the present study, these investigators found that only those patients with severe comorbidity had a significantly increased risk of death. Consistent with our results, another study, involving older patients with head and neck cancer,23 found that increasing comorbidity, determined by the NIA/NCI and ACE-27 indices, was significantly associated with decreased survival.
The ACE-27 index is comprehensive and accounts for disease severity when assessing the comorbidity burden.16 The NIA/NCI index is comprehensive, but disease severity is not taken into consideration.6 The CCI is not as comprehensive as the others but does weight conditions based on clinical impact.10 In the present study, all comorbidity indices were significantly associated with death, and the HRs were not substantially different, even though there were differences in the scoring system for the three indices. For studies involving the effect of comorbidity, the ACE-27 method might be preferred because it is comprehensive, accounts for disease severity, and is straightforward in application. The present results, however, show that all three indices are significantly associated with all-cause and colon cancer–specific mortality.
The results have other implications. Comorbidity offers a way to stratify cancer patients beyond known risk factors, such as age and tumor-associated characteristics. Older patients benefit from adjuvant therapy, but they are less likely to receive chemotherapy.24 Although advanced age is now less often an exclusionary criterion, many clinical trials are restrictive with regard to comorbidity burden.25–27 By assessing comorbidity among participants in a clinical trial, researchers would have information that would lead to more accurate projections of treatment-related toxicity, drug-drug interactions, and efficacy of chemotherapeutic regimens.7,28 Because this information is currently lacking, older patients with comorbidity may be denied treatment from which they could benefit.29
Regarding the results obtained for BMI, our results are biologically plausible when considered in the context of tumor stage and relate to frailty. Various criteria are used to identify the frail elderly, including malnutrition and the presence of comorbidity. Our unpublished findings29a suggest that being underweight is associated with an increased risk of death for those with stage III disease; being overweight or obese is associated with a decreased risk for those with stage IV disease. Stage III patients who were underweight were older (mean age, 75.7 years) and more likely to have either moderate or severe comorbidity (66.7%) as measured by the ACE-27 index.29a For these patients, being underweight could be a marker of frailty. These underweight, frail patients had decreased biologic reserve and, hence, decreased capacity to compensate for the physical demands imposed by the cancer. In contrast, being overweight/obese was a measure of better overall health for patients with stage IV disease. These overweight/obese patients with stage IV disease were younger (mean age, 63.6 years) and more likely to have no or mild comorbidity (ACE-27 index, 54.2%). Therefore, being overweight or obese for stage IV patients was simply a marker of better health, which translated into a better capacity to withstand the symptoms associated with cancer.
The present study has several strengths. The method of comorbidity assessment, comprehensive medical record review, is superior to other methods of assessment (eg, the use of administrative data).30 Another positive feature is the long follow-up period of the study population. Each participant had the potential to be observed for a minimum of more than 5 years from the end of accrual to termination of the study. A limitation of the study is the more than 20-year period for entry onto the study. In an effort to account for improvements in patient care that occurred during this time, the data were adjusted for year of surgery. Nonetheless, there may be differences in the probability of survival between patients who entered the study earlier compared with later that were not sufficiently accounted for by this adjustment.
In summary, the results support the inclusion of comorbidity information in cancer research studies. The assessment of comorbidity minimizes its impact as a potential source of bias, leading to more accurate estimates of effect. All three indices were significantly associated with mortality, and the results were similar across the three models. Also, severe comorbidity burden is associated with decreased survival after surgery. In future research, the decision to use a particular comorbidity index should be guided by the goals of the study, the data available, and the resources necessary to acquire data. Comorbid diseases impact patient survival and should be assessed early in cancer management. Although there is some variation in the comorbidity tools in predicting mortality, the ACE-27 may be most applicable for colon carcinoma.
Acknowledgment
We thank Donald L. Hill, PhD, Division of Preventive Medicine, University of Alabama at Birmingham, Birmingham, AL, for his critical review of this article.
Footnotes
Supported in part by Grants No. U54-CA118948 and RO1-CA98932-01 (U.M.) from the National Institutes of Health (NIH)/National Cancer Institute (NCI). Also supported by an NIH/NCI Grant No. 5-R25-CA47888 (R.B.H.) as part of the Cancer Prevention and Control Training Program.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Robert B. Hines, John W. Waterbor, Upender Manne
Financial support: Robert B. Hines, Upender Manne
Administrative support: Robert B. Hines, John W. Waterbor, Gerald McGwin Jr, Upender Manne
Provision of study materials or patients: Robert B. Hines, James Posey, Upender Manne
Collection and assembly of data: Robert B. Hines, Chakrapani Chatla, Harvey L. Bumpers, James Posey, Upender Manne
Data analysis and interpretation: Robert B. Hines, Chakrapani Chatla, Harvey L. Bumpers, John W. Waterbor, Gerald McGwin Jr, Ellen Funkhouser, Christopher S. Coffey, James Posey, Upender Manne
Manuscript writing: Robert B. Hines, James Posey, Upender Manne
Final approval of manuscript: Robert B. Hines, Chakrapani Chatla, Harvey L. Bumpers, John W. Waterbor, Gerald McGwin Jr, Ellen Funkhouser, Christopher S. Coffey, James Posey, Upender Manne
REFERENCES
- 1.de Groot V, Beckerman H, Lankhorst GJ, et al. How to measure comorbidity: A critical review of available methods. J Clin Epidemiol. 2003;56:221–229. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 2.Berger DA, Megwalu II, Vlahiotis A, et al. Impact of comorbidity on overall survival in patients surgically treated for renal cell carcinoma. Urology. 2008;72:359–363. doi: 10.1016/j.urology.2008.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemmens VE, Janssen-Heijnen ML, Verheij CD, et al. Co-morbidity leads to altered treatment and worse survival of elderly patients with colorectal cancer. Br J Surg. 2005;92:615–623. doi: 10.1002/bjs.4913. [DOI] [PubMed] [Google Scholar]
- 4.Extermann M. Measurement and impact of comorbidity in older cancer patients. Crit Rev Oncol Hematol. 2000;35:181–200. doi: 10.1016/s1040-8428(00)00090-1. [DOI] [PubMed] [Google Scholar]
- 5.Yancik R, Ganz PA, Varricchio CG, et al. Perspectives on comorbidity and cancer in older patients: Approaches to expand the knowledge base. J Clin Oncol. 2001;19:1147–1151. doi: 10.1200/JCO.2001.19.4.1147. [DOI] [PubMed] [Google Scholar]
- 6.Yancik R, Wesley MN, Ries LA, et al. Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: A population-based study. Cancer. 1998;82:2123–2134. [PubMed] [Google Scholar]
- 7.Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23:3112–3124. doi: 10.1200/JCO.2005.00.141. [DOI] [PubMed] [Google Scholar]
- 8.Extermann M. Measuring comorbidity in older cancer patients. Eur J Cancer. 2000;36:453–471. doi: 10.1016/s0959-8049(99)00319-6. [DOI] [PubMed] [Google Scholar]
- 9.Payne JE, Meyer HJ. The influence of other diseases upon the outcome of colorectal cancer patients. Aust N Z J Surg. 1995;65:398–402. doi: 10.1111/j.1445-2197.1995.tb01767.x. [DOI] [PubMed] [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 11.Havlik RJ, Yancik R, Long S, et al. The National Institute on Aging and the National Cancer Institute SEER collaborative study on comorbidity and early diagnosis of cancer in the elderly. Cancer. 1994;74:2101–2106. doi: 10.1002/1097-0142(19941001)74:7+<2101::aid-cncr2820741718>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 12.Piccirillo JF. Importance of comorbidity in head and neck cancer. Laryngoscope. 2000;110:593–602. doi: 10.1097/00005537-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Greene FL. ed 6. New York, NY: Springer-Verlag; 2002. AJCC Cancer Staging Manual. [Google Scholar]
- 14.Compton C, Fenoglio-Preiser CM, Pettigrew N, et al. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer. 2000;88:1739–1757. doi: 10.1002/(sici)1097-0142(20000401)88:7<1739::aid-cncr30>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 15.National Institutes of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Obes Res. 1998;6(suppl 2):51S–209S. [PubMed] [Google Scholar]
- 16.Piccirillo JF, Tierney RM, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 17.Janssen-Heijnen ML, Maas HA, Houterman S, et al. Comorbidity in older surgical cancer patients: Influence on patient care and outcome. Eur J Cancer. 2007;43:2179–2193. doi: 10.1016/j.ejca.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 18.De Marco MF, Janssen-Heijnen ML, van der Heijden LH, et al. Comorbidity and colorectal cancer according to subsite and stage: A population-based study. Eur J Cancer. 2000;36:95–99. doi: 10.1016/s0959-8049(99)00221-x. [DOI] [PubMed] [Google Scholar]
- 19.Gomez SL, O'Malley CD, Stroup A, et al. Longitudinal, population-based study of racial/ethnic differences in colorectal cancer survival: Impact of neighborhood socioeconomic status, treatment and comorbidity. BMC Cancer. 2007;7:193. doi: 10.1186/1471-2407-7-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouellette JR, Small DG, Termuhlen PM. Evaluation of Charlson-Age Comorbidity Index as predictor of morbidity and mortality in patients with colorectal carcinoma. J Gastrointest Surg. 2004;8:1061–1067. doi: 10.1016/j.gassur.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 21.Rieker RJ, Hammer E, Eisele R, et al. The impact of comorbidity on the overall survival and the cause of death in patients after colorectal cancer resection. Langenbecks Arch Surg. 2002;387:72–76. doi: 10.1007/s00423-002-0291-0. [DOI] [PubMed] [Google Scholar]
- 22.Soares M, Salluh JI, Ferreira CG, et al. Impact of two different comorbidity measures on the 6-month mortality of critically ill cancer patients. Intensive Care Med. 2005;31:408–415. doi: 10.1007/s00134-005-2554-z. [DOI] [PubMed] [Google Scholar]
- 23.Sanabria A, Carvalho AL, Vartanian JG, et al. Validation of the Washington University Head and Neck Comorbidity Index in a cohort of older patients. Arch Otolaryngol Head Neck Surg. 2008;134:603–607. doi: 10.1001/archotol.134.6.603. [DOI] [PubMed] [Google Scholar]
- 24.Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345:1091–1097. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 25.Goodwin JS, Hunt WC, Humble CG, et al. Cancer treatment protocols: Who gets chosen? Arch Intern Med. 1988;148:2258–2260. [PubMed] [Google Scholar]
- 26.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Sanoff HK, Bleiberg H, Goldberg RM. Managing older patients with colorectal cancer. J Clin Oncol. 2007;25:1891–1897. doi: 10.1200/JCO.2006.10.1220. [DOI] [PubMed] [Google Scholar]
- 28.Yates JW. Comorbidity considerations in geriatric oncology research. CA Cancer J Clin. 2008;51:329–336. doi: 10.3322/canjclin.51.6.329. [DOI] [PubMed] [Google Scholar]
- 29.Keating NL, Landrum MB, Klabunde CN, et al. Adjuvant chemotherapy for stage III colon cancer: Do physicians agree about the importance of patient age and comorbidity? J Clin Oncol. 2008;26:2532–2537. doi: 10.1200/JCO.2007.15.9434. [DOI] [PubMed] [Google Scholar]
- 29a.Hines RB, Shanmugam C, Waterbor JW, et al. Effect of comorbidity and body mass index on colon cancer survival of African American and Caucasian patients. Cancer. doi: 10.1002/cncr.24598. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kieszak SM, Flanders WD, Kosinski AS, et al. A comparison of the Charlson comorbidity index derived from medical record data and administrative billing data. J Clin Epidemiol. 1999;52:137–142. doi: 10.1016/s0895-4356(98)00154-1. [DOI] [PubMed] [Google Scholar]