Abstract
Background:
Because retinal and cerebral arterioles share similar pathologic processes, retinal microvascular changes are expected to be markers of cerebral small vessel disease (SVD). To better understand the role of SVD in cognitive function, we investigated the relationship between retinal microvascular abnormalities and longitudinal changes in cognitive function in a community-based study.
Methods:
A total of 803 participants underwent 4 cognitive assessments between 1990–1992 and 2004–2006, using the Word Fluency (WF) test, Digit Symbol Substitution (DSS), and Delayed Word Recall as well as retinal photography in 1993–1995. Covariate adjusted random effects linear models for repeated measures were used to determine the associations of cognitive change with specific retinal vascular abnormalities.
Results:
Individuals with retinopathy showed declines in executive function and psychomotor speed, with 1) an average decline in WF of −1.64 words per decade (95% confidence interval [CI] −3.3, −0.02) compared to no decline in those without retinopathy +0.06 (95% CI −0.6, 0.8) and 2) a higher frequency of rapid decliners on the DSS test.
Conclusion:
Signs of retinal vascular changes, as markers of the cerebral microvasculature, are associated with declines in executive function and psychomotor speed, adding to the growing evidence for the role of microvascular disease in cognitive decline in the elderly.
GLOSSARY
- ARIC
= Atherosclerosis Risk in Communities;
- AV
= arteriovenous;
- BP
= blood pressure;
- CES-D
= Center for Epidemiologic Studies–Depression scale;
- CI
= confidence interval;
- CRAE
= central retinal artery equivalent;
- CRVE
= central retinal vein equivalent;
- DSS
= digit symbol substitution;
- DWR
= delayed word recall;
- MABP
= mean arterial blood pressure;
- SVD
= small vessel disease;
- WF
= word fluency.
Historically, the term vascular dementia has been used to describe a stepwise deterioration in cognitive function following large vessel clinical strokes often termed multi-infarct dementia.1,2 Recently, studies with newer MRI techniques, or extensive pathologic evaluation, have led to consensus on the greater importance of cerebral small vessel disease (SVD) in the development of cognitive impairment.3–6
A noninvasive alternative method to study cerebral SVD involves evaluation of retinal blood vessels with standardized interpretation using retinal photographs.7 Retinal and cerebral arterioles have a common embryologic origin, similar autoregulation and histologic properties,8 and are subject to similar arteriolosclerosis pathology.8–11 We hypothesize, therefore, that retinal vascular markers will be associated with cognitive decline, thus providing an estimate of the effect on cerebral SVD on cognition. While cross-sectional studies have reported associations between retinal vascular abnormalities and cognitive impairment,12,13 we are not aware of any other information on cognitive decline.
In the current analysis, we examined prospectively the association of retinal vascular abnormalities with cognitive decline over 14 years of follow-up in the Atherosclerosis Risk in Communities (ARIC) Study cohort. We tested 3 different cognitive domains—word fluency, digit symbol substitution, and delayed word recall—and hypothesized that word fluency and digit symbol substitution as tests of executive function and psychomotor speed would be more sensitive to microvascular changes than delayed word recall.
METHODS
Study population.
Participants in this study were a subset of the ARIC cohort who, over a period of 14 years between 1990–1992 and 2004–2006, had 4 cognitive assessments (called CA1–CA4), 2 as part of the main ARIC study and 2 as part of an ancillary Brain MRI Study. Details of the ARIC study sampling and study design have been published.14 Institutional review boards approved the ARIC study, and all participants provided written informed consent.
The main ARIC study examined 15,792 women and men age 45 to 64 years from 4 US communities between 1987 and 1989. At the second examination (1990–1992), 14,348 individuals underwent the cognitive testing that provides the baseline cognitive assessment (CA1) for this report. Retinal photographs were offered to all 12,887 cohort members at visit 3 (1993–1995). At that time, a subset of 2,891 participants age 55 or older from 2 communities, Forsyth County, NC, and Jackson, MS, were invited to be part of the Brain MRI ancillary study and had a second cognitive assessment (CA2), and 1,920 completed the examination. All ARIC participants underwent cognitive testing (CA3) at visit 4 (1996–1998). All of the 1,920 who completed cerebral MRI scans in 1993–1995 were invited to a final cognitive testing (CA4) between 2004 and 2006, 14 years after CA1, and 1,134 successfully completed CA4, whereas 268 had died and 518 refused or were ineligible (because of MRI contraindications). Those who completed the CA4 visit were more likely to be women, healthier (less likely to have diabetes, hypertension, and incident stroke) than those excluded, had fewer retinal abnormalities, and tended to have higher cognitive scores at CA1. Of the 1,134 individuals who completed CA4, 331 were missing one or more retinal measurements or data on key covariates, leaving 803 for this study.
Retinal grading and definitions.
As described in detail,15,16 retinal photographs were graded by trained, certified graders masked to participant characteristics. “Any retinopathy” was defined as present if any of the following lesions were detected: microaneurysms, soft or hard exudates, retinal hemorrhages, macular edema, intraretinal microvascular abnormalities, venous beading, new vessels, vitreous hemorrhage, disc swelling, or laser photocoagulation scars. Focal narrowing and arteriovenous (AV) nicking were also noted. Retinal arteriolar and venular diameters were quantified via a computer-assisted technique. The photographs were digitized, and the diameters of individual arterioles and venules coursing through a specified zone surrounding the optic disc were measured. To obtain the central retinal artery equivalent (CRAE) and central retinal vein equivalent (CRVE), measurements of individual arterioles and venules were combined according to the following formulas:
In which Wc is the caliber of the trunk vessel, Wa is the caliber of the smaller branch, and Wb is the caliber of the larger branch. Pairing of vessels was done arbitrarily combining the largest with the smallest vessel and then the next largest with next smallest and so on until all vessels were accounted.15 In this study, generalized arteriolar narrowing was defined as the lowest 25% of the CRAE distribution (cutoff value of 154 μm). Venular widening was defined as the largest 25% of the CRVE distribution (cutoff value of 206.5 μm). Intragrader and intergrader K statistics were 0.89 and 0.76 for retinopathy, 0.64 and 0.59 for AV nicking, and 0.58 and 0.44 for focal narrowing, and intraclass correlation coefficients (the proportions of the total variance due to between-individual variance) were 0.80 and 0.68 for CRAE and 0.84 and 0.73 for CRVE.17
Cognitive function tests.
Participants were administered 3 neuropsychological tests at the 4 time points, CA1–CA4: the Delayed Word Recall Test (DWR), Word Fluency Test (WF), and Digit Symbol Substitution Test (DSS).18 The DWR is a test of verbal learning and recent memory that requires recall of 10 common nouns after a 5-minute interval during which another test is given. Test-retest reliability over 6 months has been reported to be 0.75 in normal elderly individuals.18 The DSS requires timed translation of numbers to symbols using a key. It measures psychomotor speed and is relatively unaffected by intellectual ability, memory, or learning. It is scored as the total number of numbers correctly translated to symbols within 90 seconds.19 Test-retest reliability over 2–5 weeks has been reported as 0.82 in middle-aged persons. The WF test is a measure of executive functioning, assessing the spontaneous production of words beginning with a given letter. Participants are asked to generate as many words as possible beginning with 3 letters and given 60 seconds for each letter. The score is the total number of acceptable words produced. Test-retest reliability is high (0.74) for up to 5 years in older adults.20
Definition of other variables.
Cardiovascular risk factors were measured at CA1 (visit 2). Education was determined at visit 1 and categorized dichotomously (≤12 vs >12 years) and in 3 groups (<12, 12–16, and >16 years). Medication usage was determined at each visit. Diabetes mellitus was defined as a fasting glucose ≥126 mg/dL, nonfasting glucose of ≥200 mg/dL, or self-reported history or current treatment for diabetes. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic BP ≥90 mm Hg, or recent use of antihypertensive medications. Mean arterial blood pressure (MABP) was calculated as the average of 1/3 systolic blood pressure (BP) + 2/3 diastolic BP at CA1 and CA2. Cigarette smoking was recorded as never, former, or current smoker. APOE genotyping was performed with the TaqMan assay (Applied Biosystems, Foster City, CA) and classified here as presence or absence of an ε4 allele.
Prevalent strokes were self-reported, and incident strokes were verified by an ARIC clinician reviewing abstracted hospital records.21 At CA4, depressive symptoms were assessed using an 11-item version of the Center for Epidemiologic Studies–Depression scale (CES-D).22
Statistical analysis.
Rates of decline for each cognitive test were distributed approximately normally. To test the association between each retinal variable and cognitive decline rate, we used random-effects linear models for repeated measures (PROC MIXED, SAS software 9.1; SAS Institute, Cary, NC). The basic model consisted of the retinal variables of interest, age (centered at 50 years), gender, race (black and non-black), time of follow-up (converted to per decade change), and interaction of time with each variable. Thus, the term for time refers to the 10-year change in cognitive score for the reference group, and the interaction of each variable with time reflects the additional effect of that variable on the rate of change on cognitive scores. The final model included cardiovascular risk factors known to be associated with retinal variables and cognitive change. Because of the importance of hypertension and diabetes, we ran models using 2 variables together to control for high BP and diabetes, namely MABP and the hypertension definition, as well as glucose level and the DM definition. The inclusion of MABP or glucose level did not alter the association of cognitive decline with retinal vascular variables, so the dichotomous definitions of hypertension and DM alone were used in final models.
To evaluate the retinal associations with larger cognitive declines, we defined rapid decliners as individuals who were among the 10% with the greatest declines, with the following cutoffs (expressed as a change in test score per decade with the more negative values indicating steeper declines): DWR test ≤−2.1, DSS test ≤−9.3, WF test ≤−8.5. For each test, we used logistic regression to determine the odds of the most rapid cognitive decline associated with specific retinal variables. Models were repeated within strata of hypertension and diabetes status.
RESULTS
The 14-year follow-up cognitive assessment visit (CA4) was attended by 1,134 participants, 803 of whom had complete data on the retinal variables and key covariates from the first visit 11–14 years earlier. Table 1 compares the characteristics of the 803 individuals included to the 331 excluded from analysis. Those included were slightly younger, less likely to have hypertension or diabetes, and more likely to be white. Baseline cognitive test scores (CA1) were similar in the 2 groups. Table 2 shows the average cognitive test scores and average rate of decline per decade for those examined on each occasion.
Table 1 Baseline characteristics of the Atherosclerosis Risk in Communities study population, 1990–1992, who were included in or excluded from analysis of 14-year cognitive change
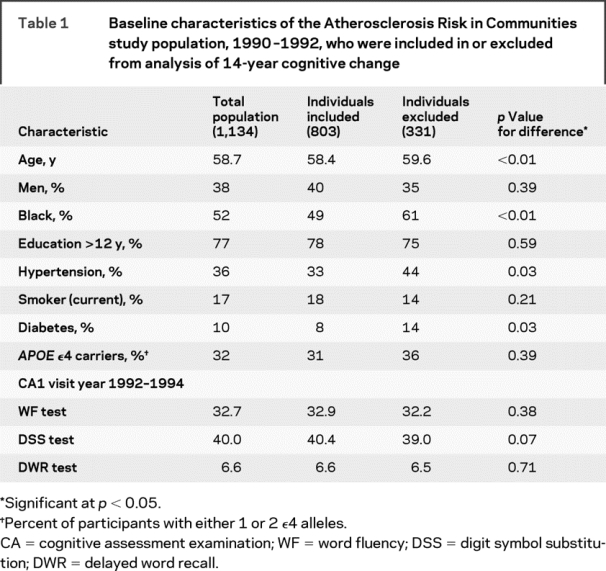
Table 2 Cognitive scores among Atherosclerosis Risk in Communities participants who underwent repeated cognitive testing over 14 years and had retinal data available
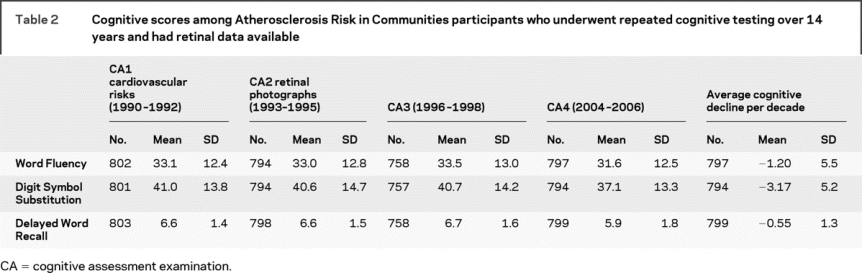
After adjustment for age, sex, race, field center, diabetes, hypertension, smoking, and APOE ε4 allele, cognitive scores on the WF test declined more in persons with than in those without retinal abnormalities (table 3). This difference was significant for any retinopathy, for retinal microaneurysms specifically, and for retinal focal arteriolar narrowing. The results were similar in a more limited model adjusted only for age, sex, race, and field center (data not shown). Retinopathy and focal narrowing were still associated with greater decline in mean WF scores in persons who were either not diabetic (16 with retinopathy) or not hypertensive (30 with retinopathy). These associations were found to be in the same direction, though not significant, in the smaller sample of persons with diabetes or with hypertension. Retinal abnormalities were not associated with significant declines in mean DWR or DSS scores. On the WF test, over 14 years of follow-up, the reference group without retinal abnormalities had no decline in score, whereas those with specific retinal changes demonstrated substantial and significant declines in mean score (figure).
Table 3 Ten-year change in scores on Word Fluency Test (WF), Digit Symbol Substitution Test (DSS), and Delayed Word Recall Test (DWR) by select retinal characteristics measured at baseline in 1,134 Atherosclerosis Risk in Communities men and women followed from 1990–1992 until 2004–2006
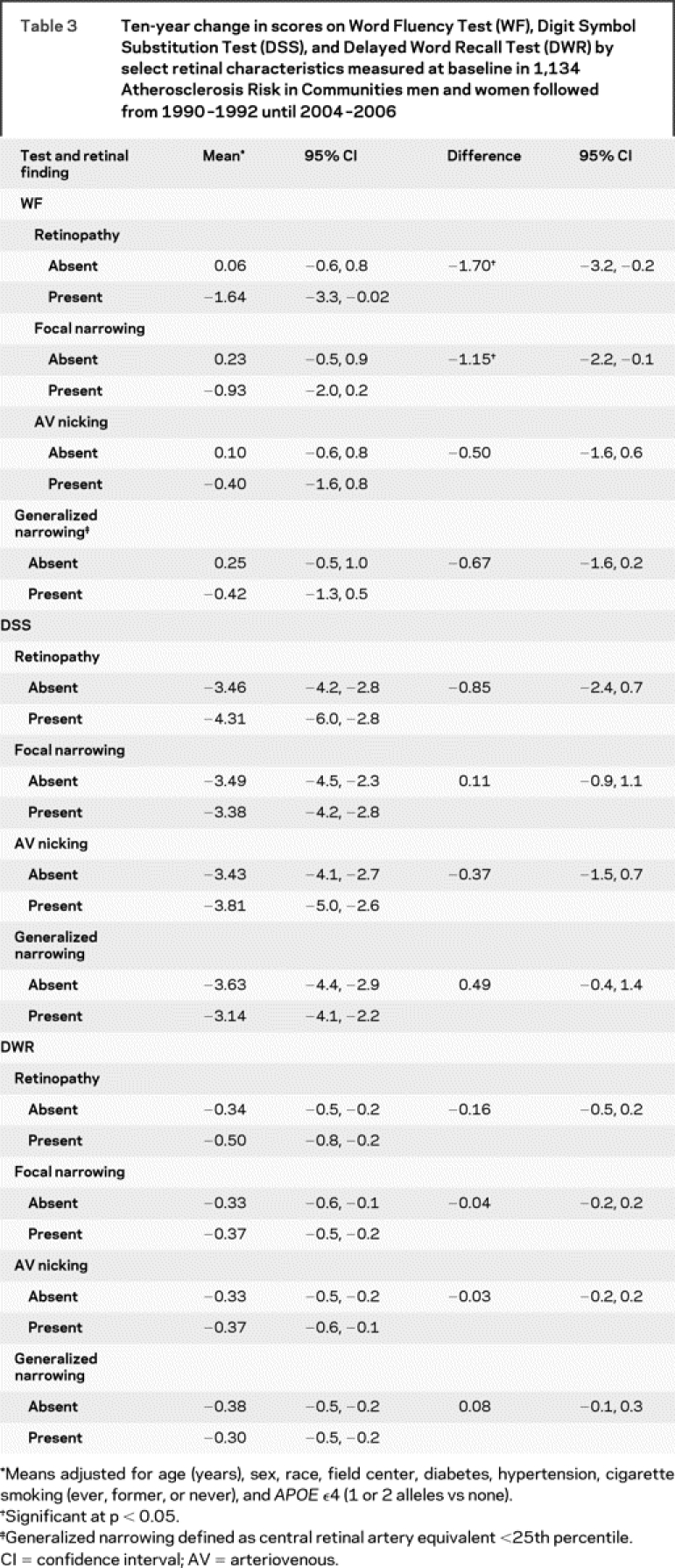
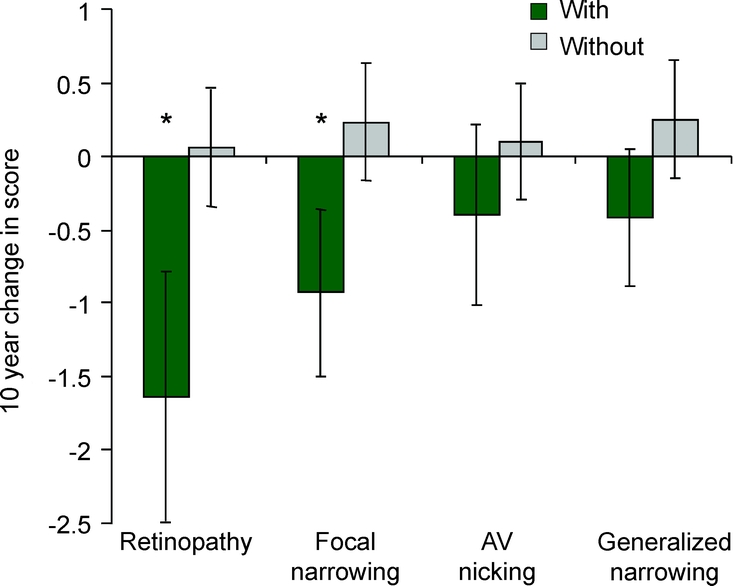
Figure Ten-year change in word fluency score in individuals with and without retinal abnormalities
*p value ≤0.05. AV = arteriovenous.
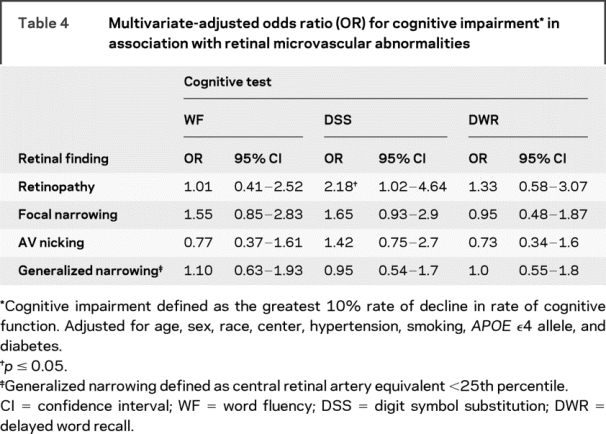
For rapid decliners, the DSS test demonstrated an association with retinopathy, with an OR of 2.18 (CI 1.02–4.64) (table 4).
Table 4 Multivariate-adjusted odds ratio (OR) for cognitive impairment* in association with retinal microvascular abnormalities
Neither the lowest quartile of arteriolar diameter nor the largest quartile of venular diameter was associated with cognitive declines (data not shown). Arteriolar and venular diameters also failed to show significant associations with cognitive decline when they were analyzed as continuous variables.
As education (classified either dichotomously or in 3 groups) was not significantly associated with either cognitive decline or retinal variables, it was not included in final models. We repeated the analyses excluding individuals with prevalent and incident stroke, those taking CNS-altering medications at either the baseline visit or CA4, those with depressive symptoms at CA4, and those with low cognitive scores (lowest 5%) at CA1. None of the associations reported was substantially altered by these exclusions.
We also repeated the analyses for subgroups stratified by age (<60 vs ≥60 years at baseline), gender, and race. Lacking a priori hypotheses, we considered interactions significant only if p ≤ 0.01. None met this level of significance.
DISCUSSION
In this prospective, population-based study, we found that retinopathy and focal retinal arteriolar narrowing in middle age were associated with significant declines in mean WF scores. In fact, in the reference group, i.e., women without retinopathy aged 50 years at baseline who did not smoke or have hypertension or diabetes, WF scores remained stable, whereas in risk factor-adjusted analyses, those with retinopathy demonstrated an average loss of 1.7 words per decade. For the DSS test, retinopathy was associated with rapid cognitive decline (OR = 2.18).
The retinal vascular signs studied are associated with diabetes and elevated blood pressure, which are established risk factors for cerebrovascular disease, especially the microvasculature.9,23,24 Of note, retinopathy is not just a disease of diabetes; 57% of ARIC participants with incident retinopathy signs did not have diabetes.25 The retinal-cognitive associations we found were not attenuated after adjusting for vascular risk factors and, despite smaller numbers, remained significant in those who did not have hypertension or did not have diabetes. The independence of these associations suggests that the degree of microvascular damage, rather than the risk factors underlying that pathology, is the more direct cause of the cognitive decline. Retinopathy signs, particularly microaneurysms, hemorrhages, and soft exudates, are believed to be the results of microvascular closure and loss of vascular integrity.26 These ischemic processes are likely in turn to damage the retinal tissues supplied.11,26 As retinal and brain microvasculature share common physiology and embryology, these retinal changes provide an indirect indicator of similar changes in the brain microvasculature, and the association we found between retinal changes and cognitive decline may also be interpreted as evidence of an ischemic effect. As our findings were not attenuated after excluding persons with recognized clinical strokes, ischemia from unrecognized cerebral SVD would appear to be the reasonable mechanistic interpretation of the cognitive associations we found.
To date, information about the microvascular contributions to cognitive impairments has arisen primarily from pathologic and brain imaging studies. MRI signs of cerebral SVD are consistently associated with cognitive impairment, even in persons with AD.27–29 However, pathologic and imaging studies have their limitations. Lacunar infarcts and white matter disease are common in elderly persons without cognitive impairment,30 and microscopic infarcts that affect cognition are not visualized by currently available neuroimaging techniques, so there are still no accepted standards for the lesion load seen on imaging that can unambiguously characterize a person’s cognitive impairment as vascular in origin.31 Microinfarcts, detectable only by direct microscopic examination of the brain,5 appear to contribute importantly to vascular dementia.4,5 But microinfarcts have been studied only recently, and it is not yet clear how to assess them adequately. Retinal vascular abnormalities have been shown to be strongly associated with lacunar infarcts, cerebral atrophy, and white matter hyperintensities,16,32 and in addition they share similar histologic properties with cerebral microvasculature; thus knowledge of their associations with cognitive change represents useful knowledge that may be added to that gained from imaging and pathology studies.
Cerebral SVD preferentially affects white matter and subcortical gray matter (thalamus and basal ganglia), with disruption of frontal-subcortical circuits. This results in difficulties with attention, planning, and response times for complex tasks, the hallmarks of executive function deficits.33,34 The greatest cognitive deficits in persons with cerebral SVD are seen in executive function and processing speed,35,36 rather than episodic memory, which is more related to AD.28,33 This SVD pattern was evident in the Rotterdam Study, where WMH and silent infarcts measured by MRI were associated with declines in processing speed and executive function.28 Our study employed 3 relevant cognitive tests. The WF test is believed to be a sensitive test of frontal lobe damage with deficits in executive functioning.37 The DSS test is a measure of psychomotor speed and executive function and has been demonstrated to show impairment in vascular dementias.33 The fact that these were the 2 tests we found associated with retinal abnormalities further supports the idea that retinal vascular changes predict the cognitive impairment which is specifically microvascular in origin. In contrast, we failed to find any association with decline in DWR, a test of memory more typically affected in AD.38
Two previous cross-sectional reports have shown associations of retinal vascular abnormalities with cognitive impairment.12,13 Interestingly, unlike our study, cross-sectional associations from the ARIC study were somewhat stronger for DWR than for DSS or WF.12 This inconsistency may pertain to the advantage of studying change in cognitive function over time. In a healthy young cohort, cognitive function is relatively stable and is heavily influenced by extrinsic factors such as socioeconomic status and education. However, longitudinal analysis tends to reduce the influence of extrinsic factors that cannot be completely accounted for with multivariate modeling. Indeed, we, like others,39 found no association of cognitive decline with educational level. Cognitive change is a better measure of currently progressing disease processes, and longitudinal analysis may explain why WF and DSS, as expected, were most strongly associated with retinopathy in the current report.
The study has several potential limitations. The exclusion of persons who tended to be black, diabetic, and older, and to have more retinal abnormalities, threatens the study’s generalizability. However, except for DSS, baseline mean test scores did not differ significantly between excluded and included groups. Patients lost to follow-up also tended to be older, black, hypertensive, and diabetic, but this may have made the associations we found conservative. The imprecision of retinal measurements results in nondifferential misclassification, which is also likely to attenuate the retinal associations we found. Severe proliferative retinopathy can produce visual impairment that could influence cognitive performance; however, this is unlikely to have affected our study (particularly the WF test): none of the participants had proliferative retinopathy, and of those with retinopathy, 90% had fewer than 5 microaneurysms and 70% had fewer than 4 retinal hemorrhages.
Our study findings suggest that retinopathy (a few small retinal microaneurysms or blot hemorrhages) and focal retinal arteriolar narrowing are indices of the type of cerebral vascular abnormalities which affect cognition in elderly persons. Of course, ongoing MRI studies will provide complementary information using a different modality. Our findings support the need for research on the potential cost-effectiveness of ophthalmoscopy in identifying persons with unexplained cognitive impairment who might benefit from risk reduction modification.
AUTHOR CONTRIBUTIONS
Statistical analysis was performed by Suzanne Lesage, MD (Assistant Professor, Department of Neurology), with guidance, selection of statistical methods, and review of results from Dianne Catellier, PhD (Research Associate Professor, Department of Biostatistics, University of North Carolina), and Stephen Cole, PhD, MPH (Associate Professor, Department of Epidemiology, University of North Carolina).
ACKNOWLEDGMENT
The authors thank the staff and participants of the ARIC study for their contributions.
DISCLOSURE
Dr. Mosley has received research support from the NIH [NHLBI R01-HL70825 (PI)]. Dr. Wong serves on scientific advisory boards of Pfizer, Novartis, and Allergan; serves on the editorial boards of Investigative Ophthalmology and Visual Science, Ophthalmic Epidemiology, the American Journal of Ophthalmology, and Diabetes Care; and receives research support from the NIH [NIH-NEI R03 EYO13939 (Co-PI) and NHLBI R21 HL077166-01 (PI)]. Dr. Szklo serves as Editor-in-Chief of the American Journal of Epidemiology and receives royalties for publishing Epidemiology: Beyond the Basics, Second Edition (Jones and Bartlett, 2007). Dr. Knopman serves as an Associate Editor for Neurology®; has served on a data safety monitoring board for Sanofi-Aventis Pharmaceuticals; is an investigator in a clinical trial sponsored by Elan Pharmaceuticals and Forest Pharmaceuticals; and receives research support from the NIH [R01-AG023195 (PI), R01-AG11378 (Co-Investigator), P50-AG 16574 (Mayo Alzheimer Disease Research Center), U01 AG 06786 (Mayo Alzheimer Disease Patient Registry), and R01 HL70825 (Co-Investigator).] Drs. Catellier and Cole report no disclosures. Dr. Klein serves on the scientific advisory board of Astra-Zeneca, the editorial advisory board of Ophthalmology, and on a data monitoring safety board for Allergan; serves as a consultant to Pfizer, Eli Lilly, Novartis, Genentech, Comentis, and Merck; and receives research support from the NIH [NEI 2 U10 EY006594-21A1 (PI), NEI 1 R01 HL69979 (PI), NEI 1 R01 EY016379 (PI), NIH/DK 1 R01 DK073217-01 (PI), and N01-AG-1-2100 (PI)]. Dr. Coresh receives research support from the NIH [N01-HC-55020 (PI), R01DK076770-01 (Co-Investigator), UL1-RR025005-01 (Co-Investigator), PA-07-070 (Co-investigator), 5U01DK067651 (PI), MM-0997-07/07 (Co-investigator), U01-HL-075572 (PI of subcontract), and T32HL007024 (PI).]. Dr. Coker receives research support from the NIH as a Co-investigator [N01-WH-4-4221 and N01HC95178]. Drs. Sharrett and Lesage report no disclosures.
Address correspondence and reprint requests to Dr. Suzanne Lesage, University of Maryland Medical Center, Department of Neurology, 22 S. Greene St., Baltimore, MD 21201 slesage@medicine.umaryland.edu
The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. This work was also supported by grant R01-HL70825.
Disclosure: Author disclosures are provided at the end of the article.
Received January 9, 2009. Accepted in final form June 16, 2009.
REFERENCES
- 1.Hachinski VC, Lassen NA, Marshall J. Multi-infarct dementia: a cause of mental deterioration in the elderly. Lancet 1974;2:207–210. [DOI] [PubMed] [Google Scholar]
- 2.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 2006;37:2220–2241. [DOI] [PubMed] [Google Scholar]
- 3.Esiri MM, Wilcock GK, Morris JH. Neuropathological assessment of the lesions of significance in vascular dementia. J Neurol Neurosurg Psychiatry 1997;63:749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White L, Petrovitch H, Hardman J, et al. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann NY Acad Sci 2002;977:9–23. [DOI] [PubMed] [Google Scholar]
- 5.Ince PG, Fernando MS. Neuropathology of vascular cognitive impairment and vascular dementia. Int Psychogeriatr 2003;15 suppl 1:71–75. [DOI] [PubMed] [Google Scholar]
- 6.Staekenborg SS, van Straaten EC, van der Flier WM, Lane R, Barkhof F, Scheltens P. Small vessel versus large vessel vascular dementia: risk factors and MRI findings. J Neurol 2008;11:1644–1651. [DOI] [PubMed] [Google Scholar]
- 7.Liew G, Wang JJ, Mitchell P, Wong TY. Retinal vascular imaging: a new tool in microvascular disease research. Circ Cardiovasc Imaging 2008;1:156–161. [DOI] [PubMed] [Google Scholar]
- 8.Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat 2005;206:319–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goto I, Kimoto K, Katasuki S, Mimatsu T, Ikui H. Pathological studies on the intracerebral and retinal arteries in cerebrovascular and noncerebrovascular diseases. Stroke 1975;6:263–29. [DOI] [PubMed] [Google Scholar]
- 10.Haritoglou C, Rudolph G, Hoops JP, Opherk C, Kampik A, Dichgans M. Retinal vascular abnormalities in CADASIL. Neurology 2004;62:1202–1205. [DOI] [PubMed] [Google Scholar]
- 11.Gardiner TA, Archer DB, Curtis TM, Stitt AW. Arteriolar involvement in the microvascular lesions of diabetic retinopathy: implications for pathogenesis. Microcirculation 2007;14:25–38. [DOI] [PubMed] [Google Scholar]
- 12.Wong TY, Klein R, Sharrett AR, et al. Retinal microvascular abnormalities and cognitive impairment in middle-aged persons: the Atherosclerosis Risk in Communities Study. Stroke 2002;33:1487–1492. [DOI] [PubMed] [Google Scholar]
- 13.Baker ML, Marino Larsen EK, Kuller LH, et al. Retinal microvascular signs, cognitive function, and dementia in older persons: the Cardiovascular Health Study. Stroke 2007;38:2041–2047. [DOI] [PubMed] [Google Scholar]
- 14.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 15.Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosis Risk in Communities Study. Ophthalmology 1999;106:2269–2280. [DOI] [PubMed] [Google Scholar]
- 16.Wong TY, Klein R, Sharrett AR, et al. Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA 2002;288:67–74. [DOI] [PubMed] [Google Scholar]
- 17.Couper DJ, Klein R, Hubbard LD, et al. Reliability of retinal photography in the assessment of retinal microvascular characteristics: the Atherosclerosis Risk in Communities Study. Am J Ophthalmol 2002;133:78–88. [DOI] [PubMed] [Google Scholar]
- 18.Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol 1989;46:141–145. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler D. The Wechsler Adult Intelligence Scale– Revised. New York: Psychological Corp.; 1981. [Google Scholar]
- 20.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 1999;14:167–177. [PubMed] [Google Scholar]
- 21.Toole JF, Lefkowitz DS, Chambless LE, Wijnberg L, Paton CC, Heiss G. Self-reported transient ischemic attack and stroke symptoms: methods and baseline prevalence: the ARIC Study, 1987–1989. Am J Epidemiol 1996;144:849–856. [DOI] [PubMed] [Google Scholar]
- 22.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index: established populations for epidemiologic studies of the elderly: study design and methodology. J Aging Health 1993;5:179–193. [DOI] [PubMed] [Google Scholar]
- 23.Sharrett AR, Hubbard LD, Cooper LS, et al. Retinal arteriolar diameters and elevated blood pressure: the Atherosclerosis Risk in Communities Study. Am J Epidemiol 1999;150:263–270. [DOI] [PubMed] [Google Scholar]
- 24.Klein R, Sharrett AR, Klein BEK, et al. Are retinal arteriolar abnormalities related to atherosclerosis? The Atherosclerosis Risk in Communities Study. Arterioscler Thromb Vasc Biol 2000;20:1644–1650. [DOI] [PubMed] [Google Scholar]
- 25.Wong TY, Klein R, mirul Islam FM, et al. Three-year incidence and cumulative prevalence of retinopathy: the atherosclerosis risk in communities study. Am J Ophthalmol 2007;143:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bresnick GH. Nonproliferative diabetic retinopathy. In: Ryan SJ, ed. Retina, 2nd ed. St. Louis: Mosby; 1994:1277–1318. [Google Scholar]
- 27.Vermeer SE, Prins ND, Den HT, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215–1222. [DOI] [PubMed] [Google Scholar]
- 28.Prins ND, Van Dijk EJ, Den HT, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain 2005;128:2034–2041. [DOI] [PubMed] [Google Scholar]
- 29.van der Flier WM, van Straaten EC, Barkhof F, et al. Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke 2005;36:2116–2120. [DOI] [PubMed] [Google Scholar]
- 30.Fernando MS, Ince PG. Vascular pathologies and cognition in a population-based cohort of elderly people. J Neurol Sci 2004;226:13–17. [DOI] [PubMed] [Google Scholar]
- 31.Jellinger KA. The enigma of vascular cognitive disorder and vascular dementia. Acta Neuropathol (Berl) 2007;113:349–388. [DOI] [PubMed] [Google Scholar]
- 32.Longstreth WT, Jr., Larsen EK, Klein R, et al. Associations between Findings on Cranial Magnetic Resonance Imaging and Retinal Photography in the Elderly. Am J Epidemiol 2007;165:78–84. [DOI] [PubMed] [Google Scholar]
- 33.O’Sullivan M, Morris RG, Markus HS. Brief cognitive assessment for patients with cerebral small vessel disease. J Neurol Neurosurg Psychiatry 2005;76:1140–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tullberg M, Fletcher E, DeCarli C, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology 2004;63:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jokinen H, Kalska H, Mantyla R, et al. Cognitive profile of subcortical ischaemic vascular disease. J Neurol Neurosurg Psychiatry 2006;77:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verdelho A, Madureira S, Ferro JM, et al. Differential impact of cerebral white matter changes, diabetes, hypertension and stroke on cognitive performance among non-disabled elderly. The LADIS study. J Neurol Neurosurg Psychiatry 2007;78:1325–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lezak M. Neuropsychological Assessment, 3rd ed. New York: Oxford University Press; 1995. [Google Scholar]
- 38.Fleisher AS, Sowell BB, Taylor C, Gamst AC, Petersen RC, Thal LJ. Clinical predictors of progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology 2007;68:1588–1595. [DOI] [PubMed] [Google Scholar]
- 39.Albert M, Blacker D, Moss MB, Tanzi R, McArdle JJ. Longitudinal change in cognitive performance among individuals with mild cognitive impairment. Neuropsychology 2007;21:158–169. [DOI] [PubMed] [Google Scholar]


