Abstract
Stress is a major driving force in reinstatement of drug-seeking behavior. The bed nucleus of the stria terminalis (BNST) has been identified as a key brain region in this behavior, and receives a dense input of the stress-neurotransmitter norepinephrine through the ventral noradrenergic bundle. Activation of α2-adrenergic receptors (α2-ARs) in the BNST blocks stress-induced reinstatement of drug-seeking, indicating a potentially important role for these receptors. Currently, it is unclear how α2-AR agonists elicit this behavioral action, or through which α2-AR subtype. Activation of α2-ARs decreases glutamatergic transmission in the BNST, an effect which is nearly absent in the α2A-AR knockout mouse. Here, we take advantage of a knockin mouse in which a hemagglutinin-tagged α2A-AR was inserted into the endogenous locus, along with the α2A-AR selective agonist guanfacine, to further study the role of the α2A-AR subtype in modulation of neurotransmission in the BNST. Using immunohistochemistry, we find that α2A-ARs are highly expressed in the BNST, and that this expression is more similar in distribution to the vesicular glutamate transporters than to either norepinephrine transporter or tyrosine hydroxylase positive terminals. Using whole cell patch-clamp recordings, we show that guanfacine causes a depression of evoked excitatory and, to a more limited extent, inhibitory fast synaptic transmission. In total, these data support a prominent heterosynaptic role for α2A-ARs in modulating fast synaptic transmission in the BNST.
Keywords: Addiction, norepinephrine, extended amygdala, anxiety, guanfacine, stress
Stress has been implicated as a major driving force in drug addiction, playing a significant role in relapse to drug- and alcohol seeking (Brown et al., 1995, Sinha et al., 1999, Le et al., 2000). Relapse to drug-seeking can be modeled in rodents using behavioral paradigms such as stress-induced reinstatement of drug-seeking (Shaham et al., 2000a) and conditioned place preference (Wang et al., 2001). The bed nucleus of the stria terminalis (BNST), part of a region known as the extended amygdala, has been suggested to play an important role in general anxiety and relapse to drug abuse (Walker et al., 2003). The BNST is well-situated to process inputs from other brain regions involved in stress and reward pathways, such as the insular and infralimbic cortices, hippocampus, and the central and basolateral nuclei of the amygdala (Weller and Smith, 1982, Chiba et al., 2001, Shin et al., 2008). The BNST sends projections to many brain regions, including the hypothalamus (Conrad and Pfaff, 1976, Swanson and Cowan, 1979), a region involved in the stress response; and the ventral tegmental area (VTA) (Swanson and Cowan, 1979, Georges and Aston-Jones, 2002), a region involved in reward and motivation. Thus, the BNST is an important center for processing inputs from stressful stimuli and integrating them into stress and reward circuitry.
The noradrenergic system is a key mediator of the stress response, and has been implicated in relapse to drug addiction. The BNST receives one of the densest noradrenergic (NE) inputs in the brain (Brownstein et al., 1974). This input arises primarily from the nucleus tractus solitaris (NTS) which makes up part of the ventral noradrenergic bundle (VNAB) (Aston-Jones et al., 1999). Lesions of the VNAB result in the inhibition of footshock-induced reinstatement of morphine-seeking (Wang et al., 2001) and heroin-seeking (Shalev et al., 2001). Withdrawal from chronic morphine treatment increases extracellular norepinephrine (NE) levels in the rat BNST (Fuentealba et al., 2000). Lesions of the VNAB have also been shown to block opiate withdrawal-induced conditioned place aversion (Aston-Jones et al., 1999). Also, mice lacking the enzyme dopamine beta-hydroxylase (DBH) do not exhibit morphine-induced conditioned place preference (CPP). Viral restoration of DBH in the NTS but not the locus coerulus (the source of NE in the dorsal noradrenergic bundle) of these mice can restore CPP for morphine, indicating the importance of NE in this paradigm (Olson et al., 2006). Importantly, NE has been shown to suppress neuronal firing in the BNST and studies indicate that the BNST output is under noradrenergic tone (Casada and Dafny, 1993, Forray et al., 1997, Dumont and Williams, 2004, Egli et al., 2005). In total, these data strongly implicate NE in the BNST in stress-reward related behaviors.
NE is known to act through β-adrenergic receptors (β-ARs), α1-adrenergic receptors (α1-ARs), and α2-adrenergic receptors (α2-ARs) in the BNST (Matsui and Yamamoto, 1984, Dumont and Williams, 2004, Egli et al., 2005, McElligott and Winder, 2008). Evidence suggests that α2-ARs within the BNST play important roles in stress-addiction interactions. Peripheral administration of blood-brain-barrier permeant α2-AR agonists blocks foot-shock induced reinstatement of heroin seeking (Shaham et al., 2000b), and alcohol-seeking in rats (Le et al., 2005). Intra-BNST injections of α2-AR agonists block morphine withdrawal-induced conditioned place aversion (Delfs et al., 2000), and stress-induced reinstatement of morphine-conditioned place preference (Wang et al., 2001). Intra-BNST injection of the α2-AR agonist clonidine also blocks stress-induced freezing behavior induced by the innate stressor trimethylthiazoline, a component of fox feces (Fendt et al., 2005).
Previous electrophysiology studies have implicated α2-ARs in the modulation of glutamatergic and potentially GABAergic transmission in the BNST. The non-subtype specific α2-AR agonists UK-14,304 and clonidine cause a decrease in evoked excitatory transmission in the BNST and this effect is absent in brain slices prepared from α2A-AR knockout (KO) mice (Egli et al., 2005). Paired-pulse ratios of evoked synaptic responses are increased by UK-14,304, suggesting a presynaptic modulation of glutamate release by α2-ARs (Egli et al., 2005). Although NE is known to modulate both glutamatergic and GABAergic transmission in the BNST through α1-AR and β-ARs (Dumont and Williams, 2004, Egli et al., 2005, McElligott and Winder, 2008), the modulation of inhibitory transmission by α2-ARs in naïve mice has not been extensively studied. The α2-AR partial agonist clonidine does not alter GABAA receptor-mediated spontaneous transmission in the ventral BNST, suggesting that α2-ARs may not modulate GABA transmission (Dumont and Williams, 2004).
There are three known α2-AR subtypes, α2A-AR, α2B-AR, and α2C-ARs, in mammalian brain tissues (Bylund et al., 1994). The α2A-AR has a widespread mRNA distribution in the brain including, but not limited to, regions involved in mediation of stress and anxiety, such as the NTS, amygdaloid complex, hypothalamus, and cortex (Scheinin et al., 1994, Wang et al., 1996). The α2C-AR has a similar though not as widespread mRNA distribution as compared to the α2A-AR, whereas α2B-AR mRNA expression is mainly limited to the thalamus (Scheinin et al., 1994, Wang et al., 1996). Studies have implicated the α2A-AR as a major subtype utilized by NE to modulate neuronal function in the brain, although the α2C-AR also plays a role in this modulation (Trendelenburg et al., 2001). Egli and others have shown that the effects of the α2-AR agonist UK-14,304 on excitatory transmission are absent in the dBNST and dramatically reduced in the vBNST of the α2A-AR knockout mouse (Egli et al., 2005). However, additional data indicate that actions of other ARs in BNST are also absent in these knockout mice, raising the possibility that these phenotypes may be more related to autoreceptor rather than heteroreceptor roles of the α2A-AR (Egli et al., 2005, McElligott and Winder, 2008). Similarly, hippocampal and cortical tissue from α2A/D-AR KO mice show substantial loss of α2-AR agonist-mediated inhibition of NE release, suggesting that these mice have disruptions in autoreceptor-mediated inhibition of NE (Trendelenburg et al., 1999, Trendelenburg et al., 2001). Recently, however, research in mice expressing the α2A-AR in only adrenergic neurons (autoreceptors), has suggested that many functions previously attributed to the role of autoreceptors such as analgesia, hypothermia, sedation, and anesthetic-sparing, may actually be mediated by α2A-ARs on non-noradrenergic neurons (heteroreceptors) (Gilsbach et al., 2009). Studies in the central nucleus of the amygdala also point to a heterosynaptic function of α2-ARs in the brain (Delaney et al., 2007). We have therefore focused our current studies on the α2A-AR subtype, with the hypothesis that heterosynaptic α2A-ARs modulate acute synaptic transmission in the BNST.
We have taken advantage of a hemagglutinin α2A-AR knockin (HA α2A-AR KI) mouse to localize the α2A-AR within the BNST. Using the α2A-AR selective agonist guanfacine, we have characterized the ability of α2A-ARs to modulate both excitatory and inhibitory transmission in the dorsal BNST. We provide evidence that the α2A-AR functions in the BNST as a heteroreceptor regulating glutamate release presynaptically, with a more modest action on GABAergic transmission.
Experimental Procedures
All procedures were performed according to Institutional Animal Care and Use Committee approved procedures.
Light Microscopy Level Immunohistochemistry Studies
Fluorescent Immunohistochemistry
A minimum of 10 adult male 6-14 week old C57BL/6J mice (Jackson Laboratory), a minimum of 10 male and female 6-14 week old HA-tagged α2A-AR knockin (KI) mice (backcrossed to C57BL/6 background for 10 generations, provided by Qin Wang at University of Alabama), and two male 6-14 week old NET KO mice (provided by Randy Blakely at Vanderbilt University) were transcardially perfused with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde. Brains were post-fixed in 4% paraformaldehyde for 2-3 days at 4°C, and were then transferred to 30% sucrose until fully impregnated. Following post-fixation, 30 μm coronal or 30 μm sagittal sections of brain were sliced on a cryostat (Leica CM3050S). Sections containing the BNST were then free-floated for immunolabeling. Sections were blocked with 4% normal donkey serum containing 0.2% Triton-X-100 in PBS. Sections were then incubated with primary antibody for 48 hours at 4°C, followed by incubation with cyanine dye- or Alexa fluorophore-conjugated secondary antibody for 24 hours at 4°C. Sections were mounted on slides, sealed with PolyAquamount, and left overnight to dry.
Reagents used
Mouse anti-HA from Chemicon was used at 1:500-1:1000, guinea-pig anti-VGLUT1 from Chemicon was used at 1:8000, mouse anti-NET contributed from Randy Blakely was used at 1:1000, rabbit anti-TH from Chemicon was used at 1:1000. Secondary antibodies used from Jackson ImmunoResearch included: cy2- and cy3-conjugated donkey anti-mouse (1:1000), cy5-conjugated donkey anti-guinea pig (1:2000), and cy3-conjugated donkey anti-rabbit (1:1000). Secondary antibodies used from Molecular Probes included: Alexa 568-conjugated goat anti-mouse IgG2b and Alexa 488-conjugated goat anti-mouse IgG1.
In the first immunohistochemistry experiment (Figure 2), mouse anti-HA was used at 1:500 and cy2-conjugated donkey anti-mouse was used at 1:1000. In the second immunohistochemistry experiment (Figure 4A-C), mouse anti-HA (IgG1) was used at 1:1000 and mouse anti-NET (IgG2b) was used at 1:1000. Alexa-488-conjugated goat anti-mouse IgG1 was used at 1:1000, and Alexa-568-conjugated goat anti-mouse IgG2b was used at 1:1000. In the third immunohistochemistry experiment (Figure 4D-F, 5D-F), mouse anti-HA was used at 1:1000 and rabbit anti-TH was used at 1:1000. Cy2-conjugated donkey anti-mouse was used at 1:2000, and cy3-conjugated donkey anti-rabbit was used at 1:1000. In the final immunohistochemistry experiment (Figure 4G-I, 5A-C), mouse anti-HA was used at 1:1000 and guinea pig anti-VGLUT1 was used at 1:8000. Cy2-conjugated donkey anti-mouse was used at 1:1000, and cy5-conjugated donkey anti-guinea pig was used at 1:2000.
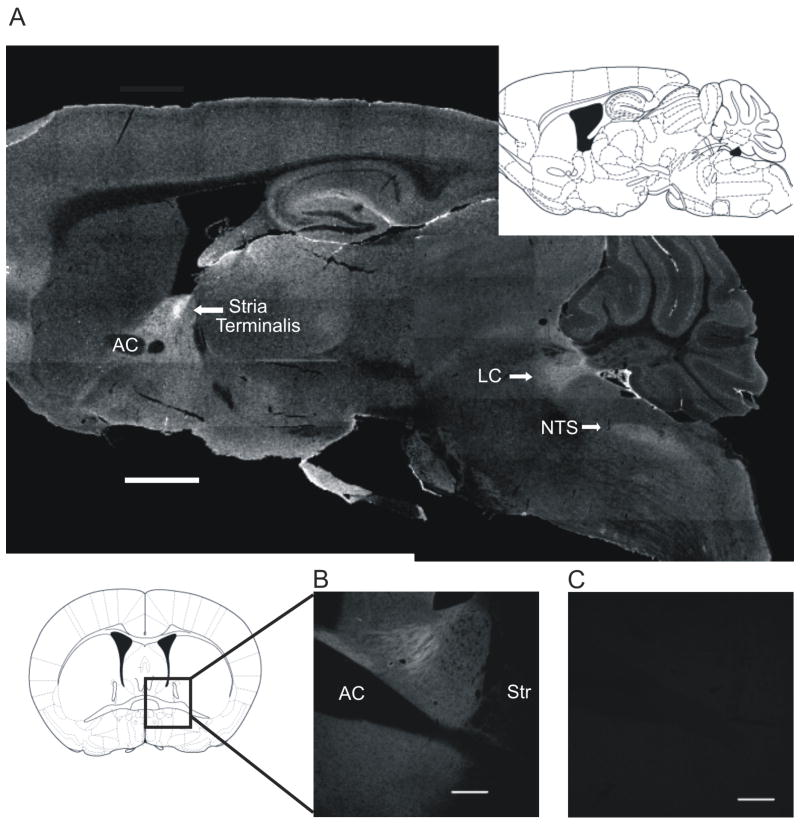
Figure 2.
α2A-ARs are enriched in the BNST. A) Parasaggital section of the HA α2A-AR knockin (KI) mouse showing HA labeling, and corresponding Franklin-Paxinos image at 0.96 mm lateral to midline (scale bar = 1 mm). B) Coronal section of the HA α2A-AR KI mouse bed nucleus of the stria terminalis (BNST) showing labeled HA (scale bar = 200 μm), and corresponding Franklin-Paxinos image at (+) 0.14 mm from bregma. C) Corresponding wildtype C57BL6/J mouse image showing lack of labeling with HA antibody (scale bar = 200 μm).
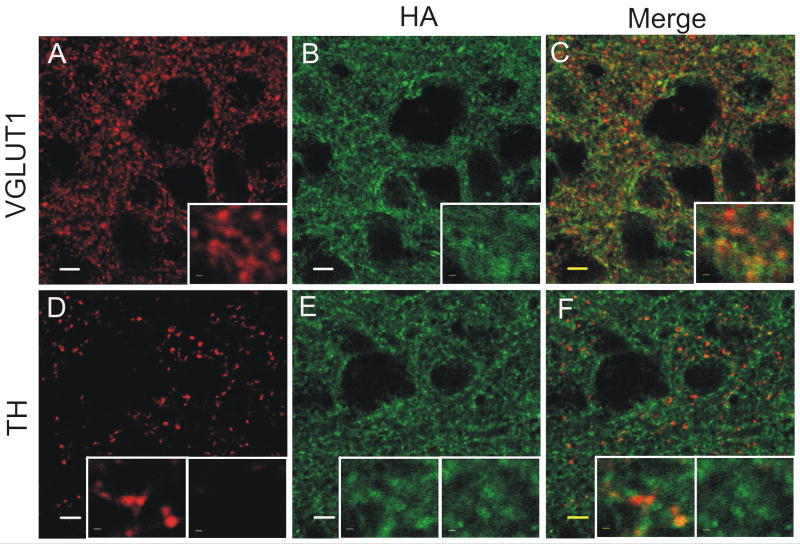
Figure 4.
α2A-AR distribution in the BNST is dissimilar to norepinephrine transporter (NET) and tyrosine hydroxylase (TH) distribution but is similar to vesicular gluatamate transporter 1 (VGLUT1) distribution. (A-I) 2×2 tiles of coronal sections containing the BNST, scale bars represent 200 μm. A) NET labeling in the BNST with inset of the NET KO mouse. B) HA labeling in the BNST. C) Merge of NET and HA. D) TH labeling in the BNST. E) HA labeling in the BNST. F) Merge of TH and HA. G) VGLUT1 labeling in the BNST. H) HA labeling in the BNST. I) Merge of VGLUT1 and HA.
Figure 5.
α2A-AR distribution is in the BNST is broader than the distribution of tyrosine hydroxylase (TH), but is similar to the distribution of vesicular glutamate transporter 1 (VGLUT1). (A-F) High power image of coronal sections containing the dorsal lateral BNST, scale bars represent 5 μm. Inset scale bars are 0.5 μm. Inset boxes measure 6.9 μm (width) × 5.6 μM (height). A) VGLUT1 labeling in the dBNST. B) HA labeling in the dBNST. C) Merge of VGLUT1 and HA. D) TH labeling in the dBNST. E) HA labeling in the dBNST. F) Merge of TH and HA.
Imaging
Images were taken with an LSM510 confocal microscope using a 10x objective and a 63x/1.4 NA planapochromat objective (Carl Zeiss, Inc.). Laser excitation was kept constant between genotypes and primary/no primary controls. High-power (63x) images (optical sections) were taken at 0.5 μm focus intervals. Under conditions identical for those used to obtain sample images, we imaged microscopic fluorescent latex beads (Multispeck, Molecular Probes, Inc.) with spectral properties similar to the three sample probes (Cy2, Cy3, and Cy5) in order to calibrate the three-channel registration and subsequently compensate for instrument-induced positional shifts in the data. Measured shifts were minor and used to apply precise offsets to the image data using the LSM software, thus providing accurate probe positions to within the diffraction limit of the microscope (less than 0.25 μm in x and y). Using CorelDraw12, images in Figure 2 were converted to monochrome, and intensity, brightness, and contrast were altered to allow clear presentation of signal. Using ImageJ software, Figures 4 and 5 were subtracted for background and were then altered in brightness and contrast to allow clear presentation of signal.
Electrophysiological recordings in BNST slices
Animals
6-12 week old male C57BL/6J mice (The Jackson Laboratory) or HA α2A-AR KI mice were housed in cages of two to five animals on a 12 h light/dark cycle with food and water ad libitum. The number of animals used in each experiment is indicated in the text and figure legends.
Brain slice preparation
Methods were as described previously (Grueter and Winder, 2005, Grueter et al., 2006b). Briefly, mice were decapitated under isofluorane anesthesia. Brains were quickly removed and placed in an ice-cold, low-sodium/high-sucrose dissecting solution. Hemisected (300 μm) coronal brain slices containing anterior portions of BNST (bregma 0.26–0.02 mm) were prepared on a Leica vibratome and transferred to either an interface chamber (field potential recordings) or a holding chamber (whole cell recordings) containing oxygenated and heated artificial cerebrospinal fluid (ACSF) composed of the following (in mM): 124 NaCl, 4.4 KCl, 2.5 CaCl2, 1.3 MgSO4, 1 NaH2PO4, 10 glucose, and 26 NaHCO3.
Field potential recordings
Field potential recordings were performed as previously reported (McElligott and Winder, 2008). Briefly, slices were transferred immediately after slicing to interface chambers where they rested for at least 30 min in a humidified and oxygenated environment while continuously being perfused with oxygenated and heated (approximately 28–30°C) ACSF at a rate of 2 ml/min. Next, 25 μM picrotoxin was added to the bath to block GABAA receptors and slices were rested another 30 min prior to recording. Picrotoxin was included during the entirety of all experiments to isolate excitatory transmission. Recording electrodes of approximately 1 MΩ resistance were pulled on a Flaming/Brown micropipette puller (Sutter Instruments, Novato, CA) and filled with ACSF. A bipolar Nichrome (A-M Systems, Carlsborg, WA) stimulating electrode was placed dorsal to the recording electrode within the dorsal lateral BNST such that stimulation of the field resulted in two distinguishable negative shifts in potential: N1 (the TTX sensitive fiber volley estimate) and N2 (CNQX sensitive synaptic response) as previously reported (Weitlauf et al., 2004, Egli et al., 2005, Grueter and Winder, 2005). The amplitude (voltage) of the N2 was measured at a stimulation intensity that resulted in a voltage approximately 50% of the maximum N2 response. Slices were stimulated at a frequency of 0.05 Hz. Field potentials were recorded using Clampex 8.2 (Molecular Devices, Sunnyvale, CA). All drugs were bath applied at their final concentrations.
Reagents used
UK-14,304 (Sigma) stock solution was made in DMSO and added directly to the ACSF.
Analysis of Field Recordings
All recorded data were analyzed via Clampfit 9.0 (Molecular Devices). All field recordings contain a 20 min baseline recording prior to agonist application (with the exception of one 10 μM UK-14,304 experiment completed in wildtype C57BL/6J, which had a 10 min baseline) and all data points were normalized to the baseline 5 min prior to the agonist application. Plotted time courses for field experiments are represented as 1 min averages. Minutes 21-25 post-drug application were also averaged for statistical analysis and comparison to baseline.
Whole-cell voltage-clamp recordings
Whole-cell recordings were performed as previously reported (Grueter and Winder, 2005, Grueter et al., 2006a, Kash and Winder, 2006). Briefly, following slicing on the vibratome, slices were allowed to recover for a minimum recovery period of 60 minutes in a submerged holding chamber (25°C) containing oxygenated ACSF. Slices were then removed from the holding chamber and placed in the recording chamber, where they were continuously perfused with oxygenated (95% O2/5% CO2) and heated (approximately 24-25°C) ACSF at a rate of 2 ml/min.
Experiments examining excitatory transmission in the BNST
Electrodes of 2.5–7.0 MΩ were filled with the following for experiments examining excitatory transmission (in mM): 117 Cs gluconate, 20 HEPES, 0.4 EGTA, 5 TEA, 2 MgCl, 4 Na2ATP, 0.3, Na2GTP (pH 7.2-7.4, Osm 290-295). Two experiments looking at the effect of 1 μM UK-14,304 were conducted using electrodes filled with (in mM): 135 K+-gluconate, 5 NaCl, 10 HEPES, 0.6 EGTA, 4 Na2ATP, 0.4 Na2GTP (pH 7.2-7.4, Osm 290-295), but no difference was noted, so results were combined. EPSCs of 100–400 pA were recorded at a frequency of 0.17 Hz while voltage-clamped at −70 mV in the presence of the GABAA receptor antagonist, picrotoxin (25 μM). After whole-cell configuration was achieved, cells were allowed to equilibrate a minimum of 10 minutes before baseline recordings were started. Postsynaptic parameters were monitored continuously throughout the duration of the experiments. Data are represented as an average of the peak amplitudes of ten sweeps (1 min). A paired-pulse ratio (PPR) was acquired by applying a second stimulus of equal intensity 50 ms after the first stimulus, where PPR = EPSC2/EPSC1.
Experiments examining inhibitory transmission in the BNST
For IPSC experiments, electrodes of 2.5–7.0 MΩ were filled with 70 K+-gluconate, 70 Cs gluconate, 1 EGTA, 5 HEPES, 4 Na2ATP, 0.3 Na2GTP (pH 7.2-7.4, Osm 290-295). IPSCs of 100-400 pA were recorded at frequency of 0.10Hz while voltage-clamped at -70 mV in the presence of 4 mM kynurenic acid to block excitatory transmission. After whole-cell configuration was achieved, cells were allowed to equilibrate a minimum of 10 min before baseline recordings were started. Postsynaptic parameters were monitored continuously throughout the duration of the experiments. Data are represented as an average of the peak amplitudes of six sweeps (1 min). A paired-pulse ratio (PPR) was acquired by applying a second stimulus of equal intensity 50 ms after the first stimulus, where PPR = EPSC2/EPSC1.
Reagents used
UK-14,304 (Sigma) stock solution was made in DMSO, guanfacine hydrochloride (Tocris) stock solution was made in water, picrotoxin (Tocris) stock solution was made in DMSO, and atipamezole hydrochloride (Pfizer Animal Health) and kynurenic acid (Sigma) were added directly to ACSF.
Analysis of whole-cell recordings
Recorded data were analyzed via Clampfit 9.2 (Molecular Devices). Recordings contain a 5-10 min baseline recording prior to agonist application, and all data points were normalized to the baseline 5 min prior to agonist application. Plotted time courses for whole-cell experiments are represented as 1 min averages. Minutes 16-20 post-drug application were also averaged for statistical analysis and comparison to baseline.
Statistics
All data points were reported as the mean +/- SEM and significance (determined by paired and unpaired Student’s t-test). Experiments examining a difference as compared to baseline were analyzed using a paired Student’s t-test. Experiments comparing relative effects of a drug across separate conditions were analyzed using an unpaired student’s t-test (Figure 1B comparing effects of UK-14,304 between HA KI and WT mice, Figure 7 comparing effects of guanfacine between excitatory and inhibitory transmission). Results are reported in the text and figure legends. Significant differences were defined as having a p < 0.05.
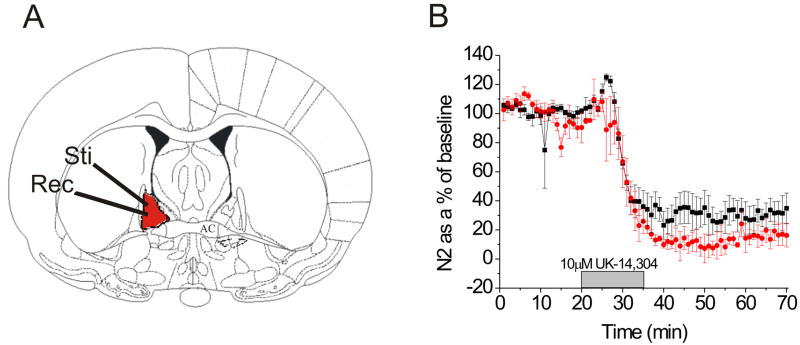
Figure 1.
The HA α2A-adrenergic receptor knockin mouse (HA α2A-AR KI) contains functional α2-ARs. A) Coronal brain section demonstrating placement of stimulating and recording electrodes for field electrophysiology experiments. B) A 10 min 10 μM UK-14,304 application causes a depression in excitatory transmission in the HA α2A-AR KI (n = 4) (red) that is not different from wildtype C57BL6/J mice (n = 4) (black).
Figure 7.
Overlay of guanfacine dose-response curves showing increased efficacy of guanfacine on excitatory transmission over inhibitory transmission.
Results
The HA α2A-AR KI mouse contains functional α2A-ARs
The HA α2A-AR KI mouse is a recently described line (Lu et al., 2009) in which an α2A-AR with an N-terminal HA tag is inserted into the endogenous allele. Lu et al. have demonstrated that the N-terminal HA tag on the α2A-AR does not appreciably alter receptor density or coupling (Lu et al., 2009). Thus this mouse affords a model for genotypically-controlled analysis of α2A-AR expression and localization.
We have previously reported that the α2-AR agonist UK-14,304 causes a depression in excitatory transmission in the BNST and that this response is absent in slices prepared from the α2A-AR KO mouse (Egli et al., 2005). To grossly assess whether α2-AR function in the BNST is normal in the knock-in line, we assessed the ability of the α2-AR agonist UK-14,304 (10 μM) to depress synaptic transmission. Using extracellularly recorded field potentials (Egli et al., 2005, Grueter and Winder, 2005, Weitlauf et al., 2005, McElligott and Winder, 2008) we show that 10 μM UK-14,304 depresses excitatory transmission in both wildtype mice (68.4 +/- 9.9% from baseline, n = 4, p < .01, t = 7.50, df = 3) and HA KI mice (90.7 +/- 5.1% from baseline, n = 4, p < .001, t = 17.47, df = 3), and that the HA KI mouse has functional α2-ARs, as the response to 10 μM UK-14,304 was not different from that observed in slices prepared from wildtype animals (p > .07, t = -2.14, df = 5) (Figure 1B).
The α2A-AR is highly expressed in the BNST
We examined the distribution of the α2A-AR in the knockin mouse at the light microscopy level utilizing an anti-HA antisera. High levels of HA labeling (presumptive α2A-AR immunoreactivity) were found in the locus coeruleus, NTS, hippocampus, and lateral septum, as expected based on previous findings (Scheinin et al., 1994, Wang et al., 1996, Lee et al., 1998, Milner et al., 1998, Glass et al., 2001) (see Figure 2A). In addition, intense labeling was observed in the BNST. The α2A-AR immunoreactivity was present throughout both the dorsal and ventral portions of the anterior BNST, with brightest labeling in the stria terminalis (Figure 2B). Wildtype tissue incubated with the mouse anti-HA antibody did not show appreciable immunolabeling (Figure 2C).
The α2A-AR selective agonist guanfacine depresses glutamatergic transmission in the dBNST
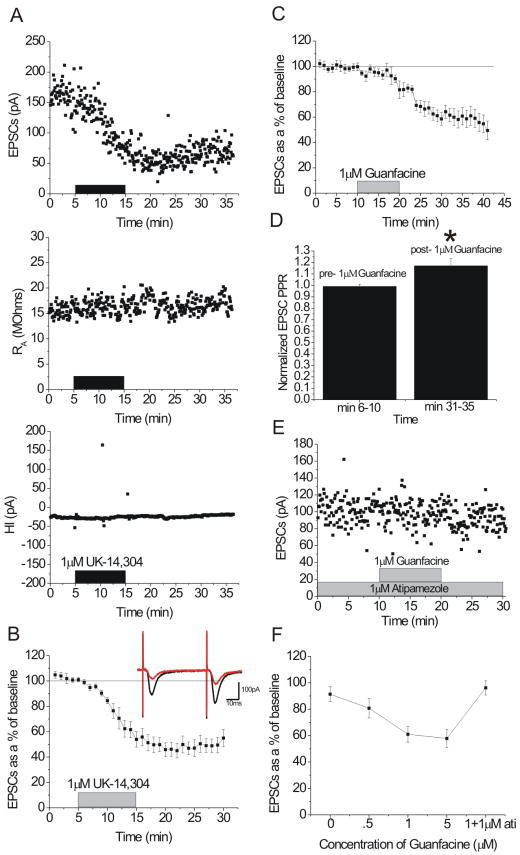
Next, we used whole cell patch clamp electrophysiology in slices prepared from wildtype mice to assess the likelihood that previously observed effects of α2-AR agonists in the BNST are mediated by the α2A-AR subtype. First, we replicated previous findings and showed that the α2-AR agonist UK-14,304 (1 μM) causes an acute depression (51.0 +/- 5.9% from baseline, n = 9, p < .0001, t = 9.55, df = 8) of excitatory transmission (Figure 3A-B). We then examined the effects of application of the α2A-AR selective agonist guanfacine. Guanfacine is an α2-AR agonist with 60-fold selectivity for the α2A-AR subtype over α2B-ARs (Uhlen and Wikberg, 1991) and 22-fold selectivity over α2C-ARs (Uhlen et al., 1992). Guanfacine elicited a concentration-dependent acute decrease in excitatory synaptic transmission in BNST (500 nM, 19.1 +/- 7.4% from baseline, n = 6, p < .05, t = 2.71, df = 5; 1 μM, 39.9 +/- 5.9% from baseline, n = 9, p < .001, t = 6.04, df = 8; 5 μM, 42.2 +/- 6.8% from baseline, n = 10, p < .001, t = 6.13, df = 9) (Figure 3C, 3F). The effect of 1 μM guanfacine was blocked by the α2-AR selective antagonist atipamezole (n = 4, p > .30, t = 1.19, df = 3) (Figure 3E-F). Thus these data further suggest a role for the α2A-AR subtype in modulation of glutamatergic transmission in the BNST.
Figure 3.
α2-AR agonists depress excitatory transmission in the dorsal BNST (dBNST). A) Representative experiment showing the effect of 1 μM UK-14,304 on excitatory transmission in the dBNST. pA = pico-amps, HI = holding current, RA = access resistance. B) 1 μM UK-14,304 depresses excitatory transmission in the dBNST (n = 9). Inset: representative 5 min average traces pre (black)- and post (red)-UK-14,304. C) 1 μM guanfacine depresses excitatory transmission in the dBNST (n = 9). D) 5 min average paired-pulse ratios pre- and post- 1 μM guanfacine. E) Representative experiment showing the effect of 1 μM guanfacine in the presence of 1 μM atipamezole in the dBNST. F) Dose-response curve for effect of guanfacine on excitatory transmission in the dBNST (n = 3 vehicle; n = 9, 500 nM; n = 9, 1 μM; n = 10, 5 μM), also effect of 1 μM guanfacine in the presence of 1 μM atipamezole (n = 4).
To begin to assess synaptic mechanisms involved in the effects elicited by guanfacine, we assessed paired-pulse responses before and after guanfacine application. Changes in the paired-pulse ratio (PPR) are indicative of alterations in the probability of transmitter release. We observed that PPR of evoked glutamate responses increased after guanfacine application (1 μM, p < .05, t = -2.55, df = 8; 500 nM, p < .01, t = -4.30, df = 5) (Figure 3D).
The α2A-AR is not distributed similarly with noradrenergic terminal markers in the BNST
We used immunohistochemistry in attempt to morphogenically localize the α2A-AR in parallel with our functional assessments. This receptor subtype has been proposed to play a role as an autoreceptor at noradrenergic synapses to modulate NE release (Altman et al., 1999, Hein et al., 1999, Stewart, 2000, Trendelenburg et al., 2001). However, the acute depression of glutamate transmission observed here would most easily be explained by a heterosynaptic role of the receptor. Alternatively, the receptor could be regulating glutamate transmission indirectly by regulating endogenous NE release. The distribution of the α2A-AR (as assessed by HA immunoreactivity in the knock-in mouse), however, was very distinct from the distribution of immunoreactivity for the NE transporter (NET) (Figure 4A-C). Additionally, the α2A-AR is much more broadly distributed throughout the BNST than tyrosine hydroxylase (TH), a marker for both dopaminergic and noradrenergic terminals (Figure 4D-F and Figure 5D-F). The broad light microscopy level distribution of α2A-AR immunoreactivity outside that of TH and NET immunoreactivity suggests that the receptor may indeed exist as a heteroreceptor on non-noradrenergic terminals.
The α2A-AR is distributed similarly to glutamatergic terminal markers in the BNST
Next, we looked at the co-distribution at the light microscopy level of the α2A-AR and immunoreactivity for the glutamatergic terminal marker vesicular glutamate transporter 1 (VGLUT1). VGLUT1 is diffusely distributed throughout the BNST in a pattern grossly similar to the α2A-AR (Figure 4G-4I, Figure 5A-C). Qualitatively, it appears that the α2A-AR colocalizes considerably with VGLUT1 (Figure 5A-C).
The α2A-AR selective agonist guanfacine depresses GABAergic transmission in the dBNST
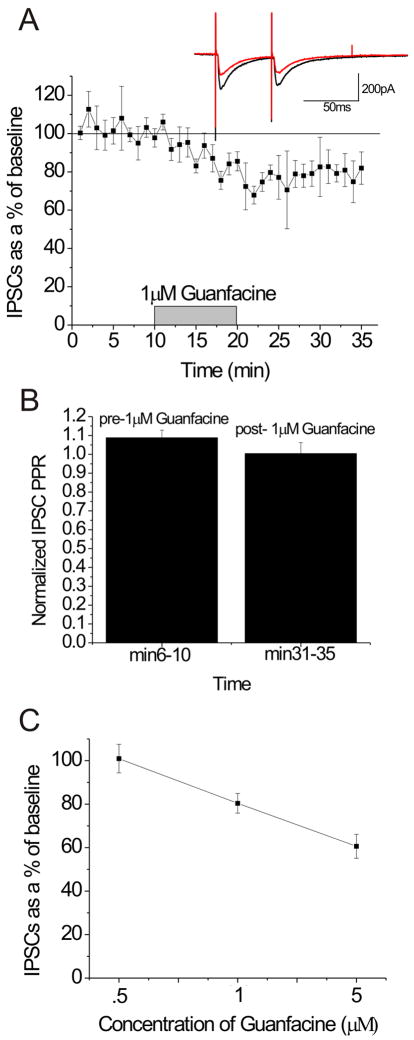
Due to the widespread expression of the α2A-AR in the BNST, we examined the ability of guanfacine to regulate fast inhibitory transmission. Here, we examined the ability of the α2A-AR specific agonist guanfacine to regulate evoked inhibitory postsynaptic currents (eISPCs) in the dBNST. We found that guanfacine elicited a concentration-dependent acute albeit modest decrease in inhibitory synaptic transmission in BNST (500 nM, 0.9 +/- 6.5% from baseline, n = 3, p > .8, t = 0.27, df = 2; 1 μM, 23.7 +/- 4.7% from baseline, n = 5, p < .01, t = 8.35, df = 4; 5 μM, 39.4 +/- 5.5% from baseline, n = 4, p < .01, t = 6.69, df = 3) (Figure 6A, 6C). Interestingly, there was no change in PPR as seen with EPSCs, with a trend for a decrease in PPR (Figure 6B).
Figure 6.
The α2A-AR agonist guanfacine depresses inhibitory transmission in the dBNST. (A) 1 μM guanfacine depresses excitatory transmission in the dBNST (n = 5) Inset: representative 5min average traces pre (black)- and post (red)- guanfacine. B) 5 min average paired-pulse ratios pre- and post- 1 μM guanfacine. C) Dose-response curve for the effect of guanfacine on inhibitory transmission in the dBNST (n = 3, 500 nM; n = 5, 1 μM; n = 4, 5 μM).
We show that guanfacine decreases both excitatory and inhibitory transmission in the dBNST. Whereas 500 nM guanfacine had a significant effect on EPSCs, it did not produce a change in IPSCs. At 1 μM guanfacine, the effect on EPSCs was significantly greater than the effect on IPSCs (p < .05, t = -2.19, df = 12) (Figure 7). Therefore, under our recording conditions, it is possible that activation of the α2A-AR produces greater changes at excitatory synapses than inhibitory synapses, thereby causing an overall reduction in BNST excitability.
Discussion
The HA α2A-AR KI mouse contains normally distributed and functional α2A-ARs in the mouse brain
The HA α2A-AR KI mouse contains a 9 amino acid N-terminal hemagglutinin tag on the α2A-AR (Lu et al., 2009). This tag does not grossly alter function of the α2A-AR in vitro, and the receptor is grossly normally distributed in the mouse brain (Lu et al., 2009). A parasaggital section of HA α2A-AR KI mouse brain demonstrates high levels of α2A-AR in the LC, NTS, hippocampus, and stria terminalis, as expected based on previous studies (Scheinin et al., 1994, Wang et al., 1996, Lee et al., 1998, Milner et al., 1998, Glass et al., 2001). Moreover, our data suggest that these receptors remain functional in the BNST, consistent with a previous report on this mouse (Lu et al., 2009).
The HA α2A-AR KI mouse provides a unique method to specifically label α2A-ARs in brain tissue
HA is an exogenous sequence not found in normal brain tissue, and thus should not cross-react with other receptors or proteins in the brain. As shown in Figure 2B-2C, mouse anti-HA specifically labels the HA presented on the HA-tagged α2A-AR and does not show appreciable immunolabeling in the WT mouse. Previous studies examining localization of the α2A-AR in rodent brain tissue have used antibodies targeting various portions of the α2A-AR. Studies conducted in the NTS by Glass and others took advantage of a goat anti-α2A-AR from Santa Cruz (Glass et al., 2001), which is an antibody against the C-terminus of the receptor. Additionally, Milner and others have published studies in the hippocampus and ventrolateral medulla using an antibody developed against a 47 amino acid chain of the 3rd intracellular loop of the rat α2A-AR fused to GST (Milner et al., 1998, Milner et al., 1999). Our data confirm aspects of the previous studies, demonstrating presence of the α2A-AR in VGLUT1-(+) and TH-(+) compartments within the BNST.
The α2A-AR selective agonist guanfacine depresses excitatory transmission in the BNST
Using in vivo microdialysis techniques, Forray and others have shown that in the BNST, the α2-AR agonist UK-14,304 depresses extracellular glutamate and K+-induced glutamate release, and that the α2-AR antagonist RX821002 has opposite effects (Forray et al., 1999). We previously reported, using field and whole-cell electrophysiology approaches, that the α2-AR agonist UK-14,304 depresses glutamatergic transmission in the BNST (Egli et al., 2005). This effect is absent in the α2A-AR knockout mouse (Egli et al., 2005), implicating the α2A AR subtype as potentially responsible for this depression of glutamatergic transmission. It is also possible though, that multiple ARs in the α2A-AR knockout mouse are desensitized due to high levels of extracellular NE in this knockout mouse, as α1-AR-mediated LTD is also absent in these mice (McElligott and Winder, 2008). Therefore, we cannot rule out that the α2C-AR contributes to some effects seen with UK-14,304 (Trendelenburg et al., 1999).
Using whole-cell electrophysiology to explore the specific role of the α2A-AR in modulation of glutamate transmission in the BNST, we applied the α2A-AR selective agonist guanfacine to cells in the dBNST. We found that guanfacine depresses glutamatergic transmission in a dose-dependent manner, and that this effect can be blocked with the α2-AR selective antagonist atipamizole. Thus, in total these data strongly suggest that activation of the α2A-AR acutely depresses glutamate release in the BNST.
α2A-ARs depress excitatory transmission by presynaptically modulating glutamate release
The α2A-AR is discussed as an autoreceptor modulating NE release (Altman et al., 1999, Hein et al., 1999, Stewart, 2000, Trendelenburg et al., 2001), and it is conceivable that effects on glutamate transmission are the result of an α2A-AR-mediated altered release of NE. To examine this possibility, we used immunohistochemistry to co-label the HA-tagged α2A-AR and markers of noradrenergic terminals, NET and TH. Interestingly, the α2A-AR is much more broadly distributed than either NET or TH in the BNST, making modulation of NE release by the α2A-AR unlikely to provide the sole, or perhaps even major, explanation for the acute effects seen with application of guanfacine. In fact, the α2A-AR has a very similar distribution to the glutamatergic terminal marker, VGLUT1. In electrophysiological experiments we observed an increase in the paired pulse ratio (PPR) upon application of guanfacine, thus suggesting that the α2A-AR regulates glutamate transmission by decreasing glutamate release probability.
Other areas of the extended amygdala, such as the central nucleus of the amygdala (CeA), also show heterosynaptic regulation of glutamate transmission by α2-ARs, but via a distinct mechanism involving direct interaction of the βγ subunit of the G-protein coupled receptor complex with release machinery (Delaney et al., 2007). In contrast, α2A-AR mediated depression of excitatory transmission in the prefrontal cortex has been theorized to be postsynaptic due to an absence of effect on PPR, although regulation similar to that seen in the CeA cannot be ruled out based on current findings (Ji et al., 2008). The α2A-ARs are also known to presynaptically modulate L-type calcium channels in the retina (Dong et al., 2007). In other areas including the NTS (Glass et al., 2001), ventrolateral medulla (Milner et al., 1999), and hippocampus (Milner et al., 1998), evidence consistent with expression of heterosynaptic presynaptic α2A-ARs has been reported. Using a combination of various techniques, we demonstrate that the α2A-AR is also located heterosynaptically on glutamate terminals and modulates excitatory transmission in the BNST by presynaptically altering glutamate release, thus adding to findings demonstrating the complex role of the α2A-AR in modulation of transmission in the brain. Our findings are consistent with the proposal by Gilsbach and others that many α2A-AR-mediated modulations of function in the brain are via α2A-AR heteroreceptors, not autoreceptors (Gilsbach et al., 2009).
α2A-ARs modulate GABAergic transmission in the BNST
NE is known to modulate both inhibitory and excitatory transmission in the BNST. For example, activation of the α1-AR causes LTD at excitatory synapses (McElligott and Winder, 2008), and also increases IPSC frequency at inhibitory synapses that are shown to project to the VTA via a retrograde label (Dumont and Williams, 2004). Therefore, we must consider the possibility that α2A-ARs regulate inhibitory transmission in the BNST in addition to their effects on excitatory transmission. Dumont and Williams saw no effect of the α2-AR agonist clonidine or the α2-AR antagonist yohimbine on spontaneous IPSCs in the ventral BNST (Dumont and Williams, 2004). Also, Forray and others saw no effect of the α2-AR agonist UK-14,304 on GABA basal extracellular levels using in vivo microdialysis approaches in the BNST (Forray et al., 1999). Here, however, we show that the α2A-AR selective agonist depresses inhibitory transmission in the BNST in a dose-dependent manner, but does not alter PPR. These data suggest that the α2A-AR may modulate inhibitory transmission in the BNST through a postsynaptic mechanism, although we cannot rule out that there is some direct interaction between the α2A-AR and release machinery as seen with excitatory transmission in the CeA (Dumont and Williams, 2004). Also, at present we cannot rule out the possibility that the greater effect of guanfacine on eIPSCs at higher concentrations is partially due to recruitment of α2C-ARs in addition to α2A-ARs.
α2A-ARs gate excitatory drive to the BNST, and regulate inhibitory transmission in the BNST via distinct mechanisms
Taken together, our data indicate that the α2A-AR plays a particularly important role in modulation of BNST output. This is not the first instance in which α2A-ARs have been suggested to be differentially localized within a region. In the NTS, ventrolateral medulla, and hippocampus, α2A-AR-like immunoreactivity has been noted on presynaptic autoreceptor and heterosynaptic terminals, as well as postsynaptically, and on glial cells (Milner et al., 1998, Milner et al., 1999, Glass et al., 2001). Here, we find that the α2A-AR is broadly associated with at least two compartments, TH(+) and VGLUT(+) terminals.
Based on current findings, guanfacine appears to have a higher potency at excitatory than inhibitory synapses. Excitatory input into the BNST comes from insular cortex, infralimbic cortex, the ventral subiculum and the basolateral amygdala (Weller and Smith, 1982, Dong et al., 2001, Massi et al., 2008), other regions known to be involved in stress-reward circuitry, and regions known to express mRNA for the α2A-AR (Scheinin et al., 1994, Wang et al., 1996). Thus, the α2A-AR potentially gates these inputs, effectively decreasing excitatory drive to the BNST during events known to release NE, such as chronic stressors, withdrawal from drugs of abuse, and other social stressors (Aston-Jones et al., 1999, Delfs et al., 2000, Cecchi et al., 2002a, Cecchi et al., 2002b, Fendt et al., 2005).
The main inhibitory projection to the BNST is from a closely related structure, the central nucleus of the amygdala (CeA), a region also involved in fear and anxiety states. Although the BNST and CeA are very homologous structures with similar afferents and efferents, it is hypothesized that the BNST is involved in unconditioned prolonged anxiety states (Walker et al., 2003) whereas the CeA is involved in conditioned fear to distinct sensory cues (Kim and Davis, 1993, Campeau and Davis, 1995, Wilensky et al., 2006). Others, however, have considered that these two structures have more anatomical similarity than differences and suggested they be grouped together as the extended amygdala (Hopkins and Holstege, 1978, Veening et al., 1984, Holstege et al., 1985, Dong et al., 2000, Dong and Swanson, 2004b, a). Therefore, the activation of α2A-ARs may modulate functional signaling between or within these two parts of the extended amygdala.
Additionally, the BNST is made of a large network of GABAergic interneurons. Thus, modulation of inhibitory transmission by activation of α2-ARs may also influence output of the BNST. For example, the BNST is thought to contain an excitatory projection to the VTA (Georges and Aston-Jones, 2002, Massi et al., 2008), and Dumont and others’ work has suggested that NE triggers GABAA-mediated inhibition of these fibers (Dumont and Williams, 2004). Here, we have demonstrated that α2-ARs do modulate inhibitory transmission in this region.
α2A-AR knockout mice have been shown to have enhanced anxiety and depression-related phenotypes (Schramm et al., 2001), leading to the hypothesis that the α2A-AR may play a protective role for an animal under stressful conditions. Studies demonstrate that α2-AR activation in the BNST mediates inhibition of stress-induced reinstatement to various drugs of abuse (Delfs et al., 2000, Wang et al., 2001) and inhibition of stress-induced behavior by predator odor (Fendt et al., 2005). These findings support a stress-protective function of α2A-ARs in the BNST. Thus, activation of the α2A-AR may in fact serve to lessen the effects of stress by dampening signaling from inputs to the BNST such as the infralimbic cortex, insular cortex, and BLA, regions known to be activated under stressful events such as withdrawal from drugs of abuse.
The BNST may play a role in the therapeutic effects of guanfacine on anxiety-related disorders
Clinical data indicate that stress is a common reason patients relapse to using drugs of abuse (Sinha et al., 1999). Finding therapeutic agents to help addicts with anxiety and craving are extremely limited at this time. Guanfacine, an α2A-AR selective agonist, is FDA-approved for treatment of some anxiety disorders including post-traumatic stress disorder (PTSD) and chronic Tic disorder. Guanfacine is also prescribed as a centrally-acting hypertensive agent to reduce blood pressure in patients struggling with hypertension (Kisicki et al., 2007), and is used for treatment of patients suffering from schizophrenia (McClure et al., 2007). Guanfacine is currently undergoing clinical trial for its role in preventing stress-induced relapse to cocaine, smoking, and alcohol use (Clinicaltrials.gov). Additional clinical trials looking at its efficacy in treating PTSD, ADHD, and schizophrenia are also underway (Posey and McDougle, 2007).
Guanfacine is a partial agonist at the α2A-AR, and data suggest that partial agonism at this receptor subtype results in fewer unwanted side effects such as sedation in the potential treatment of anxiety disorders (Tan et al., 2002). Additionally, in disorders such as addiction, PTSD, or ADHD, where chronic therapeutics are needed, an agonist that does not readily induce desensitization of its target may be beneficial. Guanfacine desensitizes α2A-ARs much less readily than clonidine, likely explaining guanfacine’s longer duration of action clinically despite the two partial agonists having a similar half-life (Lu et al., 2009). Thus, guanfacine is a promising potential therapy for anxiety disorders and relapse in addiction. We theorize that the effects of guanfacine on α2A-ARs in the BNST are partially responsible for its effectiveness as a therapeutic agent in anxiety- and addiction-related behaviors.
Acknowledgments
This work was supported by NIDA and NIAAA grant VA019112, and NIH grants HLZ2518Z and DK43852. We thank Bob Matthews and Lee Limbird for their help in reviewing the manuscript. We thank Dr. Sam Wells and the staff from the Vanderbilt Cell Imaging Shared Resource center for their expertise and instruction in confocal microscopy. We also thank Dr. Randy Blakely for the NET KO mice and NET antibody, and the Vanderbilt Medical Scientist training Program for their ongoing support.
List of Abbreviations
- α1-AR
α1-adrenergic receptor
- α2-AR
α2-adrenergic receptor
- α2A-AR
α2A-adrenergic receptor
- α2B-AR
α2B-adrenergic receptor
- α2C-AR
α2C-adrenergic receptor
- ACSF
artificial cerebral spinal fluid
- ADHD
attention-deficit hyperactivity disorder
- β-AR
β -adrenergic receptor
- BNST
bed nucleus stria terminalis
- CeA
central nucleus of the amygdala
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- CPP
conditioned place preference
- CRF
corticotrophin releasing factor
- dBNST
dorsal BNST
- HA
hemagglutinin
- KI
knock in
- KO
knock out
- LTD
long term depression
- NE
norepinephrine
- NET
norepinephrine transporter
- NTS
nucleus tractus solitarus
- PPR
paired-pulse ratio
- PTSD
post-traumatic stress disorder
- VNAB
ventral noradrenergic bundle
- TH
tyrosine hydroxylase
- TTX
tetrodotoxin
- VGLUT1
vesicular glutamate transporter 1
- VTA
ventral tegmental area
- WT
wildtype
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman JD, Trendelenburg AU, MacMillan L, Bernstein D, Limbird L, Starke K, Kobilka BK, Hein L. Abnormal regulation of the sympathetic nervous system in alpha2A-adrenergic receptor knockout mice. Mol Pharmacol. 1999;56:154–161. doi: 10.1124/mol.56.1.154. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann N Y Acad Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. J Stud Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- Brownstein M, Saavedra JM, Palkovits M. Norepinephrine and dopamine in the limbic system of the rat. Brain Res. 1974;79:431–436. doi: 10.1016/0006-8993(74)90440-5. [DOI] [PubMed] [Google Scholar]
- Bylund DB, Eikenberg DC, Hieble JP, Langer SZ, Lefkowitz RJ, Minneman KP, Molinoff PB, Ruffolo RR, Jr, Trendelenburg U. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15:2301–2311. doi: 10.1523/JNEUROSCI.15-03-02301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casada JH, Dafny N. Responses of neurons in bed nucleus of the stria terminalis to microiontophoretically applied morphine, norepinephrine and acetylcholine. Neuropharmacology. 1993;32:279–284. doi: 10.1016/0028-3908(93)90112-g. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002a;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Morilak DA. Modulatory effects of norepinephrine, acting on alpha 1 receptors in the central nucleus of the amygdala, on behavioral and neuroendocrine responses to acute immobilization stress. Neuropharmacology. 2002b;43:1139–1147. doi: 10.1016/s0028-3908(02)00292-7. [DOI] [PubMed] [Google Scholar]
- Chiba T, Kayahara T, Nakano K. Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain Res. 2001;888:83–101. doi: 10.1016/s0006-8993(00)03013-4. [DOI] [PubMed] [Google Scholar]
- Clinicaltrials.gov. [internet] Bethesda, MD: National Library of Medicine (US); January 1993-updated May 18, 2008; cited April 16, 2009 Available from: www.clinicaltrials.gov/ct2/resutls?term=guanfacine. [Google Scholar]
- Conrad LC, Pfaff DW. Efferents from medial basal forebrain and hypothalamus in the rat. I. An autoradiographic study of the medial preoptic area. J Comp Neurol. 1976;169:185–219. doi: 10.1002/cne.901690205. [DOI] [PubMed] [Google Scholar]
- Delaney AJ, Crane JW, Sah P. Noradrenaline modulates transmission at a central synapse by a presynaptic mechanism. Neuron. 2007;56:880–892. doi: 10.1016/j.neuron.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Dong CJ, Guo Y, Wheeler L, Hare WA. Alpha2 adrenergic receptor-mediated modulation of cytosolic Ca++ signals at the inner plexiform layer of the rat retina. Invest Ophthalmol Vis Sci. 2007;48:1410–1415. doi: 10.1167/iovs.06-0890. [DOI] [PubMed] [Google Scholar]
- Dong H, Petrovich GD, Swanson LW. Organization of projections from the juxtacapsular nucleus of the BST: a PHAL study in the rat. Brain Res. 2000;859:1–14. doi: 10.1016/s0006-8993(99)02246-5. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol. 2004a;468:277–298. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol. 2004b;471:396–433. doi: 10.1002/cne.20002. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Williams JT. Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci. 2004;24:8198–8204. doi: 10.1523/JNEUROSCI.0425-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli RE, Kash TL, Choo K, Savchenko V, Matthews RT, Blakely RD, Winder DG. Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2005;30:657–668. doi: 10.1038/sj.npp.1300639. [DOI] [PubMed] [Google Scholar]
- Fendt M, Siegl S, Steiniger-Brach B. Noradrenaline transmission within the ventral bed nucleus of the stria terminalis is critical for fear behavior induced by trimethylthiazoline, a component of fox odor. J Neurosci. 2005;25:5998–6004. doi: 10.1523/JNEUROSCI.1028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forray MI, Bustos G, Gysling K. Regulation of norepinephrine release from the rat bed nucleus of the stria terminalis: in vivo microdialysis studies. J Neurosci Res. 1997;50:1040–1046. doi: 10.1002/(SICI)1097-4547(19971215)50:6<1040::AID-JNR15>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Forray MI, Bustos G, Gysling K. Noradrenaline inhibits glutamate release in the rat bed nucleus of the stria terminalis: in vivo microdialysis studies. J Neurosci Res. 1999;55:311–320. doi: 10.1002/(SICI)1097-4547(19990201)55:3<311::AID-JNR6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Fuentealba JA, Forray MI, Gysling K. Chronic morphine treatment and withdrawal increase extracellular levels of norepinephrine in the rat bed nucleus of the stria terminalis. J Neurochem. 2000;75:741–748. doi: 10.1046/j.1471-4159.2000.0750741.x. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsbach R, Roeser C, Beetz N, Brede M, Hadamek K, Haubold M, Leemhuis J, Philipp M, Schneider J, Urbanski M, Szabo B, Weinshenker D, Hein L. Genetic dissection of {alpha}2-adrenoceptor functions in adrenergic versus non-adrenergic cells. Mol Pharmacol. 2009 doi: 10.1124/mol.109.054544. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Huang J, Aicher SA, Milner TA, Pickel VM. Subcellular localization of alpha-2A-adrenergic receptors in the rat medial nucleus tractus solitarius: regional targeting and relationship with catecholamine neurons. J Comp Neurol. 2001;433:193–207. doi: 10.1002/cne.1135. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Gosnell HB, Olsen CM, Schramm-Sapyta NL, Nekrasova T, Landreth GE, Winder DG. Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed nucleus of the stria terminalis is disrupted by cocaine administration. J Neurosci. 2006a;26:3210–3219. doi: 10.1523/JNEUROSCI.0170-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Winder DG. Group II and III metabotropic glutamate receptors suppress excitatory synaptic transmission in the dorsolateral bed nucleus of the stria terminalis. Neuropsychopharmacology. 2005;30:1302–1311. doi: 10.1038/sj.npp.1300672. [DOI] [PubMed] [Google Scholar]
- Grueter CE, Abiria SA, Dzhura I, Wu Y, Ham AJ, Mohler PJ, Anderson ME, Colbran RJ. L-type Ca2+ channel facilitation mediated by phosphorylation of the beta subunit by CaMKII. Mol Cell. 2006b;23:641–650. doi: 10.1016/j.molcel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Hein L, Altman JD, Kobilka BK. Two functionally distinct alpha2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402:181–184. doi: 10.1038/46040. [DOI] [PubMed] [Google Scholar]
- Holstege G, Meiners L, Tan K. Projections of the bed nucleus of the stria terminalis to the mesencephalon, pons, and medulla oblongata in the cat. Exp Brain Res. 1985;58:379–391. doi: 10.1007/BF00235319. [DOI] [PubMed] [Google Scholar]
- Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res. 1978;32:529–547. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- Ji XH, Ji JZ, Zhang H, Li BM. Stimulation of alpha2-adrenoceptors suppresses excitatory synaptic transmission in the medial prefrontal cortex of rat. Neuropsychopharmacology. 2008;33:2263–2271. doi: 10.1038/sj.npp.1301603. [DOI] [PubMed] [Google Scholar]
- Kash TL, Winder DG. Neuropeptide Y and corticotropin-releasing factor bi-directionally modulate inhibitory synaptic transmission in the bed nucleus of the stria terminalis. Neuropharmacology. 2006;51:1013–1022. doi: 10.1016/j.neuropharm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Kim M, Davis M. Electrolytic lesions of the amygdala block acquisition and expression of fear-potentiated startle even with extensive training but do not prevent reacquisition. Behav Neurosci. 1993;107:580–595. doi: 10.1037//0735-7044.107.4.580. [DOI] [PubMed] [Google Scholar]
- Kisicki JC, Fiske K, Lyne A. Phase I, double-blind, randomized, placebo-controlled, dose-escalation study of the effects on blood pressure of abrupt cessation versus taper down of guanfacine extended-release tablets in adults aged 19 to 24 years. Clin Ther. 2007;29:1967–1979. doi: 10.1016/j.clinthera.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Lee A, Rosin DL, Van Bockstaele EJ. alpha2A-adrenergic receptors in the rat nucleus locus coeruleus: subcellular localization in catecholaminergic dendrites, astrocytes, and presynaptic axon terminals. Brain Res. 1998;795:157–169. doi: 10.1016/s0006-8993(98)00266-2. [DOI] [PubMed] [Google Scholar]
- Lu R, Li Y, Zhang Y, Chen Y, Shields AD, Winder DG, Angelotti T, Jiao K, Limbird LE, Zhou Y, Wang Q. Epitope-tagged receptor knock-in mice reveal that differential desensitization of alpha2-adrenergic responses is due to ligand-selective internalization. J Biol Chem. 2009 doi: 10.1074/jbc.M807535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massi L, Elezgarai I, Puente N, Reguero L, Grandes P, Manzoni OJ, Georges F. Cannabinoid receptors in the bed nucleus of the stria terminalis control cortical excitation of midbrain dopamine cells in vivo. J Neurosci. 2008;28:10496–10508. doi: 10.1523/JNEUROSCI.2291-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H, Yamamoto C. The possible involvement of adenylate cyclase inhibition in the field potential suppression through alpha-2 adrenergic receptors in the bed nucleus of the stria terminalis. Brain Res. 1984;293:187–190. doi: 10.1016/0006-8993(84)91469-0. [DOI] [PubMed] [Google Scholar]
- McClure MM, Barch DM, Romero MJ, Minzenberg MJ, Triebwasser J, Harvey PD, Siever LJ. The effects of guanfacine on context processing abnormalities in schizotypal personality disorder. Biol Psychiatry. 2007;61:1157–1160. doi: 10.1016/j.biopsych.2006.06.034. [DOI] [PubMed] [Google Scholar]
- McElligott ZA, Winder DG. Alpha1-adrenergic receptor-induced heterosynaptic long-term depression in the bed nucleus of the stria terminalis is disrupted in mouse models of affective disorders. Neuropsychopharmacology. 2008;33:2313–2323. doi: 10.1038/sj.npp.1301635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Lee A, Aicher SA, Rosin DL. Hippocampal alpha2a-adrenergic receptors are located predominantly presynaptically but are also found postsynaptically and in selective astrocytes. J Comp Neurol. 1998;395:310–327. [PubMed] [Google Scholar]
- Milner TA, Rosin DL, Lee A, Aicher SA. Alpha2A-adrenergic receptors are primarily presynaptic heteroreceptors in the C1 area of the rat rostral ventrolateral medulla. Brain Res. 1999;821:200–211. doi: 10.1016/s0006-8993(98)00725-2. [DOI] [PubMed] [Google Scholar]
- Olson VG, Heusner CL, Bland RJ, During MJ, Weinshenker D, Palmiter RD. Role of noradrenergic signaling by the nucleus tractus solitarius in mediating opiate reward. Science. 2006;311:1017–1020. doi: 10.1126/science.1119311. [DOI] [PubMed] [Google Scholar]
- Posey DJ, McDougle CJ. Guanfacine and guanfacine extended release: treatment for ADHD and related disorders. CNS Drug Rev. 2007;13:465–474. doi: 10.1111/j.1527-3458.2007.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinin M, Lomasney JW, Hayden-Hixson DM, Schambra UB, Caron MG, Lefkowitz RJ, Fremeau RT., Jr Distribution of alpha 2-adrenergic receptor subtype gene expression in rat brain. Brain Res Mol Brain Res. 1994;21:133–149. doi: 10.1016/0169-328x(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Schramm NL, McDonald MP, Limbird LE. The alpha(2a)-adrenergic receptor plays a protective role in mouse behavioral models of depression and anxiety. J Neurosci. 2001;21:4875–4882. doi: 10.1523/JNEUROSCI.21-13-04875.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000a;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2000b;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology (Berl) 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- Shin JW, Geerling JC, Loewy AD. Inputs to the ventrolateral bed nucleus of the stria terminalis. J Comp Neurol. 2008;511:628–657. doi: 10.1002/cne.21870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. The connections of the septal region in the rat. J Comp Neurol. 1979;186:621–655. doi: 10.1002/cne.901860408. [DOI] [PubMed] [Google Scholar]
- Tan CM, Wilson MH, MacMillan LB, Kobilka BK, Limbird LE. Heterozygous alpha 2A-adrenergic receptor mice unveil unique therapeutic benefits of partial agonists. Proc Natl Acad Sci U S A. 2002;99:12471–12476. doi: 10.1073/pnas.122368499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trendelenburg AU, Hein L, Gaiser EG, Starke K. Occurrence, pharmacology and function of presynaptic alpha2-autoreceptors in alpha2A/D-adrenoceptor-deficient mice. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:540–551. doi: 10.1007/s002109900093. [DOI] [PubMed] [Google Scholar]
- Trendelenburg AU, Klebroff W, Hein L, Starke K. A study of presynaptic alpha2-autoreceptors in alpha2A/D-, alpha2B- and alpha2C-adrenoceptor-deficient mice. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:117–130. doi: 10.1007/s002100100423. [DOI] [PubMed] [Google Scholar]
- Uhlen S, Wikberg JE. Delineation of rat kidney alpha 2A- and alpha 2B-adrenoceptors with [3H]RX821002 radioligand binding: computer modelling reveals that guanfacine is an alpha 2A-selective compound. Eur J Pharmacol. 1991;202:235–243. doi: 10.1016/0014-2999(91)90299-6. [DOI] [PubMed] [Google Scholar]
- Uhlen S, Xia Y, Chhajlani V, Felder CC, Wikberg JE. [3H]-MK 912 binding delineates two alpha 2-adrenoceptor subtypes in rat CNS one of which is identical with the cloned pA2d alpha 2-adrenoceptor. Br J Pharmacol. 1992;106:986–995. doi: 10.1111/j.1476-5381.1992.tb14446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res. 1984;303:337–357. doi: 10.1016/0006-8993(84)91220-4. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Wang R, Macmillan LB, Fremeau RT, Jr, Magnuson MA, Lindner J, Limbird LE. Expression of alpha 2-adrenergic receptor subtypes in the mouse brain: evaluation of spatial and temporal information imparted by 3 kb of 5’ regulatory sequence for the alpha 2A AR-receptor gene in transgenic animals. Neuroscience. 1996;74:199–218. doi: 10.1016/0306-4522(96)00116-9. [DOI] [PubMed] [Google Scholar]
- Wang X, Cen X, Lu L. Noradrenaline in the bed nucleus of the stria terminalis is critical for stress-induced reactivation of morphine-conditioned place preference in rats. Eur J Pharmacol. 2001;432:153–161. doi: 10.1016/s0014-2999(01)01487-x. [DOI] [PubMed] [Google Scholar]
- Weitlauf C, Egli RE, Grueter BA, Winder DG. High-frequency stimulation induces ethanol-sensitive long-term potentiation at glutamatergic synapses in the dorsolateral bed nucleus of the stria terminalis. J Neurosci. 2004;24:5741–5747. doi: 10.1523/JNEUROSCI.1181-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitlauf C, Honse Y, Auberson YP, Mishina M, Lovinger DM, Winder DG. Activation of NR2A-containing NMDA receptors is not obligatory for NMDA receptor-dependent long-term potentiation. J Neurosci. 2005;25:8386–8390. doi: 10.1523/JNEUROSCI.2388-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller KL, Smith DA. Afferent connections to the bed nucleus of the stria terminalis. Brain Res. 1982;232:255–270. doi: 10.1016/0006-8993(82)90272-4. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]