Abstract
Purpose
Notch pathway activation by mutations in either NOTCH1 and/or FBXW7 is one of the most common molecular events in T-cell acute lymphoblastic leukemia (T-ALL) and, in pediatric disease, predicts for favorable outcome. Their prognostic significance in adult T-ALL is unclear. We sought to evaluate the outcome according to mutation status of patients with adult T-ALL treated on the United Kingdom Acute Lymphoblastic Leukaemia XII (UKALLXII)/Eastern Cooperative Oncology Group (ECOG) E2993 protocol.
Methods
NOTCH1 and FBXW7 were screened by a combination of denaturing high-performance liquid chromatography and sequencing in 88 adult patients with T-ALL treated on the UKALLXII/ECOG E2993 protocol and compared with clinical characteristics and outcome.
Results
NOTCH1 and FBXW7 mutations were common (60% and 18%, respectively) and were not associated with age or WBC count. NOTCH1 heterodimerization domain mutations were associated with FBXW7 mutations (P = .02), and NOTCH1 proline, glutamic acid, serine, threonine (PEST) rich domain and FBXW7 mutations were mutually exclusive. There were an equal number of high- and standard-risk patients in the NOTCH1 and FBXW7 mutated (MUT) groups. Patients wild type (WT) for both markers trended toward poorer event-free survival (EFS; MUT v WT, 51% v 27%, P = .10; hazard ratio, 0.6). Analysis by each marker individually was not significantly predictive of outcome (NOTCH1 MUT v WT, EFS 49% v 34%, P = .20; FBXW7 MUT v WT, EFS 53% v 41%, P.72).
Conclusion
NOTCH1 and FBXW7 mutant-positive patients do not fare sufficiently well to warrant an individualized treatment approach in future studies.
INTRODUCTION
A risk-adapted approach to the treatment of patients with acute lymphoblastic leukemia (ALL) has the potential of improving survival in high-risk patients and reducing therapy-related long-term sequelae in those at low risk. Despite stratifying risk on established criteria such as presenting blast count, age, and cytogenetics,1–3 it remains difficult to predict patient outcome, particularly for adult T-cell ALL (T-ALL), for which cytogenetic data are less frequently informative.3 Consequently, molecular markers that can complement or supersede current strategies are needed.
Mutations in the NOTCH1 gene are one of the most common genetic abnormalities found in T-ALL, affecting more than 50% of patients,4,5 and are thought to activate a broad range of anabolic routes6 and oncogenic pathways, including those involving c-Myc,6,7 mTOR,8,9 and NFKB.10 Mutations affect two hotspots—the extracellular heterodimerization domain (HD), where mutations lead to ligand-independent cleavage, and C-terminal proline, glutamic acid, serine, threonine (PEST) rich domain truncating mutations, where binding to the negative regulator FBXW7 is disrupted, prolonging half-life of intracellular NOTCH1 (ICN1).4,5,11 Approximately 20% of patients acquire both types of mutations in cis that synergistically activate signaling,4 suggesting there is a selective pressure on T-ALL cells to continually increase Notch signal strength. Additionally, activating mutations in the juxtamembrane (JM) domain have been reported in a minority of patients.12 Recently, mutations in the E3 ubiquitin ligase FBXW7 gene have also been described that are thought to activate Notch signaling by preventing ICN1 ubiquitination and degradation, akin to NOTCH1 PEST domain mutations.11,13–15
Pediatric studies have shown an excellent outcome in NOTCH1 mutated (MUT) patients14,16; on the Acute Lymphoblastic Leukemia-Berlin-Frankfurt-Münster (ALL-BFM) protocol, NOTCH1 MUT patients had an event-free survival (EFS) of 90%, compared with 71% in wild-type (WT) patients.16 The prognostic impact of NOTCH1/FBXW7 mutation status in adult T-ALL is controversial; a Chinese study showed adult patients with MUT NOTCH1 fared worse than WT patients,17 whereas the converse has recently been reported on the Lymphoblastic Acute Leukemia in Adults (LALA)-94 and Group for Research on Adult Acute Lymphoblastic Leukemia 2003 (GRAALL-2003) protocol.18 Considering the relatively small numbers of patients reported thus far and differences in treatment approach among different trials, we sought to address whether adult patients with NOTCH1 and/or FBXW7 mutations treated on the Medical Research Council (MRC) United Kingdom Acute Lymphoblastic Leukaemia XII (UKALLXII)/Eastern Cooperative Oncology Group (ECOG) E2993 trial also faired sufficiently well, such that they might avoid treatment intensification/transplantation in future MRC/ECOG trials.
METHODS
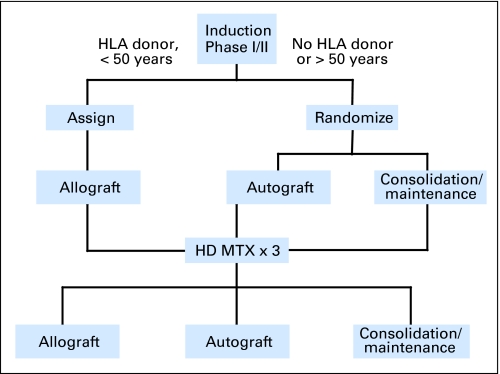
Patients were treated according to the MRC UKALLXII/ECOG E2993 protocol as previously reported (Fig 1).19 Consent was obtained from all patients at trial entry according to the Declaration of Helsinki. The study was in accordance with local and multicenter research ethics committee approval. T-cell phenotype was confirmed by flow cytometry at local centers (UKALL) or centrally (ECOG).
Fig 1.
A simplified algorithm of the United Kingdom Acute Lymphoblastic Leukaemia XII/Eastern Cooperative Oncology Group E2993 treatment protocol. HD-MTX, high-dose methotrexate.
DNA was obtained from diagnostic bone marrow samples of 88 adult patients with T-ALL (UKALLXII, n = 54; ECOG2993, n = 34) entered onto the trial between 1993 and 2005. Patients were selected according to the availability of sufficient DNA for the molecular analyses. Polymerase chain reaction products of the NOTCH1 HD-N (exon 26), HD-C (exon 27), JM (exon 28), transactivation domain (TAD), and PEST domains (exon 34), and WD40 domain of FBXW7 (exons 8 to 12) were screened by denaturing high-performance liquid chromatography (UKALL patients) or bidirectional sequencing (ECOG patients) as previously described.12,20 For denaturing high-performance liquid chromatography, polymerase chain reaction products were denatured and slow cooled to encourage heteroduplex formation and were analyzed by the WAVE DNA Fragment Analysis System (Transgenomic, Elancourt, France). Abnormal chromatograms were confirmed by repeat analysis, and samples were sequenced. One low-level mutation that could not be detected by sequencing was purified using the fragment collector facility of the WAVE and sequenced.
Statistical Analysis
The association between NOTCH1 and FBXW7 mutation status with age (< 35 years v ≥ 35 years) and WBC count (< 30 × 109/L v 30 to 99 × 109/L v ≥ 100 × 109/L) at diagnosis were investigated using Fisher's exact test in 2 × 2 tables and Mantel-Haenszel test for trend. Outcome was analyzed according to overall survival (OS) and EFS, the latter defined as time to relapse or death. Kaplan-Meier curves were used to assess survival, and differences between groups were compared using the log-rank test. Multivariate analyses were performed using the Cox model. All P values quoted are two-sided.
RESULTS
Clinical Features of Patient Cohort
The cohort analyzed was representative of all the patients with T-ALL entered onto the clinical trial in terms of sex and median age, but had a higher presenting WBC count (Table 1). Median follow-up was shorter, but complete remission rates, OS, and EFS were similar. In the cohort analyzed, complete remission was achieved in 97% of patients (33 of 35 WT and 52 of 53 NOTCH1/FBXW7 MUT patients, P= .56). The NOTCH1 WT and MUT groups received similar treatment (WT v MUT, seven [20%] v 16 [30%] sibling allografts; three [9%] v seven [13%] autografts; three [9%] v three [6%] matched unrelated donor allografts; 18 [51%] v 25 [47%] chemotherapy with maintenance alone; χ2 P = .69). There was no significant association with WBC count or age according to either NOTCH1 or FBXW7 mutational status (Mantel-Haenszel test for trend, P > .1 for each case).
Table 1.
Characteristics of Patient Cohort Versus Those Not Tested
| Characteristic | Patients With Notch1/FBXW7 Mutation Data | Other UKALLXII T-Cell Patients | P |
|---|---|---|---|
| Total, No. | 88 | 268 | |
| Male sex | .8 | ||
| No. | 65 | 195 | |
| % | 74 | 73 | |
| Age, years | .8 | ||
| Median | 30.5 | 28.5 | |
| Range | 16-60 | 15-60 | |
| WBC, × 109/L | .003 | ||
| Median | 50 | 31 | |
| Range | 1-653 | 0.6-541 | |
| Median follow-up, years | 3.6 | 7.9 | < .0001 |
| Achieved remission | .3 | ||
| No. | 85 | 251 | |
| % | 97 | 94 | |
| Survival, * | 49.4 | 41.3 | .98 |
| Event-free survival, * | 42.7 | 44.9 | .88 |
Abbreviation: UKALLXII, United Kingdom Acute Lymphoblastic Leukaemia XII.
Percent at 5 years; P value = log-rank over all follow-up.
NOTCH1/FBXW7 Mutation Incidence and Features
Of the 88 patients with T-ALL analyzed, 53 patients (60%) had at least one NOTCH1 mutation, 36 patients had a mutation in the HD only, eight patients had a mutation in the PEST domain only, six patients had mutations in both HD and PEST domains, and three patients had JM expansion mutations (Table 2). The mutation rate was similar in the UKALL and ECOG cohorts (59% v 62%). These results are comparable with previous studies in adult and pediatric patients.4,5,12,16,18 Notably, all three insertions in the JM expansion contained the amino acid sequence QLHF, as has been found in the Jurkat cell line and the majority of reported primary T-ALL cases.12
Table 2.
Association of NOTCH1 and FBXW7 Mutation Status of 88 Adult Patients With T-ALL
| NOTCH1 | FBXW7 WT | FBXW7 MUT |
|---|---|---|
| NOTCH1 WT | 30 | 5 |
| NOTCH1 HD only | 25 | 11 |
| NOTCH1 JME | 3 | 0 |
| NOTCH1 PEST only | 8 | 0 |
| NOTCH1 HD + PEST | 6 | 0 |
Abbreviations: T-ALL, T-cell acute lymphoblastic leukemia; WT, wild type; HD, heterodimerization domain; PEST, proline, glutamic acid, serine, threonine rich domain; JME, juxtamembrane expansion mutation.
Sixteen patients (18%) had an FBXW7 mutation (seven with R465C, three with R505C, two with R479Q, two with R479L, one with R465H, and one with G423V), and, of note, all except one of these mutations altered conserved arginine residues in the WD40 domain thought to be responsible for binding to the NOTCH1 PEST domain. Consistent with this finding, FBXW7 mutations and NOTCH1 PEST mutations were mutually exclusive (Table 2). There was a positive association between having a mutation in the NOTCH1 HD only and an FBXW7 mutation, the combination of which has been shown to be synergistically activating (11 of 36 patients with HD-only mutations were FBXW7 mutant v five of 52 other patients; Fisher's exact test P = .02).14
Patient Outcome According to Mutation Status
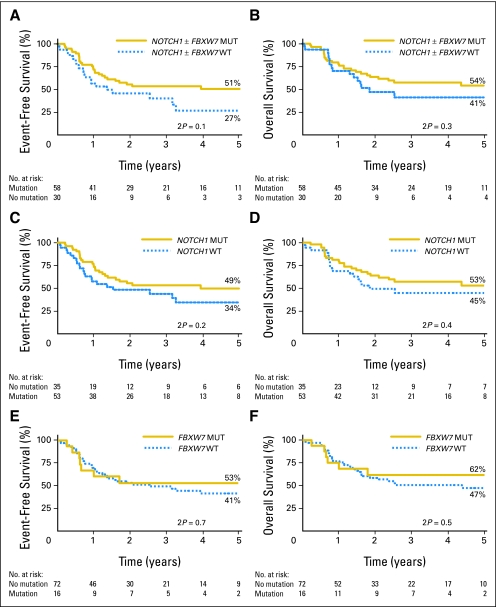
Importantly, standard- and high-risk patients were evenly distributed between the MUT and WT groups (by WBCs, 61% of patients with WBCs < 100 × 109/L were NOTCH1 MUT v 62% patients with WBCs ≥ 100 ×109/L, Fisher's exact test P = 1.0; by age, 55% of patients ≤ 35 years of age were NOTCH1 MUT v 69% of patients > 35 years of age, P = .37). The overall outcome of all patients on the trial has recently been reported.21 Comparison of outcome in patients with a mutation in the Notch pathway (NOTCH1 and/or FBXW7) with those without showed a trend toward improved outcome in those with a mutation. At 5 years, EFS and 95% CIs were 51% ± 14% versus 27% ± 19% (P = .10) in MUT and WT patients, respectively (Fig 2A; hazard ratio, 0.6; 95% CI, 0.3 to 1.1). The OS rates at 5 years were 54% ± 14% and 41% ± 20%, respectively (P= .30; Fig 2B). Analysis of patients with or without a NOTCH1 mutation revealed a 5-year EFS of 49% ± 15% versus 34% ± 18% (P= .2; Fig 2C) and OS of 53% ± 15% versus 45% ± 17% (P = .41; Fig 2D), respectively. Comparison of patients with or without an FBXW7 mutation showed a 5-year EFS at 53% ± 26% versus 41% ± 13% (P = .72; Fig 2E) and OS of 62% ± 24% versus 47% ± 12% (P = .51; Fig 2F). On the trial as a whole, treatment, age, and WBCs significantly influenced outcome.19,21 Of these, only treatment received was significant in the Cox model (P = .03) in this smaller cohort of patients with T-ALL, and inclusion of these variables did not materially effect EFS (Table 3). There were insufficient data to test the effect of transplantation by means of a donor versus no donor comparison. Standard-risk patients with a NOTCH1/FBXW7 mutation did not fair significantly differently from standard-risk WT patients (EFS = 47.7% for WBCs < 100 × 109/L v 30.3%, P = .5; 45.5% for age < 35 years v 39.3%, P = .6), and there was no interaction between effects of mutation and risk group.
Fig 2.
Outcome of adult patients with T-cell acute lymphoblastic leukemia treated on United Kingdom Acute Lymphoblastic Leukaemia XII/Eastern Cooperative Oncology Group E2993 protocol stratified by NOTCH1 and FBXW7 mutational status. (A) Event-free survival (EFS) by NOTCH1 and/or FBXW7 mutation. (B) Overall survival (OS) by NOTCH1 and/or FBXW7 mutation. (C) EFS by NOTCH1 mutation. (D) OS by NOTCH1 mutation. (E) EFS by FBXW7 mutation. (F) OS by FBXW7 mutation. MUT, mutated; WT, wild type.
Table 3.
Multivariate Analyses of Event-Free Survival
| Model | Hazard Ratio for Variable Shown in Italics | 95% CI |
|---|---|---|
| NOTCH1/FBXW7 | 0.62 | 0.34 to 1.11 |
| NOTCH1/FBXW7, age, log(WBC+1) | 0.66 | 0.34 to 1.29 |
| NOTCH1/FBXW7, age, log(WBC+1), treatment* | 0.73 | 0.37 to 1.44 |
| NOTCH1 | 0.70 | 0.39 to 1.24 |
| NOTCH1, age, log(WBC+1) | 0.77 | 0.42 to 1.42 |
| NOTCH1, age, log(WBC+1), treatment* | 0.85 | 0.45 to 1.61 |
| FBXW7 | 0.85 | 0.38 to 1.91 |
| FBXW7, age, log(WBC+1) | 0.91 | 0.40 to 2.08 |
| FBXW7, age, log(WBC+1), treatment* | 0.87 | 0.36 to 2.13 |
First remission transplantation or chemotherapy (n = 84).
Considering the marked in vitro synergistic activation of the Notch pathway by dual HD and PEST mutations or dual HD and FBXW7 mutations,4,14 we analyzed the outcome of this synergistic subgroup versus the WT patients. The EFS was 62% versus 30%, respectively, but this did not reach statistical significance (P = .17). Overall, our data show a trend toward improved EFS in patients with a Notch pathway mutation, but did not identify a subgroup of patients with a significantly favorable outcome to warrant treatment reduction on future trials.
DISCUSSION
Despite risk-stratification in adult ALL by WBC count, age, and neurologic involvement, there remains marked heterogeneity in outcome among standard- and high-risk patients. Although minimal residual disease kinetics using T-cell receptor rearrangements are likely to contribute significantly toward stratification protocols in the future,22,23 minimal residual disease in adult T-ALL is less robust at predicting prognosis and identifying early relapse than it is in adult B-ALL, or pediatric B- or T-ALL.24–26 Subsequently, simple mutation screening strategies that predict outcome could prove invaluable in clinical decision making in adult T-ALL, particularly in evaluation of patients for allogeneic hematopoietic stem-cell transplantation.
Here we show a high incidence of activating mutations in the NOTCH1 (60%) and FBXW7 genes (18%) in adult T-ALL, similar to that seen in pediatric T-ALL cohorts,7,16 and this indicates that the presence of these mutations, in themselves, is unlikely to explain the disparity in outcome seen between these age groups. There was a significant positive association between having a NOTCH1 mutation in the HD domain only and an FBXW7 mutation and a strong negative association between having a NOTCH1 PEST mutation and FBXW7 mutation. This observation is consistent with the hypothesis that NOTCH1 HD and FBXW7 mutations act in concert, similar to dual HD and PEST mutations.11,14 Although FBXW7 also targets c-Myc for degradation,11,27 the association described here favors the concept that FBXW7 mutations are acquired by T-ALL cells primarily as a means of increasing NOTCH1 signal strength. If FBXW7 mutations were acquired by tumor cells predominantly to upregulate c-Myc, they would likely be found in conjunction with PEST mutations in some cases.
Although our data did not show a significantly improved outcome in those patients with mutations in the Notch pathway, the trend is in accord with data previously presented in pediatric patients treated on the ALL-BFM protocol16 and, more recently, in adults on the French LALA-94 and GRAALL-2003 trials,18 and in contrast to the association with poor prognosis reported in adults by Zhu et al.17 Other molecular markers have been reported to have some impact on prognostic outcome in adult T-ALL. For example, TLX1 mRNA upregulation has been shown to be associated with an improved prognosis.28 It was not possible to evaluate this in our cohort because of lack of RNA samples from all patients. However, patients with TLX1 upregulation constitute a minority of patients and are strongly associated with NOTCH1 mutations (20 of 21 patients with TLX1 upregulation were NOTCH1 MUT in the French study).18 NOTCH1/FBXW7 has also been shown to be prognostically important independently of TLX1.18 Together, these data suggest it is valid to evaluate patient outcome according to NOTCH1/FBXW7 status alone. Furthermore, when the three most robust prognostic factors on the overall UKALLXII/ECOG trial were taken into account (age, WBCs, and treatment arm), the differences in EFS and OS were unaffected, and the NOTCH1/FBXW7 MUT and WT groups were equally balanced in regard to the percentages of standard- and high-risk patients.
Although we found no significant difference in outcome in NOTCH1 or FBXW7 MUT patients when analyzed individually, the combined NOTCH1/FBXW7 MUT group showed a trend toward improved outcome (P = .1), highlighting the importance of the addition of FBXW7 status to that of NOTCH1. Thus unlike the situation of FLT3 mutations in acute myeloid leukemia, where activating the same receptor by either point mutation or internal tandem duplication is associated with a diametric prognostic outcome,29 the suggestion in T-ALL may be that it is Notch pathway activation itself that is important in determining treatment response.
To date, this is the largest adult cohort of T-ALL patients treated on a single trial addressing outcome by NOTCH1/FBXW7 mutation status. Although the data show a nonsignificant difference between the MUT and WT groups, it is compatible with the MUT group having a better prognosis, as shown by Asnafi et al,18 and much larger studies of adult patients will be required to demonstrate this unequivocally. The current study has 80% power to detect a 30% increase in EFS. The data on this adult cohort do show that, even if there is a better outcome in those with a Notch pathway mutation, the magnitude of the improvement in EFS is likely to be too low to consider de-intensification of therapy in this group of patients.
Footnotes
Supported by the United Kingdom Medical Research Council (M.R.M.), Leukemia Research Fund (R.E.G. and D.C.L.), and National Institutes of Health (Grants No. R01CA120196 and R01CA129382 to A.F.); the WOLF Foundation (A.F.); the Leukemia and Lymphoma Society (Grants No. 1287-08 and 6237-08 to A.F.); and the Charlotte Geyer Foundation (A.F.). A.F. is a Leukemia & Lymphoma Society Scholar.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical Trials repository link available on JCO.org.
Clinical trial information can be found for the following: NCT00002514.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Marc R. Mansour, Rosemary E. Gale, Adolfo A. Ferrando, David C. Linch
Provision of study materials or patients: Marc R. Mansour, Maria L. Sulis, Veronique Duke, Letizia Foroni, Rosemary E. Gale, Elisabeth Paietta, Jacob M. Rowe, Martin S. Tallman, Anthony H. Goldstone, Adolfo A. Ferrando, David C. Linch
Collection and assembly of data: Marc R. Mansour, Maria L. Sulis, Veronique Duke, Letizia Foroni, Sarah Jenkinson, Kenneth Koo, Christopher G. Allen, Rosemary E. Gale, Georgina Buck, Sue Richards, Jacob M. Rowe, Martin S. Tallman, Anthony H. Goldstone, Adolfo A. Ferrando
Data analysis and interpretation: Marc R. Mansour, Maria L. Sulis, Rosemary E. Gale, Georgina Buck, Sue Richards, Jacob M. Rowe, Martin S. Tallman, Anthony H. Goldstone, Adolfo A. Ferrando, David C. Linch
Manuscript writing: Marc R. Mansour, Rosemary E. Gale, Adolfo A. Ferrando, David C. Linch
Final approval of manuscript: Marc R. Mansour, Letizia Foroni, Rosemary E. Gale, Georgina Buck, Sue Richards, Jacob M. Rowe, Martin S. Tallman, Anthony H. Goldstone, Adolfo A. Ferrando, David C. Linch
REFERENCES
- 1.Hoelzer D, Thiel E, Loffler H, et al. Prognostic factors in a multicenter study for treatment of acute lymphoblastic leukemia in adults. Blood. 1988;71:123–131. [PubMed] [Google Scholar]
- 2.Chessells JM, Hall E, Prentice HG, et al. The impact of age on outcome in lymphoblastic leukemia. MRC UKALL X and XA compared: A report from the MRC Paediatric and Adult Working Parties. Leukemia. 1998;12:463–473. doi: 10.1038/sj.leu.2400959. [DOI] [PubMed] [Google Scholar]
- 3.Moorman AV, Harrison CJ, Buck GAN, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): Analysis of cytogenetics data from patients treated on the Medical Research Council (MRC) UKALLXII/ Eastern Cooperative Oncology Group (ECOG) 2993 Trial. Blood. 2007;109:3189–3197. doi: 10.1182/blood-2006-10-051912. [DOI] [PubMed] [Google Scholar]
- 4.Weng AP, Ferrando AA, Lee W, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 5.Mansour MR, Linch DC, Foroni L, et al. High incidence of NOTCH1 mutations in adult patients with T-cell acute lymphoblastic leukemia. Leukemia. 2006;20:537–539. doi: 10.1038/sj.leu.2404101. [DOI] [PubMed] [Google Scholar]
- 6.Palomero T, Lim WK, Odom DT, et al. NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proc Natl Acad Sci U S A. 2006;103:18261–18266. doi: 10.1073/pnas.0606108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weng AP, Millholland JM, Yashiro-Ohtani Y, et al. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev. 2006;20:2096–2109. doi: 10.1101/gad.1450406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan SM, Weng AP, Tibshirani R, et al. Notch signals positively regulate activity of the mTOR pathway in T cell acute lymphoblastic leukemia. Blood. 2007;110:278–286. doi: 10.1182/blood-2006-08-039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palomero T, Sulis ML, Cortina M, et al. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nat Med. 2007;13:1203–1210. doi: 10.1038/nm1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vilimas T, Mascarenhas J, Palomero T, et al. Targeting the NF-kappaB signaling pathway in Notch1-induced T-cell leukemia. Nat Med. 2007;13:70–77. doi: 10.1038/nm1524. [DOI] [PubMed] [Google Scholar]
- 11.Thompson BJ, Buonamici S, Sulis ML, et al. The SCFFBW7 ubiquitin ligase complex as a tumor suppressor in T cell leukemia. J Exp Med. 2007;204:1825–1835. doi: 10.1084/jem.20070872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sulis ML, Williams O, Palomero T, et al. NOTCH1 extracellular juxtamembrane expansion mutations in T-ALL. Blood. 2008;112:733–740. doi: 10.1182/blood-2007-12-130096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maser RS, Choudhury B, Campbell PJ, et al. Chromosomally unstable mouse tumours have genomic alterations similar to diverse human cancers. Nature. 2007;447:966–971. doi: 10.1038/nature05886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malyukova A, Dohda T, von der Lehr N, et al. The tumor suppressor gene hCDC4 is frequently mutated in human T-cell acute lymphoblastic leukemia with functional consequences for Notch signalling. Cancer Res. 2007;67:5611–5616. doi: 10.1158/0008-5472.CAN-06-4381. [DOI] [PubMed] [Google Scholar]
- 15.Song JH, Schnittke N, Zaat A, et al. FBXW7 mutation in adult T-cell and B-cell acute lymphocytic leukemias. Leuk Res. 2008;32:1751–1755. doi: 10.1016/j.leukres.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 16.Breit S, Stanulla M, Flohr T, et al. Activating NOTCH1 mutations predict favorable early treatment response and long-term outcome in childhood precursor T-cell lymphoblastic leukemia. Blood. 2006;108:1151–1157. doi: 10.1182/blood-2005-12-4956. [DOI] [PubMed] [Google Scholar]
- 17.Zhu YM, Zhao WL, Fu JF, et al. NOTCH1 mutations in T-cell acute lymphoblastic leukemia: Prognostic significance and implication in multifactorial leukemogenesis. Clin Cancer Res. 2006;12:3043–3049. doi: 10.1158/1078-0432.CCR-05-2832. [DOI] [PubMed] [Google Scholar]
- 18.Asnafi V, Agnes Buzyn A, Sandrine Le Noir S, et al. NOTCH1/FBXW7 mutation identifies a large subgroup with favourable outcome in adult T-cell acute lymphoblastic leukemia (T-ALL): A GRAALL study. Blood. doi: 10.1182/blood-2008-10-184069. epub ahead of print on December 23, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Rowe JM, Buck G, Burnett AK, et al. Induction therapy for adults with acute lymphoblastic leukemia: Results of more than 1500 patients from the international ALL trial—MRC UKALLXII/ECOGE2993. Blood. 2005;106:3760–3767. doi: 10.1182/blood-2005-04-1623. [DOI] [PubMed] [Google Scholar]
- 20.Mansour MR, Duke V, Foroni L, et al. NOTCH1 mutations are secondary events in some patients with T-cell acute lymphoblastic leukemia. Clin Cancer Res. 2007;13:6964–6969. doi: 10.1158/1078-0432.CCR-07-1474. [DOI] [PubMed] [Google Scholar]
- 21.Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: Final results of the International ALL Trial (MRC UKALL XII/ECOG E2993) Blood. 2008;111:1827–1833. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- 22.Rafft, Gokbuget N, Luschen S, et al. Molecular relapse in adult standard-risk ALL patients detected by prospective MRD monitoring during and after maintenance treatment: Data from the GMALL 06/99 and 07/03 trials. Blood. 2007;109:910–915. doi: 10.1182/blood-2006-07-037093. [DOI] [PubMed] [Google Scholar]
- 23.Brüggemann M, Raff T, Flohr T, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107:1116–1123. doi: 10.1182/blood-2005-07-2708. [DOI] [PubMed] [Google Scholar]
- 24.Gameiro P, Mortuza FY, Hoffbrand AV, et al. Minimal residual disease monitoring in adult T-cell acute lymphoblastic leukemia: A molecular based approach using T-cell receptor and gene rearrangements. Haematologica. 2002;87:1126–1134. [PubMed] [Google Scholar]
- 25.Szczepański T, van der Velden VHJ, Raff T, et al. Comparative analysis of T-cell receptor gene rearrangements at diagnosis and relapse of T-cell acute lymphoblastic leukemia (T-ALL) shows high stability of clonal markers for monitoring of minimal residual disease and reveals the occurrence of second T-ALL. Leukemia. 2003;17:2149–2156. doi: 10.1038/sj.leu.2403081. [DOI] [PubMed] [Google Scholar]
- 26.Willemse MJ, Seriu T, Hettinger K, et al. Detection of minimal residual disease identifies differences in treatment response between T-ALL and precursor B-ALL. Blood. 2002;99:4386–4393. doi: 10.1182/blood.v99.12.4386. [DOI] [PubMed] [Google Scholar]
- 27.O'Neil J, Grim J, Strack P, et al. FBW7 mutations in leukemic cells mediate NOTCH pathway activation and resistance to gamma-secretase inhibitors. J Exp Med. 2007;204:1813–1824. doi: 10.1084/jem.20070876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrando AA, Neuberg DS, Dodge RK, et al. Prognostic importance of TLX1 (HOX11) oncogene expression in adults with T-cell acute lymphoblastic leukaemia. Lancet. 2004;363:535–536. doi: 10.1016/S0140-6736(04)15542-6. [DOI] [PubMed] [Google Scholar]
- 29.Mead AJ, Linch DC, Hills RK, et al. FLT3 tyrosine kinase domain mutations are biologically distinct from and have a significantly more favorable prognosis than FLT3 internal tandem duplications in patients with acute myeloid leukemia. Blood. 2007;110:1262–1270. doi: 10.1182/blood-2006-04-015826. [DOI] [PubMed] [Google Scholar]