A previously healthy 5-month-old girl was found face down on the bed by her grandmother. Two hours earlier she had acted normally and was placed on her back to nap. She was found to be apneic and pulseless by Emergency Medical Services and regained a pulse after 45 minutes of cardiopulmonary resuscitation.
She was born full-term via spontaneous vaginal delivery to a 30-year-old gravida 1 para 1. Her newborn screen was normal. By maternal report, she was meeting milestones. She had no recent infections and immunizations were up to date. She was breastfed exclusively. Her only medication was Poly-Vi-Sol with iron. There were no smokers or pets in her household. The parents were a mixed race (Asian/Caucasian) couple with no family history of cardiac or neurologic disease or sudden death.
Physical examination revealed reactive pupils but absent oculocephalic and corneal reflexes, gag, and cough. There was no withdrawal from pain or purposeful movement. She was not dysmorphic. Her liver was percussed 4 cm below the costal margin. A dilated ophthalmoscopic examination showed trace optic nerve head pallor but no other abnormality.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of a prolonged arrest in an infant includes respiratory, cardiac, infectious, neurologic, metabolic, and traumatic events. Cultures must be obtained for sepsis or meningoencephalitis. Cardiac conduction anomalies may be detected on EKG, although paroxysmal arrhythmias would not necessarily be detected after the event. While familial arrhythmia syndromes such as long-QT syndrome or Wolff-Parkinson-White syndrome may be revealed by family history, sudden cardiac death in children may also be due to sporadic mutations in cardiac ion channel genes and provoked by fever, as in Brugada syndrome. Structural heart disease, leading to apnea or embolism, can be evaluated by echocardiography. Metabolic or neuromuscular diseases causing respiratory distress may be associated with dysmorphisms, hypotonia, hypoglycemia, metabolic acidosis, ketosis, or hyperammonemia; evaluation should include serum amino acids, urine organic acids, and lactate and pyruvate levels. The newborn screen must be reviewed for inborn errors of metabolism; although not tested in all states, fatty acid oxidation disorders such as very-long-chain acyl-coA dehydrogenase deficiency can present with sudden death when cardiac energy metabolism becomes impaired (Pennsylvania screening information: www.perkinelmergenetics.com/newbornscreening.htm). Toxic screens are warranted even without a known ingestion. While status epilepticus may not be seen on EEG if extensive damage has occurred, neuroimaging may show underlying pathology provoking a seizure, such as strokes or hemorrhages, tumors, or traumatic brain injury. Nonaccidental trauma or smothering (unintentional or intentional) must be suspected in any sudden infant death.
CASE: MEDICAL AND PATHOLOGIC EVALUATION
Initial noncontrast head CT showed subtle midbrain hypodensities (figure). Laboratory studies revealed initial arterial pH of 7.03, arterial lactate of 4.2 mg/dL, and albumin of 2.9 mg/dL (norms: lactate 0.5–1.6 mmol/L; albumin 3.1–4.2 g/dL). The initial arterial lactate improved to 1.9 mg/dL prior to the lumbar puncture. CSF showed 8 white blood cells (47% monocytes, 33% lymphocytes), 2 red blood cells, protein 94 mg/dL, glucose 43 mg/dL (serum glucose 61 mg/dL), lactate 2.4 mmol/L (norms: CSF protein 15–40 mg/dL, glucose 32–82 mg/dL or 60% of serum, lactate 0.7–2.0 mmol/L; CSF leukocytes >6/mL is considered abnormal in children older than 3 months1). Serum ammonia was 13 μmol/L (norm: 9–33 μmol/L). Blood, urine, and CSF cultures showed no growth. CSF herpes simplex virus and enterovirus PCRs were negative. Serum and urine drug screens were negative for acetaminophen, salicylates, tricyclic antidepressants, ethyl alcohol, cannabinoids, opiates, cocaine, benzodiazepines, barbiturate, amphetamines, phencyclidine, and methadone. Her serum amino acids, urine organic acids, and acylcarnitine profile were all normal. Urine ketones were negative.
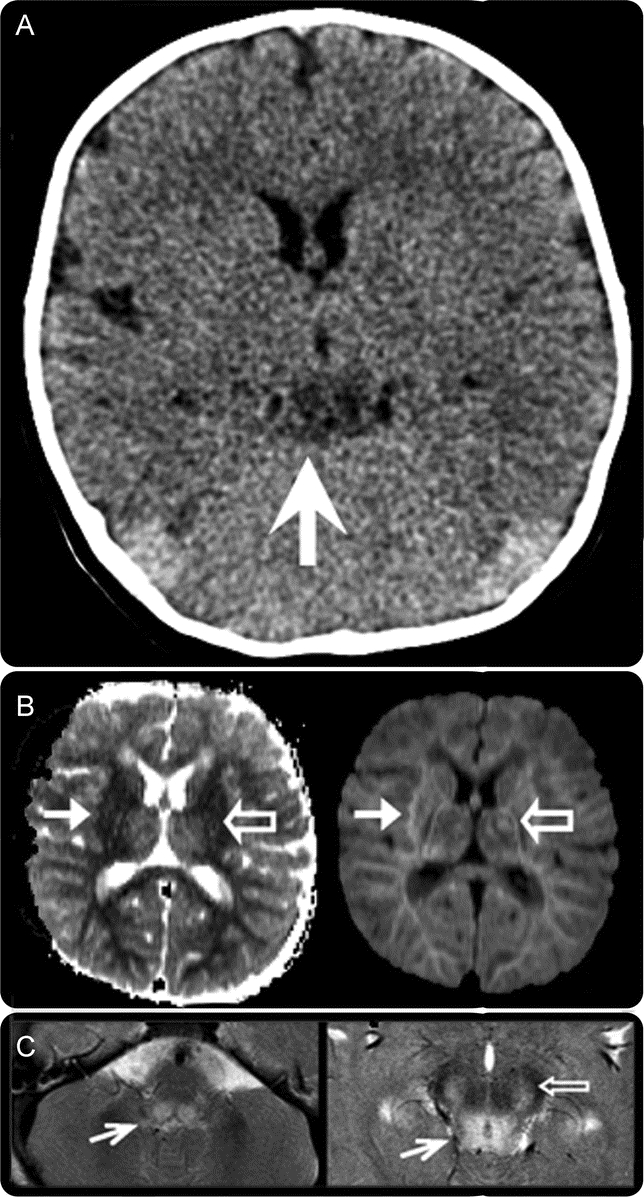
Figure Neuroimaging depicting lesions of different ages
Initial head CT showing hypodensities in the midbrain (white arrow, A). MRI of the brain showing an area of restricted diffusion in the left thalamus (open arrow, right panel, B) not correlating to the apparent diffusion coefficient map (open arrow, left panel, B), implying that this is an older lesion than the acute diffusion-restricting infarcts seen throughout the white matter (white arrows, B). Also seen are T2 hyperintensities in the dorsal midbrain (white arrow, left panel, C), periaqueductal grey matter (white arrow, right panel, C), and substantia nigra (open arrow, C).
Her initial EEG was flat with gradual return of some continuous activity over 48 hours. EKG showed no conduction defects and echocardiography revealed a structurally and functionally normal heart. Abdominal ultrasound revealed normal hepatic echotexture.
In addition to the extensive white matter infarcts typically seen after prolonged arrest, her brain MRI performed 3 days after her arrest also revealed infarcts of various ages in the left thalamus and the dorsal brainstem suspicious for a mitochondrial cytopathy (figure). A muscle biopsy was performed. Mitochondrial enzyme activities were within normal range. Quantitative mitochondrial DNA analysis showed a reduced amount of DNA, but the degree of reduction was not sufficient to diagnose a primary mitochondrial depletion syndrome (mtDNA sequencing, MitoMet oligo aCGH: Baylor College of Medicine; quantitative PCR, mitochondrial enzyme activities: Columbia University). Her parents decided to withdraw care, and her heart stopped beating shortly after extubation.
DISCUSSION
In the evaluation of an infant who has had a prolonged arrest, neurologists are frequently consulted. Determining the etiology is of critical importance given the risk to future siblings if there is genetically inherited disease or child abuse. The diagnosis of sudden infant death syndrome (SIDS) requires other causes to be ruled out; by definition, SIDS is any sudden unexplained death in an infant less than 1 year old, for which no cause can be found, despite thorough history and examination including autopsy and examination of the scene of death.2 One recent article proposed that the relative risk of recurrent SIDS, reported between 1.7 and 10.1, has been overestimated in part due to flawed investigations into these deaths.3 Too frequently the diagnosis of SIDS may be made before appropriate evaluations have been completed, and physicians may feel uncomfortable pursuing autopsies if parents object.
While the evaluation of sudden death in an infant requires autopsy, examination of the scene of death, and detailed history, the evaluation of an infant who has been resuscitated after a prolonged arrest is not clearly defined. More has been written on the diagnostic evaluation of an apparent life-threatening event (ALTE), defined as an acute change in breathing that was frightening to the caretaker including some combination of apnea, color change, change in muscle tone, choking, or gagging. While some SIDS deaths are due to respiratory causes, SIDS and ALTE are differentiated epidemiologically by multiple factors, including the decrease in SIDS, but not ALTE, after the Back to Sleep campaign.4 The differential diagnosis listed above for prolonged arrest is meant as a guide and is unlikely to be all-encompassing.
While several factors in this case suggested a metabolic disorder, including hepatomegaly, depressed albumin, and elevated lactate, these are nonspecific findings that could follow prolonged arrest. An MRI was obtained not because of any protocol necessitating neuroimaging after prolonged arrest, but because the family felt that they needed to see more evidence of brain damage before considering withdrawal of care. While profound hypoxia can produce ischemic lesions such as these, the varying ages of the diffusion-weighted imaging abnormalities in this case raised the suspicion of a mitochondrionopathy, especially given the location of these lesions in the dorsal brainstem and periaqueductal gray matter and that hypodensities were seen in the midbrain on CT on arrival.5
If resuscitation efforts had failed in our patient, she may have been classified as SIDS. She was found in the prone position, the most significant risk factor for SIDS, although she did not have other risk factors, such as male gender, African American or Native American race, prematurity, or exposure to secondhand smoke.6 In this case, an MRI that was intended to be prognostic actually widened the differential diagnosis, which had significant implications for genetic counseling. Parents should be informed that the risk of future children being affected, even when a specific genetic or metabolic cause has not been identified, is small but not trivial. This case illustrates that while the evaluation of an infant after prolonged arrest varies widely between institutions and from case to case, there may be a role for neuroimaging.
DISCLOSURE
Dr. Kranick, Dr. Ganesh, and C.R. Coughlin report no disclosures. Dr. Licht receives research support from the NIH [NINDS K23 NS052380 (Principal Investigator)] and the Dana Foundation and has served as a medical expert for defense and plaintiff attorneys.
Address correspondence and reprint requests to Dr. Sarah Kranick, Human Motor Control Section, National Institute of Neurological Disorders and Stroke, NIH, Bldg. 10, Rm. 7D42, 10 Center Dr., MSC 1428, Bethesda, MD 20892 mattes1@mail.nih.gov
Disclosure: Author disclosures are provided at the end of the article.
REFERENCES
- 1.Feigin RD, Pearlman E. Bacterial meningitis beyond the neonatal period. In: Feigin RD, Cherry JD, Demmler GJ, Kaplan SL, eds. Textbook of Pediatric Infectious Diseases, 5th ed. Philadelphia: Saunders; 2004:443. [Google Scholar]
- 2.Willinger M, James LS, Catz C. Defining the sudden infant death syndrome (SIDS): deliberation of an expert panel convened by the National Institute of Child Health and Human Development. Pediatr Pathol 1991;11:677–684. [DOI] [PubMed] [Google Scholar]
- 3.Bacon CJ, Hall DBM, Stephenson TJ, Campbell MJ. How common is repeat sudden infant death syndrome? Arch Dis Child 2008;93:323–326. [DOI] [PubMed] [Google Scholar]
- 4.Kiechl-Kohlendorfer U, Hof D, Peglow UP, Traweger-Ravanelli B, Kiechl S. Epidemiology of apparent life threatening events. Arch Dis Child 2005;90:297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saneto RP, Friedman SD, Shaw DWW. Neuroimaging of mitochondrial disease. Mitochondrion Epub 2008 May 23. [DOI] [PMC free article] [PubMed]
- 6.American Academy of Pediatrics Task Force on Sudden Infant Death Syndrome. The changing concept of sudden infant death syndrome: diagnostic coding shifts, controversies regarding the sleeping environment, and new variables to consider in reducing risk. Pediatrics 2005;116:1245–1255. [DOI] [PubMed] [Google Scholar]


